Abstract
Anhedonia is a cardinal symptom of major depression and is often refractory to standard treatment, yet no approved medication for this specific symptom exists. In this exploratory re-analysis, we assessed whether administration of rapid-acting antidepressant ketamine was associated specifically with reduced anhedonia in medication-free treatment-refractory patients with major depressive disorder in an open-label investigation. Additionally, participants received either oral riluzole or placebo daily beginning 4 hours post-infusion. A subgroup of patients underwent fluorodeoxyglucose positron emission tomography scans at baseline (1–3 days pre-infusion) and 2 hours post-ketamine infusion. Anhedonia rapidly decreased following a single ketamine infusion; this was sustained for up to three days, but was not altered by riluzole. Reduced anhedonia correlated with increased glucose metabolism in the hippocampus and dorsal anterior cingulate cortex (dACC) and decreased metabolism in the inferior frontal gyrus and orbitofrontal cortex (OFC). The tentative relationship between change in anhedonia and glucose metabolism remained significant in dACC and OFC, and at trend level in the hippocampus, a result not anticipated, when controlling for change in total depression score. Results, however, remain tenuous due to the lack of a placebo control for ketamine. In addition to alleviating overall depressive symptoms, ketamine could possess anti-anhedonic potential in major depressive disorder, which speculatively, may be mediated by alterations in metabolic activity in the hippocampus, dACC and OFC.
Keywords: Anti-anhedonic, dorsal anterior cingulate cortex, depression, 18FDG-PET, glutamate, hippocampus, NMDA, orbitofrontal cortex, reward, riluzole, subiculum
Introduction
Anhedonia, the decreased enjoyment derived from or desire for pleasurable activities, is a principal symptom of major depression. Approximately 40% of patients diagnosed with major depressive disorder (MDD) exhibit clinically significant anhedonia (Pelizza and Ferrari, 2009). The presence of anhedonia is associated with more severe depression and poorer treatment response in MDD patients treated with standard medications (Spijker et al., 2001; Uher et al., 2012). Furthermore, anhedonia in MDD is a predictor of proximal (within 1 year) suicide completion (Fawcett et al., 1990). Additionally, mounting evidence suggests that standard antidepressants may possess minimal efficacy in relieving anhedonia (Nutt et al., 2007) and may even induce emotional blunting (McCabe et al., 2010; Opbroek et al., 2002; Price et al., 2009) and sexual dysfunction (Hindmarch, 1998). Despite the importance of this symptom in psychiatry, and particularly in MDD, there is currently no approved medication specifically targeting anhedonia.
Treatment of anhedonia in patients with depression has been proposed to be feasible through manipulating signalling within the dopaminergic system (Argyropoulos and Nutt, 2013). However, few studies have tested this proposal directly. Moreover, even when effective in ameliorating broad-spectrum depressive symptoms, dopamine-modulating drugs typically take from weeks to months to induce meaningful antidepressant effects (Corrigan et al., 2000). Recent evidence suggests that targeting the glutamatergic system may provide fast-acting treatments for general depressive symptomatology (Sanacora et al., 2012). Berman et al. (2000) first reported that a single sub-anaesthetic infusion of the non- competitive N-methyl-D-aspartate (NMDA) receptor antagonist ketamine had rapid-acting antidepressant efficacy in major depression, a finding replicated in treatment-refractory patients diagnosed with MDD (Ibrahim et al., 2012; Murrough et al., 2013a, 2013b; Zarate et al., 2006;) and bipolar disorder (BD) (Diazgranados et al., 2010; Zarate et al., 2012). However, whether anhedonia is specifically treated by ketamine is currently unclear.
Given the inherent diagnostic heterogeneity in MDD, it is crucial to understand the symptom domains ameliorated by particular medications and the neural mechanisms underpinning such effects, as outlined in the National Institute of Mental Health Research Domain Criteria (RDoC) (Insel et al., 2010). Critically, evidence suggests that disruption in glutamatergic signalling may be particularly important in maintaining anhedonia. Blockade of astrocytic glutamate uptake, via both intracerebroventricular and intraprefrontal cortex dihydrokainic acid infusion, induced an anhedonia-like phenotype in rats (Bechtholt-Gompf et al., 2010; John et al., 2012). Furthermore, administration of a sub-anaesthetic dose of ketamine (Garcia et al., 2009; Li et al., 2011), and other NMDA antagonists (Papp and Moryl, 1993; Reus et al., 2012), has been shown to ameliorate anhedonia in rodent models of depression (although see Donahue et al., 2014, for a contrary finding). Using proton magnetic resonance spectroscopy, Walter et al. (2009) identified reduced glutamine (a precursor to glutamate), but not glutamate, in anhedonic patients with MDD relative to healthy volunteers, with a trend reduction relative to non-anhedonic MDD patients. Recently, in a randomized placebo-controlled crossover trial in treatment-resistant BD we demonstrated that ketamine exerted a specific anti-anhedonic effect, which was evident over and above the effects on other depressive symptoms (Lally et al., 2014). Intriguingly, the anti-anhedonic effect identified was correlated with increases in glucose metabolism in the dorsal anterior cingulate cortex (dACC).
Here in this study, we sought to extend these findings to patients with MDD. Medication-free patients received a single open-label dose of intravenous ketamine followed by oral riluzole or placebo for 28 days. The aim of the original study (Ibrahim et al., 2012) was to assess the ability of riluzole, a glutamate reuptake enhancer, to sustain the rapid-acting antidepressant effect of ketamine in MDD. In this paper we report further exploratory analyses, including 10 additional patients recruited from April 2011 to August 2013, following the submission of the initial report (Ibrahim et al., 2012), to investigate the changes following ketamine and adjunctive riluzole specifically on anhe-donia, as assessed by the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995). The result of the original report found no improvement in antidepressant response post-ketamine with adjunctive riluzole administration; therefore, we hypothesized that riluzole would also have no additional benefit in alleviating levels of anhedonia in MDD. Thus, the primary aim of this study was to assess the effect of an open-label ketamine infusion on levels of anhedonia. Additionally, in a subsample of patients, the underlying neural correlates of the effect of ketamine on anhedonia levels were assessed using [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET). The neural correlates of the general antidepressant effects of ketamine (and riluzole) in this sample have been reported previously (Carlson et al., 2013). However, the specific changes in anhedonia levels following ketamine administration, and the corresponding alterations in regional metabolism in MDD, have not been reported.
Materials and methods
Participants
Fifty-two patients diagnosed with treatment-refractory MDD without psychotic features were recruited to participate in this inpatient trial at the National Institute of Mental Health (NIMH), Bethesda, MD, USA. The Structured Clinical Interview for Axis I DSM-IV Disorders – Patient Version (First, 2002) was used to establish the psychiatric diagnosis, along with unstructured clinical interviews. Treatment resistance was defined as failure of two or more adequate antidepressant trials, as assessed by the antidepressant treatment history form (Sackeim, 2001). All patients were currently in a major depressive episode (DSM-IV-TR; First, 2002) lasting at least 4 weeks and had a score of 22 or higher on the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Åsberg, 1979). All patients were physically healthy and were free of comorbid substance abuse (including alcohol) or dependence (excluding caffeine or nicotine), lasting a minimum 3 months prior to admission. Comorbid Axis I anxiety disorders were permitted if they were not the primary diagnosis within the previous 12 months. Exclusion criteria included medication-induced mania or hypomania, other serious unstable medical conditions, previous use of either ketamine, riluzole, or phencyclidine, or electroconvulsive therapy in the 2 weeks prior to infusion, or use of fluoxetine in the 5 weeks prior to infusion; additionally, subjects could not be pregnant or nursing and were required to use approved methods of birth control during the study. The protocol was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health and all patients provided written informed consent prior to study entry.
Trial design
Following a 2-week drug-free period (5 weeks for fluoxetine), patients received a single open-label infusion of ketamine hydrochloride (0.5 mg/kg; Abbott Labs, Chicago, IL, USA) over 40 minutes via a Baxter infusion pump, administered by an anaesthesiologist or an advanced cardiac life support licensed practitioner. Between 4 and 6 hours post-infusion, patients were randomized to receive either riluzole or placebo (N in both groups = 26) twice a day for 4 weeks. Staff and subjects were blind to riluzole or placebo assignment. Riluzole was initiated at 100 mg/day, with the dose increasing in increments of 50 mg to a maximum of 200 mg/day. Dose escalations continued on a weekly basis until the appearance of treatment-limiting side effects or study completion. Dose reductions were permitted by one capsule (50 mg) in case of side effects. Subjects who were unable to tolerate the minimal allowable dose of riluzole (100 mg/day) were discontinued from the study. Inpatient nursing staff monitored medication adherence. No psychotherapy or other medications with central nervous system effects were permitted during the study.
Rating scales were administered at 60 minutes pre-infusion (baseline) and 40, 80, 120 and 230 minutes post-infusion, and thereafter daily for the subsequent 28 days. The primary outcome measure for this study was the MADRS. However, here we focus on anhedonia, which was measured using the SHAPS (e.g. item 1, ‘I would enjoy my favourite television or radio programme’). The SHAPS is a 14-item self-administered questionnaire, with each item scored from 1–4 (1 = strongly agree, 2 = agree, 3 = disagree, 4 = strongly disagree; range 14–56), with higher scores indicating greater anticipatory, not consummatory, anhedonia. The presence or absence of anhedonia was judged based on the original scoring guidelines indicated by Snaith et al. (1995), where disagreement (i.e. score of 3 or 4) on at least three items was defined as indicating clinically significant anhedonia (see Table 1). For a list of other secondary measures acquired, see Ibrahim et al. (2012).
Table 1.
Demographic information for MDD patients and the subgroup that underwent FDG-PET imaging.
| All subjects N = 52 |
FDG-PET subgroup N = 20 |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 48.29 | 12.84 | 47.80 | 11.79 |
| Age of onset of first depressive episode | 19.92 | 11.84 | 21.30 | 12.97 |
| Length of current episode (months) | 86.88 | 124.12 | 115.90 | 161.04 |
| Number of failed treatment trials | 7.54 | 4.31 | 7.70 | 4.14 |
| Baseline SHAPS | 37.69 | 6.67 | 37.20 | 6.13 |
| Baseline MADRS | 33.12 | 4.78 | 33.10 | 5.67 |
| BMI | 30.01 | 6.30 | 29.60 | 4.34 |
|
| ||||
| N | % | N | % | |
|
| ||||
| Caucasian | 49 | 94 | 20 | 100 |
| Clinically significant anhedonia (Snaith et al., 1995)a | 45 | 87 | 18 | 90 |
| Comorbid anxiety disorder | 30 | 59 | 11 | 55 |
| Female | 19 | 37 | 6 | 30 |
| Family history of alcohol use disorderb | 19 | 37 | 4 | 20 |
| Family history of mood disorder | 44 | 85 | 18 | 90 |
| Melancholic | 16 | 33 | 8 | 44 |
| Currently smoking (nicotine) | 7 | 13 | 2 | 10 |
MDD: major depressive disorder; FDG-PET: [18F]-fluorodeoxyglucose positron emission tomography; SHAPS, Snaith-Hamilton Pleasure Scale; MADRS: Montgomery-Åsberg Depression Rating Scale; BMI: body mass index.
Clinically significant anhedonia was defined as scoring three or more items on the SHAPS with a value of 3 (disagree) or 4 (strongly disagree), as per the classification suggested by Snaith et al. (1995).
Family history of alcohol use disorder was defined as having a first-degree relative meeting criterion on the Family Interview for Genetic Studies.
PET acquisition and analysis
A subgroup of 20 patients (44%) also underwent two FDG-PET scans (see Table 1), one at baseline (1–3 days pre-ketamine infusion) and another beginning 2 hours and ending 3.5 hours post-infusion (but prior to riluzole/placebo randomization). Due to limited resources, no more patients could undergo PET imaging. The precise details of the imaging parameters have been reported elsewhere (Carlson et al., 2013). Briefly, following 6 hours of fasting, PET images were acquired using a GE Advance PET scanner (GE Medical Systems, Waukesha, WI, USA) in 3D mode (35 contiguous slices, 4.25 mm plane separation; reconstructed resolution = 6 mm isotropic full width at half maximum). A bolus of 4.5 mCi of [18F]-FDG was infused following chest and heart transmission scans. Images of the ventricular blood pool were acquired. Following transmission scans of the brain, a 10-minute brain emission scan, beginning 45 minutes following FDG injection, was obtained. Beginning 15 minutes post-FDG injection, venous blood samples were acquired every 5 minutes.
To quantify regional cerebral metabolic rate of glucose (rCM-RGlu) images, dynamic cardiac imaging from the left ventricular chamber was used to calculate a cardiac input function. Venous blood samples during the brain emission scan were used to assess the blood pool radioactivity. According to methods established by Brooks (1982), these samples provided the [18F]-FDG input function used to generate parametric rCMRGlu images. To allow anatomical localization of PET activity, magnetic resonance images (MRI) were acquired on a 3 T scanner (Signa, GE Medical Systems) using a 3D MPRAGE sequence (echo time = 2.982 ms, repetition time = 7.5 ms, inversion time = 725 ms, voxel size = 0.9 × 0.9 × 1.2 mm).
PET analyses comprised both region of interest (ROI) and whole-brain investigations. The ROI analysis pipeline has been detailed elsewhere (Nugent et al., 2014). Briefly, the 3dskullstrip function of the Analysis of Functional NeuroImages (AFNI; Bethesda, MD, USA) software package was used to remove non-brain tissue; the resulting images were segmented into grey matter, white matter and cerebrospinal fluid binary mask images using the FMRIB software library automated segmentation tool (Zhang et al., 2001); Montreal Neurological Institute (MNI) 152 template-defined ROIs (ventral striatum (VS); orbitofrontal cortex (OFC)) were selected based on extant literature implicating these structures in depression and reward processing (Eshel and Roiser, 2010; Gorwood, 2008; Robinson et al., 2012; Treadway and Zald, 2011). The VS ROI was two bilateral 8 × 6 mm ellipsoids that covered three 2 mm axial slices and straddled both the caudate and putamen; the OFC ROI was a bilateral irregular shape that followed the grey matter of the orbital cortex, beginning medially at the gyrus rectus and extending laterally 48 mm and dorsally 24 mm. ROIs were transferred to the individual normalized anatomical MRIs and positioned to accommodate inter-individual anatomical variation; ROIs were then transferred back to native MRI space, multiplied by a binary grey matter mask, and applied to the rCMRGlu PET images. Mean glucose metabolism rate values, normalized by the mean metabolism throughout grey matter, were then calculated.
For the whole-brain analysis, raw FDG images, which were not modelled using the cardiac input function, were pre-processed and relationships with baseline and change in anhedonia scores were assessed using Statistical Parametric Mapping (SPM5; Wellcome Trust Centre for Neuroimaging, London, UK), within Matlab (MathWorks Inc, Natick, MA, USA). Baseline and post-ketamine images were first co-registered to the anatomical image, which was then normalized to the MNI152 anatomical template, and this transformation was then applied to the co-registered PET images. PET images were smoothed with an 8 mm (full width at half maximum) Gaussian kernel. To create the difference image, PET images were normalized by the SPM5-calculated global mean and the baseline image was subtracted from the post-ketamine image. A custom cerebral mask, which encompassed grey and white matter but reduced the inclusion of cerebrospinal fluid and non-brain tissue, was applied to all whole-brain analyses to limit the number of intracerebral voxels.
For both the ROI and whole-brain change relationship analyses, the percentage change between SHAPS levels at baseline and 230 minutes post-infusion ([ketamine – baseline]/baseline) was calculated and used as a regression variable. Before creating the percentage change score, the number 14 was subtracted from each total SHAPS score to generate a minimum score of 0. The 230-minute time point was selected for three reasons: the lack of psychotomimetic effect, the large effect on depressive symptoms noted at this time and the temporal proximity to the PET scan. To validate that this time point (230 minutes) was appropriate for inclusion in this analysis (i.e. free from the acute psychotomimetic effects of the ketamine infusion), we compared scores on the clinician-administered dissociative states scale (CADSS) at 230 minutes to scores at baseline and 24 hours post-infusion.
The main effect of ketamine (i.e. the difference in rCMRGlu between baseline and post-ketamine) has been reported elsewhere (Carlson et al., 2013).
Statistical analyses
For all statistical analyses, two-tailed statistical significance was determined as p < .05, with p < .1 considered a trend towards significance. All analyses, except where specified, were performed with SPSS 21 (IBM SPSS, 2010, Chicago, IL, USA). For the analysis of symptom changes, all available time points for each subject were included in the analysis. Due to the preliminary and exploratory nature of the imaging approach, in conjunction with the small sample size, adjustment for multiple comparisons was not conducted for this analysis.
Psychometrics
To explore the role of drug tapering and the medication-free period on depression and anhedonia, we compared MADRS scores, and specifically the MADRS anhedonia question (item 8, ‘inability to feel’), from consensus ratings (acquired between 1 and 20 days pre-infusion, mean = 6.5, SD = 4.36 days) and the baseline (60 minutes pre-ketamine infusion) using paired-samples t-tests. These data were available for only 46 of 52 subjects. Unfortunately, SHAPS scores were not collected at this time point.
Primary analyses
To examine the change in the total SHAPS score over time, linear mixed models were estimated with a restricted maximum likelihood procedure using an autoregressive moving averages covariance structure. We first examined whether riluzole had an anti-anhedonic effect by using study day (1–28) as a factor, baseline SHAPS score as a covariate, and randomization to riluzole or placebo as a between-subjects factor. Due to a non-significant effect of riluzole on levels of anhedonia, subsequent models explored the change (relative to baseline) in anhedonia levels following ketamine only and included all available time points with no between-subjects drug factor (riluzole or placebo); baseline SHAPS score was not entered as a covariate for these analyses as the focus was on whether the symptoms changed from baseline. Post-hoc simple effects tests, Bonferroni corrected for 32 time points, were used to assess differences between baseline and post-ketamine levels of anhedonia over the 28-day period.
A subsequent model, including total MADRS score (minus anhedonia item 8) as a time point-specific covariate, was constructed to explore whether the change in anhedonia levels following ketamine occurred over and above the effect on other depressive symptoms. However, unlike in our previous report of an anti-anhedonic effect in BD (Lally et al., 2014), here we were unable to account for variation in total MADRS score due to the lack of a placebo-controlled comparison. Without a placebo condition, the baseline is the only time point against which it is possible to compare post-ketamine scores. However, differences in variance between the baseline and follow-up points precluded accurate estimation of the model including total MADRS score as a covariate. The total depression score covariate accounted for substantially more variance at baseline than at other time points, making accurate estimations of fixed effects and comparisons between baseline and post-baseline time points untenable.
Secondary analyses
Additionally, to explore whether ketamine exerted specific anti-anhedonic effects, we examined whether the diagnosis of melancholic MDD subtype was associated with enhanced response in comparison to other subtypes (atypical, neither). We also explored whether a comorbid diagnosis of anxiety was associated with an improved anti-anhedonic response to ketamine. Finally, based on previous research demonstrating an effect of family history of alcohol use disorder on ketamine antidepressant effects (Luckenbaugh et al., 2012; Phelps et al., 2009), we assessed an additional model to explore whether family history of alcohol use disorder (in a first-degree relative) predicted enhanced anti-anhedonic response to ketamine. These three additional models (melancholia, anxiety and alcohol) all included baseline SHAPS score as a covariate to account for individual differences at baseline in these between-subjects analyses; including baseline in other analyses (within subjects, e.g. the effect of ketamine) was not appropriate, as the focus was on whether the symptoms changed from baseline.
PET imaging (ROI)
We assessed whether rCMRGlu in the VS and the OFC was related to hedonic capacity at baseline and the change following ketamine ([ketamine – placebo]/placebo). Pearson product moment correlations were used to assess all relationships. Partial correlations (including MADRS minus item 8) were used to examine the specificity of associations between anhedonia and rCMRGlu. Finally, based on our recent finding that the anti-anhedonic effect of ketamine in BD was associated with increased rCMRGlu in the dACC (Lally et al., 2014), we performed small volume correction (SVC) on the whole-brain images discussed directly below using a 10 mm radius sphere upon the peak voxel identified in our previous study (MNI: [X = −8, Y = 40, Z = 28]).
PET imaging (whole brain)
Complementing the ROI analysis, we also examined whether baseline rCMRGlu was related to baseline SHAPS score, and whether change in SHAPS score (230 minutes post-infusion relative to baseline) was related to change in rCMRGlu, across the whole brain, in SPM5. Additionally, where significant associations between anhedonia levels and rCMRGlu were detected, we orthogonalized SHAPS score against the depression score (total MADRS minus item 8) and regressed this variable against rCMRGlu to examine the specificity of the relationship to anhedonia. Orthogonalization was performed using the SPM function spm_orth, which removes overlapping variance between two variables from one of them; here, the variance in change in MADRS was removed from the variance in change in SHAPS. These analyses used an uncorrected voxel threshold of p < .001, with whole-brain correction (WBC) for multiple comparisons at the cluster level (family-wise error rate p < .05, p < .1 for trend significance).
Results
Psychometrics
Almost all patients (87%) reported clinically significant levels of anhedonia at baseline, as measured by the SHAPS. There was a significant positive correlation between total SHAPS score and MADRS score at baseline (r(52) = .47, p < .001), indicating that patients with higher levels of anhedonia also exhibited higher levels of depressive symptoms overall.
There was no significant change in levels of depression (total MADRS score; t(45) = 0.993, p = .326) or anhedonia (MADRS item 8; t(45) = −0.2985, p = .767) between the time of the consensus ratings and the baseline time point, suggesting that the drug tapering and drug-free period did not significantly induce changes in anhedonia.
In comparison to baseline, levels of dissociation, as measured by the CADSS, were significantly lower at 230 minutes post-ketamine (t(51) = 3.74, p < .001). Furthermore, there was no significant difference between CADSS scores at 230 minutes and 24 hours post-infusion (t(51) = 0.861, p = .393).
Primary analyses
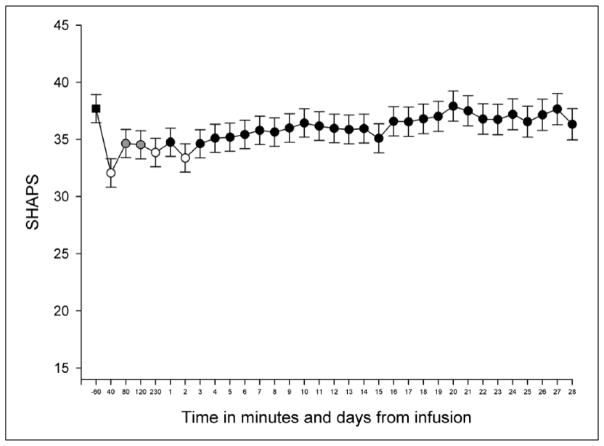
We investigated the effect of riluzole on anhedonia levels on days 1–28 to explore whether the results necessitated separate models for the pre- and post-riluzole phases. We found a significant effect of time (F(27,610) = 1.910, p < .004) but no effect of drug (F(1,51) = 0.201, p = .656) and no interaction between time and drug (F(27,610) = 1.204, p < .221), indicating a lack of an additive effect of riluzole to the anti-anhedonic effect of ketamine. Due to the lack of an effect of riluzole on SHAPS scores, we conducted a model with an effect of time but without the effect of drug (i.e. riluzole vs placebo) to explore the anti-anhedonic changes following ketamine. We found a significant effect of time (F(32, 642) = 3.355, p < .001), indicating that there was a reduction in SHAPS scores across the 28-day period following a single ketamine infusion (Figure 1). Stringent post-hoc Bonferroni-corrected multiple comparisons indicated that, relative to baseline, anhedonia was significantly reduced, or at trend level, at 40 (t(1010) = 8.57, pcorr < .001), 80 (t(1312) = 4.55, pcorr < .005), 120 (t(1278) = 4.53, pcorr < .005) and 230 (t(1073) = 5.28, pcorr < .001) minutes post-ketamine, and on days 1 (t(867) = 3.91, pcorr = .053), 2 (t(723) = 5.51, pcorr < .001) and 3 (t(614) = 3.79, pcorr = .087) following the single ketamine infusion. Of note, degrees of freedom derived from linear mixed models are parameters estimated through SPSS and are adjusted by the software package to offer a less biased test of the null hypothesis, and thus do not accurately reflect the number of participants.
Figure 1.
Change in anhedonia levels (SHAPS) across time (minutes = −60 to 230, days = 1 to 28) following open-label ketamine. The values presented here are derived from the estimated marginal means from the linear mixed model using all subjects (placebo and riluzole combined). The baseline value, to which the other values are compared, is represented by a square (time point −60). White and grey circles indicate time points at which statistically significant reductions were detected relative to baseline at p < .001 and p < .005, respectively (corrected for multiple comparisons). Error bars represent 1 SEM.
Secondary analyses
There was no significant main effect of depression subtype (melancholic vs other subtypes; F(1, 51) = 2.001, p = .163) or interaction between subtype and time (F(31, 574) = 0.792, p = .783). There was no main effect of comorbid anxiety diagnosis (F(1, 53) = 0.743, p = .393) and no interaction between anxiety diagnosis and time (F(31, 577 ) = 1.221, p = .194). There was a significant main effect of family history of alcohol use disorder (F(1, 54) = 4.538, p = .038), with improved anti-anhedonic response to ketamine in subjects with a positive (versus a negative) history; however, the interaction between time and family history of alcohol use disorder was non-significant (F(31, 590) = 0.934, p = .572).
PET imaging
ROI
Due to technical difficulties, venous blood sampling was not possible and ROI modelling could not be completed for one patient, leaving a sample size of 19 for this analysis only. There was no relationship between rCMRGlu in either of our ROIs and SHAPS score at baseline, and baseline metabolism in these regions did not correlate with change in anhedonia following ketamine (see Table 2). However, consistent with our previous report (Lally et al., 2014), a trend towards a negative association between increased rCMRGlu and decreased anhedonia was found in the VS (r(19) = −.422, p = .072), although this relationship was non-significant when controlling for total depression score (total MADRS score minus item 8: r(19) = −.319, p = .197). No relationship between change in rCMRGlu in the OFC and change in SHAPS score was found (see Table 2).
Table 2.
Correlations between SHAPS scores and rCMRGlu in the two ROIs.
| ROI | r(19) | p |
|---|---|---|
| Baseline rCMRGlu and baseline SHAPS | ||
| VS | .018 | .940 |
| OFC | .326 | .173 |
| Baseline rCMRGlu and %Δ SHAPS | ||
| VS | .094 | .703 |
| OFC | −.339 | .156 |
| Δ rCMRGlu and %Δ SHAPS | ||
| VS | −.422 | .072 |
| OFC | −.155 | .527 |
SHAPS: Snaith-Hamilton Pleasure Scale; rCMRGlu: regional cerebral metabolic rate of glucose; ROI: region of interest; VS: ventral striatum; OFC: orbitofrontal cortex; %Δ: percentage change.
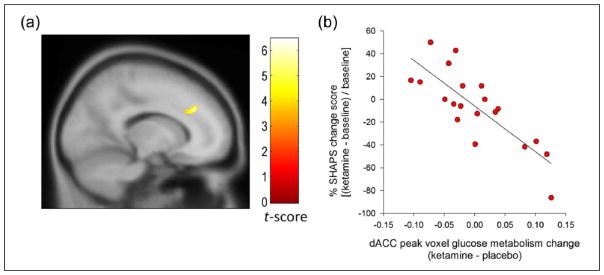
Finally, based on our recent report in which we identified that increased rCMRGlu in the dACC was associated with decreased anhedonia levels following ketamine (Lally et al., 2014), we performed SVC using the peak voxel from our prior analysis. There was a significant negative association between decreases in anhedonia and increases in rCMRGlu in the dACC following ketamine (X = −14, Y = 34, Z = 32, t(19) = 5.78, voxel-level pSVC = .002) (Figure 2(a) and (b)). This finding remained significant when controlling for change in total MADRS (X = −14, Y = 34, Z = 30, t(19) = 5.15, pSVC = .007).
Figure 2.
Increased dorsal anterior cingulate cortex (dACC) metabolism correlated with anti-anhedonic response following ketamine administration. (a) Presented in a sagittal plane, the analysis, which survived small volume correction, entailed a 10 mm radius sphere centered on a peak derived from our previous investigation demonstrating the anti-anhedonic effect of ketamine in bipolar depression (Lally et al., 2014). Colour bar indicates t-values and the image is displayed at an uncorrected threshold of p < .001. (b) Scatter plot depicting increased dACC glucose metabolism at the peak voxel associated with decreased anhedonia levels.
Whole brain
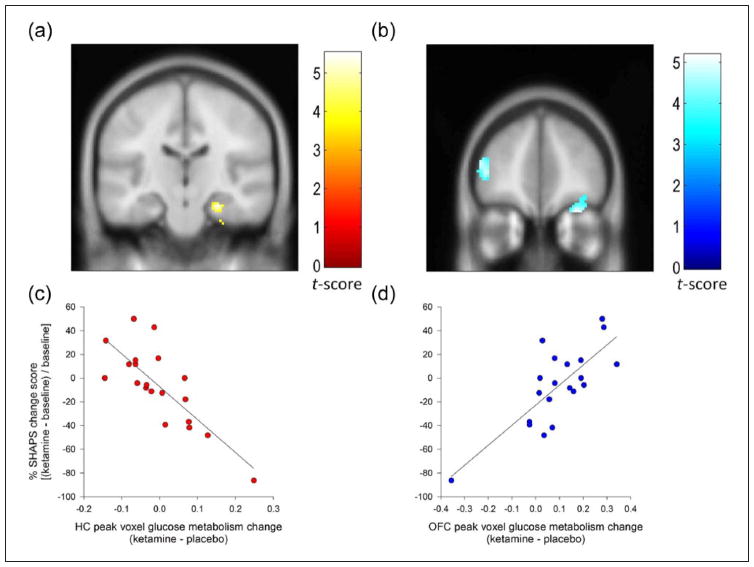
There were no significant whole-brain corrected associations between baseline levels of SHAPS and baseline glucose metabolism, nor was baseline rCMRGlu predictive of the anti-anhedonic response to ketamine. However, examination of the relationship between change in SHAPS and change in rCMRGlu revealed that increased glucose metabolism in the right hippocampus was associated with decreased anhedonia (cluster-level pWBC = .01) (Figure 3(a) and (c)); this extended into the entorhinal cortex (Table 3). A trend towards a similar association was present in the contralateral hemisphere (cluster-level pWBC = .058). This analysis also revealed that decreased rCMRGlu in the right OFC (cluster-level pWBC = .01) (Figure 3(b) and (d)) and the left inferior frontal gyrus (IFG) (cluster-level pWBC = .045) was associated with decreased anhedonia. The relationships between hippocampal and OFC rCMRGlu, but not IFG rCMRGlu, were significant or at trend level when controlling for change in MADRS score (minus item 8) (see Table 3).
Figure 3.
Results of percentage change in SHAPS regressed against FDG-PET difference images. Associations (whole-brain corrected, cluster-level) were identified in (a) the hippocampus (yellow) and (b) both the inferior frontal and orbitofrontal cortex (blue). (c) Increased glucose metabolism in the hippocampus and (d) decreased metabolism in the orbitofrontal cortex (OFC) and inferior frontal gyrus were associated with the greatest anti-anhedonic response. Images are displayed at an uncorrected threshold of p < .001, and at an extent threshold such that only clusters surviving whole-brain correction are presented. Colour bars indicate t-values.
Table 3.
FDG-PET imaging results. Changes in glucose metabolism associated with decreased anhedonia levels. All p values are whole-brain cluster-corrected for multiple comparisons following an initial uncorrected threshold of p < .001. Where possible, up to three sub-peaks (regions without a corresponding cluster significance and extent) are given for each cluster. Only clusters with a minimum extent of 50 voxels are reported.
| Region | MNI coordinatea
|
Peak t(19)-value
|
Cluster pWBC
|
Extent | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Ketamine baseline PET vs SHAPS % improvement | ||||||
| rCMRGlu increases | ||||||
| Lingual gyrus | 28 | −52 | −4 | 6.07 | .304 | 75 |
| Dorsal ACC | −14 | 34 | 32 | 5.78 | .445 | 62 |
| Hippocampus (subiculum) | 26 | −20 | −20 | 5.51 | .010 | 193 |
| Entorhinal cortex | 22 | −26 | −24 | 5.21 | ||
| Entorhinal cortex | 28 | −18 | −34 | 3.79 | ||
| Pregenual ACC | −2 | 46 | 2 | 5.26 | .374 | 68 |
| Middle frontal gyrus | 30 | 24 | 38 | 4.65 | .408 | |
| Calcarine gyrus | 22 | −76 | 4 | 4.58 | .286 | |
| Entorhinal cortex | −28 | −18 | −28 | 4.54 | .058 | 130 |
| Hippocampus (subiculum) | −20 | −10 | −30 | 4.05 | ||
| Superior frontal gyrus | 14 | 50 | 48 | 4.14 | .470 | 60 |
| Fusiform gyrus | −24 | −80 | 0 | 4.13 | .539 | 55 |
| rCMRGlu decreases | ||||||
| Orbitofrontal cortex | 28 | 46 | −24 | 5.18 | .010 | 193 |
| Orbitofrontal cortex | 36 | 52 | −18 | 4.39 | ||
| IFG (pars orbitalis) | 40 | 34 | −16 | 4.01 | ||
| Middle cingulate cortex | −8 | −18 | 34 | 5.09 | .323 | 73 |
| IFG (pars triangularis) | −44 | 44 | 6 | 4.83 | .045 | 139 |
| IFG (pars triangularis) | −48 | 46 | 14 | 4.79 | ||
| Cerebellum (lobule VIIa) | −52 | −52 | −36 | 4.67 | .323 | 73 |
| Medial temporal pole | 58 | 10 | −32 | 3.96 | .484 | 59 |
| Ketamine baseline PET vs SHAPS % improvement orthogonalized against % MADRS improvement | ||||||
| rCMRGlu increases | ||||||
| Calcarine gyrus | 22 | −76 | 6 | 4.97 | .397 | 66 |
| Fusiform gyrus | 26 | −32 | −22 | 4.91 | .091 | 115 |
| Hippocampus (subiculum) | 26 | −20 | −20 | 4.22 | ||
| Middle frontal gyrus | 28 | 20 | 38 | 4.90 | .333 | 72 |
| Entorhinal cortex | −26 | −10 | −36 | 4.73 | .067 | 125 |
| Hippocampus (subiculum) | −28 | −20 | −26 | 4.67 | ||
| Superior medial gyrus | 8 | 38 | 52 | 4.68 | .138 | 101 |
| rCMRGlu decreases | ||||||
| Orbitofrontal cortex | 28 | 46 | −24 | 5.14 | .032 | 151 |
| Orbitofrontal cortex | 34 | 44 | −14 | 4.25 | ||
| Inferior temporal gyrus | 56 | −50 | −26 | 4.67 | .584 | 52 |
FDG-PET: [18F]-fluorodeoxyglucose positron emission tomography; MNI: Montreal Neurological Institute; SHAPS: Snaith-Hamilton Pleasure Scale; rCMRGlu: regional cerebral metabolic glucose metabolism; ACC: anterior cingulate cortex; IFG: inferior frontal gyrus; MADRS: Montgomery-Åsberg Depression Rating Scale; WBC: whole brain corrected.
MNI coordinates indicate the distance (in millimeters) from the stereotaxic origin (anterior commissure), with X representing the lateral distance from the origin (positive numbers to the right), Y representing the anterior–posterior dimension (positive numbers anterior) and Z representing the dorsal–ventral dimension (positive numbers dorsal).
Discussion
This re-analysis assessed the anti-anhedonic efficacy of a single open-label intravenous infusion of ketamine in the treatment of medication-refractory MDD. These patients also received either placebo or oral riluzole as a daily adjunctive treatment for 28 days, beginning between 4 and 6 hours post-infusion. Ketamine rapidly reduced levels of anhedonia in this sample, with a substantial effect within 40 minutes that remained 3 days post-infusion. Riluzole had no additional benefit. In a subsample of patients, we acquired rCMRGlu images to assess the neurobiological correlates of anhedonia at baseline and post-ketamine infusion. No association between anhedonia and baseline glucose metabolism was found. However, decreased anhedonia was most strongly associated with rCMRGlu increases in the hippocampus and dACC, and decreases in the OFC, which remained significant or at trend level after controlling for change in overall depressive symptoms. These results, while promising, remain tentative due to the lack of a placebo-controlled comparison for the effects of ketamine.
Given the prevalence and debilitating nature of anhedonia across neuropsychiatric disorders (Gard et al., 2007; Lemke et al., 2005; Pizzagalli et al., 2008), the absence of approved treatments for this symptom is surprising. In particular, evidence suggests that standard medications for depression are poor at alleviating anhedonia, with anhedonia often being the last symptom improved by selective serotonin reuptake inhibitors (SSRIs) (Boyer et al., 2000; Shelton and Tomarken, 2001). Moreover, some reports suggest that SSRIs may in fact induce some aspects of anhedonia in depressed patients (Hindmarch, 1998; Price et al., 2009). Unlike our previous investigation in BD where we demonstrated an anti-anhedonic effect of ketamine independent of its broad-spectrum antidepressant efficacy (Lally et al., 2014), in this study it was not possible to examine the specificity of the reduction. Nevertheless, the rapid and sustained anti-anhedonic response shown here following a single infusion of ketamine in treatment-resistant patients diagnosed with MDD is promising and consistent with our prior report (6.45 vs 7.36 point reduction in SHAPS score at 40 minutes post-ketamine, respectively).
Consistent with our previous report finding a lack of an adjunctive effect post-ketamine, riluzole had no apparent additional benefit in further reducing the anhedonia decrease induced by ketamine (Ibrahim et al., 2012). A detailed explanation as to why riluzole failed to improve the antidepressant response in our sample has recently been reported (Niciu et al., 2014b).
Echoing previous investigations (Luckenbaugh et al., 2012; Phelps et al., 2009), we found that patients with a family history of an alcohol use disorder had an enhanced anti-anhedonic response to ketamine. This supports the hypothesis of a biological disposition to NMDA receptor antagonists associated with a family history of an alcohol use disorder (Krystal et al., 2003; Petrakis et al., 2004). Indeed, a family history of alcohol dependence has been reported to be associated with polymorphisms in subunits of the NMDA receptor (Edwards et al., 2012; Schumann et al., 2008), on which ketamine acts. While the precise mechanism behind the antidepressant efficacy of ketamine is presently undetermined, it is believed to occur via an enhancement in neuroplasticity hypothesized to be triggered by increased synaptic glutamate and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-to-NMDA receptor throughput (Niciu et al., 2014a).
At the systems level, the broad-spectrum improvements in depression symptomatology following ketamine in patients with MDD have been correlated with rCMRGlu increases in the temporal gyrus and cerebellum, and decreases in the parahippocampal gyrus and ventral ACC (amongst others), respectively (Carlson et al., 2013). Consistent with the notion that anhedonia has a neural basis distinct from that of other depressive symptoms, the present study identified a specific set of neurobiological correlates disjunct to the previous broad-spectrum investigation of the anti-depressive effects of ketamine in MDD (Carlson et al., 2013). Contrary to our hypotheses, we identified no significant relationships between metabolic activity and either baseline or change levels of anhedonia in our ROIs when controlling for change in other depressive symptoms, a finding consistent with our recent report in BD patients (Lally et al., 2014). However, the whole-brain investigation revealed that change from baseline in rCMRGlu in both the hippocampal subiculum (increases; an association not hypothesized) and right OFC (decreases) were related to reductions in anhedonia levels following ketamine. Furthermore, we also identified increased dACC rCMRGlu associated with reduced anhedonia, consistent with our previous investigation of the anti-anhedonic effect of ketamine in BD (Lally et al., 2014). The relationships between change in glucose metabolism in the OFC and dACC and the anti-anhedonic response to ketamine were predicted.
The hippocampus has been implicated in reward processing and in particular in reward anticipation (Schott et al., 2008), motivation and learning (Wittmann et al., 2005). The subiculum, the most inferior component and major output centre of the hippocampal formation, contains excitatory glutamatergic neurons that directly innervate the nucleus accumbens in the rat brain (Sweet and Neill, 1999). Direct activation of the subiculum through electrical stimulation or the application of NMDA causes an increase in dopamine levels in the nucleus accumbens (an effect that is glutamatergic receptor dependent (Blaha et al., 1997; Brudzynski and Gibson, 1997) and also in the ventral tegmental area (Legault and Wise, 1999). The increased glucose metabolism in the subiculum shown here may be a potential mechanism for increased dopamine release by ketamine (Moghaddam et al., 1997; Vollenweider et al., 2000). However, it should be noted that Blaha et al. (1997) found that subiculum stimulation did not result in a temporally linear relationship to dopamine release in the nucleus accumbens. Subiculum stimulation initially caused dopamine increases in the nucleus accumbens, then decreases, and finally increases once more; this non-linear relationship may help to explain why changes in VS rCMRGlu were not found to be significantly associated with changes in anticipatory anhedonia levels.
In reward processing, the OFC appears to be critical for the representation of value across the sensorium (Kringelbach et al., 2003). Evidence suggests that there may be distinct functional specialization within the OFC, with the medial area responsible for reward and the lateral area for punishment (Elliott et al., 2000; O’Doherty et al., 2001). While our OFC ROI analysis, which comprised only the posterior sections, found no association between glucose metabolism and response, our whole-brain corrected voxel-wise analysis found a significant relationship. The rCMRGlu reduction found in anterolateral OFC here could reflect a decrease in punishment or cost associated with the decisions related to the anticipation of activities assessed by the SHAPS. Indeed, patients with MDD have frequently been found to exhibit both hyposensitivity to reward and hypersensitivity to punishment during decision-making tasks (Eshel and Roiser, 2010). Furthermore, physiological abnormalities, such as increased cerebral blood volume, have been identified in the OFC in patients with MDD (Drevets, 2007). Although the precise function of this region and the relationship to MDD remains unknown, our results are suggestive of a potential link between reductions in anhedonia and decreased OFC rCMRGlu, which may relate to punishment or cost sensitivity.
In BD dACC rCMRGlu was negatively correlated with the anti-anhedonic response to ketamine (Lally et al., 2014). Corroborating this finding, we again found evidence for an increase in glucose metabolism in this region in conjunction with a decrease in anhedonia levels. Shidara and Richmond (2002) determined that reward expectancy, or motivation, was highly correlated with single unit neuronal signals in the dACC of the macaque. In healthy humans, activity in the dACC has also been found to be associated with reward anticipation (Benoit et al., 2011). The increases in dACC rCMRGlu seen in the present study might reflect changes in motivation towards, and anticipation of, or ability to anticipate, pleasurable activities.
No association between rCMRGlu and levels of anhedonia, as measured by the SHAPS, was found at baseline, nor was baseline metabolism predictive of the anti-anhedonic response to ketamine. One possible explanation for these negative findings is the lack of variance within our sample before medication administration. Indeed, the SD for the SHAPS score at 230 minutes (9.65) was higher than at baseline (6.67), potentially suggesting that there was not enough variability in our sample at baseline to detect subtle relationships between brain and behaviour. As anhedonia is a cardinal symptom of depression, selecting patients who currently fit the criteria for a major depressive episode almost directly ensures a lack of variance at baseline.
This study has several limitations that need to be addressed by future research. First, the lack of a placebo control for ketamine lessens the potential to extrapolate fully the direct influence of the drug on both brain and behaviour in patients. It is possible that some of changes in anhedonia levels following ketamine were driven by the subjects’ knowledge and expectation surrounding the infusion. However, the treatment-refractory nature of the sample here, in conjunction with the lack of evidence for a placebo effect in previous randomized placebo-controlled studies of ketamine in treatment-resistant depression (Murrough et al., 2013a, 2013b; Zarate et al., 2006), is perhaps contradictory to this possibility. Second, due to the lack of a placebo control for ketamine, it was not possible to appropriately estimate the independence of the change in anhedonia levels from the change in total depression score. In our previous study we compared placebo and ketamine at corresponding time points; in the present study, the only possible comparison to post-infusion time points, baseline, was untenable as a comparator due to substantial differences in score variability between baseline and post-infusion assessments. Controlling for total depression score removed substantially more variance from the baseline than post-infusion times, rendering the baseline comparison flawed.
Third, the baseline scan occurred 1–3 days pre-infusion, while the baseline SHAPS was administered 60 minutes prior to the infusion on day 0; this discrepancy may have reduced the sensitivity of our analyses. Fourth, the validity of the anti-anhedonic reduction induced by ketamine at 40 minutes post-infusion commencement is questionable. A recent publication explored the effect of ketamine infusion on levels of dissociation and psychotomimesis in depressed patients (unipolar and bipolar) and found that at 40 minutes post-infusion, patients experienced the greatest side effects in both of these areas (Luckenbaugh et al., 2014). Nevertheless, Luckenbaugh et al. (2014) also found that the individuals who experienced the most dissociation at 40 minutes post-infusion had the greatest reduction in depressive symptoms at 230 minutes and 7 days post-infusion, suggesting that the acute ‘high’ effects induced by ketamine may be an important component of its antidepressant mechanism of action.
Fifth, while we attempted to home in on the specific underlying biomarkers of reductions in anhedonia by controlling for total depression score, it is possible that our rCMRGlu FDG-PET change results may reflect alterations in variables not assessed by the MADRS. For example, Behrens et al. (2007) found that blood-oxygen level-dependent signals in the dACC, in a similar coordinate to our finding, were strongly related to environmental levels of volatility in a reward decision-making task. Although there are no data describing differing estimates of environmental volatility in patients with MDD after ketamine or even at baseline, this example illustrates that other interpretations of our results not relating specifically to anhedonia exist.
Finally, replication and extension of our brain imaging findings are required due to the relatively small number of patients that could be included in PET imaging and the lack of appropriate correction for multiple comparisons, which renders the imaging portion of this study highly exploratory. Future studies examining the neural correlates of anhedonia may also benefit from studying a wider spectrum of levels of anhedonia, as suggested by the RDoC (Insel et al., 2010), and the inclusion of non-treatment-refractory patients, particularly in the examination of pre- treatment biomarkers.
In summary, our results suggest that a single infusion of ketamine is potentially efficacious in rapidly ameliorating levels of anhedonia in MDD. Riluzole, however, was not found to be an effective adjunctive anhedonia treatment post-ketamine. We found no relationship between baseline brain metabolism and anhedonia, either at baseline or following ketamine. The anti-anhedonic effects found post-ketamine infusion were associated with changes in the hippocampus (not hypothesized), dACC and OFC. However, our findings remain tentative due to the lack of a placebo control and, in particular, the lack of correction for multiple comparisons for the imaging analyses. Nevertheless, our results add increasing weight to the promise of NMDA receptor antagonists and other glutamatergic compounds in treating cardinal symptoms of depression. Given the safety of ketamine, the potential for treating patients with residual anhedonic symptomatology is high. Future efforts to understand its antidepressant effects should aim to parse the improvements into symptom domains to determine which patient subgroups may be most likely to benefit from treatment.
Acknowledgments
The authors would like to thank the clinical staff of the 7-SE unit, without whom this work could not have been possible.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; grant number 04-M-0222).
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A patent application for the use of ketamine in depression has been submitted listing Dr Carlos A Zarate among the inventors; he has assigned his rights on the patent to the U.S. government, but will share a percentage of any royalties that may be received by the government. All other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Argyropoulos SV, Nutt DJ. Anhedonia revisited: Is there a role for dopamine-targeting drugs for depression? J Psychopharmacol. 2013;27:869–877. doi: 10.1177/0269881113494104. [DOI] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Walther HV, Adams MA, et al. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology. 2010;35:2049–2059. doi: 10.1038/npp.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, et al. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, et al. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Boyer P, Tassin JP, Falissart B, et al. Sequential improvement of anxiety, depression and anhedonia with sertraline treatment in patients with major depression. J Clin Pharm Ther. 2000;25:363–371. doi: 10.1046/j.1365-2710.2000.00302.x. [DOI] [PubMed] [Google Scholar]
- Brooks RA. Alternative formula for glucose utilization using labeled deoxyglucose. J Nucl Med. 1982;23:538–539. [PubMed] [Google Scholar]
- Brudzynski SM, Gibson CJ. Release of dopamine in the nucleus accumbens caused by stimulation of the subiculum in freely moving rats. Brain Res Bull. 1997;42:303–308. doi: 10.1016/s0361-9230(96)00290-0. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: A preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, et al. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety. 2000;11:58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, et al. Effects of striatal deltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Aliev F, Bierut LJ, et al. Genome-wide association study of comorbid depressive syndrome and alcohol dependence. Psychiatr Genet. 2012;22:31–41. doi: 10.1097/YPG.0b013e32834acd07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuro-imaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute: Biometrics Research; 2002. [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, et al. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I. The behavioural toxicity of antidepressants: Effects on cognition and sexual function. Int Clin Psychopharmacol. 1998;13(Suppl 6):S5–S8. [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: Results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- John CS, Smith KL, Van’t Veer A, et al. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology. 2012;37:2467–2475. doi: 10.1038/npp.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, et al. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, et al. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh D, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Translational Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lemke MR, Brecht HM, Koester J, et al. Anhedonia, depression, and motor functioning in Parkinson’s disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci. 2005;17:214–220. doi: 10.1176/jnp.17.2.214. [DOI] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh DA, Ibrahim L, Brutsche N, et al. Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord. 2012;14:880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, et al. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010;67:439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry. 2013a;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013b;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, et al. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: Ketamine and other compounds. Annu Rev Pharmacol Toxicol. 2014a;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, et al. Riluzole likely lacks antidepressant efficacy in ketamine non-responders. J Psychiatr Res. 2014b;58:197–199. doi: 10.1016/j.jpsychires.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Diazgranados N, Carlson PJ, et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2014;16:119–128. doi: 10.1111/bdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: The role of catecholamines in causation and cure. J Psychopharmacol. 2007;21:461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Opbroek A, Delgado PL, Laukes C, et al. Emotional blunting associated with SSRI-induced sexual dysfunction. Do SSRIs inhibit emotional responses? Int J Neuropsychopharmacol. 2002;5:147–151. doi: 10.1017/S1461145702002870. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E. New evidence for the antidepressant activity of MK-801, a non-competitive antagonist of NMDA receptors. Pol J Pharmacol. 1993;45:549–553. [PubMed] [Google Scholar]
- Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, et al. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, et al. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: Qualitative study. Br J Psychiatry. 2009;195:211–217. doi: 10.1192/bjp.bp.108.051110. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Abelaira HM, Stringari RB, et al. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis. 2012;27:175–182. doi: 10.1007/s11011-012-9281-2. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, et al. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012;169:152–159. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Johann M, Frank J, et al. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52:1469–1478. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, et al. A scale for the assessment of hedonic tone: The Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, et al. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: Results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- Sweet KL, Neill DB. Amphetamine injections into the nucleus accumbens enhance the reward of stimulation of the subiculum. Ann N Y Acad Sci. 1999;877:828–830. doi: 10.1111/j.1749-6632.1999.tb09332.x. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: Replicable evidence for interest-activity symptoms. Psychol Med. 2012;42:967–980. doi: 10.1017/S0033291711001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, et al. Effects of (S)-ketamine on striatal dopamine: A [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, et al. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]