Abstract
Aspirin-exacerbated respiratory disease (AERD) is explained in part by over-expression of pro-inflammatory mediators including 5-lipoxygenase and leukotriene C4 synthase (LTC4S) that results in constitutive over-production of cysteinyl leukotrienes (CysLTs). Mast cells and eosinophils are two cell types that have important roles in mediating many of the effects observed in this disease. Increased levels of both interleukin (IL-4) and interferon (IFN)-γ are present in the tissue of AERD subjects. Previous studies demonstrated that IL-4 is primarily responsible for the upregulation of LTC4S by mast cells. Our studies demonstrate that IFN-γ, but not IL-4 drives this process in eosinophils. We also extend to both IL-4 and IFN-γ the ability to upregulate CysLT receptors. Prostaglandin E2 (PGE2) acts to prevent CysLT secretion by inhibiting mast cell and eosinophil activation. PGE2 concentrations are reduced in AERD and studies confirm that this reflects diminished expression of cyclooxygenase (COX)-2, a process again that is driven by IL-4. Thus, IL-4 and IFN-γ acting on eosinophils and mast cells together play an important pathogenic role in generating the phenotype of AERD. This review will examine the overall role that eosinophils and mast cells contribute to the pathophysiology of AERD.
Keywords: eosinophil, mast cell, leukotriene, cyclooxygenase, prostaglandin, aspirin-exacerbated respiratory disease, arachidonic acid
Introduction
In 1968, the term Samter’s triad was coined and was defined by the presence of nasal polyps, aspirin sensitivity, and asthma 1, however this disease is now referred to as aspirin-exacerbated respiratory disease (AERD) as asthma is not always present despite reactions to aspirin. AERD comprises as many as 7% of adult-onset asthmatics and up to 12–14% of adult asthmatics with severe asthma 2, 3. This disorder is characterized by the unique intolerance to aspirin and other non-selective cyclooxygenase (COX) inhibitors 4–6. Other characteristics include hypereosinophilia, both in circulation and in the tissue, a tendency to develop de novo in adulthood 5, 7–9 and often an absence of identifiable atopy 5, 7. Sinusitis is present in this disorder, the degree of which is often severe and associated with complete or near complete opacification of the sinus cavity 9. Though not a requirement, when asthma is present it often progresses in severity and is associated with aggressive airway remodeling 10.
During aspirin reactions, many mediators are released including cysteinyl leukotrienes (CysLT), tryptase, eosinophil cationic protein (ECP) and prostaglandin D2 (PGD2) suggesting both mast cell and eosinophil activation 11–13. Recently, aspirin was shown to directly activate both of these cell types ex vivo potentiating mediator release 14. A predominant physiological feature of AERD is the robust over-production and over-responsiveness to CysLTs (inflammatory) 12, 15 while at the same time there is under-production and under-responsiveness to the anti-inflammatory lipid mediator PGE2 16–18. These CysLTs have important pro-inflammatory and pro-fibrotic effects that contribute to the asthma severity and to the extensive hyperplastic sinusitis and nasal polyposis 9, 19, 20. And, conversely, the down-regulation of PGE2 pathways reduces the constraints that would normally act to attenuate these pro-inflammatory pathways 21. This review will focus on the role that eosinophils and mast cells play in contributing to these cardinal features of AERD.
Eosinophil and mast cell numbers in AERD
Chronic sinusitis is now recognized as a collection of disorders that result from inflammation of the sinuses and in many cases can be separated into different types based on the cellular infiltrate. One distinguishing feature in the nasal polyps that often form in association with chronic sinusitis is the presence or absence of eosinophils and amongst eosinophilic polyps a distinction can be made between AERD, allergic fungal sinusitis (AFS) and chronic hyperplastic eosinophilic sinusitis (CHES) 22, 23. Within the eosinophilic polyps, AERD has more than twice the number of eosinophils in the polyp tissue than AFS or CHES, implicating them as important cells in the disease process 23. Examination of bronchial biopsies from AERD subjects also revealed highly elevated eosinophil numbers in comparison to aspirin-tolerant asthmatics and non-asthmatics 24. These eosinophils were in an activated state as evidenced by the presence of secretory ECP 24.
We have reported lower numbers of mast cells in nasal polyps from eosinophilic sinus disease as compared to healthy tissue by both toluidine blue and chloroacetate (chymase) staining, and there was no difference between aspirin tolerant and AERD groups 23. This contrasts somewhat with a previous report that found no difference in mast cell numbers in nasal polyps from AERD groups when compared to allergic or non-allergic subjects via tryptase staining 25. The differences in the results of these studies may reflect the use of different markers of mast cells (chymase vs. tryptase) or the stratification of the groups, the latter study not taking into account eosinophilic infiltration into the polyp tissue. Regardless, there do not appear to be more mast cells in nasal polyps in subjects with AERD. However, this result may be erroneous – given the high expression of mast cell-derived mediators – and perhaps reflects the inability to stain for activated, granule-depleted mast cells (so-called “phantom” mast cells). Similarly, when the lungs have been examined, as with NPs, fewer numbers of mast cells have been found in AERD subjects compared to non-asthmatic controls using immunohistochemistry to stain for tryptase positive cells 24. Another study examining the bronchial mucosa found increased numbers of tryptase-positive mast cells only in subjects with non-aspirin sensitive asthma: again, AERD and healthy controls paradoxically had similar numbers 26.
Development of Eosinophils and Mast Cells
Eosinophils develop from pluripotent hematopoietic stem cells in bone marrow that initially differentiate into eosinophil/basophil progenitors or colony forming units (Eo/B CFU) (Figure 1). Eo/B CFU are mononuclear cells that express CD34, CD35, and interleukin (IL)-5 receptors (CD125) that are capable of responding to appropriate cytokine signals allowing differentiation into mature basophils and eosinophils 27, 28. Eo/B CFU are increased in numbers in both the blood and bone marrow of allergic patients and further increases in their number are observed following allergen exposure 28. These progenitors are also observed in nasal polyp tissue 29. Several transcription factors including GATA-1, PU.1 and C/EBP are induced in response to appropriate cytokine signals and become involved in the development of the eosinophil lineage and eosinophil-associated genes 30–32. In vitro eosinophil differentiation experiments have demonstrated that GATA-1 is the primary transcription factor responsible for this eosinophil lineage specification 33.
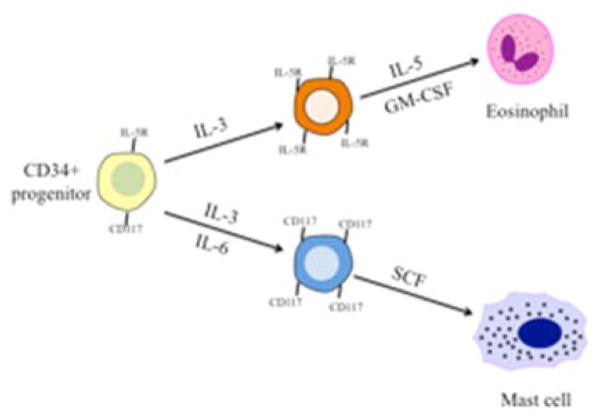
Figure 1. Eosinophil and mast cell development from CD34 progenitor cell.
Through the actions of IL-3, IL-5 and GM-CSF, CD34 progenitor cells mature into eosinophils. Mast cells develop following stimulation with IL-3, IL-6 and SCF from CD34 progenitor cells.
Three cytokines, IL-3, IL-5 and granulocyte macrophage colony-stimulating factor (GM-CSF) play the most important roles in the regulation of eosinophil development (Figure 1). The function of IL-3 is the broadest as it leads to the expansion of a variety of cell types including monocytes, megakaryocytes, erythrocytes, basophils, neutrophils and eosinophils 27. GM-CSF acts in a similar fashion, albeit with more mature precursor cells, inducing the formation of macrophages, neutrophils and eosinophils 34. IL-5 is the cytokine responsible for selective terminal differentiation of eosinophils 35 and stimulates the release of eosinophils from the bone marrow into peripheral circulation 36. GM-CSF, IL-3 and the chemokines CCL11, CCL24, and CCL26 (eotaxins) are also involved in eosinophil homeostasis and play an important role upon arrival of the eosinophil at a tissue location. In addition, an IFN-γ-induced transcription factor ICSBP can also drive the differentiation of eosinophils 37. As IFN-γ is routinely present in allergic inflammation and, in our studies, was particularly upregulated in AERD 38, this led us to speculate on the role of IFN-γ being able to contribute to eosinophilia. Using an in vitro model with CD34+ hematopoietic progenitors, we demonstrated the capacity of IFN-γ, acting in synergy with IL-5, to promote the survival and differentiation of mature bi-lobed, CCR3- and Siglec-8-expressing eosinophils 38 confirming prior studies 39 regarding the influence of this cytokine on eosinophil-mediated inflammation.
As with eosinophils, mast cells are derived from pluripotent hematopoietic CD34+ cells from the bone marrow 40, however mast cells do not fully mature until they reach their final tissue destination with the exception of mast cell leukemia. IL-3 and IL-6 increase early CD34+ progenitor cell numbers and begin the differentiation process, however it is binding of stem cell factor (SCF) to its receptor c-Kit (CD117) that is the master growth and differentiation factor for human mast cells (Figure 1) 41. SCF is produced primarily by stromal cells 42 and it can either be released as a soluble growth factor or expressed on the cell surface of these cells. It is the expression and binding of SCF at tissue sites that cause the CD34+ precursor cells to arrest and terminally differentiate into mast cells.
Cysteinyl leukotriene over-production and over-responsiveness in AERD
Arguably, the best-characterized molecules associated with AERD are the CysLTs. A unique characteristic of the disease is the over-production of CysLTs in the resting state and a tremendous surge in CysLT production in response to aspirin and other non-selective cyclooxygenase inhibitors that target COX-1 43. Included in this list are the non-selective non-steroidal anti-inflammatory drugs (NSAIDs) as well as other inhibitors of COX-1 44, 45. The high CysLT levels in AERD reflect increased expression of the primary synthesis enzymes 5-lipoxygenase (5-LO) and more importantly leukotriene C4 synthase (LTC4S) (Figure 2). Increased expression of these enzymes is observed in the lungs, sinuses and nasal polyps of AERD subjects with eosinophils and resident mast cells being the primary cells expressing the enzymes 16, 19, 24, 46.
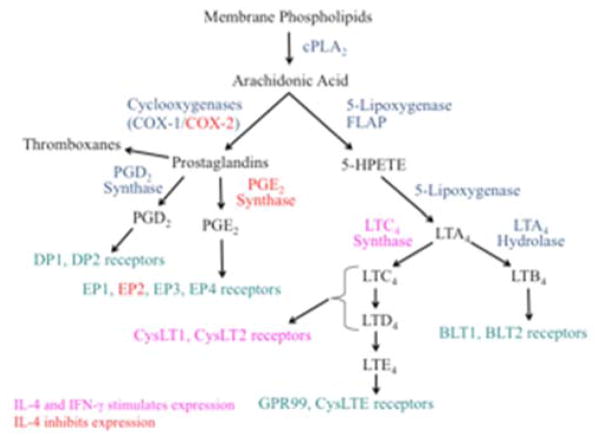
Figure 2. Pathway depicting metabolites of arachidonic acid important in AERD.
Following conversion to arachidonic acid by phospholipase A2, further processing occurs via either the prostaglandin pathway mediated by COX-1/COX-2 or the leukotriene pathway mediated by 5-lipoxygenase. Red lettering shows genes inhibited by IL-4 and pink lettering show genes stimulated by IL-4 and IFN-γ.
Not only do AERD subjects produce more CysLTs, but they also demonstrate an increased sensitivity to CysLTs 47. Initially, two CysLT receptors were identified and were distinguished from each other by their differing potency for the CysLTs: CysLT1 receptors primarily respond to LTD4 whereas CysLT2 receptors respond equally to LTD4 and LTC4. Neither receptor responds well to LTE4. Acting through CysLT1 and inhibited by the CysLT2 receptor, CysLTs induce mast cell proliferation through activation of c-kit and extracellular signal-regulated kinase 48. In AERD sinus tissue, high levels of CysLT1 were found in comparison to healthy tissue and following aspirin desensitization, the CysLT1 levels returned to normal 49. CysLT1 receptors are also prominently expressed on airway smooth muscle 50 and these receptors mediate a portion of the CysLT-induced bronchospasm associated with aspirin challenges or desensitizations 51–54 as demonstrated by the ability of leukotriene receptor antagonists to attenuate much of the bronchospasm that occurs during these procedures.
While these findings made it appear that CysLT1 was the only important leukotriene receptor in AERD, several observations suggested they were only partially involved in the pathogenesis of disease. The most abundant leukotriene found in the circulation and airway is LTE4 with C4 and D4 being rapidly converted to E4, thus limiting their duration of action in vivo. Inhalation of LTE4 by asthmatic and AERD subjects potentiates airway hyperresponsiveness to subsequent challenges with histamine, the effect of which could be blocked with indomethacin 47, 55, 56. In the bronchial mucosa of asthmatics, inhalation of LTE4, but not LTD4, causes recruitment of eosinophils, basophils and mast cells into the tissue 57, 58. Mice in which both the CysLT1 and CysLT2 receptors have been deleted show enhanced skin swelling in response to intracutaneous LTE4 in comparison to mice with these genes intact 59. These studies led to the exploration for and ultimate identification of additional CysLT receptors that selectively respond to LTE4 (GPR99 and P2Y12) 59–62. The functional role of these LTE4 receptors in AERD and the utility in targeting them as a therapeutic option are areas of active research, although diminished synthesis of CysLTs and, by extension, of LTE4, could explain the superior efficacy of 5-lipoxygenase inhibitors in treating this disorder 63.
Underappreciated role for PGD2 in AERD
PGD2 and its metabolites have been found in blood and urine following aspirin challenge 11, 13, 64 and the conventional thought is that it is primarily mast cell derived, however reports demonstrate that eosinophils are also a source 65, 66. Synthesis of PGD2 is regulated by hematopoietic prostaglandin D2 synthase (hPGDS) (Figure 2) and when secreted it binds two receptors CRTH2 (DP2) and DP1 that are expressed on numerous cell types. Activities of PGD2 binding include stimulation of cell migration (including eosinophils and innate lymphoid type 2 (ILC2) cells), bronchoconstriction, vasodilation (flushing), and cellular activation and differentiation 67–70. A role for PGD2 in the pathogenesis of AERD is supported by studies in chronic sinusitis. Expression of hPGDS has been observed in polyps, specifically in eosinophils 66, 71, 72. The degree of hPGDS expression correlated with eosinophil number and severity of disease. These studies did not examine AERD subjects, but given the high levels of eosinophilic infiltrate in AERD tissue, it is likely that hPGDS levels would have correlated as well. Recent work from our lab demonstrated that amongst CRS syndromes, in AERD the highest levels of hPGDS transcripts and proteins expression were observed and with eosinophils being the predominant cell type where expression was localized 66. As discussed in other chapters, aspirin desensitization followed by high-dose aspirin therapy is often used to treat AERD, however not all patients tolerate the desensitization protocol. It has recently been shown that those subjects who cannot be desensitized express higher basal levels of PGD2 (and thromboxane) in their serum and urine 64. During the desensitization procedure, these patients had a surge of PGD2 release but not thromboxane, whereas those who were successfully desensitized had decreased thromboxane and unchanged PGD2 levels 64. In this study, the correlation between PGD2 and eosinophil number in the polyp tissue was not performed. Baseline PGD2 levels may serve as a marker to identify those who will successfully undergo desensitization.
PGE2 and PGE2 receptor dysregulation in AERD
PGE2 displays both pro- and anti-inflammatory functions reflecting its ability to interact with 4 distinct receptors (EP1-4) each having various activating or inhibitory functions. However, it is the role of PGE2 acting through anti-inflammatory EP2 receptors to block eosinophil and mast cell degranulation that is central to the pathogenesis of AERD. AERD patients constitutively produce low levels of PGE2 16, 73 attenuating the anti-inflammatory constraints provided by this lipid in this basal state. The further reduction of tissue PGE2 concentrations by aspirin and other NSAIDs through COX-1 inhibition precipitates the activation of eosinophils and mast cells in AERD, as demonstrated by the ability of inhaled PGE2 to protect against these reactions 74, 75. This sensitivity of AERD patients to low tissue PGE2 concentrations is amplified by their reduced expression of the anti-inflammatory EP2 receptor 18. Serra-Pages and colleagues demonstrated that the ratio of EP2 to EP3 receptors on the surface of a mast cell influences the activation potential of these cells when the high affinity IgE receptor (FcεRI) is stimulated in a PGE2-containing milieu 76. Through examination of various mast cell lines, the authors found that those with high levels of EP2 could suppress FcεRI activation in the presence of PGE2, but when EP3 levels were high FcεRI activation of mast cells was enhanced. It is likely that the low EP2/EP3 ratio on mast cells and possibly eosinophils in AERD contributes to this disease as any PGE2 that is available would preferentially signal through the EP3 receptor and activate these cells, thus contributing to the pro-inflammatory cascade.
Several studies have investigated the mechanism behind the reduced levels of PGE2 in AERD and, perhaps not surprisingly, have correlated this with a decrease in the relevant upstream metabolic enzymes. The production of PGE2 from arachidonic acid (Figure 2) involves the sequential synthesis of PGG2/PGH2 by the two cyclooxygenase enzymes (COX-1 and COX-2) followed by the synthesis of PGE2 by the microsomal PGE2 synthases (mPGES-1, mPGES-2) and cytosolic PGE2 synthase (cPGES). It is mPGES-1 that is most relevant to PGE2 production in inflammatory disorders such as AERD as it is the enzyme primarily functionally coupled to COX-2 77. COX-2 mRNA and protein expression are markedly diminished in AERD 16, 17, 78. Our studies have confirmed this diminished expression of COX-2 79. We found no significant change in COX-1 and a trend towards diminished mPGES-1 expression and that this is driven in part by IL-4. Diminished COX-2 expression and the reduced capacity to synthesize PGE2 contributes to the severity of inflammation observed in AERD and accentuates the sensitivity of these individuals to the further inhibition of PGE2 synthesis associated with aspirin and other NSAIDs. With this relative absence of COX-2, AERD subjects become dependent upon COX-1 for the PGE2 that is necessary to restrain mast cell and eosinophil activation. Thus, most AERD patients tolerate selective COX-2 inhibitors 80, supporting this concept regarding the unique importance of COX-1-derived PGE2.
Cytokine expression in AERD
Numerous studies have examined the cytokine milieu found in the tissue of eosinophilic sinusitis and AERD. Many of these studies have reported expression of a Th2-like immune profile (IL-4, IL-13 and IL-5), similar to other allergic diseases with eosinophilic infiltrate, though few have specifically focused on AERD 81–88. However, numerous observations suggest that in contrast to eosinophilic sinusitis patients who tolerate aspirin, AERD appears to have a mixed Th2- and Th1-like cytokine milieu characterized by prominent expression of IFN-γ. The first study to suggest this examined non-allergic sinusitis patients and demonstrated enhanced IFN-γ expression in this cohort: a group in which AERD is likely to be over-expressed 89. Since this initial report, high levels of IFN-γ in chronic sinusitis have been reported by other investigators, although AERD subjects were not specifically separated or recruited for the studies 90. One study specifically addressed IFN-γ expression in AERD and found enhanced levels of IFN-γ in circulating CD8+ cells compared to aspirin-tolerant controls 91. The concept that AERD reflects a mixed Th2- and Th1-like cytokine profile was confirmed in our recent study in which NP tissue derived from AERD subjects was contrasted with those obtained from aspirin tolerant and control subjects by their over-expression of IFN-γ mRNA transcripts and protein 38. To our surprise, we found that eosinophils themselves were the most important source for this cytokine, which is consistent with previous studies that demonstrated IFN-γ can be expressed by eosinophils in substantial quantities 92–94. In contrast to our findings, a recent report did not find elevated levels of IFN-γ protein in nasal polyps from AERD subjects, however this group did not perform flow cytometry, immunohistochemistry or quantitative PCR to verify their results 88.
Cytokine dysregulation of LTC4S and CysLT receptors
Under non-inflammatory conditions, mast cells express moderate levels of LTC4S that can be increased greatly following stimulation by IL-4 (but not by IL-5 or IL-13) 95. It has also been observed that IFN-γ also has the capability of upregulating LTC4S expression in umbilical cord-derived mast cell progenitors (Joshua Boyce, personal communication). While mast cells are capable of synthesizing CysLTs, previous studies 24 have demonstrated that eosinophils are the more important cell type over-expressing LTC4S in AERD. Examining a battery of cytokines (including IL-3, IL-4, IL-5, GM-CSF, IL-1, TNF-α and IFN-γ), we were unable to demonstrate an ability of any of these cytokines to modulate LTC4S expression on circulating eosinophils. This may be due to their terminal differentiation state and short life span. We have also failed to demonstrate the ability of IL-4 to increase LTC4S expression on eosinophils differentiated from progenitors in the presence of IL-3 and IL-5. However, when the progenitors were incubated with IFN-γ during the differentiation stage, a significant increase in LTC4S expression was observed 38. The increase in LTC4S expression translated into increased capacity of these newly differentiated eosinophils to secrete CysLTs upon activation by aspirin 14. Another mechanism of CysLT production involves the transcellular conversion of LTA4 by adherent platelets expressing LTC4S 96. The frequency of platelet-adherent eosinophils, neutrophils and monocytes are markedly elevated in the blood of AERD subjects. Any LTA4 that is released into the extracellular space can be captured by the platelets and converted to CysLTs. It has been estimated that adherent-platelets contribute up to 50% of the total LTC4S activity in blood and thus would represent a significant source of the CysLTs found in AERD 96.
CysLT receptor expression is regulated by numerous cytokines including IL-4 and IFN-γ, but also IL-5 and IL-13. IL-4 increases expression of CysLT1 on mast cells 97, 98 and monocytes 99. In our studies, IL-4 also increased the expression of both CysLT1 and CysLT2 on T and B lymphocytes and eosinophils 100. We also demonstrated robust upregulation of CysLT1 and CysLT2 receptors in response to IFN-γ on T cells and eosinophils 100. In recent studies, examination of eosinophils derived from CD34+ progenitors have also demonstrated the ability of IFN-γ to upregulate CysLT1 and CysLT2 receptor expression 38. There have been no reports of cytokine modulation of other CysLT receptors. In summary, in the AERD cytokine environment, both mast cells and eosinophils are primed to produce and respond to the CysLTs that are produced during the disease process.
Cytokine dysregulation of PGE2 Synthesis and EP2 Receptors
Cytokine regulation of the PGE2 synthesis pathway in AERD has not been thoroughly investigated. However, in other model systems, the influence of IL-4 on COX-2 expression has been reported. Inhibition of COX-2 expression by IL-4 was noted using peripheral blood monocytes, alveolar macrophages and non-small cell lung cancer cells 101–103. We performed studies on nasal polyp-derived fibroblasts and mononuclear phagocytic cells. Monocytes were utilized both as representative inflammatory cells, but also because PGE2 is their dominant prostaglandin product. Similar to other findings, significant inhibition of COX-2 and also mPGES-1 (but not COX-1) mRNA and protein expression was observed following stimulation with IL-4 on both the monocytes and nasal polyp-derived fibroblasts 79. Inhibition of COX-2 and mPGES-1 synergize to result in dramatically less stimulated PGE2 secretion by monocytes 79. IL-13 has been reported to have a similar effect on airway epithelial cells 104. Thus, in addition to enhancing the CysLT pathways, IL-4 and IL-13 contribute to the AERD phenotype through inhibition of the PGE2 pathway. The role of IFN-γ modulation of the prostaglandin pathway is unclear as its action appears to be cell-type specific. IFN-γ can induce COX-2 mRNA in most inflammatory cells 105, 106 whereas it decreases COX-2 expression in placental 107 and intestinal epithelial cancer cells 108. Actions of IFN-γ on other parts of the PGE2 pathway have not been studied in detail.
Activation of eosinophils and mast cells by aspirin
It was unknown in AERD how aspirin triggered the release of pro-inflammatory mediators. While, as noted, inhibition of COX releases the protective constraints provided by PGE2, this alone does not explain the positive signaling driving cell activation. In a particularly robust murine model of AERD, aspirin sensitivity is induced by the knocking out of the mPGES-1 gene 109. In this model, the positive – activating – signal is provided by allergic inflammation, a mechanism not likely to be relevant in AERD, at least in those AERD patients who are not atopic. We speculated that aspirin and other NSAIDs had the inherent capacity to directly activate eosinophils and mast cells. When tested, both eosinophils and mast cells generated Ca+2 fluxes following stimulation with water soluble lysine aspirin (LysASA) 14. Similar results were observed with eosinophil activation measured by EDN release and eosinophil and mast cell secretion of PGD2 14, 66. To our surprise, when eosinophils from control, aspirin tolerant, and AERD subjects were compared, no differences were observed in levels of mediator release. Our explanation as to why hypersensitivity reactions due to mediator release caused by aspirin/NSAIDs are not observed in these control cohorts reflects alterations in their PGE2 sensitivity, specifically the decreased capacity to produce and respond to this anti-inflammatory mediator observed in AERD. We speculate that the higher expression of PGE2 as well as its anti-inflammatory EP2 receptor acts to prevent the acute reactions to aspirin/NSAIDs in controls and aspirin tolerant asthmatics 21, 74. An additional explanation for the absence of clinical symptoms in these control cohorts is that when activated with aspirin, their circulating eosinophils produced very low levels of CysLTs 14 in contrast to the robust levels found in AERD subjects following aspirin ingestion 43. As mentioned earlier, AERD sinonasal and lung tissue is characterized by high numbers of eosinophilic hematopoietic progenitor (CD34+IL-5Rα+) cells 29, 110. We therefore investigated whether eosinophils differentiated from progenitor cells in the presence of IFN-γ would recapitulate the sensitivity to aspirin displayed by tissue eosinophils in vivo in AERD. After maturation with IFN-γ, the mature eosinophils displayed increased gene expression for both LTC4S and hPGDS 14, 66. Consistent with the increase in LTC4S gene expression, CysLT secretion was dramatically increased upon LysASA activation 14. In addition to increased CysLT production, these IFN-γ matured eosinophils displayed enhanced PGD2 production when stimulated with LysASA 66.
Summary: Towards a generalized model for the induction of the AERD phenotype
While our understanding that aspirin causes reactions and that AERD is a debilitating disease have been known for many years, the exact mechanisms driving AERD and how to treat it are still largely unknown. Our work and that of others have demonstrated the importance of both eosinophils and mast cells as drivers of the disease leading to increased expression of pro-inflammatory mediators and reflecting the loss of protective PGE2. The ultimate result is constitutive over-production of and over-responsiveness to mediators by eosinophils and mast cells in the basal state in AERD, with the uncontrolled release of mediators when these cells are directly triggered by aspirin. The increased recognition of the cellular components and mechanisms of action in AERD provides an opportunity to develop alternative targeted therapeutic approaches aimed at dampening the severe impacts of this disease.
Key Points.
AERD is a disease of overproduction and hyper-responsiveness to lipid mediators.
Mast cells and eosinophils are key driver of AERD pathogenesis through production of pro-inflammatory mediators following aspirin stimulation.
Due to their involvement, therapies that target mast cells and eosinophils may be useful in providing clinical benefit in AERD.
Acknowledgments
Supported by NIH grants R01AI057438, R56AI120055, and U01AI100799
Abbreviations
- 5-LO
5-lipoxygenase
- AERD
aspirin exacerbated respiratory disease
- AFS
allergic fungal sinusitis
- CHES
chronic hyperplastic eosinophilic sinusitis
- COX
cyclooxygenase
- CysLT
cysteinyl leukotriene
- ECP
eosinophil cationic protein
- Eo/B CFU
eosinophil/basophil progenitors or colony forming units
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IFN
interferon
- IL
interleukin
- LT
leukotriene
- LTC4S
leukotriene C4 synthase
- NP
nasal polyposis
- NSAID
non-steroidal anti-inflammatory drugs
- PG
prostaglandin
- PGDS
prostaglandin D2 synthase
- PGES
prostaglandin E2 synthase
- SCF
stem cell factor
- STAT
signal transducer and activator of transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–83. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- 2.Mattos JL, Woodard CR, Payne SC. Trends in common rhinologic illnesses: analysis of U.S. healthcare surveys 1995–2007. Int Forum Allergy Rhinol. 2011;1:3–12. doi: 10.1002/alr.20003. [DOI] [PubMed] [Google Scholar]
- 3.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–81. e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Szczeklik A, Stevenson DD. Aspirin-induced asthma: Advances in pathogenesis and management. J Allergy Clin Immunol. 1999;104:5–13. doi: 10.1016/s0091-6749(99)70106-5. [DOI] [PubMed] [Google Scholar]
- 5.Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax. 2000;55:S42–S4. doi: 10.1136/thorax.55.suppl_2.S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2002;89:474–8. doi: 10.1016/S1081-1206(10)62084-4. [DOI] [PubMed] [Google Scholar]
- 7.Vally H, Taylor ML, Thompson PJ. The prevalence of aspirin intolerant asthma (AIA) in Australian asthmatic patients. Thorax. 2002;57:569–74. doi: 10.1136/thorax.57.7.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinke JW, Huyett P, Payne SC, Negri J, Borish L. Cytokines and lymphocytes in eosinophilic sinusitis: prominent role for interferon-γ. 2011 submitted. [Google Scholar]
- 9.Mascia K, Borish L, Patrie J, Hunt J, Phillips CD, Steinke JW. Chronic hyperplastic eosiniphilic sinusistis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;94:652–7. doi: 10.1016/S1081-1206(10)61323-3. [DOI] [PubMed] [Google Scholar]
- 10.Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116:970–5. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin n aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–56. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 12.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of resiratory responses. J Allergy Clin Immunol. 1999;104:559–64. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 13.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. The Journal of allergy and clinical immunology. 2003;111:743–9. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 14.Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol. 2014;193:41–7. doi: 10.4049/jimmunol.1301753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antczak A, Montuschi P, Kharitonov S, Gorski P, Barnes PJ. Increased exhaled cysteinyl-leukotrienes and 8-isoprostane in aspirin-induced asthma. American Journal of Respiratory and Critical Care Medicine. 2002;166:301–6. doi: 10.1164/rccm.2101021. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Novo CA, Watelet JB, Claeys C, van Cauwenberge P, Bachert C. Prostaglandin, leukotiene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–96. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Picado C, Fernandez-Morata JC, Juan M, Roca-Ferrer J, Fuentes M, Xaubet A, et al. Cyclooxygenase-2 mRNA is downexpressed in nasal polyps from aspirin-sensitive asthmatics. Am J Respir Crit Care Med. 1999;160:291–6. doi: 10.1164/ajrccm.160.1.9808048. [DOI] [PubMed] [Google Scholar]
- 18.Ying S, Meng Q, Scadding G, Parikh A, Corrigan CJ, Lee TH. Aspirin-sensitive rhinosinusitis is associated with reduced E-prostanoid 2 receptor expression on nasal mucosal inflammatory cells. J Allergy Clin Immunol. 2006;117:312–8. doi: 10.1016/j.jaci.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Steinke JW, Bradley D, Arango P, Crouse CD, Frierson H, Kountakis SE, et al. Cytseinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: Importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–9. doi: 10.1067/mai.2003.67. [DOI] [PubMed] [Google Scholar]
- 20.Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci USA. 2004;101:3047–52. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinke JW. Editorial: Yin-Yang of EP receptor expression. Journal of leukocyte biology. 2012;92:1129–31. doi: 10.1189/jlb.0812374. [DOI] [PubMed] [Google Scholar]
- 22.Adamjee J, Suh YJ, Park HS, Choi JH, Penrose JF, Lam BK, et al. Expression of 5-lipoxygenase and cyclooxygenase pathway enzymes in nasal polyps of patients with aspirin-intolerant asthma. J Pathol. 2006;209:392–9. doi: 10.1002/path.1979. [DOI] [PubMed] [Google Scholar]
- 23.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. The Laryngoscope. 2011;121:2262–7. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–46. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HS, Nahm DH, Park K, Suh KS, Yim HE. Immunohistochemical characterization of cellular infiltrate in nasal polyp from aspirin-sensitive asthmatic patients. Ann Allergy Asthma Immunol. 1998;81:219–24. doi: 10.1016/s1081-1206(10)62815-3. [DOI] [PubMed] [Google Scholar]
- 26.Corrigan CJ, Napoli RL, Meng Q, Fang C, Wu H, Tochiki K, et al. Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. J Allergy Clin Immunol. 2012;129:1636–46. doi: 10.1016/j.jaci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Boyce JA, Friend D, Matsumoto R, Austen KF, Owen WF. Differentiation in vitro of hybrid eosinophil/basophile granulocytes: autocrine function of an eosinophil intermediate. J Exp Med. 1995;182:49–57. doi: 10.1084/jem.182.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O’Bryne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000;106:S242–S6. doi: 10.1067/mai.2000.110156. [DOI] [PubMed] [Google Scholar]
- 29.Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer RP, et al. Immunolocalization of CD34 in nasal polyp. Effect of topical corticosteroids. Am J Respir Cell Mol Biol. 1999;20:388–97. doi: 10.1165/ajrcmb.20.3.3060. [DOI] [PubMed] [Google Scholar]
- 30.Nerlov C, Graf T. PU. 1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12:2403–12. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nerlov C, McNagny KM, Doderlein G, Kowenz-Leutz E, Graf T. Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev. 1998;12:2413–23. doi: 10.1101/gad.12.15.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNagny K, Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med. 2002;195:F43–F7. doi: 10.1084/jem.20020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirasawa R, Shimizu R, Takahashi S, Osawa M, Takayanagi S, Kato Y, et al. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med. 2002;195:1379–86. doi: 10.1084/jem.20020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishijima I, Nakahata T, Hirabayashi Y, Inoue T, Kurata H, Miyajima A, et al. A human-GM-CSF receptor expressed in transgenic mice stimulates proliferation and differentiation of hemopoietic progenitors to all lineages in response to human GM-CSF. Mol Cell Biol. 1995;6:497–508. doi: 10.1091/mbc.6.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–9. [PubMed] [Google Scholar]
- 36.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accummulation in vivo. J Exp Med. 1995;182:1169–74. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milanovic M, Terszowski G, Struck D, Liesenfeld O, Carstanjen D. IFN consensus sequence binding protein (Icsbp) is critical for eosinophil development. J Immunol. 2008;181:5045–53. doi: 10.4049/jimmunol.181.7.5045. [DOI] [PubMed] [Google Scholar]
- 38.Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent Role of Interferon-γ in Aspirin-Exacerbated Respiratory Disease. Journal of Allergy and Clinical Immunology. 2013;132:856–65. e3. doi: 10.1016/j.jaci.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bruin AM, Buitenhuis M, van der Sluijs KF, van Gisbergen KP, Boon L, Nolte MA. Eosinophil differentiation in the bone marrow is inhibited by T cell-derived IFN-gamma. Blood. 2010;116:2559–69. doi: 10.1182/blood-2009-12-261339. [DOI] [PubMed] [Google Scholar]
- 40.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–42. [PubMed] [Google Scholar]
- 41.Kirshenbaum AS, Goff JP, Kessler SW, Mican JM, Zsebo KM, Metcalfe DD. Effect of IL-3 and stem cell factor on the appearance of human basophils and mast cells from CD34+ pluripotent progenitor cells. J Immunol. 1992;148:772–7. [PubMed] [Google Scholar]
- 42.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–43. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 43.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–9. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 44.Cardet JC, White AA, Barrett NA, Feldweg AM, Wickner PG, Savage J, et al. Alcohol-induced respiratory symptoms are common in patients with aspirin exacerbated respiratory disease. The journal of allergy and clinical immunology In practice. 2014;2:208–13. doi: 10.1016/j.jaip.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payne SC. Alcohol’s effect in AERD related to polyphenols. J Allergy Clin Immunol Pract. 2014 in press. [Google Scholar]
- 46.Sampson AP, Cowburn AS, Sladek K. Profound overexpression of leukotriene C4 synthase in bronchial biopsies from aspirin-intolerant asthmatic patients. Int Archives Allergy Immunology. 1997;113:355–7. doi: 10.1159/000237600. [DOI] [PubMed] [Google Scholar]
- 47.Arm JP, O’Hickey S, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989;140:148–53. doi: 10.1164/ajrccm/140.1.148. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–70. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in asprin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–9. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 50.Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 51.Christie PE, Smith CM, Lee TH. The potent and selective sulfidopeptide leukotriene antagonists, SK&F 104353, inhibits aspirin-induced asthma. Am Rev Resir Dis. 1991;144:957–8. doi: 10.1164/ajrccm/144.4.957. [DOI] [PubMed] [Google Scholar]
- 52.Dahlen B. Treatment of aspirin-intolerant asthma with antileukotrienes. Am J Respir Crit Care Med. 2000;161:S137–S41. doi: 10.1164/ajrccm.161.supplement_1.ltta-27. [DOI] [PubMed] [Google Scholar]
- 53.Walch L, Norel X, Back M, Gascard JP, Dahlen SE, Brink C. Pharmacological evidence for a novel cysteinyl-leukotriene receptor subtype in human pulmonary artery smooth muscle. Br J Pharmacol. 2002;137:1339–45. doi: 10.1038/sj.bjp.0704991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White A, Bigby T, Stevenson D. Intranasal ketorolac challenge for the diagnosis of aspirin-exacerbated respiratory disease. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2006;97:190–5. doi: 10.1016/S1081-1206(10)60012-9. [DOI] [PubMed] [Google Scholar]
- 55.Christie PE, Hawksworth R, Spur BW, Lee TH. Effect of indomethacin on leukotriene4-induced histamine hyperresponsiveness in asthmatic subjects. Am Rev Respir Dis. 1992;146:1506–10. doi: 10.1164/ajrccm/146.6.1506. [DOI] [PubMed] [Google Scholar]
- 56.Christie PE, Schmitz-Schumann M, Spur BW, Lee TH. Airway responsiveness to leukotriene C4 (LTC4), leukotriene E4 (LTE4) and histamine in aspirin-sensitive asthmatic subjects. Eur Respir J. 1993;6:1468–73. [PubMed] [Google Scholar]
- 57.Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet. 1993;341:989–90. doi: 10.1016/0140-6736(93)91073-u. [DOI] [PubMed] [Google Scholar]
- 58.Gauvreau GM, Parameswaran KN, Watson RM, O’Byrne PM. Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. American Journal of Respiratory and Critical Care Medicine. 2001;164:1495–500. doi: 10.1164/ajrccm.164.8.2102033. [DOI] [PubMed] [Google Scholar]
- 59.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–8. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 61.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–55. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. The Journal of biological chemistry. 2013;288:10967–72. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahlen B, Nizankowska E, Szczeklik A. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Critc Care Med. 1998;157:1187–94. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 64.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–52. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. Journal of immunology. 2011;187:6518–26. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng X, Negri J, Baker MG, Payne SC, Borish L, Steinke JW. Eosinophil production of PGD2 in aspirin-exacerbated respiratory disease. 2016 doi: 10.1016/j.jaci.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–9. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- 69.Schratl P, Royer JF, Kostenis E, Ulven T, Sturm EM, Waldhoer M, et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179:4792–9. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 70.Stinson SE, Amrani Y, Brightling CE. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J Allergy Clin Immunol. 2015;135:395–406. doi: 10.1016/j.jaci.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okano M, Fujiwara T, Yamamoto M, Sugata Y, Matsumoto R, Fukushima K, et al. Role of prostaglandin D2 and E2 terminal synthases in chronic rhinosinusitis. Clin Exp Allergy. 2006;36:1028–38. doi: 10.1111/j.1365-2222.2006.02528.x. [DOI] [PubMed] [Google Scholar]
- 72.Hyo S, Kawata R, Kadoyama K, Eguchi N, Kubota T, Takenaka H, et al. Expression of prostaglandin D2 synthase in activated eosinophils in nasal polyps. Arch Otolaryngol Head Neck Surg. 2007;133:693–700. doi: 10.1001/archotol.133.7.693. [DOI] [PubMed] [Google Scholar]
- 73.Schmid M, Gode U, Schafer D, Wigand ME. Arachidonic acid metabolism in nasal tissue and peripheral blood cells in aspirin intolerant asthmatics. Acta Otolaryngol. 1999;119:277–80. doi: 10.1080/00016489950181819. [DOI] [PubMed] [Google Scholar]
- 74.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, et al. Inhaled PgE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. American J Respiratory Critical Care Medicine. 1996;153:572–5. doi: 10.1164/ajrccm.153.2.8564100. [DOI] [PubMed] [Google Scholar]
- 75.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–50. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serra-Pages M, Olivera A, Torres R, Picado C, de Mora F, Rivera J. E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J Leukoc Biol. 2012 doi: 10.1189/jlb.0212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murakami M, Nakashima K, Kamei D, Masuda S, Ishikawa Y, Ishii T, et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. The Journal of biological chemistry. 2003;278:37937–47. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 78.Gosepath J, Brieger J, Mann WJ. New immunohistologic findings on the differential role of cyclooxygenase 1 and cyclooxygenase 2 in nasal polposis. Am J Rhinol. 2005;19:111–6. [PubMed] [Google Scholar]
- 79.Steinke JW, Culp JA, Kropf E, Borish L. Modulation by aspirin of nuclear phospho-signal transducer and activator of transcription 6 expression: Possible role in therapeutic benefit associated with aspirin desensitization. J Allergy Clin Immunol. 2009;124:724–30. e4. doi: 10.1016/j.jaci.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 80.Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol. 2006;118:773–86. doi: 10.1016/j.jaci.2006.07.024. quiz 87–8. [DOI] [PubMed] [Google Scholar]
- 81.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–42. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 82.Bachert C, Gevaert P, van Cauwenberge P. Nasal polyposis- A new concept on the formation of polyps. ACI International. 1999;11:130–5. [Google Scholar]
- 83.Minshall EM, Cameron L, Lavigne F, Leung DYM, Hamilos D, Garcia-Zepada A, et al. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Amer J Resp Cell Mol Biol. 1997;17:683–90. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- 84.Hamilos DL, Leung DYM, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5, and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis. Clin Exp Allergy. 1998;28:1145–52. doi: 10.1046/j.1365-2222.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 85.Kamil A, Ghaffar O, Lavigne F, Taha R, Renzi PM, Hamid Q. Comparison of inflammatory cell profile and Th2 cytokine expression in the ethmoid sinuses, maxillary sinuses, and turbinates of atopic subjects with chronic sinusitis. Otolaryngol Head Neck Surg. 1998;18:804–9. doi: 10.1016/S0194-5998(98)70273-6. [DOI] [PubMed] [Google Scholar]
- 86.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Burner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35:1186–91. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 87.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. 41 e1–3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 88.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015;192:682–94. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamilos DL, Leung DYM, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–44. doi: 10.1016/s0091-6749(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 90.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 91.Shome GP, Tarbox J, Shearer M, Kennedy R. Cytokine expression in peripheral blood lymphocytes before and after aspirin desensitization in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2007;28:706–10. doi: 10.2500/aap.2007.28.3052. [DOI] [PubMed] [Google Scholar]
- 92.Kanda A, Driss V, Hornez N, Abdallah M, Roumier T, Abboud G, et al. Eosinophil-derived IFN-γ induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J Allergy Clin Immunol. 2009;124:573–82. doi: 10.1016/j.jaci.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 93.Akuthota P, Xenakis JJ, Weller PF. Eosinophils: Offenders or General Bystanders in Allergic Airway Disease and Pulmonary Immunity? J Innate Immun. 2011;3:113–9. doi: 10.1159/000323433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spencer LA, Szela CT, Perez SAC, Kirchhoffer CL, Neves JS, Radke AL, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–23. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C4 synthase expression by interleukin 4. J Exp Med. 2001;193:123–33. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–8. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195:583–92. doi: 10.1084/jem.20020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF, et al. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: Functional distinction from CysLT1R. Proc Natl Acad Sci U S A. 2003;100:11589–93. doi: 10.1073/pnas.2034927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thivierge M, Stankova J, Rola-Pleszczynski M. IL-13 and IL-4 up-regulate cysteinyl leukotriene 1 receptor expression in human monocytes and macrophages. J Immunol. 2001;167:2855–60. doi: 10.4049/jimmunol.167.5.2855. [DOI] [PubMed] [Google Scholar]
- 100.Early SB, Barekzi E, Negri J, Hise K, Borish L, Steinke JW. Concordant modulation of cysteinyl leukotriene receptor expression by IL-4 and IFN-g on peripheral immune cells. Am J Respir Cell Mol Biol. 2007;36:715–20. doi: 10.1165/rcmb.2006-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yano T, Hopkins HA, Hempel SL, Monick M, Hunninghake GW. Interleukin-4 inhibits lipopolysaccharide-induced expression of prostaglandin H synthase-2 in human alveolar macrophages. J Cell Physiol. 1995;165:77–82. doi: 10.1002/jcp.1041650110. [DOI] [PubMed] [Google Scholar]
- 102.Dworski R, Sheller JR. Differential sensitivites of human blood monocytes and alveolar macrophages to the inhibition of prostaglandin endoperoxide synthase-2 by interleukin-4. Prostaglandins. 1997;53:237–51. doi: 10.1016/s0090-6980(97)89598-6. [DOI] [PubMed] [Google Scholar]
- 103.Cui X, Yang SC, Sharma S, Heuze-Vourc’h N, Dubinett SM. IL-4 regulates COX-2 and PGE2 production in human non-small cell lung cancer. Biochem Biophys Res Commun. 2006;343:995–1001. doi: 10.1016/j.bbrc.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 104.Trudeau J, Hu H, Chibana K, Chu HW, Wescott JY, Wenzel SE. Selective downregulation of prostaglandin E2-related pathways by the TH2 cytokine IL-13. J Allergy Clin Immunol. 2006;117:1446–54. doi: 10.1016/j.jaci.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 105.Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, et al. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1999;274:29138–48. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Yoon Y, Jeoung D, Kim YM, Choe J. Interferon-gamma stimulates human follicular dendritic cell-like cells to produce prostaglandins via the JAK-STAT pathway. Mol Immunol. 2015;66:189–96. doi: 10.1016/j.molimm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Hanna N, Bonifacio L, Reddy P, Hanna I, Weinberger B, Murphy S, et al. IFN-gamma-mediated inhibition of COX-2 expression in the placenta from term and preterm labor pregnancies. Am J Reprod Immunol. 2004;51:311–8. doi: 10.1111/j.1600-0897.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 108.Klampfer L, Huang J, Kaler P, Sasazuki T, Shirasawa S, Augenlicht L. STAT1-independent inhibition of cyclooxygenase-2 expression by IFNgamma; a common pathway of IFNgamma-mediated gene repression but not gene activation. Oncogene. 2007;26:2071–81. doi: 10.1038/sj.onc.1210015. [DOI] [PubMed] [Google Scholar]
- 109.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16987–92. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Denburg JA. Haemopoietic mechanisms in nasal polyposis and asthma. Thorax. 2000;55:S24–S5. doi: 10.1136/thorax.55.suppl_2.S24. [DOI] [PMC free article] [PubMed] [Google Scholar]