Abstract
Background
The role of alcohol in the development of subclinical cardiovascular disease is unclear. We examined the association between alcohol consumption and markers of subclinical cardiac damage and wall stress.
Methods
We studied the cross-sectional and prospective associations of alcohol consumption with high-sensitivity cardiac troponin (hs-cTnT) and N-terminal pro B-type natriuretic peptide (NT-proBNP) measured at two time points, 6 years apart (baseline, 1990-1992; follow-up, 1996-1998), in over 11,000 participants of the Atherosclerosis Risk in Communities (ARIC) Study with no history of cardiovascular disease. Alcohol was categorized as: never, former, current: ≤1, 2-7, 8-14, and ≥15 drinks/week.
Results
Compared to never drinkers, persons who consumed 2-7 drinks per week were less likely to have increased hs-cTnT (≥ 14 ng/L) at baseline (OR= 0.67, 95%CI 0.46-0.96), and had a lower risk of incident increases in hs-cTnT at follow up (RR=0.70, 95%CI 0.49-1.00). Conversely, there was a positive association between alcohol intake and NT-proBNP concentrations at baseline. Consumption of ≥15 drinks/week was positively associated with incident increases in NT-proBNP (≥300 pg/mL) at the 6-year follow-up visit (RR=2.38, 95%CI 1.43-3.96).
Conclusion
In this community-based study of middle-aged adults without a history of cardiovascular disease, moderate drinking was associated with lower concentrations of hs-cTnT, a marker of chronic subclinical myocardial damage, and positively associated with NT-proBNP, a biomarker of cardiac wall stress. Our results suggest that the cardiac effects of alcohol are complex. Cardiac biomarkers may help improve our understanding of the full cardiovascular effects of alcohol consumption.
Keywords: troponin T, brain natriuretic peptide, alcohol, epidemiology
Epidemiologic studies have demonstrated a benefit of low to moderate alcohol consumption on cardiovascular disease (CVD), in particular on coronary artery disease morbidity and mortality (1). However, there are also numerous adverse consequences of alcohol consumption such as increased risk of liver disease, cancer and injuries (2), that may offset potentially beneficial effects and make it difficult to provide clear clinical recommendations regarding regular alcohol consumption.
The association of alcohol with subclinical cardiovascular disease is relatively uncharacterized and may shed some light into the mechanisms for the putative cardiovascular effects of alcohol consumption. Several studies have examined the association between alcohol consumption and measures of atherosclerosis, but these results have been inconsistent (3-6). Beyond its effects on measures of atherosclerosis or atherosclerotic risk factors (7), the effects of alcohol on the myocardium are unclear. While prolonged high levels of alcohol consumption (>100 grams/day >10 years) have been associated with overt myocardial damage (8), it is yet to be determined if cardiac damage occurs at lower levels of alcohol consumption.
Novel cardiac biomarkers can detect chronic subclinical myocardial damage and wall stress in the general population, and these biomarkers may help to further characterize the cardiac effects of alcohol consumption. Cardiac Troponin-T (cTnT), is a highly specific measure of myocardial damage and is a standard protein used to identify acute myocardial infarction.(9) New, highly sensitive assays for cardiac troponin-T (hs-cTnT) have much lower detection limits than standard clinical cTnT assays (10) and have been shown to improve the prediction of cardiovascular morbidity and mortality in persons without clinically evident cardiovascular disease (11-13). To our knowledge, the association between alcohol consumption and hs-cTnT has not been formally examined in a population based study, with the exception of a small study of ∼500 individuals (14).
B-type natriuretic peptide (BNP) is a hormone secreted by the heart in response to hemodynamic stress with natriuretic, diuretic, and vaso-relaxant activity (15). BNP is closely associated with left ventricular mass index (16) and is clinically used for heart failure diagnosis and prognosis (17). N-terminal pro B-type natriuretic peptide (NT-proBNP), a stable prohormone of BNP, has been demonstrated to reflect subclinical left ventricular dysfunction, and is also associated with cardiovascular risk and mortality in the general population(18-21). Few studies, have examined the association between alcohol and natriuretic peptides and their findings were inconsistent (14, 22, 23).
We undertook the present study to comprehensively examine the association of alcohol consumption with subclinical myocardial damage and wall stress, as assessed by hs-cTnT and NT-proBNP, respectively, in 11,597 participants of the community-based Atherosclerosis Risk in Communities (ARIC) Study. The availability of hs-cTnT and NT-proBNP measurements at two time points, 6 years apart, permitted us to evaluate both cross-sectional and prospective associations of these biomarkers with alcohol consumption.
Methods
The ARIC Study is an ongoing community-based, predominantly bi-racial cohort of 15,792 middle-aged adults from 4 U.S. communities: Forsyth Country, North Carolina (white and black participants); Jackson, Mississippi (black participants); suburbs of Minneapolis, Minnesota (white participants); and Washington County, Maryland (white participants)(24). The first examination of participants took place from 1987 to 1989, with 3 subsequent follow-up visits each taking place, approximately 3 years apart, and a fifth visit conducted in 2011-2013. The current analyses are based on information obtained at two visits, 6-years apart: visit 2 in 1990-1992 and visit 4 in 1996-1999. For the present study, visit 2 (1990-1992) was the baseline. All participants signed written informed consent and the institutional review boards (IRB) at each site approved the study.
Assessment of Alcohol Consumption
Information on alcohol consumption status and average quantity of alcohol consumption among current drinkers was obtained at ARIC visit 2 (1990-1992). During the exam, participants were asked the following questions: Do you presently drink alcoholic beverages?”, and “Have you ever consumed alcoholic beverages?”. Individuals replying no to both questions were classified as never drinkers. Those who replied “no” to the first question and “yes” to the second question were classified as former drinkers. For those reporting presently drinking alcoholic beverages, 3 separate questions were asked to assess the usual number of drinks (4 oz. wine,12 oz. beer, or 1.5 oz. liquor) per week, with open ended responses. Given that prior ARIC studies (25) and others (7) have found uniform associations across beverage type, we used total weekly alcohol consumption as the main independent variable. Based on the answer to these questions we categorized individuals into 6 mutually exclusive groups: never, former, current: ≤1 drink/week, 2-7, 8-14, ≥15 drinks/week. History of excessive alcohol consumption (yes/no) was only assessed during ARIC visit 3 using the following question: “Was there ever a time in your life when you consumed 5 or more drinks of any kind of alcoholic beverage almost every day?”. We used this variable only for further description of the study sample.
Measurement of High Sensitivity Cardiac Troponin T (hs-cTnT)
Cardiac troponin T was measured at the two visits, 6 years apart (1990-1992 and 1996-1998), using a high sensitivity (pre-commercial) sandwich immunoassay method (Roche Elecsys T; Roche Diagnostics, Indianapolis, Indiana). Stored serum samples collected at visit 2 (1990-1992) were assayed for hs-cTnT using a Roche Elecys 2010 Analyzer (Roche Diagnostics) at the University of Minnesota in 2012-2013. Stored plasma samples collected at visit 4 (1996-1998) were assayed for hs-cTnT using a Cobas e411 analyzer (Roche Diagnostics) at Baylor College of Medicine.
Measurement of NT-proBNP
NT-proBNP was also measured at visits 2 and 4. Stored serum from samples collected at visit 2 were analyzed using a sandwich immunoassay method (Roche Diagnostics) implemented on a Roche Elecys 2010 Analyzer at the University of Minnesota. Stored plasma from samples collected at visit 4 were analyzed using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics) at Baylor College of Medicine.
We conducted a formal calibration study of both hs-cTnT and NT-proBNP (N=200 paired samples) to evaluate possible differences across specimen type and laboratory. No significant differences were observed and statistical correction was not indicated for either assay (26).
Other Measurements
Socio-demographic characteristics, smoking status, medical history and medication use, were assessed during a home interview or during the visit to the ARIC field center according to the original ARIC protocol. Using standardized methods, height, weight, and blood pressure were measured. We defined history of cardiovascular disease (CVD) as self-reported myocardial infarction or stroke before visit 1, or any of the following: silent myocardial infarction (diagnosed by electrocardiographic changes) at or before visit 4, myocardial infarction (adjudicated) or revascularization (at or before visit 4), or hospitalization for congestive heart failure (at or before visit 4). Diabetes was defined as a self-reported physician diagnosis of diabetes, current use of diabetic medications or hemoglobin A1c >6.5% or fasting plasma glucose ≥126 mg/dl (≥6.99 mmol/L).
ECG evidence of left ventricular hypertrophy was defined using the Cornell voltage criteria. Fasting blood samples were obtained, and using standard methods the following assays were performed: serum glucose, cholesterol (total, and HDL-cholesterol), triglycerides and creatinine. LDL cholesterol was calculated using the Friedewald equation (27). Gamma-glutamyl transferase (GGT) and hs-C-reactive protein were measured in 2012-13 in stored serum samples (Roche Diagnostics). Glomerular filtration rate was estimated from serum creatinine, sex, race and age using the CKD-EPI 2009 equation (28).
Study Populations
Of the total 14,348 ARIC participants who attended visit 2, we excluded participants with history of coronary heart disease (including silent myocardial infarction detected by ECG) or heart failure (n=1,365), those with missing cardiac biomarkers (n=844), those without information on alcohol consumption (n=24), those with missing covariates (n=437) and finally those with race/ethnicity other than black or white, or blacks from the Washing County and Minnesota field centers,owing to small numbers (n=81). The final analytic sample for our cross-sectional analyses consisted of 11,597 participants. In our prospective analyses of incident increased hs-cTnT at the 6 year follow-up visit, we further excluded 454 participants with increased hs-cTnT (≥14 ng/L) at baseline and those with missing hs-cTnT at visit 4 who had not had a cardiovascular event or death between visits. Among the 9,476 persons included in our prospective analyses for hs-cTnT, there were 632 cardiovascular events and 284 deaths. Similarly, for our prospective analyses of incident increased NT-proBNP (≥300 ng/L) at the 6 year follow-up visit, we excluded 308 participants with increased NT-proBNP (≥300 ng/L) at baseline and those with missing NT-proBNP at visit 4 who had not had a cardiovascular event or death between visits. Among the 9,587 persons included in our prospective analyses for NT-proBNP, there were 643 cardiovascular events and 296 deaths. In secondary analyses using a lower cut-point to define 6-year incident increased NT-proBNP (≥100 ng/L), we necessarily excluded 2,439 participants with increased NT-proBNP (≥100 ng/L) at baseline. Among the 7,785 persons included in this prospective analysis, there were 475 cardiovascular events and 211 deaths. (Supplemental Figure 1)
Statistical Methods
For the cross-sectional analyses, the main outcomes were: 1) increased hs-cTnT (≥14 ng/L), and 2) increased NT-proBNP (≥300 ng/L) at baseline (13, 29). We conducted secondary analyses using a lower cut-point of 100 ng/L of NT-proBNP (30). We also modeled hs-cTnT and NT-proBNP continuously (log transformed). We used multivariable logistic regression models to examine the association of alcohol categories with increased hs-cTnT and NT-proBNP concentrations. For the continuous analyses, we used robust regression models to examine the association of alcohol categories with hs-cTnT concentrations among those with measurable concentrations of hs-cTnT (>3 ng/dl), n=5,562. Similarly, we used robust regression models to examine the association of alcohol consumption categories with NT-proBNP concentrations using data from 11,221 participants with detectable concentrations of NT-proBNP (≥5 ng/L). For the main analyses, all multivariable models were adjusted for confounders not thought to be in the causal pathway: age (years), race-center (whites, Washington County; whites, Minneapolis; blacks, Jackson; blacks, Forsyth County; or whites Forsyth County), sex, education (less than high school), smoking (current, former, never). Further analyses adjusted for other cardiovascular risk factors potentially in the causal pathway: body mass index, diabetes, systolic blood pressure, use of anti-hypertensive medications, left-ventricular hypertrophy, HDL-cholesterol, triglycerides and c-reactive protein.
Among current drinkers, to further examine the shape of the continuous association between alcohol consumption and the two cardiac biomarkers, we used restricted cubic splines with knots placed at 1, 7, 14, and 21 drinks/week (31).
In prospective analyses, we used multinomial logistic regression and restricted cubic splines (in current drinkers with knots at 1, 7, 14 and 21 drinks/weeks) to examine the association of alcohol consumption at baseline with incident increased hs-cTnT (≥14 ng/L) or incident increased NT-proBNP (≥100 or ≥300 ng/L) at the 6 year follow-up visit. The multinomial regression approach allowed us to include in the analyses those individuals with intervening fatal or non-fatal CVD, and those non-CVD deaths as additional outcomes. For these analyses, we created mutually exclusive categories in the following sequence: participants with a CVD event between visit 2 and 4, participants with non-CVD death between visit 2 and 4, participants with incident increased hs-cTnT or NT-proBNP at visit 4, and participants without intervening CVD or death, without an incident increase in the biomarker. In addition, we used robust regression models to examine the association between alcohol consumption categories and 6-year absolute changes in NT-proBNP and hs-cTnT using data from participants with detectable concentrations of each biomarker at each visit (n=3,689 in analyses of hs-cTnT and n=8,703 in analyses of NT-proBNP).
For both the cross-sectional analyses and the prospective analyses, we conducted analyses stratified by sex and formally testes for the presence of sex-by-alcohol consumption interaction.
Results
The characteristics of ARIC participants at baseline (1990-1992) by alcohol consumption group are shown in Table 1. Overall, 23% of the participants were never drinkers, 20% were former drinkers and 58% were current drinkers, with only 4.8% reporting heavy drinking. History of excessive drinking was more prevalent among former drinkers (14.9%) and those reporting drinking ≥15 drinks per week (33.3%), compared to those with more moderate consumption (4.9%). Both, never and former drinkers had in general a worse cardiometabolic profile, including a much higher prevalence of increased hs-cTnT, compared to current drinkers. Among current drinkers, higher alcohol consumption was associated with less obesity, more hypertension, higher HDL-cholesterol and triglycerides concentrations, higher prevalence of diabetes, smoking and left ventricular hypertrophy, and higher GGT, and higher C-reactive protein concentrations.
Table 1. Characteristics of study participants without clinical cardiovascular disease according to categories of alcohol consumption at baseline, the Atherosclerosis Risk in Communities Study, 1990-1992.
| Former drinker | Never drinks | Current Drinkers (drinks/week) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| ≤1 | 2-7 | 8-14 | ≥15 | |||
| N (%) | 2,284 (19.7%) | 2633 (22.7%) | 3295 (28.4%) | 2013 (17.4%) | 812 (7.0%) | 560 (4.8%) |
|
| ||||||
| Mean (SD) Age (years) | 57.0 (5.7) | 57.3 (5.7) | 56.2 (5.7) | 56.4 (5.7) | 56.2 (5.5) | 56.9 (5.7) |
| African-American, % | 36.6 | 37.3 | 13.5 | 15.0 | 14.3 | 17.0 |
| Female, % | 50.0 | 78.8 | 63.9 | 48.2 | 34.5 | 16.3 |
| Family income <$35000, % | 62.9 | 64.4 | 41.8 | 35.1 | 34.6 | 42.3 |
| Education less than high school, % | 13.7 | 10.6 | 3.5 | 4.0 | 4.7 | 8.2 |
| Obesity (BMI≥30), % | 33.9 | 35.3 | 26.5 | 19.4 | 20.2 | 17.1 |
| Hypertension, % | 37.6 | 38.0 | 26.3 | 29.0 | 32.4 | 37.3 |
| Mean (SD) HDL-cholesterol (mg/dL) | 46.8 (15.2) | 51.4 (15.9) | 50.1 (16.2) | 52.3 (17.6) | 54.1 (19.3) | 53.4 (19.9) |
| Mean (SD) LDL-cholesterol (mg/dL) | 136.0 (36.3) | 135.5 (37.7) | 132.1 (35.5) | 130.5 (35.4) | 130.7 (37.4) | 128.7 (39.5) |
| Median [P25-p75] Triglycerides (mg/dL) | 112.0 | 111.0 | 113.0 | 106.0 | 112.0 | 116.0 |
| Mean (SD) C-reactive protein (mg/L) | 4.9 (8.0) | 4.6 (6.6) | 3.9 (7.2) | 3.4 (5.2) | 3.5 (6.2) | 3.8 (6.6) |
| Diabetes, % | 21.1 | 19.0 | 11.4 | 9.9 | 10.1 | 16.3 |
| Median [P25-p75] GGT (U/L) | 22.0 [15.0-33.0] | 20.0 [14.0-30.0] | 18.0 [13.0-28.0] | 21.0 [14.0-31.0] | 27.0 [17.5-44.0] | 34.0 [21.0-55.0] |
| Current smoker, % | 24.0 | 10.6 | 21.0 | 25.2 | 33.1 | 39.3 |
| Left ventricular hypertrophy, % | 2.6 | 3.4 | 1.2 | 1.4 | 1.6 | 2.7 |
| Median [P25-p75] hs-cTnT (ng/L) | 3 [1.5-6] | 1.5 [1.5-6] | 1.5 [1.5-5] | 1.5 [1.5-5] | 1.5 [1.5-6] | 3.5 [1.5-6] |
| Increased hs-cTnT (≥ 14 ng/L), % | 7.1 | 3.8 | 2.6 | 2.8 | 3.6 | 3.8 |
| Median NT-proBNP (ng/L) [p25, p75] | 45.8 [14.5-87.0] | 54.1 [28.8-95.6] | 51.9 [28.6-89.2] | 49.2 [24.9-89.6] | 43.6 [24.0-84.5] | 44.9 [23.9-80.5] |
| NT-proBNP ≥300 ng/L, % | 3.1 | 2.7 | 2.2 | 2.8 | 2.2 | 3.5 |
| NT-proBNP ≥100 ng/L, % | 20.5 | 22.4 | 20.6 | 21.0 | 28.8 | 19.6 |
| History of excessive drinking §, % | 12.7 | 0.2 | 2.7 | 5.0 | 10.6 | 29.1 |
All variables significantly different across groups. §Self-reported at Visit 3. Was there ever a time in your life when you consumed 5 or more drinks of any kind of alcoholic beverage almost every day?
To convert cholesterol concentrations in mg/dL to mmol/L multiply by 0.2586.
To convert triglyceride concentrations in mg/dL to mmol/L multiply by 0.01129
We did not find evidence for a robust association at baseline between number of drinks per week and concentrations of hs-cTnT among current drinkers (Figure 1, Panel A). However, when never and formers drinkers were included in the analysis, current drinking was associated with a lower likelihood of having increased hs-cTnT (≥14 ng/L) [e.g. OR=0.67, 95% CI, 0.46-0.96 for 2-7 drinks/week (moderated drinking) compared never drinkers] (Table 2). In contrast, former drinkers were significantly more likely to have increased hs-cTnT concentrations (OR=1.37, 95% CI 1.03-1.83) compared to never drinkers.
Figure 1.
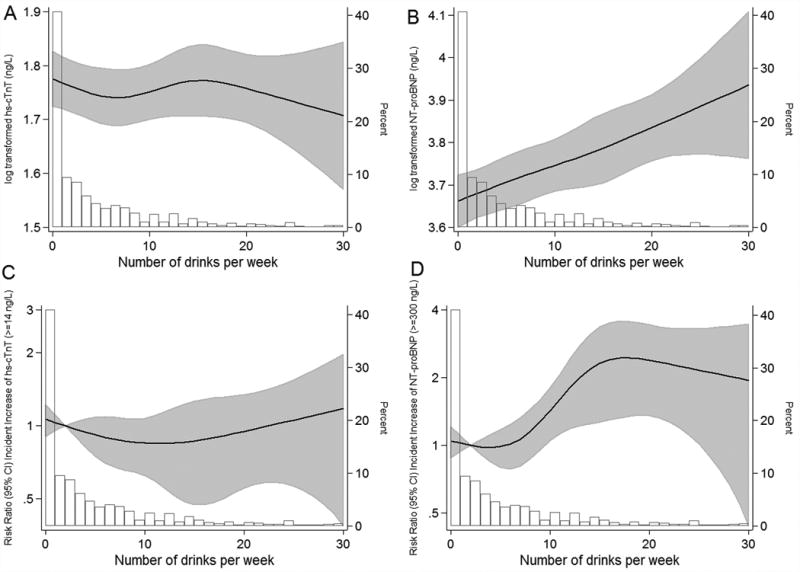
Cross-sectional and prospective* association of baseline alcoholic drinks per week and baseline log-transformed hs-cTnT (Panel A), log transformed NT-proBNP (Panel B), incident increase of hs-cTnT (≥14 ng/L) (Panel C), and incident increase of NT-proBNP (≥300 ng/L) (Panel D) among current drinkers. ARIC 1990-1992
*Only among current drinkers. Adjusted for age, race-center, education (less than high school or not), and smoking (current; former; never). Black lines are restricted cubic splines with knots at 1, 7, 14 and 21 drinks per week. Shaded gray areas are 95% CIs. Open bars are a histogram of the distribution of drinks per week (% in the group)
Table 2. Cross-sectional associations* of baseline alcohol consumption groups and increased baseline hs-cTnT or NT-proBNP. N=11,579. ARIC 1990-1992.
| Alcohol consumption category | Increased hs-cTnT OR (95% CI) | Increased NT-proBNP (≥100 ng/L) OR (95% CI) | Increased NT-proBNP (≥300 ng/L) OR (95% CI) |
|---|---|---|---|
| Former drinker | 1.37 (1.03-1.83) | 1.17 (1.00-1.36) | 1.12 (0.78-1.60) |
| Never drinker | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Current, ≤1 drink/week | 0.78 (0.57-1.08) | 0.99 (0.86-1.14) | 0.94 (0.65-1.35) |
| Current, 2-7 drinks/week | 0.67 (0.46-0.96) | 1.17 (1.00-1.38) | 1.23 (0.83-1.83) |
| Current, 8-14 drinks/week | 0.78 (0.49-1.24) | 1.33 (1.07-1.65) | 0.99 (0.56-1.75) |
| Current, ≥15 drinks/week | 0.64 (0.38-1.07) | 1.49 (1.16-1.93) | 1.47 (0.83-2.59) |
Adjusted for age, sex, race-center, education (less than high school or not), and smoking (current; former; never).
We observed a log-linear dose-response relationship of alcohol consumption and NT-proBNP at baseline among current drinkers (Figure 1, Panel B). When we included former and never drinkers into the analysis (Table 2), the results were largely consistent, and further demonstrated a moderate but positive association between former drinking and increased NT-proBNP (≥100 ng/L). Using a higher cut-point to define increased NT-proBNP the results were consistent in the direction but no longer statistically significant (Table 2).
Further adjusting for risk factors potentially in the causal pathway did not appreciably alter our results for either hs-cTnT or NT-proBNP (Supplemental Table 1).
In prospective analyses, 4.6% of the population with hs-cTnT <14 ng/L at baseline had incident increased hs-TnT concentrations (≥14 ng/L) at the 6-year follow-up visit. Among those persons with NT-proBNP <300 ng/L at baseline, 3.9% developed incident increased NT-proBNP concentrations (≥300 ng/L), and among persons with NT-proBNP <100 ng/L at baseline, 19.9% had NT-proBNP ≥100 ng/L at the 6-year follow–up visit. In the multinomial models, examining the association between baseline alcohol consumption with 6-year increases of hs-cTnT, moderate drinking (i.e. drinking 2-7 drinks/week) was associated with a significantly reduced risk of incident increase in hs-cTnT (Table 3). When limited to current drinkers, we observed some suggestion of a possible U-shaped association for alcohol consumption (drinks/week) with incidence of increase in hs-cTnT (Figure 1, Panel C). In contrast, drinking 8-14 and ≥15 drinks/week were strongly and positively associated with incident increase in NT-proBNP (≥300 ng/L) (Figure1, Panel D and Table 3). We did not observe significant associations of alcohol consumption with incident increased NT-proBNP using the lower cut-point to define our outcome (NT-proBNP ≥ 100 ng/L).
Table 3. Adjusted* risk ratios (95% confidence intervals) for the association of alcohol consumption categories with incident increased hs-cTnT (≥14 ng/L), incident increased NT-proBNP (≥100 ng/L), and incident increased NT-proBNP (≥300 ng/L). ARIC 1990-1992.
| Alcohol consumption category | Incident increase in hs-cTnT N=9476 | Incident increase in NT-proBNP ≥100 ng/L N=7785 | Incident increase in NT-proBNP ≥300 ng/L N=9587 |
|---|---|---|---|
| Former drinker | 0.98 (0.71-1.36) | 0.97 (0.80-1.18) | 1.37 (0.96-1.97) |
| Never drinker | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Current, ≤1 drink/week | 0.91 (0.66-1.24) | 0.99 (0.83-1.18) | 1.22 (0.87-1.70) |
| Current, 2-7 drinks/week | 0.70 (0.49-1.00) | 0.84 (0.68-1.03) | 1.07 (0.72-1.58) |
| Current, 8-14 drinks/week | 0.73 (0.46-1.14) | 0.92 (0.69-1.21) | 2.01 (1.28-3.16) |
| Current, ≥15 drinks/week | 0.83 (0.52-1.32) | 1.10 (0.80-1.50) | 2.38 (1.43-3.96) |
Adjusted for age, race-center, education (less than high school or not), and smoking (current; former; never). The analysis of incident increase in hs-cTnT was limited to 9,476 individuals with hs-cTnT <14 ng/L at baseline. The analysis of incident increase in NT-proBNP (≥100 ng/L) was limited to 7,785 individuals with NT-proBNP <100 ng/L at baseline; and the analysis of incident increase in NT-proBNP defined as ≥300 ng/L was limited to 9,587 individuals with NT-proBNP <300 ng/L at baseline
Our results were not appreciably altered with adjustment for additional risk factors (Supplemental Table 2).
The results of the multinomial models showed an inverse association between alcohol consumption and cardiovascular deaths, and a borderline increased risk of cardiovascular event and a significant increased risk of non-CVD death for former drinkers are shown in Supplemental Tables 3, 4 and 5.
When we examined the association of alcohol use with 6-year change (absolute) in hs-cTnT and NT-proBNP as continuous variables, former drinkers had a greater increase in hs-cTnT (β=+0.37, P value=0.053), compared to never drinkers (Supplemental Table 6).
Sex-stratified analyses showed similar patterns of association in men and women with no evidence for interaction (all P -values-for-interaction >0.05, Supplemental Tables 7-9 and Supplemental Figure 2).
Discussion
In this large community-based population of adults without cardiovascular disease, there was a significant inverse association between moderate drinking and presence or development of subclinical myocardial damage, as indicated by increases in hs-cTnT. On the other hand, we found positive cross-sectional and prospective associations between alcohol consumption and increased concentrations of NT-proBNP.
Existing evidence suggests that moderate consumption of alcohol (1-2 drinks per day) is associated with decreased risk of coronary heart disease and, to a lesser degree, a reduced risk of ischemic stroke (1). Several studies have examined effects of moderate on alcohol consumption on cardiovascular risk factors (e.g. lipids, inflammation, coagulation), and have demonstrated favorable effects on HDL cholesterol, adiponectin, and fibrinogen (7). Our results evaluating the association between alcohol consumption and two cardiac biomarkers that reflect different processes in the myocardium expand these findings and highlight the complexities of the relationship between alcohol and the cardiovascular system. While there may be a protective effect of regular and moderate alcohol consumption on the myocardium, as measured by hs-cTnT, we observed adverse associations with myocardial wall-stress, reflected by NT-proBNP.
Among asymptomatic individuals, increases of hs-cTnT detected using the newer highly sensitive assays are thought to reflect subclinical myocardial damage of any nature (e.g. ischemic or due to hypertrophy). Our finding of higher concentrations of hs-cTnT among former drinkers lend support to prior studies demonstrating a U-shaped association between alcohol consumption, heart failure (32) and CHD (1), often with highest CVD risk seen among former drinkers (as compared to never drinkers). Indeed, the “sick quitter effect” has been previously described (1) and our results provide additional evidence to support this phenomenon. Former drinkers not only had increased hs-cTnT at baseline but, in the prospective analyses, we demonstrated a significant increased risk of death, and borderline significant increased risk of CVD, as compared to never drinkers.
While the mechanisms by which alcohol consumption may contribute to increases in NT-proBNP are unclear, it has been postulated that ethanol increases plasma osmolality which can then lead to an increase in the release of natriuretic peptides (22). Regardless of the mechanisms, however, the association between alcohol consumption and increased natriuretic peptides sheds some light into the potential mechanisms of the observed adverse effects of increased alcohol consumption on blood pressure, subsequent stroke, and alterations in cardiac structure and function, as determined using cardiac echography (1, 33-36). In fact, the discrepant findings of the prospective analyses with the 2 different cutpoints to define increased NT-proBNP (≥ 300 vs ≥ 100) that showed that drinking 8-14 and ≥15 drinks/week were strongly and positively associated with the incidence of large increase in NT-proBNP (≥300 ng/L) but not significantly associated with incident increase in NT-proBNP defined using the lower cutpoint suggest the possibility of alcohol cardiomyopathy with heavy alcohol consumption.
We found a positive log-linear association between alcohol consumption and NT-proBNP, and in the prospective analyses we found an inverse association between alcohol consumption and CVD deaths, largely consistent with epidemiological studies. These contradictory findings highlight the possibility of confounding and the need for large, long-term, randomized controlled trials of alcohol consumption to more conclusively characterize the effects of alcohol on CVD.
To our knowledge only three studies have examined the association between alcohol consumption and natriuretic peptides. One was a cross-over clinical trial of 6 normotensive individuals who received placebo or 2 different doses of ethanol (equivalent to one or 2 drinks, respectively) and evaluated atrial natriuretic peptide (ANP) measured at multiple times post drink. The investigators found that shortly after consumption of alcohol (any dose) there was a significant increase in ANP which peaked at 15 minutes but the levels returned to baseline concentrations 2 hours afterwards (22). A second, cross-sectional epidemiological study of 1,345 individuals found that higher levels of alcohol consumption were associated with higher geometric ANP (23). A third cross-sectional study of 519 women without cardiovascular disease showed that those who consumed ≥1 drink per day, were less likely to have increased BNP compared to those who rarely or never drank (OR=0.85, 95% CI 0.74-0.96). The authors validated these findings using a large subsample of the JUPITER trial (n=11,052) with measurements of BNP and hs-cardiac troponin I (hs-cTnI) (14). The reasons for the differences between our findings and the JUPITER study in particular are unclear but there were a number of differences between the studies including the type of BNP assay, the categorization of increased BNP, and the characteristics of the study population that limit the comparability.
Our study has some limitations: there were few heavy drinkers (n=560), and thus we had limited ability to examine the associations for this group. We relied on self-reported alcohol consumption which may have resulted in misclassification (especially underreporting); however, we found the expected dose-response relationship of alcohol with HDL-cholesterol and GGT among drinkers. In addition, although the observed associations may be the result of direct effects of the alcohol itself on the myocardium, the possibility of residual confounding remains. Strengths of our study include the large sample size, the inclusion of two contemporary cardiac biomarkers, both of which have previously been shown to be very powerful predictors of long-term outcomes. Our analyses included both cross-sectional and prospective components that allowed for a more comprehensive examination of the research question. The study was nested in one of the most well characterized community-based studies of cardiovascular disease in the U.S and all data were collected using rigorous and standardized procedures.
In conclusion, in a large sample of individuals without clinically evident cardiovascular disease, we found a positive association between moderate and increased alcohol consumption and NT-proBNP, and a moderate protective (inverse) association of current alcohol consumption with hs-cTnT. Together, these results highlight the complexity of the relationship of alcohol consumption with cardiovascular disease that hinders universal recommendations regarding alcohol consumption in the general population. Our results highlight the need for comprehensive investigation to determine the relative risks and benefits of alcohol use on the cardiovascular system.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions. Reagents for the NT-proBNP and hs-cTnT assays were donated by Roche Diagnostics. The authors thank Usama Bilal, MD, MPH, for his contributions with the graphs.
Sources of Funding: This work was supported by NIH/NIDDK grant s K24DK106414 and 2-R01 DK089174 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Mariana Lazo has received support from the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Footnotes
Conflict of interest:
Mariana Lazo has received support from the Alcohol and Beverage Foundation (ABMRF). Reagents for the high sensitivity cardiac troponin assays were donated by Roche Diagnostics. Dr. Ballantyne has received personal fees and grant support from Roche Diagnostics and is co-investigator on a provisional patent filed by Roche for use of biomarkers in heart failure prediction.
Dr. Selvin reports personal fees from Roche Diagnostics, outside the submitted work.
The other authors declare no commercial conflicts of interest.
References
- 1.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671.:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–30. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 3.McClelland RL, Bild DE, Burke GL, Mukamal KJ, Lima JA, Kronmal RA. Multi-Ethnic Study of Atherosclerosis. Alcohol and coronary artery calcium prevalence, incidence, and progression: Results from the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88:1593–601. doi: 10.3945/ajcn.2008.26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demirovic J, Nabulsi A, Folsom AR, Carpenter MA, Szklo M, Sorlie PD, Barnes RW. Alcohol consumption and ultrasonographically assessed carotid artery wall thickness and distensibility. The Atherosclerosis Risk In Communities (ARIC) study investigators Circulation. 1993;88:2787–93. doi: 10.1161/01.cir.88.6.2787. [DOI] [PubMed] [Google Scholar]
- 5.Janszky I, Mukamal KJ, Orth-Gomer K, Romelsjo A, Schenck-Gustafsson K, Svane B, et al. Alcohol consumption and coronary atherosclerosis progression--the stockholm female coronary risk angiographic study. Atherosclerosis. 2004;176:311–9. doi: 10.1016/j.atherosclerosis.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Vliegenthart R, Oei HH, van den Elzen AP, van Rooij FJ, Hofman A, Oudkerk M, Witteman JC. Alcohol consumption and coronary calcification in a general population. Arch Intern Med. 2004;164:2355–60. doi: 10.1001/archinte.164.21.2355. [DOI] [PubMed] [Google Scholar]
- 7.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636.:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballester M, Marti V, Carrio I, Obrador D, Moya C, Pons-Llado G, et al. Spectrum of alcohol-induced myocardial damage detected by indium-111-labeled monoclonal antimyosin antibodies. J Am Coll Cardiol. 1997;29:160–7. doi: 10.1016/s0735-1097(96)00425-1. [DOI] [PubMed] [Google Scholar]
- 9.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 10.Tate JR. Troponin revisited 2008: Assay performance. Clin Chem. 2008;46:1489–500. doi: 10.1515/CCLM.2008.292. [DOI] [PubMed] [Google Scholar]
- 11.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin t measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava PK, Pradhan AD, Cook NR, Ridker PM, Everett BM. Impact of modifiable risk factors on b-type natriuretic peptide and cardiac troponin t concentrations. The Am J Cardiol. 2016;117:376–81. doi: 10.1016/j.amjcard.2015.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 16.Nishikimi T, Yoshihara F, Morimoto A, Ishikawa K, Ishimitsu T, Saito Y, et al. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996;28:22–30. doi: 10.1161/01.hyp.28.1.22. [DOI] [PubMed] [Google Scholar]
- 17.Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Sutton GC. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350:1349–53. doi: 10.1016/S0140-6736(97)06031-5. [DOI] [PubMed] [Google Scholar]
- 18.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–76. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenning BA, Raymond I, Hildebrandt PR, Nilsson JC, Baumann M, Pedersen F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma n-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart. 2004;90:297–303. doi: 10.1136/hrt.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, c-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–16. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 21.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-b-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–75. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 22.Gianoulakis C, Guillaume P, Thavundayil J, Gutkowska J. Increased plasma atrial natriuretic peptide after ingestion of low doses of ethanol in humans. Alcoholism, clinical and experimental research. 1997;21:162–70. [PubMed] [Google Scholar]
- 23.Djousse L, Hunt SC, Eckfeldt JH, Arnett DK, Province MA, Ellison RC. Alcohol consumption and plasma atrial natriuretic peptide (from the HyperGEN study) The American journal of cardiology. 2006;98:628–32. doi: 10.1016/j.amjcard.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 24.The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The aric investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 25.Volcik KA, Ballantyne CM, Fuchs FD, Sharrett AR, Boerwinkle E. Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: Differences between whites and african americans of the aric study. Ann Epidemiol. 2008;18:101–7. doi: 10.1016/j.annepidem.2007.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, et al. American Heart Association Epidemiology and Prevention and Nutrition, Physical Activity and Metabolism 2014 Scientific Sessions, Vol. San Francisco, CA: 2014. Calibration of analytes over twenty-five years in the atherosclerosis risk in communities study. [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The international collaborative of nt-probnp study. Eur Heart J. 2006;27:330–7. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 30.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2016;133:849–58. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrell F. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer; 2001. [Google Scholar]
- 32.Djousse L, Gaziano JM. Alcohol consumption and heart failure: A systematic review. Current atherosclerosis reports. 2008;10:117–20. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bower JK, Lazo M, Matsushita K, Rubin J, Hoogeveen RC, Ballantyne CM, Selvin E. N-terminal pro-brain natriuretic peptide (NT-proBNP) and risk of hypertension in the atherosclerosis risk in communities (ARIC) study. Am J Hypertens. 2015;28:1262–6. doi: 10.1093/ajh/hpv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, et al. N-terminal pro-b-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45:1646–50. doi: 10.1161/STROKEAHA.114.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: The atherosclerosis risk in communities study. Stroke. 2013;44:961–7. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension. 2001;37:1242–50. doi: 10.1161/01.hyp.37.5.1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


