I. Virology
Human papillomavirus (HPV) is the most common sexually transmitted infection worldwide. It is an important cause of cervical, vaginal, vulvar, penile, anal and head and neck cancers in kidney transplant recipients (1). These are small DNA viruses, each comprising 7900 base pairs. There are over 100 distinct HPV subtypes. Of these, there are high-risk and low-risk types, distinguished by their association with invasive cancer – high in “high-risk” and low in “low-risk”. Types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 are designated as high-risk types. Although low-risk types such as 6 and 11 do not cause invasive cancer, they are associated with anogenital warts. Types 16 and 18 (included in all three commercially available HPV prophylactic vaccines) are the most common HPV types found in cervical cancer, accounting for 70% of these malignancies (2, 3).
II. Epidemiology
Anogenital cancer
In the general population, HPV infection is associated with 90% of cervical and anal cancers, and a large proportion of vulvar, vaginal and penile cancers (4). HPV is also linked to a subset of head and neck cancers (5). Among kidney and other transplant recipients, HPV infection is associated with an increased risk of malignancies compared to the general population. In one systematic review, Grulich and others (N=31,977) reported higher standardized incidence ratios (SIR) of HPV-associated cancers in transplant patients compared to the general population (1). Cancers of the cervix (SIR 2.1), vulva and vagina (SIR 22.8), penis (SIR 15.7), and anus (SIR 4.9) were overrepresented in transplant recipients. Transplant recipients with HPV cancers were also found to have these cancers at an earlier average age compared to the general population.
Smaller studies in other countries have also demonstrated the striking increased prevalence of HPV-associated malignancies in this population. Although not adjusted for age, a rough comparison with the general population in the Netherlands in one study of 1023 patients showed a 5-fold increased risk for cervical, a 41-fold increased risk for vulvar, and a 122-fold increased risk for anal cancer among kidney transplant recipients (6). A study of 453 renal transplant patients from 1990 to 2008 in South Korea showed an incidence of 58.1 cervical carcinomas per 100,000 patient-years, 3.5 fold higher than a referent general population (7).
Head and neck and other cancers
There is increasing evidence that HPV infection is associated with head and neck cancers in the general population, although there have been few studies that focus specifically on renal transplant recipients. HPV-associated head and neck cancers are thought to arise in the base of the tongue and the tonsils. The epidemiology is distinct from the head and neck cancers associated with older age, smoking and alcohol consumption. One case-control study by D'Souza and colleagues (5) showed that seropositivity for HPV-16 (odds ratio 32.2) and detection of HPV in the oral cavity (odds ratio 14.6) had strong associations with head and neck cancer. Although there have not been many studies in the transplant population, one systematic review of several large cohorts showed that transplant recipients had a 3-fold increased risk of oropharyngeal cancer (1).
There is less evidence for a direct role between HPV infection and squamous carcinoma (SCC) of the skin. Nevertheless, there is an intriguing association between HPV infection and corresponding SCC. Among SCC patients, one study found high-risk HPV types in 46% of the epithelium of renal transplant patients compared to 24% in an immunocompetent control group (8). Although there is a clear causal route between low-risk HPV types and the benign upper airway neoplasm, respiratory papillomatosis, the role in lung cancer is more speculative. Studies so far are conflicted in whether HPV is associated with adenocarcinomas of the lungs (9, 10). There is little information in the renal transplant population.
Cervical and anal intraepithelial neoplasia
There are several studies that report the high prevalence of HPV-associated cervical (CIN) and anal intraepithelial neoplasia (AIN) among kidney transplant recipients. One study showed that CIN as well as high-risk HPV types 16 and 18 were more common among Scottish renal transplant patients compared to age-matched controls (11). In another report, this time from Italy, 7% of 151 kidney transplant recipients were found to have CIN (12). A larger retrospective study from the Netherlands evaluated 1023 women who underwent kidney transplants between 1968 and 2008. The investigators reported 1.6% HPV-associated malignancies in this cohort including 6 vulvar, 5 cervical and 6 anal carcinomas. Of 24 pre-malignant lesions examined, 22 were found to have detectable HPV, and 55% were HPV type 16 (6). Part of the reason why transplant recipients have a higher prevalence is that these precancer lesions do not regress as spontaneously as in the general population (13).
Cutaneous and anogenital warts
The prevalence of warts is linked directly to the duration of immunosuppression. In patients who have been transplanted 4-5 years ago, the proportion of patients with warts reaches as high as 50-92% (14). Compared to the general population, renal transplant patients have more numerous warts with a higher diversity of HPV types (15). Given that most lesions appear in sun exposed areas among transplant patients, ultraviolet light is thought to be a risk factor in this population. The prevalence of anogenital warts as a subset of warts may be lower, at least in published studies. However, these are not often easy to visualize or feel by patients and are often overlooked by providers unless systematically assessed. One French study of organ transplant recipients attending a dermatology clinic described a prevalence of anogenital warts of 2.3% (16).
III. Clinical Manifestations
In contrast to anogenital and cutaneous warts, HPV-associated cervical and anal cancer as well as cervical intraepithelial neoplasia (CIN) and anal intraepithelial neoplasia (AIN) do not usually present with clinically identifiable lesions. Many of these lesions can only be visualized by colposcopy or high resolution anoscopy. These comprise a powerful light source, binocular lens and reagents such as iodine or acetic acid to aid in visualization (Figure 1). Because of this, Pap screening (see below) is the most important strategy for diagnosis.
Figure 1.

High-grade anal intraepithelial neoplasia (AIN) in a female kidney transplant recipient with a history of cervical intraepithelial neoplasia (CIN).
Cutaneous warts are easily identifiable in general. They appear as well circumscribed exophytic papules with normal adjacent skin, and may be pedunculated or flat. They range in size from a few millimeters to several centimeters, with some coalescing to form plaques. Warts occur on any epithelial surface: plantar, palmar and anogenital areas are common. Anogenital warts typically present with a characteristic “cauliflower” appearance (condyloma acuminata). Kidney transplant patients have a similar appearance as in immunocompetent individuals. However, they may be more common, recur more often, appear larger and more numerous. Patients may complain of itching, burning, bleeding and pain. Often patients may have no symptoms.
IV. Diagnosis
General principles
A thorough external examination of the skin (including feet) at each visit is important to determine whether cutaneous warts are present in the kidney transplant recipient. Similarly, most external anogenital warts can be easily found by a thorough clinical inspection of the entire genital tract. A bright light with a hand lens or a colposcope may be helpful. Note that the appearance of any anogenital wart in the kidney transplant recipient should warrant further investigation for a number of reasons: 1) External anogenital lesions (which are usually benign) may indicate the presence of HPV-associated lesions that are internal. Women with external lesions, for example, should have a speculum examination to look for possible vaginal or cervical lesions; 2) any lesion with an atypical appearance (external or internal) should be biopsied. This is because immunocompromised patients may have preneoplastic lesions that are clinically indistinguishable from usually benign anogenital warts (17).
Cytology
The Papanicolau (Pap) test has been the traditional backbone of the screening strategy for cervical cancer in the general population including renal transplant recipients. Since the 1950s, it is estimated that the routine use of cervical Pap testing has decreased the incidence of invasive cervical cancer by about 70% (18).
Molecular-based methods
More recently molecular based methods such as DNA hybrid capture and PCR on clinical specimens have become more widely available, less costly and hence more widely used. There are now multiple FDA-cleared tests to detect high-risk HPV DNA (19, 20). Use of these methods should not be used for the diagnosis and management of anogenital warts. Rather, they should be used in a screening strategy for cervical cancer (see below).
Cervical cancer and cervical intraepithelial neoplasia (CIN) screening
All patients should first undergo a though examination of the external genitalia with the aid of a magnifying glass and a bright light source. The foundation of any cervical cancer screening program is the Pap test (Figure 2). Any program that incorporates Pap testing has been successful as a cancer screening strategy. This is because there is a long preinvasive state with CIN before the onset of cervical cancer.
Figure 2.

Screening for cervical intraepithelial neoplasia (CIN) and cervical cancer in the kidney transplant patient.
ASC-H, atypical squamous cells, cannot exclude HSIL; ASC-US, atypical squamous cells of undetermined significance; HR HPV, high-risk HPV test; HSIL, high-grade squamous intraepithelial lesions; LSIL, low-grade squamous intraepithelial lesions.
In the general population, guidelines have been issued by the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, the American Society for Clinical Pathology, the American College of Obstetricians and Gynecologists and the U.S. Preventive Services Task Force (21-24). In general, these guidelines recommend screening in women from ages 21 until 65. However, many of these guidelines do not apply to immunocompromised women. Many transplant centers recommend that kidney transplant recipients undergo the same screening protocol as HIV-infected women. For the first year following transplantation, a cervical Pap test should be performed every 6 months. If these are both normal, the screening interval can be increased to once yearly. Although there is little guidance from published studies, it may be reasonable to reset the screening intervals back to twice yearly for one year if the patient has been treated for rejection, particularly if anti-thymocyte agents are used.
Use of high-risk HPV testing is recommended for women aged 30 years or older in conjunction with a Pap test in the general population. If both tests are negative, the screening interval can be increased from every 3 years to every 5 years. Primary HPV testing may also be useful as a stand-alone test in resource poor areas without a good infrastructure for Pap testing. Among immunocompromised women such as kidney transplant recipients, most are screened every 6-12 months. In this patient population, there is no consensus as to the utility of HPV testing. Some clinicians recommend more frequent screening if high-risk HPV DNA is detected (every 6 months instead of once yearly).
Anal cancer and anal intraepithelial neoplasia (AIN) screening
The strategy for anal cancer and AIN screening is based on cervical cancer screening given the embryological and histological similarities between the two organs (25). Both cervical and anal cancers arise in the transformation zone, are caused by high-risk HPV types and are preceded by pre-cancer lesions. Given these similarities and the high burden of disease in the HIV-infected population, Chin-Hong and Palefsky proposed a screening strategy for anal cancer and AIN screening in the HIV-infected population (26). Given the high incidence of anal cancer and precursor lesions in the kidney transplant population, we recommend a similar approach (Figure 3). This would naturally also apply to HIV-infected kidney transplant recipients (25). The anal Pap test is the first step. We use a water-moistened polyester swab (Fisher Scientific, Pittsburgh, PA, USA). We prefer a polyester swab over a cotton swab given that epithelial cells cling to cotton therefore decreasing the yield of the Pap test. Compared to sampling the cervix, the anal Pap is more straightforward because the anal canal transformation zone does not have to be visualized during the procedure. Therefore there is no need for a speculum exam. To perform an anal Pap test, providers should first insert the polyester swab in the anal canal. As the swab is withdrawn, the swab is rotated and pressure maintained against the anal canal. Like the cervical Pap test, either a glass slide or liquid-based media is used to collect and submit the specimens for cytologic analysis by the pathologist.
Figure 3.
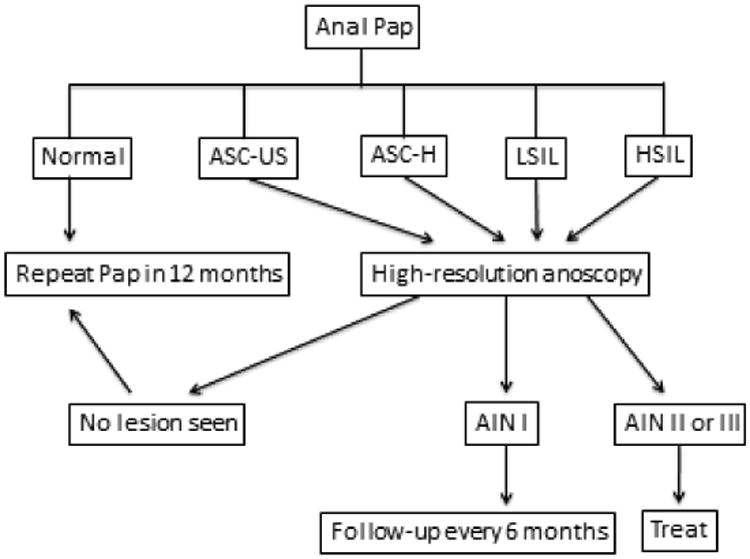
Screening for AIN and anal cancer in the kidney transplant patient ASC-H, atypical squamous cells, cannot exclude HSIL; ASC-US, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesions; LSIL, low-grade squamous intraepithelial lesions.
If patients are found to have abnormal anal cytology by the cytopathologist (ASC-US, ASC-H, LSIL and HSIL), the next step is to determine where these abnormal cells originated via direct visualization of the anal canal. Analogous to colposcopy, high-resolution anoscopy employs a powerful light and a binocular lens. Anoscopists use Lugol's (iodine) solution and 3% acetic acid (as is done in the cervix) to increase the ability of the eye to detect abnormal tissue. Once abnormal areas are identified and delineated, these suspicious lesions can be biopsied for histologic characterization.
It must be emphasized that the screening strategy for anal cancer and anal precursor lesions can only work if the infrastructure at all levels is available at the transplant center. The anal Pap test itself is very straightforward. In addition, most pathologists who interpret cervical cytology can be easily trained to interpret anal cytology. However, there must also be trained high-resolution anoscopists who are also experienced in treating limited disease, with a colorectal surgeon as backup and available for treatment of more extensive disease. If these elements are not present, a periodic digital examination of the anal canal may have some benefit (27). This will likely only be able to detect cancer (anal precancer lesions are generally not palpable) and may be helpful if an early stage of cancer. Detection of cancer of an earlier stage can maximize the chance of rectal preservation treatment modalities.
V. Treatment and Prevention
General principles
In general, treatment options for HPV-associated disease in kidney transplant recipients depend on the size, location and grade of the lesion (26, 28). Overall we prioritize treatment of high-grade pre malignant disease (CIN II or III and AIN II or III) over treatment of low-grade lesions. However, there may be some circumstances in the kidney transplant patient where treatment of low risk lesions may be recommended: 1) For relief of anxiety in the patient, or for cosmesis; and 2) To ameliorate blockage (if large), bleeding, itching or to prevent superinfection if ulcerated.
There is little evidence but some providers would also try to reduce immunosuppression if possible. This may be done in refractory disease, or if therapy is not tolerated (adverse effects) or practical (disease is extensive). Theoretically a switch from calcineurin inhibitors to mTOR inhibitors such as sirolimus can help particularly if malignant transformation has occurred (29). However, there are few studies that examine the outcomes of such a switch among kidney transplant recipients with HPV-associated neoplasia.
Cutaneous warts
In order to maximize cure, first pare down presoaked skin using a pumice stone, nail file, emery board or scalpel. Therapy may be either patient applied (salicylic acid, imiquimod), or provider applied (cryotherapy) (30, 31). Cryotherapy can be repeated every three weeks. Imiquimod is typically applied once daily before bedtime, three times a week. This can be repeated for up to 16 weeks. In our experience, kidney transplant and other immunocompromised hosts may require additional cycles of therapy, or may have incomplete response. Given the high incidence of nonmelanoma and other cancers, we recommend biopsy if any lesions look atypical or do not respond to therapy (32).
Cervical intraepithelial neoplasia (CIN)
In general CIN II and CIN III are treated in all women to prevent cancer. Clinicians use a range of excisional and ablative therapies. Loop electrosurgical excision procedure (LEEP) is the mainstay of CIN II and CIN III treatment. LEEP is comprised of an adjustable wire loop to excise lesions of different sizes. It is a very safe procedure. Tissue is also well preserved permitting histopathologic diagnosis (33). Cryotherapy via an applied probe is also used but the disadvantage is that tissue cannot be obtained to assess clear margins and for pathologic diagnosis (34). Laser therapy (carbon dioxide under colposcopy)(35) and cold-knife conization (scalpel used to excise a cone sized shape of the transformation zone) are other less common options for treatment (36).
Cervical cancer
Women with uterus-limited cervical cancer are classified as having early-stage disease (37). There are several choices of therapy based on a variety of factors. These include modified radical hysterectomy (38), fertility-sparing surgery (uterus preserved), or primary radiation therapy (with or without chemotherapy)(39). In general, surgery is preferred over primary radiation therapy unless the patient has poor functional status or medical comorbidities. For women with locally advanced cervical cancer, primary chemoradiation is preferred over either primary surgery or primary radiation therapy.
Anal intraepithelial neoplasia (AIN)
Given the anatomical challenges of the anal canal compared with the cervical canal, AIN is generally more difficult to treat than CIN (26). In general AIN I is not treated as there is low malignant potential. However, some patients with AIN I may be treated for symptomatic or psychological benefit. In general AIN II and AIN III are treated to prevent anal cancer. Size and location influence the modality of treatment. Intraanal lesions <1 cm2 at the base can be treated with 80% trichloroacetic acid, topical 5-fluorouracil or cryotherapy. AIN can sometimes with treated with imiquimod 5% cream (40, 41). If the lesion is larger or of higher grade, outpatient infrared coagulation (42, 43) or HRA-assisted intraoperative fulguration (44) are options used. If lesions of any grade are very large and not causing the patients symptoms, we may decide to follow patients closely rather than remove disease. This is because of the associated morbidity of removing proportionately large areas of tissue such as pain, anal stenosis and anal incontinence.
Anal cancer
The main modality of treatment for anal cancer is chemoradiation as it can cure the majority of patients while preserving the anal sphincter. Common chemotherapy regimens include fluorouracil plus mitomycin (45). A cisplatin based regimen is reserved for the uncommon patient with metastatic disease (46).
Prevention
Prophylactic HPV vaccines have been shown to be very effective in clinical trials (47, 48). There are three prophylactic HPV vaccines currently available (Table). These include Cervarix, a bivalent vaccine, targeting HPV types 16 and 18 (49); Gardasil, a quadrivalent vaccine, targeting types 16 and 18, as well as types 6 and 11 (50); and Gardasil 9 which covers the same types as in the quadrivalent vaccine (6, 11, 16, 18) as well as 5 additional types (high-risk HPV types 31, 33, 45, 52, 28) (51). All vaccines have shown >90% efficacy in preventing CIN and genital warts from the types included in the vaccine. Trials for the quadrivalent vaccine have also shown almost 80% efficacy in preventing incident AIN among men who have sex with men (52). In general, guidelines recommend HPV vaccination of girls and young women, with a target age range of 11-12 years old with a range of 9-26 year olds that can be immunized. Routine vaccination should also be offered to all boys with a target age group of 11-12 years old. Boys and men could be vaccinated in the age range of 9-21, with permissive use for ages 22-26 years old. The schedule is generally three doses – time 0, and at months 2 and 6 (53, 54).
Table. HPV prophylactic vaccines for kidney transplant recipients.
| Vaccine | Who to give | When to give | How to give | Adverse effects |
|---|---|---|---|---|
| Nonavalent (HPV types 6, 11, 16, 18, 31, 33, 45, 52, 58; Gardasil 9, Merck, Whitehouse Station, New Jersey) | Routinely offer to boys and girls 11-12 years old Can vaccinate ages 9-26 |
Pre-transplant preferred. Also safe post transplant (non-infectious) | Three doses at months 0, 2 and 6 | Minimal Mild to moderate localized pain, erythema, swelling |
| Quadrivalent (HPV types 6, 11, 16, 18; Gardasil, Merck, Whitehouse Station, New Jersey) | Routinely offer to boys and girls 11-12 years old Can vaccinate ages 9-26 |
Pre-transplant preferred. Also safe post transplant (non-infectious) | Three doses at months 0, 2 and 6 | Minimal Mild to moderate localized pain, erythema, swelling |
| Bivalent (HPV types 16, 18; Cervarix, GlaxoSmithKline, Rixensart, Belgium) | Routinely offer girls 11-12 years old Can vaccinate ages 9-26 |
Pre-transplant preferred. Also safe post transplant (non-infectious) | Three doses at months 0, 1 and 6 | Minimal Mild to moderate localized pain, erythema, swelling |
There is limited data about the safety and efficacy of the HPV vaccine in kidney transplant patients (55), and there is some evidence that it is safe among HIV-infected individuals (56, 57). Given that the HPV prophylactic vaccines do not contain any live virus, we suggest administration of the HPV vaccines in transplant candidates as above. Of course vaccination of eligible patients prior to transplantation is preferred given the higher probability of developing a robust antibody response.
HPV prophylactic vaccines form part of a menu of options for patients for preventing HPV infection and disease. Other options include limiting the numbers of sexual partners, condoms and circumcision (58, 59).
VI. Summary and Conclusion
HPV is a common sexually transmitted infection in the general population and among kidney transplant recipients. HPV causes a host of anogenital and head and neck cancers. Because of reduced immune surveillance, kidney transplant patients have a disproportionate burden of persistent infection and disease compared to the general population. Fortunately most HPV-associated malignancies are preventable because of the long pre-invasive state of precursor lesions. Using a foundation of Pap testing, and careful and methodical routine physical examination, many precancer lesions can be identified and treated before progression to cancer. Nevertheless, it is unfortunate that screening guideline uptake for HPV cancers in the renal transplant patient population is low (60). This is a silent epidemic that deserves our close attention and advocacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 2.Tilston P. Anal human papillomavirus and anal cancer. J Clin Pathol. 1997;50(8):625–34. doi: 10.1136/jcp.50.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hildesheim A, Schiffman M, Bromley C, Wacholder S, Herrero R, Rodriguez A, et al. Human papillomavirus type 16 variants and risk of cervical cancer. J Natl Cancer Inst. 2001;93(4):315–8. doi: 10.1093/jnci/93.4.315. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 6.Meeuwis KA, Melchers WJ, Bouten H, van de Kerkhof PC, Hinten F, Quint WG, et al. Anogenital malignancies in women after renal transplantation over 40 years in a single center. Transplantation. 2012;93(9):914–22. doi: 10.1097/TP.0b013e318249b13d. [DOI] [PubMed] [Google Scholar]
- 7.Park ST, Song MJ, Park JS, Hur SY, Lee CW. Incidence and clinicopathologic behavior of uterine cervical carcinoma in renal transplant recipients. World J Surg Oncol. 2011;9:72. doi: 10.1186/1477-7819-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho WL, Murphy GM. Update on the pathogenesis of post-transplant skin cancer in renal transplant recipients. Br J Dermatol. 2008;158(2):217–24. doi: 10.1111/j.1365-2133.2007.08363.x. [DOI] [PubMed] [Google Scholar]
- 9.Klein F, Amin Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer. 2009;65(1):13–8. doi: 10.1016/j.lungcan.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Koshiol J, Rotunno M, Gillison ML, Van Doorn LJ, Chaturvedi AK, Tarantini L, et al. Assessment of human papillomavirus in lung tumor tissue. J Natl Cancer Inst. 2011;103(6):501–7. doi: 10.1093/jnci/djr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alloub MI, Barr BB, McLaren KM, Smith IW, Bunney MH, Smart GE. Human papillomavirus infection and cervical intraepithelial neoplasia in women with renal allografts. BMJ. 1989;298(6667):153–6. doi: 10.1136/bmj.298.6667.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paternoster DM, Cester M, Resente C, Pascoli I, Nanhorngue K, Marchini F, et al. Human papilloma virus infection and cervical intraepithelial neoplasia in transplanted patients. Transplant Proc. 2008;40(6):1877–80. doi: 10.1016/j.transproceed.2008.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Ueda Y, Kakuda M, Kubota S, Matsuzaki S, Nakagawa S, et al. Clinical outcomes of abnormal cervical cytology and human papillomavirus-related lesions in patients with organ transplantation: 11-year experience at a single institution. Int J Clin Oncol. 2015 doi: 10.1007/s10147-015-0940-2. [DOI] [PubMed] [Google Scholar]
- 14.Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30(11):785–9. doi: 10.1111/j.1365-4362.1991.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 15.Martelli-Marzagao F, Santos Junior GF, Ogawa MM, Enokihara MM, Porro AM, Tomimori J. Human papillomavirus detected in viral warts of renal transplant recipients. Transpl Infect Dis. 2016;18(1):37–43. doi: 10.1111/tid.12479. [DOI] [PubMed] [Google Scholar]
- 16.Euvrard S, Kanitakis J, Chardonnet Y, Noble CP, Touraine JL, Faure M, et al. External anogenital lesions in organ transplant recipients. A clinicopathologic and virologic assessment. Arch Dermatol. 1997;133(2):175–8. [PubMed] [Google Scholar]
- 17.Sillman FH, Sentovich S, Shaffer D. Ano-genital neoplasia in renal transplant patients. Ann Transplant. 1997;2(4):59–66. [PubMed] [Google Scholar]
- 18.Vaccarella S, Franceschi S, Engholm G, Lonnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111(5):965–9. doi: 10.1038/bjc.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carozzi FM, Del Mistro A, Confortini M, Sani C, Puliti D, Trevisan R, et al. Reproducibility of HPV DNA Testing by Hybrid Capture 2 in a Screening Setting. Am J Clin Pathol. 2005;124(5):716–21. doi: 10.1309/84E5-WHJQ-HK83-BGQD. [DOI] [PubMed] [Google Scholar]
- 20.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 21.Practice Bulletin No. 157: Cervical Cancer Screening and Prevention. Obstet Gynecol. 2016;127(1):e1–e20. doi: 10.1097/AOG.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 22.Wilt TJ, Harris RP, Qaseem A High Value Care Task Force of the American College of P. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):718–25. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 23.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–91. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 24.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–42. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 25.Chin-Hong PV, Kwak EJ Practice ASTIDCo. Human papillomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):189–200. doi: 10.1111/ajt.12142. [DOI] [PubMed] [Google Scholar]
- 26.Chin-Hong PV, Palefsky JM. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis. 2002;35(9):1127–34. doi: 10.1086/344057. [DOI] [PubMed] [Google Scholar]
- 27.Long KC, Menon R, Bastawrous A, Billingham R. Screening, Surveillance, and Treatment of Anal Intraepithelial Neoplasia. Clin Colon Rectal Surg. 2016;29(1):57–64. doi: 10.1055/s-0035-1570394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh JC, Kuohung V, Palefsky JM. Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with men. J Acquir Immune Defic Syndr. 2009;52(4):474–9. doi: 10.1097/QAI.0b013e3181bc0f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77(12):1777–82. doi: 10.1097/01.tp.0000120181.89206.54. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;(3):CD001781. doi: 10.1002/14651858.CD001781.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165(2):233–46. doi: 10.1111/j.1365-2133.2011.10218.x. [DOI] [PubMed] [Google Scholar]
- 32.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 33.Bigrigg MA, Codling BW, Pearson P, Read MD, Swingler GR. Colposcopic diagnosis and treatment of cervical dysplasia at a single clinic visit. Experience of low-voltage diathermy loop in 1000 patients. Lancet. 1990;336(8709):229–31. doi: 10.1016/0140-6736(90)91746-w. [DOI] [PubMed] [Google Scholar]
- 34.Hatch KD. Cryotherapy. Baillieres Clin Obstet Gynaecol. 1995;9(1):133–43. doi: 10.1016/s0950-3552(05)80362-1. [DOI] [PubMed] [Google Scholar]
- 35.Ferenczy A. Comparison of cryo- and carbon dioxide laser therapy for cervical intraepithelial neoplasia. Obstet Gynecol. 1985;66(6):793–8. [PubMed] [Google Scholar]
- 36.Duncan ID. Cold coagulation. Baillieres Clin Obstet Gynaecol. 1995;9(1):145–55. doi: 10.1016/s0950-3552(05)80363-3. [DOI] [PubMed] [Google Scholar]
- 37.Bisseling KC, Bekkers RL, Rome RM, Quinn MA. Treatment of microinvasive adenocarcinoma of the uterine cervix: a retrospective study and review of the literature. Gynecol Oncol. 2007;107(3):424–30. doi: 10.1016/j.ygyno.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 38.Morice P, Rouanet P, Rey A, Romestaing P, Houvenaeghel G, Boulanger JC, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete response after chemoradiation therapy for stage IB2 or II cervical cancer. Oncologist. 2012;17(1):64–71. doi: 10.1634/theoncologist.2011-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ACOG practice bulletin. Diagnosis and treatment of cervical carcinomas, number 35, May 2002. Obstet Gynecol. 2002;99(5 Pt 1):855–67. [PubMed] [Google Scholar]
- 40.Fox PA, Nathan M, Francis N, Singh N, Weir J, Dixon G, et al. A double-blind, randomized controlled trial of the use of imiquimod cream for the treatment of anal canal high-grade anal intraepithelial neoplasia in HIV-positive MSM on HAART, with long-term follow-up data including the use of open-label imiquimod. AIDS. 2010;24(15):2331–5. doi: 10.1097/QAD.0b013e32833d466c. [DOI] [PubMed] [Google Scholar]
- 41.Kreuter A, Potthoff A, Brockmeyer NH, Gambichler T, Stucker M, Altmeyer P, et al. Imiquimod leads to a decrease of human papillomavirus DNA and to a sustained clearance of anal intraepithelial neoplasia in HIV-infected men. J Invest Dermatol. 2008;128(8):2078–83. doi: 10.1038/jid.2008.24. [DOI] [PubMed] [Google Scholar]
- 42.Cranston RD, Hirschowitz SL, Cortina G, Moe AA. A retrospective clinical study of the treatment of high-grade anal dysplasia by infrared coagulation in a population of HIV-positive men who have sex with men. Int J STD AIDS. 2008;19(2):118–20. doi: 10.1258/ijsa.2007.005665. [DOI] [PubMed] [Google Scholar]
- 43.Stier EA, Goldstone SE, Berry JM, Panther LA, Jay N, Krown SE, et al. Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. J Acquir Immune Defic Syndr. 2008;47(1):56–61. doi: 10.1097/QAI.0b013e3181582d93. [DOI] [PubMed] [Google Scholar]
- 44.Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Dis Colon Rectum. 2008;51(6):829–35. doi: 10.1007/s10350-008-9233-4. discussion 35-7. [DOI] [PubMed] [Google Scholar]
- 45.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14(9):2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 46.Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, et al. Anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii10–20. doi: 10.1093/annonc/mdu159. [DOI] [PubMed] [Google Scholar]
- 47.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 48.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 49.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 50.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garland SM, Cheung TH, McNeill S, Petersen LK, Romaguera J, Vazquez-Narvaez J, et al. Safety and immunogenicity of a 9-valent HPV vaccine in females 12-26 years of age who previously received the quadrivalent HPV vaccine. Vaccine. 2015;33(48):6855–64. doi: 10.1016/j.vaccine.2015.08.059. [DOI] [PubMed] [Google Scholar]
- 52.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 53.FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–9. [PubMed] [Google Scholar]
- 54.Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–8. [PubMed] [Google Scholar]
- 55.Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. 2013;13(9):2411–7. doi: 10.1111/ajt.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA, 3rd, Read JS, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chin-Hong PV. Cutting human papillomavirus infection in men. J Infect Dis. 2008;197(6):781–3. doi: 10.1086/528380. [DOI] [PubMed] [Google Scholar]
- 59.Diaz ML. Human papilloma virus: prevention and treatment. Obstet Gynecol Clin North Am. 2008;35(2):199–217. vii–viii. doi: 10.1016/j.ogc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Courtney AE, Leonard N, O'Neill CJ, McNamee PT, Maxwell AP. The uptake of cervical cancer screening by renal transplant recipients. Nephrol Dial Transplant. 2009;24(2):647–52. doi: 10.1093/ndt/gfn607. [DOI] [PubMed] [Google Scholar]


