Abstract
Objective
The Critical Illness Stress-Induced Immune Suppression (CRISIS) Prevention Trial was a randomized, masked trial of zinc, selenium, glutamine and metoclopramide compared to whey protein in delaying nosocomial infection in pediatric intensive care unit (PICU) patients. One fourth of study subjects were diagnosed with nosocomial lower respiratory infection (LRI), which contributed to subjects receiving antibiotics 74% of all patient days in the PICU. We analyzed diagnostic and treatment variability among the participating institutions and compared outcomes between nosocomial LRI subjects (n=74) and intubated subjects without nosocomial infection (n=155).
Design
Post-hoc analysis
Setting
Eight hospitals in the Collaborative Pediatric Critical Care Research Network
Patients
CRISIS study subjects.
Interventions
None
Measurements and Main Results
Variability across institutions existed in the frequency and manner by which respiratory secretion cultures were obtained, processed and results reported. Most results were reported semi-quantitatively and both Gram stains and antibiotic sensitivities were frequently omitted. The nosocomial LRI diagnosis was associated with increased PICU lengths of stay compared to those who were intubated without nosocomial infection (24±19 vs 9±6 days; p<0.001) and antibiotic use (38±29 vs. 15±20 antibiotics days; p<0.001). Despite antibiotic treatment, the same bacteria persisted in 45% of follow-up cultures.
Conclusions
The CRISIS data demonstrate that the nosocomial LRI diagnosis is associated with longer lengths of stay and increased antibiotic use, but there is considerable diagnostic and treatment variability across institutions. More rigorous standards for when and how respiratory cultures are obtained, processed, and reported are necessary. Bacterial persistence also complicates the interpretation of follow-up cultures.
Introduction
Hospital-acquired infections have a higher mortality than any of the top ten leading illnesses in the United States (1) and this fosters a low threshold for initiating antibiotics in critically ill patients. While early and aggressive use of antibiotics can be lifesaving, their indiscriminate use contributes to the emergence of multi-resistant microbes. Thus, accurate and expedient diagnosis of nosocomial infection in the intensive care unit is imperative to assure adequate treatment while avoiding unnecessary antibiotic exposure.
Ventilator-associated infections (VAIs) are the most common nosocomial infections in the Pediatric Intensive Care Unit (PICU) and the most frequent reason for antibiotic use (2). The high degree of variability in reported incidence (3–5), however, reflects the ambiguity and subjectivity of the Centers for Disease Control (CDC) diagnostic criteria (6). No gold standard exists for the diagnosis of VAI and the recent adoption of the ventilator-associated events (VAE) criteria (7) in adult medicine is in large part responsive to the inadequacies of the previously established CDC criteria (6). Unfortunately, despite their greater objectivity, the new VAE criteria do not address the indications for antibiotics (8).
The Critical Illness Stress-Induced Immune Suppression (CRISIS) Prevention Trial (9) illustrates the problems with the CDC VAI criteria and their impact on antibiotic use. CRISIS was a randomized, masked comparative effectiveness trial of a combination of zinc, selenium, glutamine and metoclopramide (ZSGM) compared to whey protein in delaying the onset of nosocomial infection in PICU patients. While the study failed to demonstrate benefit with ZSGM, one fourth (26%) of the study subjects were diagnosed with one or more episodes of VAI, specifically lower respiratory infection (LRI), based on CDC criteria. The CDC LRI criteria require only isolation of a pathogen from a respiratory secretion culture in the presence of fever, hypothermia, chills, or hypotension (6). The frequency of this diagnosis was a major contributor to the fact that subjects in the study spent 74% of all patient days in the PICU on one or more antibiotics.
We questioned the significance of the nosocomial LRI diagnosis and frequent use of antibiotics in the CRISIS study. Consequently, we analyzed the study data to evaluate diagnostic and treatment variability among the participating institutions, to identify the putative pathogens isolated from respiratory secretion cultures and their response to antibiotics, and to assess whether the nosocomial LRI diagnosis was associated with morbidity or mortality relative to subjects who required mechanical ventilation, but were not diagnosed with nosocomial infection.
Methods
The study was an analysis of the limited data set from the CRISIS study made available and approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network. No private health information was included in the data set and, as such, the study did not constitute human research and Institutional Review Board approval was not sought.
The CRISIS Prevention Trial was a randomized, masked comparative effectiveness trial of the combination of zinc, selenium, glutamine and metoclopramide (ZSGM) compared to whey protein alone in delaying the onset of nosocomial infection in critically ill children. Entry criteria included ages 1 to 18 years, enrollment within 48 hours of PICU admission, the presence of one or more invasive devices (central venous catheter, urinary catheter, or endotracheal tube), and the expectation that the subject would have venous or arterial access for the purpose of blood draws during the first 3 days of the study. Subjects were randomized to receive daily enteral whey protein powder or ZSGM for 28 days or until PICU discharge, whichever came first. The subjects were followed for the development of nosocomial infection in the PICU and up to 5 days after PICU discharge for a maximum of 33 days. A total of 293 subjects were randomized across 8 hospitals affiliated with the Collaborative Pediatric Critical Care Research Network (CPCCRN). The study was conducted from April 2007 to November 2009.
CDC criteria were used to define nosocomial infection (9). Clinical sepsis was diagnosed when a subject (> 1 year of age) had fever (≥ 38° C), hypotension (≤ 90 mm Hg systolic pressure), or oliguria (≤ 20 ml/hr) and the clinician initiated antibiotics without a positive culture or other recognized cause. Nosocomial infection was diagnosed when microbiologically proven infection (culture, antigen, PCR, or antibody) was observed in a subject hospitalized beyond 48 hours with fever, hypothermia, chills, or hypotension. If the culture was obtained from respiratory secretions, it was deemed “lower respiratory infection” (LRI) irrespective of chest x-ray or any additional clinical findings. CPCCRN members blinded to treatment arm adjudicated all infections at the Network quarterly meetings. Other than the administration of the study intervention, decisions regarding all aspects of clinical care remained the purview of the treating physicians. Specifically, there were no protocols regarding when or how cultures or other studies were to be obtained or for the use of antibiotics.
Three investigators (DW, KM, and SH) extracted data from subject case report forms (CRFs). To confirm reliability of data extraction, two of the investigators independently reviewed one in ten of the CRFs chosen at random and compared results. Specific elements extracted included results of all respiratory cultures and their method of collection (tracheal aspiration vs. bronchoalveolar lavage), identification and sensitivities (if performed) of the microorganism, and antibiotic treatment. Subject demographics and outcomes had been previously entered into the study database. To determine the significance of the nosocomial LRI diagnosis, we compared clinical characteristics and outcomes between subjects diagnosed with nosocomial LRI and those intubated but not diagnosed with LRI or any other nosocomial infections or sepsis. In this exploratory analysis, we excluded subjects with less than 3 days of follow up in the PICU after PICU admission. Specifically, 289 out of the 293 randomized children were included. Out of these 289 subjects, 155 (54%) were intubated, but did not develop nosocomial infection or sepsis, and 74 (26%) were diagnosed with nosocomial LRI (Figure 1).
Figure 1. Subject Flow Diagram.
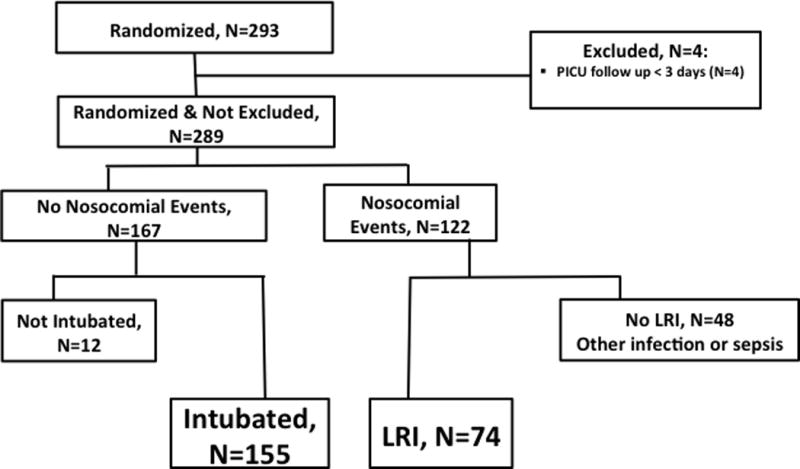
PICU = pediatric intensive care unit
LRI = lower respiratory infection
Statistical Analysis
We compared subject characteristics, outcomes, antibiotic usage, respiratory cultures, and intubated days between the two cohorts (LRI; Intubated without nosocomial infection). We also described the diagnostic and treatment variability among the participating institutions using descriptive statistics. Categorical variables are summarized as absolute counts and percentages. Continuous variables are summarized as means and standard deviations. Statistical significance of differences between cohorts with respect to each characteristic was assessed by the Pearson chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. Reported significance levels are not adjusted for multiple comparisons, as this analysis is considered exploratory. Analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
As previously reported (9), a total of 93 nosocomial LRIs were diagnosed in 74 (26%) of the study subjects. Table 1 compares the clinical characteristics of subjects diagnosed with LRI (n=74) compared to intubated subjects without nosocomial infection (n=155). The two groups were comparable demographically and in severity of illness, but fewer LRI subjects were immune compromised (1% vs. 9%) and fewer had endotracheal tubes in place (88% vs. 97%) at randomization. The diagnosis of nosocomial LRI was associated with significantly longer durations of intubation and lengths of hospital and PICU stay (Table 2). There was no difference in mortality. Because subjects were intubated an average of 5 days prior to the development of an LRI, we also separately compared subjects with a duration of intubation > 5 days. In that subgroup of subjects, the average duration of intubation was 9 (± 5) days for intubated subjects without nosocomial infection (n=50) compared to 15 (±8) days for intubated subjects with nosocomial LRI (n=64) (p<0.0001).
Table 1.
Subject Characteristics
| Intubated w/o nosocomial infection (n=155) |
Nosocomial LRI (n=74) |
p value | |
|---|---|---|---|
|
| |||
| Age (years), mean (SD) | 8 (6) | 9 (6) | 0.16 |
|
| |||
| Gender (% male) | 50% | 47% | 0.67 |
|
| |||
| Race | n=149 | n=72 | 0.99 |
| % White | 66% | 67% | |
| % Black | 28% | 28% | |
| % Asian or other | 6% | 6% | |
|
| |||
| Ethnicity | n=149 | n=69 | |
| %Hispanic | 20% | 23% | 0.61 |
|
| |||
| Pediatric Risk of Mortality, mean (SD) | 9 (6) | 10 (7) | 0.34 |
|
| |||
| Pediatric Logistic Organ Dysfunction, mean (SD) | 11 (9) | 12 (10) | 0.51 |
|
| |||
| Organ Failure Index, mean (SD) | 2 (1) | 2 (1) | 0.35 |
|
| |||
| Existing infection or clinical sepsis | 72% | 59% | 0.07 |
|
| |||
| Present at randomization | |||
| ETT | 97% | 88% | 0.01 |
| Central line | 83% | 86% | 0.53 |
| Urinary catheter | 86% | 82% | 0.42 |
|
| |||
| Immune Compromised* | 9% | 1% | 0.04 |
SD=standard deviation
ETT=endotracheal tube
as defined by CRISIS study criteria9
Table 2.
Outcomes
| Intubated w/o nosocomial infection (n=155) |
Nosocomial LRI (n=74) |
p value | |
|---|---|---|---|
| 28-Day Mortality* | 12 (8%) | 9 (12%) | 0.30 |
| PICU LOS, mean (SD) | 9 (6) | 24 (19) | < 0.0001 |
| Hospital LOS, mean (SD) | 18 (19) | 34 (24) | < 0.0001 |
| Intubated days, mean (SD) | 5 (4) | 14 (9) | < 0.0001 |
Three subjects in the ‘intubated without nosocomial infection’ group are excluded because their mortality status was unknown.
PICU=Pediatric Intensive Care Unit
LOS=Length of stay
Thirteen subjects were diagnosed with two or more LRI events. The average duration of intubation was 12 days for subjects with only one LRI event compared to 20 days for those with 2 or more events (p=0.01), and the average PICU stay 23 days and 26 days (p=0.08), respectively.
The study protocol did not dictate how respiratory cultures were to be obtained. In subjects diagnosed with LRI, 80% of the initial culture specimens were obtained by simple tracheal aspirate and 20% by bronchoalveolar lavage (BAL). One center routinely obtained cultures by BAL (8/12 initial cultures) while most did simple tracheal aspirates. Overall, 33% (178/536) of all the respiratory cultures obtained were “positive” in that organisms judged to be pathogenic were identified on culture. Staphylococcus aureus, Candida, and Pseudomonas were the most common organisms isolated (Table 3). Of the 93 diagnosed LRIs, 59 (63%) had one or more subsequent respiratory secretion cultures performed at times ranging from one to 17 days after the initial positive culture. In 75/166 (45%) of these follow-up cultures, the same organism was identified as in the previous culture (Table 4). In nearly all of these infection episodes, subjects were on antibiotics for which the organisms had either demonstrated sensitivity or are generally considered to be appropriate treatment (e.g., vancomycin for S. aureus; piperacillin tazobactam for Pseudomonas). Pseudomonas (40%) and S. aureus (29%) were the most common persistent bacteria in follow-up cultures. In several subjects, the same bacteria persisted in cultures despite multiple changes in antibiotics (data not shown).
Table 3.
Microbes in initial positive cultures*
| Bacteria | # cultures | % |
|---|---|---|
| S. aureus | 22 | 21 |
| Candida/“Yeast” | 20 | 19 |
| Pseudomonas | 20 | 19 |
| H. influenza | 11 | 11 |
| Enterobacter | 6 | 6 |
| Moraxella | 5 | 5 |
| Klebsiella | 3 | 3 |
| Stenotrophomonas | 3 | 3 |
| Serratia | 3 | 3 |
| Strep species | 2 | 3 |
| Acinetobacter | 2 | 2 |
| Eikenella | 1 | 1 |
| Influenza | 1 | 1 |
| Enterococcus | 1 | 1 |
| Cryptococcus | 1 | 1 |
| S. pneumoniae | 1 | 1 |
| Citrobacter | 1 | 1 |
numbers add to > 93 because some cultures had more than one identified organism
Table 4.
Follow-up Cultures of LRI Subjects
| Institution* | #Subjects | #LRI | #Subjects w/follow up Cultures** | #Cultures | #positive |
|---|---|---|---|---|---|
| Hospital 1 | 12 | 14 | 6 | 9 | 3 |
| Hospital 2 | 13 | 14 | 11 | 37 | 22 |
| Hospital 3 | 21 | 29 | 16 | 41 | 15 |
| Hospital 4 | 5 | 5 | 5 | 17 | 3 |
| Hospital 7 | 5 | 5 | 4 | 8 | 4 |
| Hospital 8 | 18 | 26 | 17 | 56 | 28 |
| TOTALS | 74 | 93 | 59 | 168 | 75(45%) |
hospitals 5 & 6 had no LRIs
Pseudomonas: 30/75 = 40%; Staphylococcus: 22/75 = 29%
Laboratory processing and reporting of culture and Gram stain results was variable both across and within institutions. BAL-obtained culture results were routinely reported quantitatively whereas tracheal aspirate-obtained cultures and Gram stains were reported semi-quantitatively (i.e. 1+, 2+, etc., or “few, moderate, many”) with respect to both numbers of organisms and white blood cells. None of the laboratories related the semi-quantitative reports to any standard for comparison purposes. Of the initial “positive” cultures (n=74), Gram stain identified bacteria in only 66%, with “many” or “3+” PMNs reported in 41%. Antibiotic sensitivities for bacteria identified from the initial cultures were reported in only 34% of the isolates. Certain bacteria, such as Moraxella and Klebsiella, were always tested for antibiotic sensitivity; however, the two most commonly isolated bacteria, S. aureus and Pseudomonas, were only tested 33% and 53% of the time, respectively.
The frequency with which respiratory cultures were obtained varied across the 8 participating centers (Table 5). One site rarely performed respiratory cultures (total of 14 respiratory cultures for 31 subjects) and identified no episodes of LRI.
Table 5.
Site Variability in Cultures Performed
| Institution | # Subjects | Intubated days | # Respiratory cultures | Respiratory cultures/100 intubated days | LRI/100 intubated days |
|---|---|---|---|---|---|
| Hospital 1 | 45 | 379 | 45 | 11.9 | 3.7 |
| Hospital 2 | 32 | 260 | 56 | 21.5 | 5.4 |
| Hospital 3 | 71 | 595 | 136 | 22.9 | 4.9 |
| Hospital 4 | 31 | 224 | 77 | 34.4 | 2.2 |
| Hospital 5 & 6* | 31 | 161 | 14 | 8.7 | 0.0 |
| Hospital 7 | 13 | 96 | 19 | 19.8 | 5.2 |
| Hospital 8 | 66 | 643 | 189 | 29.4 | 4.0 |
| Overall | 289 | 2358 | 536 | 22.7 | 3.9 |
Note that hospitals 5 & 6 are part of the same institution
Antibiotics were used frequently, with subjects enrolled in the study receiving one or more antibiotics 74% of all patient days in the PICU. Most subjects (66%) were already receiving antibiotics on admission for an existing infection or a diagnosis of clinical sepsis. In subjects diagnosed with LRI, 80% were started on an average of 2.7 antibiotics at the time the respiratory culture associated with the LRI was obtained. The three most common initial antibiotics were cephalosporins (36%), vancomycin (23%), and piperacillin/tazobactam (8%). Subjects diagnosed with LRI received an average of 38 (±29) antibiotic days in the PICU compared to 15 (±20) antibiotic days in intubated subjects with no nosocomial infection events (p<0.0001).
Discussion
The CRISIS study protocol dictated that identification of a putative pathogen from respiratory secretion culture be considered a LRI if accompanied by clinical symptoms that included fever, hypothermia, chills, or hypotension. While the study protocol did not mandate antibiotic treatment, subjects diagnosed with LRI received, on average, more than double the number of antibiotics relative to subjects intubated without nosocomial infection. This behavior is consistent with a recently reported survey of pediatric intensivists (10) and is illustrative of why suspected ventilator-associated infection is the most common reason for antibiotic use in the PICU (2).
The necessity and efficacy of antibiotics in the setting of a “positive” respiratory secretion culture is open to question. While the lung is generally considered “sterile” (although not completely so (11)), endotracheal tube placement provides a direct route for bacterial contamination from aspiration of secretions (12,13) and the endotracheal tube biofilm (14). Colonization of the airway with oropharyngeal flora occurs rapidly and identification of bacteria on culture cannot distinguish colonization from actual infection. We previously demonstrated that a majority of tracheal aspirate cultures in intubated children grew > 104 bacteria by day 4 of intubation, many of them considered pathogens, and this did not correlate with clinical signs or symptoms of infection (15). The CDC criteria defines > 25 white blood cells per low power field as indicative of infection but the same study (15) showed this was seen in the majority of subjects. At present no laboratory or other studies are able to distinguish colonization from infection. Studies in adults have demonstrated similar findings (16,17). The indications for antibiotic treatment in the face of a positive culture are unclear, but antibiotics have not been found to prevent progression from so-called “ventilator-associated tracheitis” to “ventilator-associated pneumonia” (18,19). Indeed, the persistence of positive cultures with the same organism despite appropriate antibiotics in 45% of follow-up cultures in this study demonstrates that antibiotics may not eradicate even sensitive bacteria. Even assuming antibiotics are able to transiently eradicate bacteria from the trachea, re-inoculation from the endotracheal biofilm is likely whenever the patient is suctioned because antibiotics are unable to penetrate the biofilm.
The current terminology for ventilator-associated infections is confusing and imprecise. The CDC LRI criteria require only isolation of a pathogen from a respiratory secretion culture in the presence of fever, hypothermia, chills, or hypotension (6). This could equally define “ventilator-associated tracheitis (VAT)”, as the term is currently applied (18–22), and which is now thought to be more common than ventilator-associated pneumonia (VAP) in pediatric patients (23). VAT is distinguished from VAP presumably by the absence of a “new or progressive pulmonary infiltrate” (24), although the ability to distinguish “new or progressive pulmonary infiltrates” on portable chest X-rays is problematic (25). The ambiguity of many of the criteria in conjunction with the imprecision of the terminology undoubtedly contributes to indiscriminate use of antibiotics.
This study demonstrates the variability with which cultures are obtained, processed, and reported in PICUs. BAL is standard in adult medicine, but limitations in size preclude its routine use in children. In the CRISIS study, practice was variable with one center using primarily BAL to obtain cultures whereas the other 7 centers obtained cultures almost exclusively by tracheal aspiration. The manner by which tracheal aspiration was performed was also not protocolized. Our own studies suggest that technique may differ across institutions10 and can significantly affect rates of positivity (15). The lack of standardization of culture and Gram stain results was also remarkable. The CDC ventilator-associated pneumonia criterion for purulence of secretions is quantitative (> 104 bacteria and/or > 25 white blood cells/low power field (7)), but tracheal aspirate results in this study were reported semi-quantitatively (1+, 2+, etc.). However, the lack of standardization in how cultures were obtained and reported may have been inconsequential because clinicians elected to treat with antibiotics if any pathogenic bacteria were identified irrespective of the quantity. The lack of antibiotic sensitivity testing is also notable, since subjects were frequently administered several antibiotics for the presumed infection and antibiotics broadened or changed when the same bacteria persisted in subsequent cultures.
Previous studies have demonstrated the association between VAI and prolongation of ventilation (19,23,26,27)., In this study, subjects diagnosed with LRI had significantly longer durations of intubation even after considering the average five-day time to onset. As with previous studies, however, the data do not elucidate whether the LRI was the cause or the effect of the longer duration of intubation. Both colonization and infection rates increase with duration of ventilation (15–17). Any child intubated longer than 3 or 4 days is likely colonized and, consequently, a respiratory culture obtained at that time for evaluation of fever or other signs or symptoms has a high probability of being “positive” (15). The specificity of a “positive” culture for infection is questionable and, consequently, the impact of the LRI diagnosis as well as the antibiotic treatment on outcomes remains uncertain.
Several of the study findings merit emphasis: (1) there is considerable variability across institutions in how often respiratory cultures are performed, how they are obtained, and how laboratories process and report them. This renders the interpretation of results difficult and antibiotic treatment decisions somewhat arbitrary; (2) the diagnosis of “lower respiratory infection” (now more likely termed “ventilator-associated tracheitis”) is common, but appropriate treatment is still in question. The CRISIS study data confirm its association with longer duration of intubation, but it is not clear if this association is causative; (3) antibiotic treatment frequently may not eradicate pathogenic bacteria from tracheal secretions even if the organism is demonstrated to be sensitive. This may relate less to the sensitivity of the microorganism than to re-inoculation from the endotracheal tube biofilm. Irrespective of the reason, broad-spectrum antibiotic therapy is associated with increasingly resistant microorganisms both in patients and in our ICUs.
Post-hoc analysis of study data is subject to significant limitations, particularly when the analysis pertains to aspects that were not the focus of the original study. Our purpose was to document the variability regarding the diagnosis of VAI even within the context of a prospective study and the consequences of the diagnosis with regard to antibiotic use. The intent was to elucidate the need for a more rigorous approach to this diagnosis and its associated therapy.
Conclusions
Based on these data, there is need for a more rigorous approach to the diagnosis of nosocomial LRI and other ventilator-associated infections. Identification of a putative pathogenic organism alone may be insufficient justification for antibiotic treatment because it does not discriminate infection from simple colonization. Discrimination is further hampered by inconsistencies in the manner by which respiratory cultures are obtained, processed and reported. Given the increasing emergence of resistant microbes, particularly in the intensive care unit, methods for the diagnosis of VAI should be standardized and greater discrimination in the use of antibiotics may be needed.
Acknowledgments
This work was supported by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS): U10HD050096, U10HD049981, U10HD050009, U10HD049945, U10HD049983, U10HD050012 and U01HD049934.
References
- 1.Institute of Medicine. To err is human. Washington: National Academy Press; 1999. [Google Scholar]
- 2.Fischer JF, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med. 2000;26:959–966. doi: 10.1007/s001340051288. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam V, Hendley JO, Willson DF. The diagnostic dilemma of ventilator-associated pneumonia in critically ill children. Pediatr Crit Care Med. 2011;12:286–96. doi: 10.1097/PCC.0b013e3181fe2ffb. [DOI] [PubMed] [Google Scholar]
- 4.Marrow BM, Mowzer R, Pitcher R, Argent AC. Investigation into the effect of closed-system suctioning on the frequency of pediatric ventilator-associated pneumonia in a developing country. Pediatr Crit Care Med. 2012;13:e25–e32. doi: 10.1097/PCC.0b013e31820ac0a2. [DOI] [PubMed] [Google Scholar]
- 5.Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: Risk factors and outcomes. Pediatrics. 2002;109:758–764. doi: 10.1542/peds.109.5.758. [DOI] [PubMed] [Google Scholar]
- 6.Klompas M. Complications of mechanical ventilation—The CDC’s new surveillance paradigm. N Engl J Med. 2013;368:1472–1475. doi: 10.1056/NEJMp1300633. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2016 Jan;:21. Accessed at CDC.gov, November, 2015.
- 8.Klowenberg PMCK, van Mourik MSM, Ong DSYH, et al. Electronic implementation of a novel surveillance paradigm for ventilator-associated events. Feasibility and validation. Am J Respir Crit Care Med. 2014;189(8):947–956. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]
- 9.Carcillo JA, Dean JM, Holubkov R, et al. The randomized comparative pediatric critical illness stress-induced immune suppression (CRISIS) prevention trial. Pediatr Crit Care Med. 2012;13:165–173. doi: 10.1097/PCC.0b013e31823896ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willson DF, Kirby A, Kicker JS. Respiratory secretion analyses in the evaluation of ventilator-associated pneumonia: A survey of current practice in pediatric critical care. Pediatr Crit Care Med. 2014;15:715–719. doi: 10.1097/PCC.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;122:2115–2121. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 13.Amantea SL, Piva JP, Sanches PRS, Palombini BC. Oropharyngeal aspiration in pediatric patients with endotracheal intubation. Pediatr Crit Care Med. 2004;5:152–156. doi: 10.1097/01.pcc.0000112375.03516.70. [DOI] [PubMed] [Google Scholar]
- 14.Adair CG, Forman SP, Feron BM, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25:1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 15.Willson DF, Conaway M, Kelly R, Hendley JO. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014;15:299–305. doi: 10.1097/PCC.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 16.Dennesen PJ, van der Ven AJ, Kessels AG, et al. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371–1375. doi: 10.1164/ajrccm.163.6.2007020. [DOI] [PubMed] [Google Scholar]
- 17.Baram D, Hulse G, Palmer LB. Stable patients receiving prolonged mechanical ventilation have a high alveolar burden of bacteria. Chest. 2005;127:1353–1357. doi: 10.1378/chest.127.4.1353. [DOI] [PubMed] [Google Scholar]
- 18.Tamma PD, Turnbull AE, Milstone AM, et al. Ventilator-associated tracheitis in children: Does antibiotic duration matter? Clin Infect Dis. 2011;52:1324–1331. doi: 10.1093/cid/cir203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven DE, Chroneou A, Zias N, Hjalmarson KI. Ventilator-associated tracheobronchitis: The Impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135:521–528. doi: 10.1378/chest.08-1617. [DOI] [PubMed] [Google Scholar]
- 20.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Palmer LB, Smaldone GC, Chen JJ, et al. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med. 2008;36:2008–2013. doi: 10.1097/CCM.0b013e31817c0f9e. [DOI] [PubMed] [Google Scholar]
- 22.Simpson VS, Bailey A, Higgersn RA, Christie LM. Ventilator-associated tracheobronchitis in a mixed medical/surgical pediatric ICU. Chest. 2013;144(1):32–38. doi: 10.1378/chest.12-2343. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler DS, Whitt JD, Lake M, et al. A case-control study on the impact of ventilator-associated tracheobronchitis in the PICU. Pediatr Crit Care Med. 2015;16:565–571. doi: 10.1097/PCC.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 24.Dallas J, Kollef M. VAT vs VAP. Are we heading toward clarity or confusion? (editorial) Chest. 2009;135(2):252–256. doi: 10.1378/chest.08-2247. [DOI] [PubMed] [Google Scholar]
- 25.Wunderink RG, Woldenberg LS, Zeiss J, et al. The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest. 1992;101:458–463. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan R, Asselin J, Gildengorin G, et al. A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009;123:1108–1115. doi: 10.1542/peds.2008-1211. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Boville BM, Blanton R, et al. A multi-centered prospective analysis of diagnosis, risk factors and outcome associated with pediatric VAP. Pediatr Crit Care Med. 2015;16(3):e65–73. doi: 10.1097/PCC.0000000000000338. [DOI] [PubMed] [Google Scholar]


