Abstract
The paper focuses on the in vitro antimicrobial activity of Lavandula angustifolia Mill. (lavender) essential oil in combination with four commercial antimicrobial agents. Stock solutions of chloramphenicol, ciprofloxacin, nystatin, and fusidic acid were tested in combination with L. angustifolia essential oil. The antimicrobial activities of the combinations were investigated against the Gram-positive bacterial strain Staphylococcus aureus (ATCC 6538) and Gram-negative Pseudomonas aeruginosa (ATCC 27858) and Candida albicans (ATCC 10231) was selected to represent the yeasts. The antimicrobial effect was performed using the minimum inhibitory concentration (MIC) microdilution assay. Isobolograms were constructed for varying ratios. The most prominent interaction was noted when L. angustifolia essential oil was combined with chloramphenicol and tested against the pathogen P. aeruginosa (ΣFIC of 0.29). Lavendula angustifolia essential oil was shown in most cases to interact synergistically with conventional antimicrobials when combined in ratios where higher volumes of L. angustifolia essential oil were incorporated into the combination.
1. Introduction
The art of using essential oils for therapeutic practice has an extensive history reaching many continents around the world [1]. The practice of aromatherapy and the use of essential oils for therapeutic purposes has maintained its popularity through the ages and continued today with essential oils used as a popular alternate therapy for antimicrobial effects. The increased interest in aromatherapy for these purposes has launched essential oils into the international markets, where they are often sold as “natural antibiotics” [2, 3].
Lavendula angustifolia, considered the most versatile and popular of all essential oils used in aromatherapy, is well documented [4–8]. Within the field of aromatherapy, L. angustifolia essential oil is believed to have antimicrobial value for a plethora of conditions ranging from skin inflictions such as boils and yeast infections to respiratory complaints such as bronchitis and whooping cough [9–11]. The therapeutic use of L. angustifolia essential oil could be traced back to as early as Roman and Greek times, where it was frequently used medicinally due to the apparent lack of toxicity [11]. Lavandula species have also been proven in literature to have the potential to treat bacterial infections commonly associated with resistance to conventional antimicrobial agents. At a concentration of less than 1%, L. angustifolia essential oil inhibits the growth of microorganisms such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis [12].
There have been a number of studies on the antimicrobial activities of essential oils in combination with conventional antibacterial agents [13–20]. A study conducted in 2014 aimed to identify the antimicrobial properties of 35 essential oil-antimicrobial combinations. The study investigated the effects of some popular essential oils such as lavender (Lavandula angustifolia) and tea-tree (Melaleuca alternifolia (Maiden & Betche) Cheel.), and less popular oils such as cinnamon bark (Cinnamomum zeylanicum Blume) and marjoram (Origanum majorana L.) [21]. These oils were tested in combination with common β-lactam penicillin antibiotics and the structurally similar cephalosporins. Of the 35 combinations tested, only four were identified as synergistic. L. angustifolia essential oil in combination with piperacillin against Escherichia coli showed the greatest level of synergy with an ΣFIC value of 0.26.
Despite the popularity and proven activity of L. angustifolia essential oil, no studies could be found that have focused on the antimicrobial potential of L. angustifolia essential oil in combination with non-beta lactam antimicrobial agents. With this in mind, the essential oil of L. angustifolia was investigated in combination with four commercially available antimicrobial agents (nystatin, chloramphenicol, ciprofloxacin, and fusidic acid) to determine what potential interaction may occur should they be used in combination.
2. Materials and Methods
The composition of L. angustifolia Mill. (Robertet®) was confirmed using gas chromatography coupled to a mass spectrometer and flame ionization detector (GC-MS-FID). The GCMS-FID (Agilent 6890N GC system and 5973 MS) was equipped with a HP-Innowax polyethylene glycol column (60 m × 250 μm i.d. × 0.25 μm film thickness). A volume of 1 μL of the essential oil was injected (using a split ratio of 200 : 1) with an autosampler at 24.79 psi and an inlet temperature of 250°C. The GC oven temperature was set at 60°C for 10 minutes and then 220°C at a rate of 4°C/minute for 10 minutes and followed by a temperature of 240°C at a rate of 1°C/minute. Helium was used as a carrier gas at a constant flow of 1.2 mL/minute. Spectra were obtained on electron impact at 70 eV, scanning from 35 to 550 m/z. The percentage composition of the individual components was quantified by integration measurements, using flame ionization detection (FID, 250°C). Component identifications were made by comparing mass spectra from the total ion chromatogram and retention indices using NIST and Mass Finder GC-MS libraries.
For the antimicrobial analysis of L. angustifolia essential oil in combination with conventional antimicrobial agents, stock solutions of ciprofloxacin (≥98.0% purity, Sigma-Aldrich), nystatin (70.0% purity, Sigma-Aldrich), and fusidic acid (≥98.0% purity, Sigma-Aldrich) were made up to a concentration of 0.01 mg/mL using sterile water as the diluent. A 70% ethanol solution was used initially as a solvent for chloramphenicol (≥98.0% purity, Sigma-Aldrich) and thereafter diluted in sterile water. These antimicrobial agents were selected for analysis due to their indication in respiratory and topical infections. Chloramphenicol was selected due to its indication in the treatment of serious Gram-negative bacterial infections [22]. Chloramphenicol was also investigated against C. albicans as it has been indicated in previous literature as having substantial antifungal activity [23]. Ciprofloxacin, a first-generation fluoroquinolone antibiotic, was selected due to its broad-spectrum activity. Fusidic acid was selected due to its specific use in the treatment of Gram-positive bacterial infections, while nystatin was chosen to represent the class of antifungal agents [22]. The oil of L. angustifolia was prepared to yield a stock concentration of 32 mg/mL in acetone. The following ratios (in μL) of L. angustifolia essential oil and the antimicrobial agents under analysis were investigated (L. angustifolia essential oil: antimicrobial agent): 90 : 10, 80 : 20, 70 : 30, 60 : 40, 50 : 50, 40 : 60, 30 : 70, 20 : 80, and 10 : 90. For each ratio tested, the essential oil and antimicrobial concentration varied and the exact concentration for each ratio is given in Table 1. The antimicrobial activities of the combinations were investigated against the Gram-positive bacterial strain Staphylococcus aureus (ATCC 6538) and Gram-negative strain Pseudomonas aeruginosa (ATCC 27858) and Candida albicans (ATCC 10231) was selected to represent the yeasts. The antimicrobial effect was determined by means of the minimum inhibitory concentration (MIC) microdilution assay [24]. The microtitre plates were prepared by adding 100 μL of sterile, distilled water into each of the wells. The antimicrobial agents and L. angustifolia essential oil were added to the first row, at a volume of 100 μL (where they were tested individually) and 50 : 50 μL (where they were tested in equal volume combinations). Negative controls (water/acetone) were included in each assay to determine the antimicrobial activity of the solvent. Sterile media, such as Tryptone Soya Broth (TSB), and culture controls were included to confirm sterility and viability, respectively. The Clinical and Laboratory Standards Institute (CLSI) [25] and KnowledgeBase [26] were used to compare antimicrobial break points of the MIC values obtained from antimicrobial testing. This is undertaken to ensure that the pathogen responded to the tested antimicrobial in a reproducible manner and that the MIC values were within the recommended ranges (Table 3). Cultures were added to all the wells of their respective microtitre plates, at a concentration of 1 × 106 colony forming units (CFU)/mL. The microtitre plates were incubated under optimal conditions of 37°C for 24 hours for bacteria and 37°C for 48 hours for the yeast. After incubation, p-iodonitrotetrazolium violet solution (INT) at a concentration of 0.4 mg/mL was added into each well (40 μL), upon which colour changes were noted approximately six hours later for the bacteria and 24 hours later for the yeast. The lowest concentration identified with no growth or colour change was determined as the MIC. The fractional inhibitory concentration (FIC) and the FIC index (ΣFIC) were calculated to determine the interaction between L. angustifolia essential oil and the selected antimicrobial agents. The ΣFIC for each combination was interpreted as synergistic where ΣFIC was less than or equal to 0.50. For additive properties, ΣFIC was interpreted as greater than 0.50 but less than or equal to 1.00. For indifference, ΣFIC values are greater than 1.00 but less than or equal to 4.00 and antagonism occurs when ΣFIC greater than 4.00 is observed [27].
Table 1.
The concentrations of essential oil and antimicrobial agent associated with the volume ratios studied.
| Plot number∗ | Volume ratio of essential oil : antimicrobial agent | Concentration of antimicrobial agent in combination | Concentration of essential oil in combination |
|---|---|---|---|
| μL | μg/mL | mg/mL | |
| 1 | 90 : 10 | 0.25 | 7.2 |
| 2 | 80 : 20 | 0.50 | 6.4 |
| 3 | 70 : 30 | 0.75 | 5.6 |
| 4 | 60 : 40 | 1.00 | 4.8 |
| 5 | 50 : 50 | 1.25 | 4.0 |
| 6 | 40 : 60 | 1.50 | 3.2 |
| 7 | 30 : 70 | 1.75 | 2.4 |
| 8 | 20 : 80 | 2.00 | 1.6 |
| 9 | 10 : 90 | 2.25 | 0.8 |
∗Referring to points on isobologram graphs.
Table 3.
Mean MIC values in μg/mL (n = 3) for the antimicrobial agents selected for combination analysis.
| Antimicrobial agent | C. albicans | S. aureus | P. aeruginosa | |||
|---|---|---|---|---|---|---|
| ATCC 10231 | ATCC 6538 | ATCC 27858 | ||||
| Value obtained | Break point range∗ | Value obtained | Break point range | Value obtained | Break point range | |
| Chloramphenicol | 0.63 | <4.00 | 0.31 | 0.10–15.60 | 0.31 | 0.06–32.00 |
| Ciprofloxacin | NA† | NA | 0.11 | 0.12–0.50 | 0.04 | <1.00–2.50 |
| Fusidic acid | NA | NA | 0.63 | 0.10–>32.00 | 0.31 | ≤1.00 |
| Nystatin | 0.16 | ≤4.00 | NA | NA | NA | NA |
∗Break point ranges for commercial antimicrobials as determined by the KnowledgeBase antimicrobial index, CLSI guidelines, and EUCAST antimicrobial index; †NA: not applicable as microorganisms are not susceptible to these antimicrobial agents.
For the varied concentrations of L. angustifolia essential oil, antibiotic combination, isobolograms were constructed using GraphPad Prism, version five® software to present the mean MIC values of the combinations as ratios [28]. The isobolograms were interpreted by examining the data points for each ratio in relation to the MIC values for the oils independently. All points between the 0.5 : 0.5 and 1.0 : 1.0 line were interpreted as additive and above the 1.0 : 1.0 line and below the 4.0 : 4.0 line were classified as noninteractive. The points below or on the 0.5 : 0.5 line on the isobologram were interpreted as synergistic with antagonism noted for data points above the 4.0 : 4.0 line [27]. All tests were undertaken in duplicate and when results differed by more than one dilution factor, a third replicate was undertaken.
3. Results and Discussion
The chemical composition of L. angustifolia essential oil was determined to confirm the specific chemotype (Table 2). A full chemical profile was established for this essential oil, with the major constituents, linalyl acetate (36.7%), linalool (31.4%), and terpinen-4-ol (14.9%), identified. The chemical composition of L. angustifolia essential oil has been studied extensively in literature and the composition reported here is congruent with literature [29–32].
Table 2.
The chemical composition of Lavandula angustifolia Mill. essential oil.
| RRI | Constituents | Percentage abundance |
|---|---|---|
| 1016 | α-Pinene | 0.1 |
| 1019 | α-Thujene | t.a.∗ |
| 1057 | Camphene | 0.1 |
| 1104 | β-Pinene | t.a.∗ |
| 1159 | Myrcene | 0.2 |
| 1193 | α-Terpinene | t.a.∗ |
| 1194 | Limonene | 0.1 |
| 1202 | Eucalyptol | 0.5 |
| 1232 | β-trans-Ocimene | 3.0 |
| 1242 | γ-Terpinene | 0.1 |
| 1250 | β-cis-Ocimene | 2.1 |
| 1319 | 3-Octanone | 0.4 |
| 1270 | p-Cymene | 0.1 |
| 1281 | Terpinolene | t.a.∗ |
| 1331 | Hexyl butyrate | 0.1 |
| 1372 | Allo-ocimene | 0.5 |
| 1376 | 1-Octenyl acetate | 0.8 |
| 1385 | 3-Octanol | 0.1 |
| 1411 | n-Hexyl butyrate | 0.3 |
| 1441 | Hexyl-2-methylbutyrate | t.a.∗ |
| 1447 | cis-Linalool oxide | 0.2 |
| 1471 | cis-Linalool oxide | 0.1 |
| 1521 | Camphor | 0.3 |
| 1541 | Linalool | 31.4† |
| 1563 | Linalyl acetate | 36.7† |
| 1572 | α-Cedrene | t.a.∗ |
| 1573 | α-Santalene | 0.4 |
| 1584 | α-Bergamotene | 0.3 |
| 1602 | Terpinen-4-ol | 14.9† |
| 1665 | β-Farnesene | 1.4 |
| 1677 | Lavandulol | 1.2 |
| 1680 | Cryptone | 0.2 |
| 1701 | α-Terpineol | 0.3 |
| 1702 | Borneol | 0.7 |
| 1741 | Carvone | t.a.∗ |
| 1763 | γ-Cadinene | 0.2 |
| 1797 | Cuminaldehyde | 0.1 |
| 1855 | p-Cymen-8-ol | 0.3 |
| 2010 | Caryophyllene epoxide | t.a.∗ |
| 2225 | Thymol | 0.1 |
|
| ||
| Total | 97.30% | |
∗t.a. indicates trace amounts; † indicates major chemical constituents.
Initially, confirmation of the antimicrobial activity of the four antimicrobial agents was undertaken against the three test microorganisms (Table 3). Where possible, the MIC values determined were congruent with breakpoint expectations. Only S. aureus demonstrated a marginally higher susceptibility pattern (0.11 μg/mL) than the expected range (0.12–0.50 μg/mL) for ciprofloxacin, but given that the assay is working with doubling dilutions, this is not significant.
The mean MIC values obtained for L. angustifolia essential oil against the tested pathogens were 3.00 mg/mL, 2.00 mg/mL, and 2.00 mg/mL for C. albicans, S. aureus, and P. aeruginosa, respectively. Synergy was noted for two interactions, L. angustifolia essential oil in combination with ciprofloxacin against S. aureus (ΣFIC of 0.49) and L. angustifolia essential oil in combination with chloramphenicol against P. aeruginosa (ΣFIC of 0.29) (Table 4). Before this study, no research has been conducted on the antimicrobial interaction between L. angustifolia essential oil and chloramphenicol, nor chloramphenicol in combination with other natural therapies against P. aeruginosa. Chloramphenicol has, however, been placed in combination with other essential oils and tested against other microorganisms. A study conducted in 2013 determined the antimicrobial effect of chloramphenicol in combination with Coriandrum sativum L. essential oil against Acinetobacter baumannii using the ΣFIC analysis [33]. This combination also demonstrated considerable synergistic antimicrobial potential with a ΣFIC value of 0.28. The antimicrobial potential of this agent in combination with essential oil compounds is further augmented by a study conducted by Halawani [34]. According to Halawani [34], chloramphenicol in combination with thymoquinone and thymohydroquinone (the major chemical constituents of the essential oil Nigella sativa L.) demonstrated synergistic antimicrobial effects against E. coli, S. aureus, P. aeruginosa, and Salmonella typhimurium. These studies further demonstrate the antimicrobial potential of this antimicrobial agent in combination with essential oils.
Table 4.
The antimicrobial effect of the individual components within the essential oil : antimicrobial combination.
| Microorganism | MIC individual | ΣFIC | Interpretation | ||
|---|---|---|---|---|---|
| LA | Antimicrobial | ||||
| (mg/mL) | (μg/mL) | ||||
| C. albicans (ATCC 10231) | 3.00 | CH | 0.63 | 1.00 | Additive |
| N | 0.16 | 1.14 | Noninteractive | ||
|
| |||||
| S. aureus (ATCC 6538) | 2.00 | CH | 0.31 | 0.75 | Additive |
| C | 0.11 | 0.49 | Synergistic | ||
| FA | 0.63 | 0.65 | Additive | ||
|
| |||||
| P. aeruginosa (ATCC 27858) | 2.00 | CH | 0.31 | 0.29 | Synergistic |
| C | 0.04 | 0.74 | Additive | ||
| FA | 0.31 | 1.13 | Noninteractive | ||
LA indicates L. angustifolia essential oil, CH indicates chloramphenicol, N indicates nystatin, C indicates ciprofloxacin, and FA indicates fusidic acid.
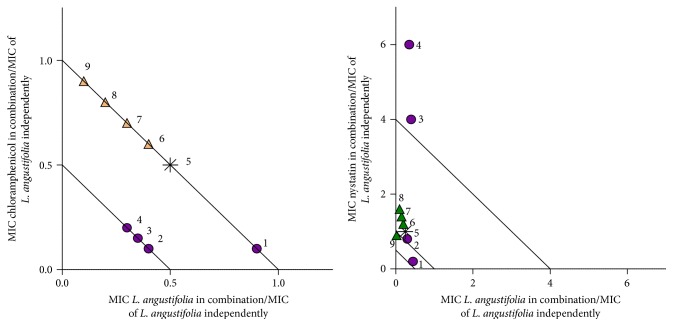
In order to determine what interactions could be apparent when the concentration of oil and conventional antibiotic varies, further investigation was undertaken and plotted on isobolograms (Table 1, Figures 1 –3). When investigating the combination of chloramphenicol and nystatin with L. angustifolia essential oil (Figure 1), synergy was noted for three of the nine ratios with chloramphenicol : L. angustifolia essential oil (20 : 80, 30 : 70, and 40 : 60) and one synergistic interaction (20 : 80) for nystatin : L. angustifolia essential oil. For the combination with chloramphenicol, synergy was more apparent where L. angustifolia essential oil was present in higher volume ratios. Indifference was mostly observed for the combinations of nystatin with L. angustifolia essential oil. Two ratios demonstrated antagonism (where nystatin is combined with L. angustifolia essential oil) at ratios of 20 : 80 and 30 : 70.
Figure 1.
Isobologram representation of L. angustifolia essential oil in combination with chloramphenicol and nystatin at various ratios against C. albicans (ATCC 10231). ● indicates L. angustifolia essential oil in majority volume, ▲ indicates the antimicrobial agent in majority volume, and ∗ indicates equal volume of L. angustifolia essential oil to antimicrobial agent. Points 1–9 (Table 1) provide exact concentrations for antimicrobial and lavender oil.
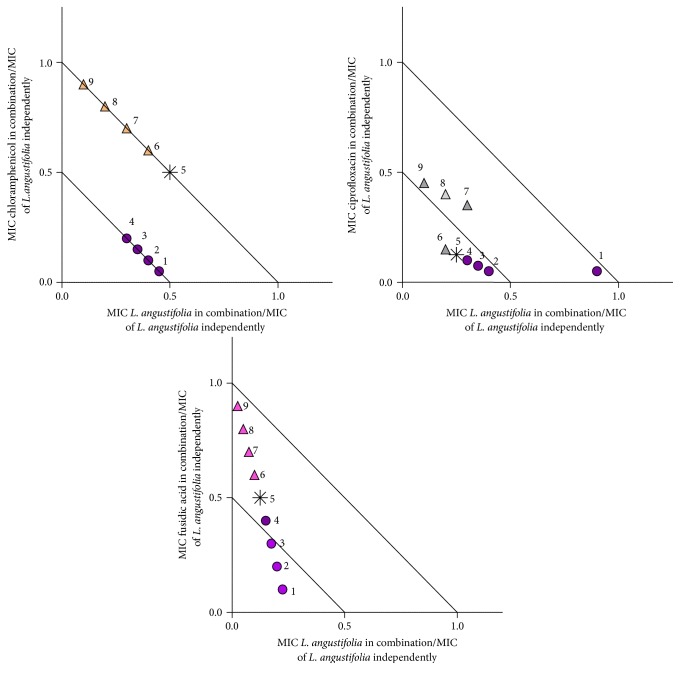
Figure 2.
Isobologram representation of L. angustifolia essential oil in combination with chloramphenicol, ciprofloxacin, and fusidic acid at various ratios against S. aureus (ATCC 6538). ● indicates L. angustifolia essential oil in majority volume, ▲ indicates the antimicrobial agent in majority volume, and ∗ indicates equal volume of L. angustifolia essential oil to antimicrobial agent. Points 1–9 (Table 1) provide exact concentrations for antimicrobial and lavender oil.
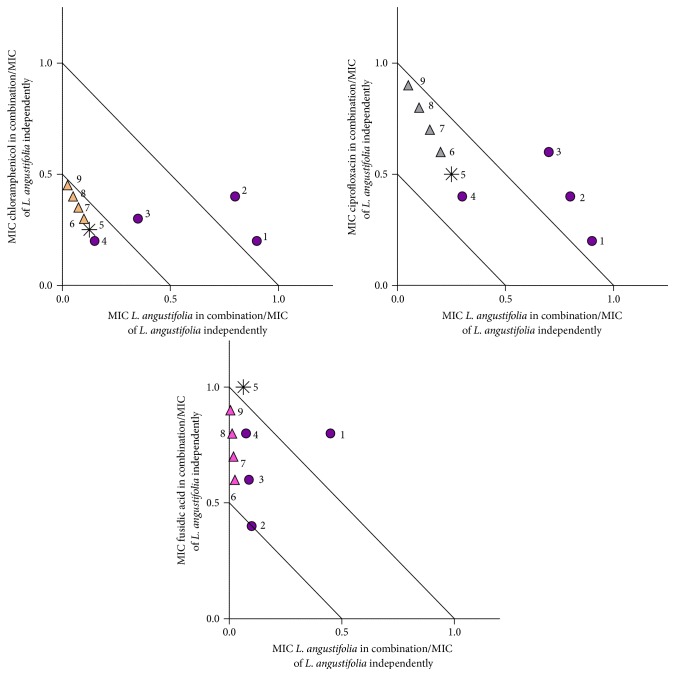
Figure 3.
Isobologram representation of L. angustifolia essential oil in combination with chloramphenicol, ciprofloxacin, and fusidic acid at various ratios against P. aeruginosa (ATCC 27858). ● indicates L. angustifolia essential oil in majority volume, ▲ indicates the antimicrobial agent in majority volume, and ∗ indicates equal volume of L. angustifolia essential oil to antimicrobial agent. Points 1–9 (Table 1) provide exact concentrations for antimicrobial and lavender oil.
Against S. aureus, L. angustifolia essential oil was combined with the antimicrobial agents chloramphenicol, ciprofloxacin, and fusidic acid (Figure 2). Synergy was observed for the combination of chloramphenicol and L. angustifolia essential oil in four of the ratios investigated (chloramphenicol : L. angustifolia essential oil 10 : 90, 20 : 80, 30 : 70, and 40 : 60), with a predominantly additive relationship identified for the remainder of the other combinations tested. When placed in combination with ciprofloxacin at varying ratios, five of the nine ratios investigated were identified as synergistic (40 : 60, 50 : 50, 60 : 40, 70 : 30, and 20 : 80). The combination of fusidic acid and L. angustifolia essential oil varied between additive and synergistic, depending on the ratio. Synergy was observed for three of the ratios investigated (10 : 90, 20 : 80, and 30 : 70).
When investigated against P. aeruginosa, the L. angustifolia essential oil combinations with chloramphenicol, ciprofloxacin, and fusidic acid demonstrated varied interactions (Figure 3). Mainly synergistic antimicrobial interactions occurred with six of the nine ratios investigated for the combination of L. angustifolia essential oil with chloramphenicol. When placed in combination with ciprofloxacin, additive effects were predominant. Similarly, the combination of fusidic acid and L. angustifolia essential oil in various combinations demonstrated mainly additive interactions, with synergy identified for one ratio (20 : 80).
Potentiation of an antimicrobial agent results when a technique is applied to the antimicrobial agent causing it to demonstrate a greater antimicrobial effect at a lower concentration [35]. A number of studies have been conducted on the potential of plants to potentiate antimicrobial agents [36–45]. One study determined that Rosmarinus officinalis L. essential oil potentiates the antimicrobial activity of erythromycin 16–32-fold against S. aureus [40].
The most promising combination is noted for L. angustifolia essential oil with fusidic acid against S. aureus in which the antimicrobial effect of this agent is potentiated 5-fold. Fusidic acid is primarily applied in the treatment of topical Staphylococcal infections; therefore the potentiation of this antimicrobial agent suggests promise for the use of this combination within future pharmaceutical preparations.
When combined with chloramphenicol, it was noted that ratios higher in L. angustifolia essential oil were responsible for the synergistic antimicrobial effects of the combination; however, an antagonistic interaction was observed with the combination of nystatin and L. angustifolia essential oil. This demonstrates that, in spite of the positive results obtained against some pathogens, caution should still be exercised. The safety of combining complementary therapy and conventional prescription medications has been of concern [46]. A study conducted by Van Vuuren et al. [47] substantiates this point as the combination of M. alternifolia essential oil and ciprofloxacin against the microorganism S. aureus was shown to yield an antagonistic antimicrobial effect with ΣFIC values ranging from 5.17 to 7.70, dependant on the concentration at which these agents were combined.
Ciprofloxacin is indicated in the treatment of many bacterial infections including S. aureus related infections [22], while L. angustifolia essential oil is indicated mainly for the treatment of superficial skin infections, many of which originate from a Staphylococcal source [9–11]. Interestingly, this combination showed that L. angustifolia essential oil potentiated the antimicrobial effects of ciprofloxacin suggesting some therapeutic benefit in combining these agents. When investigated against P. aeruginosa, L. angustifolia essential oil was placed in combination with chloramphenicol, ciprofloxacin, and fusidic acid.
The outcomes obtained indicate that L. angustifolia essential oil has the potential to improve the antibacterial effects of some commercial antibiotics. This may be as a result of the interactions occurring between the chemical compounds of L. angustifolia essential oil and the antimicrobial agents used. Due to the complexity of essential oil chemistry it has been determined that essential oils have the ability to affect any number of antimicrobial pathways. Studies on the effects of the major chemical constituents of L. angustifolia essential oil, linalool, linalyl acetate, and terpinen-4-ol, indicate the mechanism of action of these components to comprise mainly of damage to the lipid layer of the cell membrane, resulting in bacterial cell leakage [48–50]. Although some individual chemical compounds have been determined as having antimicrobial potential independently, the effects of the essential oils are predominantly due to the interactions of the chemical entities [51]. The antimicrobial agent most potentiated by L. angustifolia essential oil was fusidic acid. Fusidic acid causes damage to bacterial cells by reducing protein synthesis [22]. The combination of L. angustifolia essential oil and fusidic acid highlights the potential of multitarget effects between essential oils and their chemistry when used in combination with conventional antimicrobial agents. In order for antimicrobial resistance to be overcome, such a mechanism is required in proposed combinations in order to render a greater antimicrobial effect [52]. This interaction is further observed amongst the combinations eliciting additive and synergistic effects such as L. angustifolia essential oil in combination with ciprofloxacin (inhibiting DNA enzymes [22]) and L. angustifolia essential oil in combination with chloramphenicol (inhibiting protein synthesis [22]). One needs to remain cognisant that synergistic interactions may not necessarily be as a result of the major compounds (only) but minor compounds may also play a role in the positive interactions observed.
4. Conclusion
Antimicrobial resistance has been on the rise over recent years with an unknown number of resistant bacterial strains developed. The microorganism Enterococcus faecium, responsible for causing infections such as endocarditis, cellulitis, and bacteremia [22], had, as of 2009, developed a high level of resistance to aminoglycoside antibiotics. No known alternative for treatment currently exists [53]. Due to this growing level of resistance, research has been undertaken to develop new products with antimicrobial potential for use independently and in combination with current antibiotics, with researchers looking towards natural products and plants for solutions [54]. The lack of antagonism and indication of possible drug potentiation within this study further augments the potential for the use of essential oils in combination with antimicrobial agents for a synergistic antimicrobial effect. This study suggests some therapeutic potential in combining L. angustifolia essential oil with common antimicrobial agents in the future.
Acknowledgments
The authors thank the National Research Foundation for their generous contribution towards bursary funding. They appreciate the assistance of Dr. Guy Kamatou with the analysis of the essential oil which was kindly supplied by Robertet (France).
Competing Interests
The authors declare no conflict of interests.
References
- 1.Buckle J. Clinical Aromatherapy: Essential Oils in Practice. 2nd. Churchill Livingstone, LDN; 2003. [Google Scholar]
- 2.Hili P. The Antimicrobial Properties of Essential Oils. Winter Press, KNT; 2001. [Google Scholar]
- 3.van Vuuren S. F. Antimicrobial activity of South African medicinal plants. Journal of Ethnopharmacology. 2008;119(3):462–472. doi: 10.1016/j.jep.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Grieve M. A Modern Herbal. New York, NY, USA: Harcourt, Brace & Co; 1931. [Google Scholar]
- 5.Gattefosse R. M. Gattefosse's Aromatherapy. Saffron Walden, UK: Ebury Publishing; 1937. [Google Scholar]
- 6.Cassella S., Cassella J. P., Smith I. Synergistic antifungal activity of tea tree (Melaleuca alternifolia) and lavender (Lavandula angustifolia) essential oils against dermatophyte infection. International Journal of Aromatherapy. 2002;12(1):2–15. doi: 10.1054/ijar.2001.0127. [DOI] [Google Scholar]
- 7.Cavanagh H. M. A., Wilkinson J. M. Biological activities of lavender essential oil. Phytotherapy Research. 2002;16(4):301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 8.Saviuc C.-M., Drumea V., Olariu L., Chifiriuc M.-C., Bezirtzoglou E., Lazăr V. Essential oils with microbicidal and antibiofilm activity. Current Pharmaceutical Biotechnology. 2015;16(2):137–151. doi: 10.2174/138920101602150112151549. [DOI] [PubMed] [Google Scholar]
- 9.Sellar W. The Directory of Essential Oils. C.W. Daniel Company Limited, LDN; 1992. [Google Scholar]
- 10.Lawless J. The Illustrated Encyclopedia of Essential Oils: The Complete Guide to the use of oils in Aromatherapy and Herbalism. Rockport, Mass, USA: Element Books Limited; 1995. [Google Scholar]
- 11.Curtis S. Essential Oils. Aurum Press, LDN; 1996. [Google Scholar]
- 12.Nelson R. R. S. In-vitro activities of five plant essential oils against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium . Journal of Antimicrobial Chemotherapy. 1997;40(2):305–306. doi: 10.1093/jac/40.2.305. [DOI] [PubMed] [Google Scholar]
- 13.Avenirova E. L., Ashmarin I. P., Movchan N. A., Lapina I. K. Combination of novoimanine with antibiotics with a different mechanism of action. Antibiotiki. 1975;20(7):636–639. [PubMed] [Google Scholar]
- 14.Suresha B., Sriram S., Dhanaraj S. A., Elango K., Chinnaswamy K. Anticandidal activity of Santolina chamaecyparissus volatile oil. Journal of Ethnopharmacology. 1997;55(2):151–159. doi: 10.1016/s0378-8741(96)01490-0. [DOI] [PubMed] [Google Scholar]
- 15.Shiota S., Shimizu M., Mizusima T., et al. Restoration of effectiveness of β-lactams on methicillin-resistant Staphylococcus aureus by tellimagrandin I from rose red. FEMS Microbiology Letters. 2000;185(2):135–138. doi: 10.1016/s0378-1097(00)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.Shin S., Kang C.-A. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Letters in Applied Microbiology. 2003;36(2):111–115. doi: 10.1046/j.1472-765x.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 17.Giordani R., Trebaux J., Masi M., Regli P. Enhanced antifungal activity of ketoconazole by Euphorbia characias latex against Candida albicans . Journal of Ethnopharmacology. 2001;78(1):1–5. doi: 10.1016/s0378-8741(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 18.Hemaiswarya S., Kruthiventi A. K., Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzi V., Muselli A., Bernardini A. F., et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrobial Agents and Chemotherapy. 2009;53(5):2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadli M., Chevalier J., Saad A., Mezrioui N.-E., Hassani L., Pages J.-M. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. International Journal of Antimicrobial Agents. 2011;38(4):325–330. doi: 10.1016/j.ijantimicag.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Yap P. S. X., Lim S. H. E., Hu C. P., Yiap B. C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013;20(8-9):710–713. doi: 10.1016/j.phymed.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Beers M. H., Porter R. S., Jones T. V., Kaplan J. L., Berkwits M. The Merck Manual of Diagnosis and Therapy. 18th. Rahway, NJ, USA: Merck Research Laboratories; 2006. [Google Scholar]
- 23.Logemann W., Almirante L., Galimberti S., De Carneri I. Influence of dichloroacetylation on the antimicrobial activity of chloramphenicol derivatives and of various amines. British Journal of Pharmacology and Chemotherapy. 1961;17:286–296. doi: 10.1111/j.1476-5381.1961.tb01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Rapper S., Van Vuuren S. F., Kamatou G. P. P., Viljoen A. M., Dagne E. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils—a combination from the pharaonic pharmacopoeia. Letters in Applied Microbiology. 2012;54(4):352–358. doi: 10.1111/j.1472-765x.2012.03216.x. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Laboratory and Science Institute (CLSI) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard M7-A6. 6th. Wayne, Pa, USA: National Committee for Clinical Laboratory Standards; 2003. [Google Scholar]
- 26.KnowledgeBase. KnowledgeBase: The antimicrobial index. http://antibiotics.toku-e.com/
- 27.van Vuuren S., Viljoen A. Plant-based antimicrobial studies—methods and approaches to study the interaction between natural products. Planta Medica. 2011;77(11):1168–1182. doi: 10.1055/s-0030-1250736. [DOI] [PubMed] [Google Scholar]
- 28.Suliman S., van Vuuren S. F., Viljoen A. M. Validating the in vitro antimicrobial activity of Artemisia afra in polyherbal combinations to treat respiratory infections. South African Journal of Botany. 2010;76(4):655–661. doi: 10.1016/j.sajb.2010.07.003. [DOI] [Google Scholar]
- 29.Daferera D. J., Ziogas B. N., Polissiou M. G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum . Journal of Agricultural and Food Chemistry. 2000;48(6):2576–2581. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- 30.Behnam S., Farzaneh M., Ahmadzadeh M., Tehrani A. S. Composition and antifungal activity of essential oils of Mentha piperita and Lavandula angustifolia on post-harvest phytopathogens. Communications in Agricultural and Applied Biological Sciences. 2006;71(3):1321–1326. [PubMed] [Google Scholar]
- 31.Roller S., Ernest N., Buckle J. The antimicrobial activity of high-necrodane and other lavender oils on methicillin-sensitive and -resistant Staphylococcus aureus (MSSA and MRSA) Journal of Alternative and Complementary Medicine. 2009;15(3):275–279. doi: 10.1089/acm.2008.0268. [DOI] [PubMed] [Google Scholar]
- 32.Soković M., Glamočlija J., Marin P. D., Brkić D., van Griensven L. J. L. D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15(11):7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duarte A., Ferreira S., Silva F., Domingues F. C. Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii . Phytomedicine. 2012;19(3-4):236–238. doi: 10.1016/j.phymed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Halawani E. Antibacterial activity of thymoquinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Advanced Biomedical Research. 2009;3:148–152. [Google Scholar]
- 35.Hare J. H. Antibiotic potentiation—a review. Canadian Journal of Comparative Medicine and Veterinary Science. 1960;24:171–176. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W.-H., Hu Z.-Q., Okubo S., Hara Y., Shimamura T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2001;45(6):1737–1742. doi: 10.1128/aac.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Z.-Q., Zhao W.-H., Asano N., Yoda Y., Hara Y., Shimamura T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2002;46(2):558–560. doi: 10.1128/aac.46.2.558-560.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibbons S., Oluwatuyi M., Veitch N. C., Gray A. I. Bacterial resistance modifying agents from Lycopus europaeus . Phytochemistry. 2003;62(1):83–87. doi: 10.1016/s0031-9422(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 39.Gibbons S., Moser E., Kaatz G. W. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus . Planta Medica. 2004;70(12):1240–1242. doi: 10.1055/s-2004-835860. [DOI] [PubMed] [Google Scholar]
- 40.Oluwatuyi M., Kaatz G. W., Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis . Phytochemistry. 2004;65(24):3249–3254. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Stapleton P. D., Shah S., Anderson J. C., Hara Y., Hamilton-Miller J. M. T., Taylor P. W. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. International Journal of Antimicrobial Agents. 2004;23(5):462–467. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Shibata H., Kondo K., Katsuyama R., et al. Alkyl gallates, intensifiers of β-lactam susceptibility in methicillin-resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2005;49(2):549–555. doi: 10.1128/aac.49.2.549-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marquez B., Neuville L., Moreau N. J., et al. Multidrug resistance reversal agent from Jatropha elliptica . Phytochemistry. 2005;66(15):1804–1811. doi: 10.1016/j.phytochem.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Al-hebshi N., Al-haroni M., Skaug N. In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms. Archives of Oral Biology. 2006;51(3):183–188. doi: 10.1016/j.archoralbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Smith E. C. J., Williamson E. M., Wareham N., Kaatz G. W., Gibbons S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana . Phytochemistry. 2007;68(2):210–217. doi: 10.1016/j.phytochem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Hübsch Z., Van Zyl R. L., Cock I. E., van Vuuren S. F. Interactive antimicrobial and toxicity profiles of conventional antimicrobials with Southern african medicinal plants. South African Journal of Botany. 2014;93:185–197. doi: 10.1016/j.sajb.2014.04.005. [DOI] [Google Scholar]
- 47.Van Vuuren S. F., Suliman S., Viljoen A. M. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Letters in Applied Microbiology. 2009;48(4):440–446. doi: 10.1111/j.1472-765X.2008.02548.x. [DOI] [PubMed] [Google Scholar]
- 48.Carson C. F., Mee B. J., Riley T. V. Mechanism of action of Melaleuca alternifolia (Tea Tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrobial Agents and Chemotherapy. 2002;46(6):1914–1920. doi: 10.1128/aac.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trombetta D., Castelli F., Sarpietro M. G., et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrobial Agents and Chemotherapy. 2005;49(6):2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Romero J. C., González-Ríos H., Borges A., Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus . Evidence-Based Complementary and Alternative Medicine. 2015;2015:9. doi: 10.1155/2015/795435.795435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyldgaard M., Mygind T., Meyer R. L. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology. 2012;3, article 12 doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langeveld W. T., Veldhuizen E. J., Burt S. A. Synergy between essential oil components and antibiotics: a review. Critical Reviews in Microbiology. 2013;40(1):76–94. doi: 10.3109/1040841x.2013.763219. [DOI] [PubMed] [Google Scholar]
- 53.Arias C. A., Murray B. E. Antibiotic-resistant bugs in the 21st century-a clinical super-challenge. New England Journal of Medicine. 2009;360(5):439–443. doi: 10.1056/nejmp0804651. [DOI] [PubMed] [Google Scholar]
- 54.Othman M., Loh H. S., Wiart C., Rhoo T. J., Lim K. H., Ting K. N. Optimal methods for evaluating antimicrobial activities from plant extracts. Journal of Microbiological Methods. 2011;80:161–166. doi: 10.1016/j.mimet.2010.11.008. [DOI] [PubMed] [Google Scholar]