Studies that evaluate agents with potentially rapid antidepressant effects are critical to developing new, improved treatments for major mood disorders. In particular, such studies may offer key insights into the mechanism of action of rapid-acting antidepressants as well as the neurobiology of mood disorders (1). In their excellent article published in this issue of Biological Psychiatry, Nagele et al. (2) investigated the antidepressant effects of nitrous oxide (N2O), largely because its mechanism of action is similar to the mechanism of action of the N-methyl-D-aspartate (NMDA) antagonist ketamine. Multiple studies found that subanesthetic doses of ketamine have rapid and sustained antidepressant effects in individuals with treatment-resistant major depressive disorder and bipolar disorder (1). In this blinded, placebo-controlled, crossover trial, 20 patients with treatment-resistant depression were randomly assigned to 1-hour inhalation of 50% nitrous oxide/50% oxygen or 50% nitrogen/50% oxygen (placebo control) (2). Compared with placebo, nitrous oxide significantly reduced depressive symptoms at 2 hours and 24 hours.
Humphry Davy was the first to describe the properties of nitrous oxide, an oxide of nitrogen (3). At room temperature, nitrous oxide is a nonflammable, colorless gas, with a slightly sweet taste and odor. In use since the early 1840s, nitrous oxide—commonly known as laughing gas—is one of the oldest anesthetic drugs. However, its use as a general anesthetic was not widely accepted in hospital settings because of its lower anesthetic potency (4). The main medical uses of nitrous oxide are in dentistry and obstetrics because of its analgesic and anxiolytic properties, and it is part of the World Health Organization List of Essential Medicines (5). Nitrous oxide acts as a noncompetitive inhibitor of the NMDA receptor, and because it is minimally metabolized in humans, it has a wide range of other potential therapeutic targets.
Nagele et al. (2) found that nitrous oxide was safe; no serious side effects were reported. Nevertheless, the scientific literature notes that nitrous oxide has been associated with numerous side effects. In early experiments conducted in the 1840s, Davy self-administered nitrous oxide and reported “a sensation analogous to gentle pressure of all the muscles, attended by a highly pleasurable thrilling, particularly in the chest and the extremities …. Nothing exists but thoughts! … as the pleasurable sensations increased, I lost all connection with external things, traces of vivid images rapidly passing through my mind ….” (3). Daly’s experiences and that of others evoke the dissociative effects associated with anesthetics such as ketamine. Later studies, which confirmed Davy’s initial characterization of the properties of nitrous oxide, described a variety of subjective experiences characteristic of psychedelic drugs, such as euphoria, altered body awareness and image, altered time and perception, and dreamy, detached experiential states (6). These properties led to the recreational use of nitrous oxide.
Paracelsus was the first to say that dose is what makes any drug either a poison or a treatment. In this context, an issue of potential concern is that nitrous oxide irreversibly depletes vitamin B12 levels. This depletion is dose-dependent and may cause serious neurotoxicity due to accumulation of homocysteine. In contrast, at lower doses, nitrous oxide is neuroprotective. Specifically, nitrous oxide inhibits NMDA-activated currents in a dose-dependent manner in cultured neurons and limits excitotoxic neurodegeneration mediated by NMDA receptors, underscoring its NMDA antagonism. However, as with ketamine (7), nitrous oxide seems to have a much broader mechanistic profile, which may play a role in its putative rapid antidepressant effects.
These results also invite comparison with other rapid-acting agents such as ketamine. Although ketamine and nitrous oxide have similar mechanisms of action, the efficacy of nitrous oxide was not as robust or durable as that of ketamine (50%–70% with ketamine vs. 20% with nitrous oxide at day 1). Nagele et al. (2) also observed no euphoria, dissociative effects, or psychotomimetic symptoms in their study, although no formal rating scales were used to assess these symptoms. However, the absence of these symptoms might reflect a dosing issue; if a higher dose of nitrous oxide had been used, a higher response rate might have been obtained, although at the expense of increased dissociative/psychotomimetic side effects or even toxicity. Until we conduct dose-response studies with these drugs, we may be unable to answer these questions. A National Institute of Mental Health dose finding study with ketamine is currently underway (https://clinicaltrials.gov; ClinicalTrials.gov Identifier NCT01920555).
Blinding is another issue of potential concern in this study, although the investigators used an elaborate system to mask subjects and raters to drug. However, whether nitrogen at 50% was an adequate active control for mimicking these properties remains to be determined.
In addition to NMDA antagonism, nitrous oxide also acts as a retrograde messenger by modulating signal generation and targeting neuronal membranes. It also alters membrane fluidity via its effects at ionic channels. Nitrous oxide also modulates a broad range of ligand-gated ion channels, weakly inhibiting alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, kainate, gamma-aminobutyric acid receptor C (GABAC), and 5-hydroxytryptamine 3 receptors (5-HT3), and slightly potentiating glycine and GABAA receptors. The anesthetic and analgesic effects of nitrous oxide are mainly related to its neuronal release of endogenous opioid peptides and inhibition of NMDA receptor–mediated currents. The effects at the opioid system also involve direct and partial opioid receptor agonism (mu, kappa, and delta receptors), whereas its euphoric effects appear to be mediated by activation of the mesolimbic reward pathway and dopamine release. Nitrous oxide also has anxiolytic effects that involve activation of the GABAA receptor (8).
Nitrous oxide gives rise to nitric oxide (NO) in the central nervous system; because NO has been shown to produce antidepressant, analgesic, and anxiolytic effects, it may represent a potential therapeutic target in mood disorders (Figure 1) (9). Also, NO is a naturally occurring gas that was identified as a key endogenous biological regulator central to neuroenergetics. Similar to nitrous oxide, NO has dose-dependent effects; it is neurotoxic at higher concentrations and has neuromodulatory and neuroprotective effects at physiologic concentrations. Also, NO modulation affects neurotransmitter release and induces synaptic plasticity. One postmortem study of unmedicated subjects with major depressive disorder found that the subjects had lower levels of neuronal nitric oxide synthase and NO than healthy control subjects. Nitric oxide synthase is highly active in the limbic system and colocalizes in the same neuron with key neurotransmitters involved in mood disorders, including serotonin, noradrenaline, and GABA. The mood stabilizer lithium and various antidepressants act directly at this system, increasing NO levels in subjects with mood disorders (10).
Figure 1.
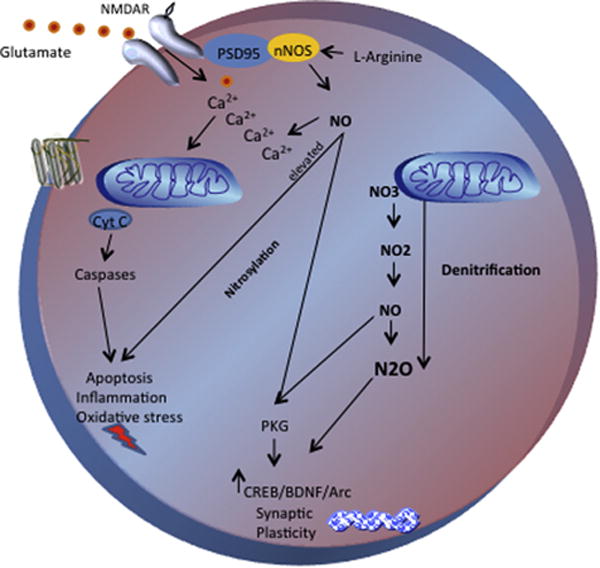
Potential pathways involved in the rapid antidepressant effects of nitrous oxide. Neuronal nitric oxide synthase is localized to N-methyl-D-aspartate receptors by the PDZ-domain adapter protein postsynaptic density protein 95. Nitric oxide (NO) acts intracellularly through different mechanisms such as nitrosylation and denitrification. The role of the NO pathway in neuroprotection has also been associated with decreased calcium influx and consequent inhibition of cell death, also increasing downstream neuroprotective proteins such as protein kinase B, cyclic AMP response element binding protein, brain-derived neurotrophic factor, and activity-regulated cytoskeleton-associated protein. NO diffuses into adjacent cells, where it activates guanylyl cyclase and long-term potentiation of protein kinase G, controls blood flow and neuronal transmission, and regulates pain (and possibly mood). The NO pathway plays a key role in cellular resilience, and NO and nitrous oxide have dual concentration-dependent effects. Physiologic levels induce plasticity, and higher concentrations activate lipid peroxidation, mitochondrial dysfunction, and apoptosis. The reactions of nitrification and denitrification involve electron transport systems and are linked to cellular energetics and adenosine triphosphate production. Arc, activity-regulated cytoskeleton-associated protein; BDNF, brain-derived neurotrophic factor; Ca2+, calcium; CREB, cyclic AMP response element binding protein; Cyt C, cytochrome C; NMDAR, N-methyl-D-aspartate receptor; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; NO2, nitrogen dioxide; NO3, nitrate; N2O, nitrous oxide; PKG, protein kinase G; PSD95, postsynaptic density protein 95.
The findings of Nagele et al. further support the role of the glutamatergic system in general—and NMDA receptor antagonism in particular—as a key property of agents with a rapid antidepressant effect. Nevertheless, this study also raises many important questions that need to be addressed in future research; many of these are reminiscent of questions raised during the development of ketamine as a treatment for treatment-resistant depression. For instance, can nitrous oxide be developed as a new treatment in mood disorders, or will it be limited to use as an investigational tool for exploring the mechanism of action of rapid antidepressant compounds? What are the right dose and duration of administration? How can we maintain the initial response of nitrous oxide? How can we maximize its tolerability? How safe is nitrous oxide when given repeatedly over time? Is NMDA antagonism alone key to the acute antidepressant effects of nitrous oxide? Might other off-site targets be involved in sustained antidepressant effects?
An urgent need exists for target engagement studies capable of testing the validity of methods and targets, particularly because other NMDA antagonists such as memantine and some NMDA subunit modulators have shown little to no efficacy in mood disorders. In addition to the obvious need to replicate these exciting preliminary findings, a better definition of the dose-response curve and an evaluation of additional time points for depression ratings are required. Such studies will likely provide new insights to determine whether nitrous oxide can become a novel treatment for treatment-resistant depression.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, by a NARSAD Independent Investigator Award (CAZ), and by a Brain & Behavior Mood Disorders Research Award (CAZ). The opinions expressed in this article are the authors' own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
We thank the 7SE research unit and staff for their support. Ioline Henter, MA (National Institute of Mental Health) provided invaluable editorial assistance.
Footnotes
Disclosures
CAZ is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression; he has assigned his rights in the patent to the U.S. Government but will share a percentage of any royalties that may be received by the Government. RM-V reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: A new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, et al. Nitrous oxide for treatment-resistant major depression: A proof-of-concept trial. Biol Psychiatry. 2015;78:10–18. doi: 10.1016/j.biopsych.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 3.West JB. Humphry Davy, nitrous oxide, the Pneumatic Institution, and the Royal Institution. Am J Physiol Lung Cell Mol Physiol. 2014;307:L661–L667. doi: 10.1152/ajplung.00206.2014. [DOI] [PubMed] [Google Scholar]
- 4.Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, et al. Neuroprotective gases—fantasy or reality for clinical use? Prog Neurobiol. 2014;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Gillman MA, Lichtigfeld FJ. Enlarged double-blind randomised trial of benzodiazepines against psychotropic analgesic nitrous oxide for alcohol withdrawal. Addict Behav. 2004;29:1183–1187. doi: 10.1016/j.addbeh.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Block RI, Ghoneim MM, Kumar V, Pathak D. Psychedelic effects of a subanesthetic concentration of nitrous oxide. Anesth Prog. 1990;37:271–276. [PMC free article] [PubMed] [Google Scholar]
- 7.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: A mechanistic and toxicologic review. Anesthesiology. 2008;109:707–722. doi: 10.1097/ALN.0b013e3181870a17. [DOI] [PubMed] [Google Scholar]
- 9.Ghasemi M, Dehpour AR. The NMDA receptor/nitric oxide pathway: A target for the therapeutic and toxic effects of lithium. Trends Pharmacol Sci. 2011;32:420–434. doi: 10.1016/j.tips.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.de Sousa RT, Zanetti MV, Busatto GF, Mouro MG, Zarate CA, Jr, Gattaz WF, et al. Lithium increases nitric oxide levels in subjects with bipolar disorder during depressive episodes. J Psychiatr Res. 2014;55:96–100. doi: 10.1016/j.jpsychires.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


