Abstract
Background
Ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists (NMDAR antagonists) recently demonstrated antidepressant efficacy for the treatment of refractory depression, but effect sizes, trajectories and possible class effects are unclear.
Method
We searched PubMed/PsycINFO/Web of Science/clinicaltrials.gov until 25 August 2015. Parallel-group or cross-over randomized controlled trials (RCTs) comparing single intravenous infusion of ketamine or a non-ketamine NMDAR antagonist v. placebo/pseudo-placebo in patients with major depressive disorder (MDD) and/or bipolar depression (BD) were included in the analyses. Hedges’ g and risk ratios and their 95% confidence intervals (CIs) were calculated using a random-effects model. The primary outcome was depressive symptom change. Secondary outcomes included response, remission, all-cause discontinuation and adverse effects.
Results
A total of 14 RCTs (nine ketamine studies: n = 234; five non-ketamine NMDAR antagonist studies: n = 354; MDD = 554, BD = 34), lasting 10.0 ± 8.8 days, were meta-analysed. Ketamine reduced depression significantly more than placebo/pseudo-placebo beginning at 40 min, peaking at day 1 (Hedges’ g = −1.00, 95% CI −1.28 to −0.73, p < 0.001), and loosing superiority by days 10–12. Non-ketamine NMDAR antagonists were superior to placebo only on days 5–8 (Hedges’ g = −0.37, 95% CI −0.66 to −0.09, p = 0.01). Compared with placebo/pseudo-placebo, ketamine led to significantly greater response (40 min to day 7) and remission (80 min to days 3–5). Non-ketamine NMDAR antagonists achieved greater response at day 2 and days 3–5. All-cause discontinuation was similar between ketamine (p = 0.34) or non-ketamine NMDAR antagonists (p = 0.94) and placebo. Although some adverse effects were more common with ketamine/NMDAR antagonists than placebo, these were transient and clinically insignificant.
Conclusions
A single infusion of ketamine, but less so of non-ketamine NMDAR antagonists, has ultra-rapid efficacy for MDD and BD, lasting for up to 1 week. Development of easy-to-administer, repeatedly given NMDAR antagonists without risk of brain toxicity is of critical importance.
Keywords: Bipolar depression, depression, ketamine, meta-analyses, N-methyl-D-aspartate receptor antagonists, trajectories
Introduction
Mood disorders and accompanying suicidality result in great personal suffering and public expenditure. In 2010, major depressive disorder (MDD) rose from 15th to 11th rank in its contribution to disability-adjusted life years (Murray et al. 2012). Although for decades antidepressants that act via monoamine pathways have dominated the treatment of depression, efficacy is often unsatisfactory. For example, in the large, randomized, multi-step National Institute of Mental Health (NIMH)-funded Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, only 47% of patients responded to standard antidepressant treatment and only 33% achieved remission (Warden et al. 2007). Moreover, the onset of clinically noticeable efficacy usually takes ≥ 2 weeks (Kasper et al. 2006). Further, the efficacy of antidepressants in bipolar depression (BD) has been challenged (Sachs et al. 2007; Pacchiarotti et al. 2013) and fewer treatment options are available than for MDD (Vieta et al. 2010). Thus, interventions with fast efficacy and efficacy for patients not responding to available antidepressants are sorely needed.
Recent studies demonstrated the role of glutamate-mediated neuroplasticity in the pathophysiology of mood disorders and antidepressant effects of glutamatergic agents (Tardito et al. 2006; Pittenger & Duman, 2008; Sanacora et al. 2008). Ketamine, a non-selective N-methyl-D-aspartic acid receptor (NMDAR) antagonist, used for decades as an anesthetic, has shown anti-depressant efficacy in subanesthetic doses within hours of administration in placebo-controlled crossover studies for MDD (Berman et al. 2000; Zarate et al. 2006; Sos et al. 2013) and BD (Diazgranados et al. 2010a; Zarate et al. 2012). In these trials, ketamine showed quick and dramatic antidepressant effects for refractory and non-refractory depression. Furthermore, ketamine reduced suicidal thoughts in both open (Price et al. 2009; Diazgranados et al. 2010b; Larkin & Beautrais, 2011) and controlled (Zarate et al. 2012; Price et al. 2014) trials.
Ketamine’s primary mechanism of action is NMDAR blockade at the phencyclidine site within the ionotropic channel. Ketamine induces presynaptic glutamate release by activating GABAergic inputs leading to increased glutamatergic neuronal firing (Machado-Vieira et al. 2009). Thus, a relevant question is whether non-ketamine NMDAR antagonists could be similarly efficacious for depression. In this context, five randomized trials of non-ketamine NMDAR antagonists, Traxoprodil CP-101,606 (Preskorn et al. 2008), AZD6765 (Zarate et al. 2013; Sanacora et al. 2014) and GLYX-13 (Preskorn et al. 2015) have been conducted. Traxoprodil (CP-101,606) is a selective antagonist of the NR2B subunit of NMDARs. AZD6765 (lanicemine) is a non-selective NMDAR channel blocker like ketamine, but with lower trapping channel blockade (54% v. 86%) (Monaghan & Larsen, 1997). GLYX-13 is a NMDAR glycine site partial agonist, producing NMDA functional antagonism, with long-term efficacy without psychotomimetic effects after a single intravenous dose in animal models (Burch et al. 2010).
There are systematic and/or narrative reviews (Aan Het Rot et al. 2012; Covvey et al. 2012; Mathew et al. 2012; Caddy et al. 2014; Fond et al. 2014; Coyle & Laws, 2015; McGirr et al. 2015; Newport et al. 2015) including five meta-analyses to date that summarized the efficacy of ketamine and non-ketamine NMDAR antagonists. However these meta-analyses have some deficits, such as not assessing the efficacy change over time for all studies (Fond et al. 2014) or for some studies (Newport et al. 2015), including only ketamine studies (Caddy et al. 2014; Fond et al. 2014; Coyle & Laws, 2015), mixing pre-post data comparison with placebo-controlled studies (Coyle & Laws, 2015), mixing intranasal with injection studies (Newport et al. 2015), missing some relevant studies (McGirr et al. 2015; Newport et al. 2015), and/or mixing in electrocon-vulsive therapy studies (Fond et al. 2014). Here, we conducted a meta-analysis of ketamine and non-ketamine NMDAR antagonists in patients with depression. We included all studies conducted to date that examined the efficacy of NMDAR antagonists compared with placebo in randomized trials and examined the time course of efficacy after a single NMDAR antagonist infusion.
Method
Search and inclusion criteria
Two investigators independently searched PubMed, PsycINFO, ISI Web of Science, and the US National Institutes of Health clinical trials registry (http://www.clinicaltrials.gov), from database inception until 25 August 2015, for, parallel-group or cross-over randomized controlled trials (RCTs), comparing single-dose, intravenous NMDAR antagonist infusion v. placebo (saline infusion) or pseudo-placebo (non-antidepressant anesthetic) for MDD and/or BD. We also included multiple injection studies, but only if data before the second injection were available. We excluded RCTs of NMDAR antagonists administered orally or intranasally. The following search string was used: (ketamine OR N-methyl-D-aspartic acid OR NMDA OR glutamat*) AND (depression OR depressive OR depressed OR bipolar OR suicidal) AND (random* OR placebo), supplementing the electronic search by hand-searching reference lists of identified studies, review articles and major meeting proceedings.
Data extraction and outcomes
The primary outcome was symptom change measured by the Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960) or the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979) at study-defined time points post-infusion. When both the HAM-D and MADRS were reported, we used HAM-D scores. Secondary outcomes included response (≥ 50% reduction in HAM-D/MADRS score), study-defined remission, all-cause discontinuation, and adverse effects, including psychotic, manic and dissociative symptoms, assessed by the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962), the Young Mania Rating Scale (YMRS; Young et al. 1978) and the Clinician Administered Dissociative States Scale (CADSS; Bremner et al. 1998), among others. When assessment time points were similar but not identical, we combined these (e.g. days 3–4). When ≥ 2 doses were examined in a single study, we combined multiple doses into one experimental arm, given that the ideal dose of such agents has not been established. However, in one phase 2, dose-finding study of GLYX-13 (Preskorn et al. 2015), the mean and S.D. of HAM-D were combined across the 1, 5 and 10 mg doses, but the 30 mg dose was excluded, being a clear outlier, suggesting an inverted U-shaped dose–response curve with an ineffective high GLYX-13 dose. Conversely, in the three-arm phase IIB study (Sanacora et al. 2014) of AZD6765, the mean and S.D. of the MADRS scores were combined for the 100 and 150 mg doses. Data were extracted independently by two or three reviewers (J.M.C., K.H. and T.K.), calculating results from graphs if needed and resolving inconsistencies by consensus.
Risk assessment including publication bias
Two reviewers independently assessed risk of bias for each study using the Cochrane Collaboration’s risk-of-bias tool, rating studies as having low, high, or unclear risks of bias on seven predefined criteria (Higgins & Green, 2011; Higgins et al. 2011).
Publication bias was assessed inspecting funnel plots for depressive symptom change, response and remission.
Meta-analytic calculations
For continuous outcomes, standardized mean difference between the intervention and placebo/pseudo-placebo was calculated as Hedges’ g with 95% confidence intervals (CIs), using random-effects models. For dichotomous outcomes, relative risk (RR) was calculated with 95% CIs, and with number-needed-to-treat/harm (NNT/NNH) when appropriate. Heterogeneity is expressed by τ2, I2, Q and p values. All-cause discontinuation was analysed both in the intent-to-treat sample and in a sensitivity analysis afterexcluding patients discontinuingdue to significant improvement in the first phase of cross-over trials to avoid biasing against the more efficacious treatment. A second sensitivity analysis focused on the three AZD6765 studies.
Results
Search results
The search yielded 1574 hits. Altogether, 1548 articles were excluded based on abstract/title. Of the remaining 26 full-text articles, 14 articles were removed (for reasons, see online Supplementary Fig. S1), resulting in 12 articles reporting on 14 trials (ketamine = 9 trials, NMDAR antagonists = 5 trials) that were meta-analysed.
Study design, population, treatment and outcomes
Of 14 trials (Berman et al. 2000; Zarate et al. 2006, 2012, 2013; Diazgranados et al. 2010a; Murrough et al. 2013a; Sos et al. 2013; Lai et al. 2014; Sanacora et al. 2014; Singh et al. 2014; Preskorn et al. 2008, 2015), which lasted 10.0 ± 8.8 days, seven were placebo-controlled cross-over studies (duration = 8.4 ± 4.1 days, interval until crossover = 9.0 ± 3.4 days), andsevenwere parallel-group studies (duration = 11.6 ± 12.1 days) (online Supplementary Table S1). Participants were 45.8 ± 3.8 years old, 40.7 ± 8.7% were male, 77.1 ± 9.2% were white (studies = 7). The current episode duration was 45.1 ± 49.0 months (studies = 9), and patients had failed 6.0 ± 1.1 anti-depressant trials (studies = 3). Nine studies investigated single-dose intravenous ketamine (n = 234), five used intravenous non-ketamine NMDAR antagonists (n = 354), i.e. CP-101,606 (studies = 1, n = 30), AZD6765 (studies = 3 including one repeated infusion study, n = 158) and GLYX-13 (n = 116). Although technically not an NMDAR antagonist, we included GLYX-13, as it pharmacodynamically reduces NMDA transmission. Placebo was the comparator in all but one parallel-group ketamine study (Murrough et al. 2013a), which used midazolam, an anesthetic without known anti-depressant effect, as active pseudo-placebo.
Ketamine studies
Of nine ketamine studies (n = 234, range = 4–73/study), seven were independently funded, six were placebo-controlled cross-over studies (duration = 8.7 ± 4.4 days, interval before cross-over = 9.5 ± 3.5 days) (Berman et al. 2000; Zarate et al. 2006, 2012; Diazgranados et al. 2010a; Sos et al. 2013; Lai et al. 2014), and three were parallel-group studies (duration = 4.0 ± 2.6 days) (Murrough et al. 2013a; Lai et al. 2014; Singh et al. 2014) (online Supplementary Table S1). There were three monotherapy studies and six add-on studies (to lithium or valproate = 2, to antidepressants = 3, to tranylcypromine and second-generation antipsychotics = 1). Of nine studies, five washed out antidepressants for ≥ 12.6 ± 3.1 days, while prior antidepressants were maintained throughout the study in the add-on keta-mine studies (Sos et al. 2013; Lai et al. 2014; Singh et al. 2014).
Seven RCTs (Berman et al. 2000; Zarate et al. 2006; Murrough et al. 2013a; Sos et al. 2013; Lai et al. 2014; Singh et al. 2014) studied MDD patients (n = 200), two trials (Diazgranados et al. 2010a; Zarate et al. 2012) studied BD patients (n = 25), and one trial included both BD and MDD patients (n = 9) (Berman et al. 2000).
In five studies with information (Zarate et al. 2006, 2012; Diazgranados et al. 2010a; Sos et al. 2013; Singh et al. 2014), subjects were hospitalized for the duration of the study. In the Murrough et al. (2013a) study, subjects were hospitalized for the first 24 h after infusion only. In the remaining two studies, the treatment setting was either unclear (Berman et al. 2000) or subjects were out-patients treated in a day-hospital setting (Lai et al. 2014).
Co-morbid anxiety disorders were permitted if not requiring current treatment in three studies; no study permitted recent substance use, unstable medical illness, serious/imminent suicidal or homicidal risk. Five of seven MDD studies included patients with inadequate response to antidepressants (the number of prior failed trials varied) (Zarate et al. 2006; Murrough et al. 2013a; Lai et al. 2014; Singh et al. 2014); whereas in BD studies, patients had to have failed ≥ 1 adequate antidepressant trial plus one prospective open trial of either lithium or valproate for ≥ 4 weeks at therapeutic levels (lithium = 0.6–1.2 mEq/l; valproic acid = 50–125 μg/ml) (Diazgranados et al. 2010a; Zarate et al. 2012) (online Supplementary Table S1).
Seven studies randomized patients to ketamine single infusion at 0.1–0.5 mg/kg per h for 40 min or saline; in one study (Sos et al. 2013), patients received 0.27 mg/kg for the first 10 min using the same dose over next 20 min. Patients were crossed over after 7–14 days in six studies, except that five patients were not crossed over because of marked responses.
Response was defined as a ≥ 50% decrease in either HAM-D (Berman et al. 2000; Zarate et al. 2006; Diazgranados et al. 2010a) or MADRS score (Diazgranados et al. 2010a; Zarate et al. 2012; Murrough et al. 2013a; Sos et al. 2013; Lai et al. 2014; Singh et al. 2014). Three studies reported remission data, i.e. MADRS < 10 (Diazgranados et al. 2010a; Zarate et al. 2012) or HAM-D < 7 (Zarate et al. 2006).
Non-ketamine NMDAR antagonist
In five studies (n = 354, range = 22–168/study), three non-ketamine NMDAR antagonists (n = 354) were studied: CP-101,600 (n = 30) (Preskorn et al. 2008), GLYX-13 (n = 116) (Preskorn et al. 2015) and AZD6765 (n = 208) (Zarate et al. 2013; Sanacora et al. 2014) (online Supplementary Table S1). Four RCTs were parallel-group, industry-sponsored RCTs (n = 332) (Preskorn et al. 2008, 2015; Sanacora et al. 2014); one was a non-industry sponsored, 7-day cross-over study of AZD6765 (Zarate et al. 2013). There were three monotherapy studies and two add-on studies [paroxetine = 1 (Tardito et al. 2006), non-tricyclic anti-depressants = 1 (Sanacora et al. 2014)]. All patients had MDD and had failed either ≥ 1 antidepressant in the current episode (Preskorn et al. 2015), ≥ 2 anti-depressant trials (Zarate et al. 2013; Sanacora et al. 2014); or ≥ 1 selective serotonin reuptake inhibitor trial, without non-responsiveness to adequate trials of ≥ 3 different antidepressant classes, plus failure to a 6-week prospective paroxetine lead-in treatment (Preskorn et al. 2008). In the three monotherapy studies, antidepressants were washed out for 11.7 ± 4.0 days (Zarate et al. 2013; Sanacora et al. 2014; Preskorn et al. 2015). One adjunctive study added CP-101,600 to paroxetine after a 6-week lead-in trial (Preskorn et al. 2008) and a second study added AZD6765 to anti-depressant, sedative and hypnotic treatment (study 9) (Sanacora et al. 2014).
Single CP-101,606 infusion was added to paroxe-tine at 0.75 mg/kg per h for 1.5 h followed by 0.15 mg/kg per h for 6.5 h for the first seven patients. Due to dissociative symptoms, the infusion dose and duration were lowered to 0.5 mg/kg per h for 1.5 h for the remaining 23 patients (online Supplementary Table S1). AZD6765 was given as a single fixed dose of 100 mg (Sanacora et al. 2014) and/or 150 mg (Zarate et al. 2013; Sanacora et al. 2014) over 60 min. In the one cross-over study (Zarate et al. 2013), one patient who responded to AZD6765 was not crossed over.
In one study, response was defined as a ≥ 50% decrease in HAM-D score from baseline at day 5 and remission was defined as an HAMD score of ≤7 (Preskorn et al. 2008). In the second study, response was defined as a ≥ 50% MADRS score decrease and remission was defined as a MADRS score of <10 (Zarate et al. 2013). Two studies did not report response or remission results (Sanacora et al. 2014; Preskorn et al. 2015) and data from the last study (Sanacora et al. 2014, study 9) could not be used, as information for the individual included study arms was not available.
Change in depressive symptoms
Ketamine
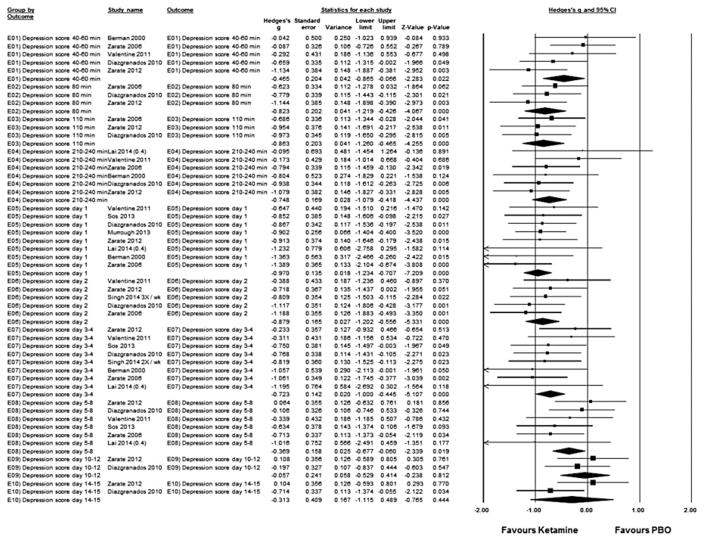
Pooled together, single ketamine infusion resulted in superior reduction of depressive symptoms compared with placebo/pseudo-placebo starting at 40–60 min (studies = 4, Hedges’ g = −0.50, 95% CI −1.00 to −0.00, p = 0.05; heterogeneity: τ2 = 0.11, I2 = 44.3, Q = 5.39, p = 0.15), peaking at day 1 (studies = 7, Hedges’ g = −1.00, 95% CI −1.28 to −0.73, p < 0.001; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 2.14, p = 0.91) and lasting until days 5–8 (studies = 5, Hedges’ g = −0.38, 95% CI −0.73 to −0.03, p = 0.036; heterogeneity: τ2 = 0.02, I2 = 9.38, Q = 4.41, p = 0.35), with non-significant group differences on days 10–12 and days 14–15 (Fig. 1).
Fig. 1.
Hedges’s g in change in depression rating scale score between ketamine-treated and placebo (PBO) control subjects in the articles analysed. Squares are effect sizes of single studies, diamonds of pooled results. CI, Confidence interval.
Non-ketamine NMDAR antagonist
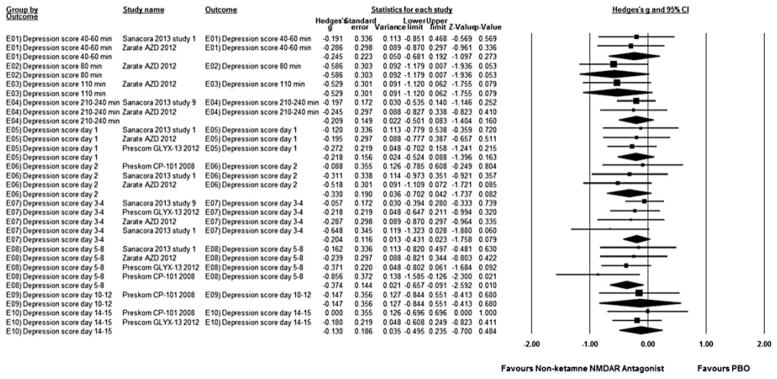
Pooled together, non-ketamine NMDAR antagonists resulted in superior reduction of depressive symptoms compared with placebo on days 5–8 (studies = 4, Hedges’ g = −0.37, 95% CI −0.66 to −0.09, p = 0.01; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 2.28, p = 0.52), without significant group differences at any other time point (Fig. 2). Repeating the analyses for the three AZD6765 studies yielded no significant group differences at any time points (data not shown).
Fig. 2.
Hedges’s g in change in depression rating scale score between non-ketamine N-methyl-D-aspartate receptor (NMDAR) antagonist-treated and placebo (PBO) control subjects in the articles analysed. Squares are effect sizes of single studies, diamonds of pooled results. CI, Confidence interval.
Response and remission
Ketamine
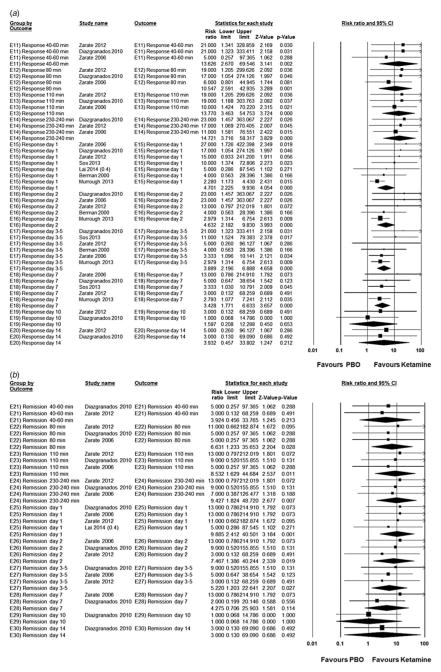
Compared with placebo/pseudo-placebo, ketamine was associated with significantly greater response starting at 40–60 min (studies = 3, ketamine = 43.1% v. placebo = 0.00%; RR = 13.6, 95% CI 2.67–69.6, p = 0.00; NNT = 3; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.63, p = 0.73), peaking at 230–240 min (studies = 3, ketamine = 58.8% v. placebo = 2.00%; RR = 14.7, 95% CI 3.72–58.3, p < 0.001; NNT = 2; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.20, p = 0.91) and lasting until day 7 (studies = 5, ketamine = 34.4% v. placebo = 7.77%; RR = 3.43, 95% CI 1.77–6.63, p < 0.001; NNT = 5; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 1.19, p = 0.88) (Fig. 3a).
Fig. 3.
Risk ratio in treatment response (a) (≥50% reduction in Hamilton Depression Rating Scale/Montgomery–Åsberg Depression Rating Scale score) and remission (b) between ketamine-treated and placebo (PBO) control subjects in the articles analysed. Squares are effect sizes of single studies, diamonds of pooled results. CI, Confidence interval.
Similarly, ketamine was associated with significantly greater remission starting at 80 min (studies = 3, keta-mine = 17.6% v. placebo = 0.00%; RR = 6.63, 95% CI 1.23–35.7, p = 0.03; NNT = 7; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.19, p = 0.91), peaking at day 1 (studies = 4, ketamine = 34.0% v. placebo = 0.00%; RR = 9.89, 95% CI 2.4–40.5, p = 0.00; NNT = 3; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.30, p = 0.96) and lasting until days 3–5 (studies = 3, ketamine = 19.6% v. placebo = 1.96%; RR = 5.22, 95% CI 1.20–22.6, p = 0.03; NNT = 7; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.26, p = 0.88) (Fig. 3b).
Non-ketamine NMDAR antagonists
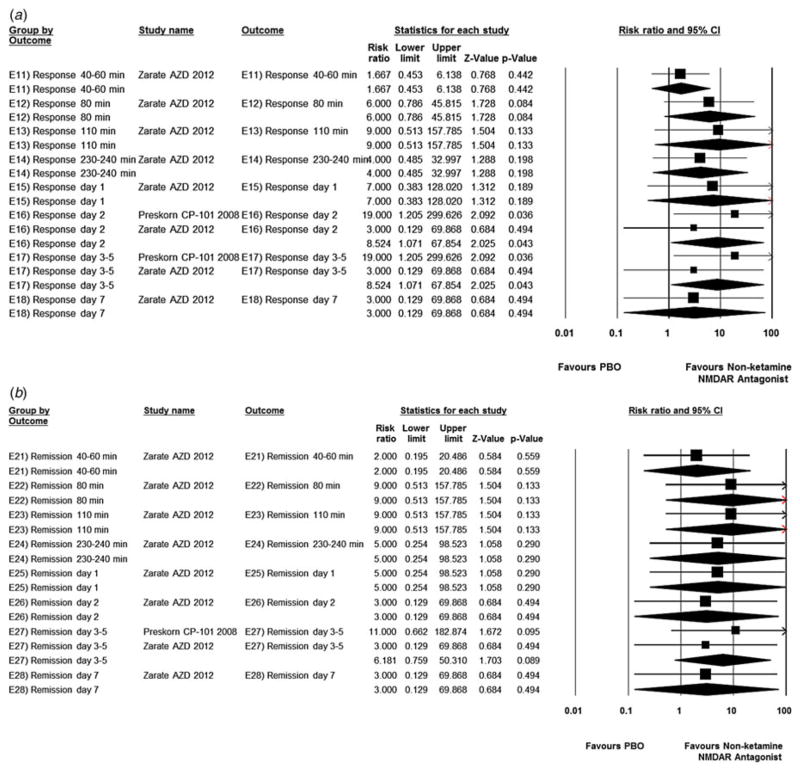
Compared with placebo, non-ketamine NMDAR antagonists were associated with significantly greater response on day 2 and days 3–5 (studies = 2, non-ketamine NMDAR antagonists = 27.0%, placebo = 0.00%; RR = 8.52, 95% CI 1.07–67.9, p = 0.04; NNT = non-significant; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.75, p = 0.39) (Fig. 4a). However, remission was not significantly different from placebo on days 3–5 (studies = 2, non-ketamine NMDAR antagonists = 16.2%, placebo = 0.00%; RR = 6.18, 95% CI 0.76–50.3, p = 0.089; NNT = non-significant; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.36, p = 0.55) (Fig. 4b).
Fig. 4.
Risk ratio in treatment response (a) (≥50% reduction in Hamilton Depression Rating Scale/Montgomery–Åsberg Depression Rating Scale score) and remission (b) between non-ketamine N-methyl-D-aspartate receptor (NMDAR) antagonist-treated and placebo (PBO) control subjects in the articles analysed. Squares are effect sizes of single studies, diamonds of pooled results. CI, Confidence interval.
All-cause discontinuation
Ketamine
All-cause discontinuation was not significantly different between ketamine and placebo (studies = 6, keta-mine = 12.1% v. placebo = 7.8%; RR = 1.52, 95% CI 0.64–3.58, p = 0.34; NNT = non-significant; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 4.38, p = 0.50), remaining non-significant after removal of five patients ‘dropping out’ during the first cross-over phase for marked improvement to ketamine (studies = 6, ketamine = 8.66% v. placebo = 6.86%; RR = 1.14, 95% CI 0.42–3.10, p = 0.81; NNT = non-significant; heterogeneity: τ2 = 0.14, I2 = 8.83, Q = 5.48, p = 0.36) (online Supplementary Fig. S2).
Non-ketamine NMDAR antagonists
All-cause discontinuation did not differ between placebo and non-ketamine NMDAR antagonists (studies = 2, non-ketamine NMDAR antagonists = 12.3% v. placebo = 20.0%; RR = 0.92, 95% CI 0.11–8.09, p = 0.94; NNT = non-significant; heterogeneity: τ2 = 1.47, I2 = 52.6, Q = 2.11, p = 0.15). When one patient on AZD6765 ‘dropping out’ due to marked response to AZD6765 during the first cross-over phase was excluded, results did not change (studies = 2, non-ketamine NMDAR antagonists = 11.1% v. placebo = 20.0%; RR = 0.62, 95% CI 0.14–2.66, p = 0.52; NNT = non-significant; heterogeneity: τ2 = 0.35, I2 = 19.2, Q = 1.24, p = 0.27) (online Supplementary Fig. S3).
Changes in psychopathology scales
Ketamine
The BPRS score was significantly higher in the keta-mine group than with placebo at 40–60 min (studies = 5, Hedges’ g = 0.90, 95% CI 0.58–1.22, p < 0.001; heterogeneity: τ2 = 0.02, I2 = 10.8, Q = 4.48, p = 0.35), becoming significantly lower on day 3 (studies = 3, Hedges’ g = −0.48, 95% CI −0.86 to −0.09, p = 0.015; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.23, p = 0.89) (online Supplementary Fig. S4). The YMRS score was significantly lower in the ketamine group than placebo at all time points until day 14, except at 40–60 min (studies = 3, Hedges’ g = 0.29, 95% CI −0.10 to 0.68, p = 0.15; heterogeneity: τ2 = 0.03, I2 = 22.5, Q = 2.58, p = 0.28), 80 min (studies = 2, Hedges’ g = −0.59, 95% CI −1.24 to 0.06, p = 0.076; heterogeneity: τ2 = 0.10, I2 = 44.1, Q = 1.79, p = 0.18) and day 7 (studies = 2, Hedges’ g = −0.57, 95% CI −1.43 to 0.30, p = 0.20; heterogeneity: τ2 = 0.27, I2 = 68.3, Q = 3.15, p = 0.08) (online Supplementary Fig. S5). The CADSS score was only significantly higher in the ketamine group than with placebo at 40–60 min post-ketamine infusion (studies = 5, Hedges’ g = 2.42, 95% CI 1.13–3.73, p < 0.001; heterogeneity: τ2 = 1.96, I2 = 92.3, Q = 52.1, p < 0.001) (online Supplementary Fig. S6).
Non-ketamine NMDAR antagonists
The BPRS score was significantly lower in the non-ketamine NMDAR antagonists than placebo at 110 min (studies = 2, Hedges’ g = −0.37, 95% CI −0.72 to −0.03, p = 0.035; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 0.38, p = 0.54) and 230–240 min (studies = 3, Hedges’ g = −0.32, 95% CI −0.63 to −0.02, p = 0.04; heterogeneity: τ2 = 0.00, I2 = 0.00, Q = 1.40, p = 0.50) (online Supplementary Fig. S7). Regarding YMRS scores, there was no difference between non-ketamine NMDAR antagonists and placebo at any post-baseline time points. The CADSS score was significantly higher in non-ketamine NMDAR antagonists than placebo at 230–240 min (studies = 1, Hedges’ g = −0.66 95% CI −1.26 to −0.07, p = 0.03; heterogeneity: not applicable) and at day 1 (studies = 1, Hedges’ g = −0.69, 95% CI −1.29 to −0.09; heterogeneity: not applicable), whereas the CADSS score was lower than placebo at day 3 (studies = 1, Hedges’ g = 0.67, 95% CI 0.07–1.26, p = 0.03; heterogeneity: not applicable) and day 7 (studies = 1, Hedges’ g = 0.68, 95% CI 0.08–1.28, p = 0.03; heterogeneity: not applicable).
Other adverse effects
Ketamine
Among adverse events reported by ≥2 studies, no significant differences emerged between ketamine and placebo: tiredness/fatigue (p = 0.37), feeling ‘woozy/loopy’ (p = 0.95), dizziness/faintness (p = 0.22), nausea (p = 0.30) and vivid dreams (p = 0.23) (online Supplementary Fig. S8).
Non-ketamine NMDAR antagonists
Adverse events were not significantly different between non-ketamine NMDAR antagonists and placebo: tiredness/fatigue (p = 0.65), dizziness/faintness (p = 0.054), anxiety (p = 0.70), nausea (p = 0.12), drowsiness/sedation (p = 0.40), irritability (p = 0.36), stomach/abdominal discomfort (p = 0.65), muscle/bone/joint pain (p = 0.96), tingling (p = 0.96), diarrhea (p = 0.75), headache (p = 0.72), insomnia/interrupted sleep (p = 0.38) and vomiting (p = 0.60) (online Supplementary Fig. S9).
Risk assessment including publication bias
Out of seven risk-of-bias categories, most studies had incomplete outcome data; i.e. they did not report results for all outcomes listed in the clinical trial registrations. Moreover, Lai et al. (2014) used ascending doses to which participants were blinded, and a placebo infusion was inserted at some point to which both raters and participants were blinded. We considered that these procedures might have compromised blinding, rating this study as being at high risk for multiple risk of bias categories. Although there has been concern of functional unblinding due to the euphorogenic and dissociative effects of sub-anesthetic doses of ketamine, we considered this effect as inevitable and regarded this fact as low risk, similar to many other agents that have substantial side effects that could be noticed by participants and raters (e.g. sedation, weight gain, muscle stiffness, restlessness, etc.) and that are generally regarded as having low risk of bias in clinical trials (online Supplementary Table S2).
Inspecting funnel plots did not indicate publication bias regarding depressive symptom reduction, response or remission.
Discussion
In this meta-analysis of randomized, placebo/pseudo-placebo-controlled trials of single-dose, intravenous ketamine or non-ketamine NMDAR antagonists for patients with MDD and BD refractory/unresponsive to trials with standard antidepressants, we examined the time trajectory of efficacy in greater detail than previous meta-analyses. Pooling six crossover trials and three parallel-group studies, single keta-mine infusion was significantly superior to placebo/pseudo-placebo regarding antidepressant efficacy. The significantly greater reduction in depressive symptoms started as early as within 40–60 min, peaking on day 1, and lasting until days 5–8, with maintenance of superior remission and response status until days 3–5 and 7, respectively. Effect sizes ranged from medium to large (−0.38 to −1.00) for the reduction in depressive symptoms, being large for response (NNT = 2–5, peaking at 230–240 min) and remission (NNT = 3–7, peaking at 1 day). At 24 h, 54.1% responded and 34.0% remitted on ketamine compared with only 7.8% and 0% on placebo. Furthermore, the findings were homogeneous throughout. In contrast to keta-mine, single infusion of non-ketamine NMDAR antagonists was only significantly superior to placebo at one assessment time point (days 5–8) with a small to medium effect size (−0.37). Although non-ketamine NMDAR antagonists had significantly higher response rates on days 2 and 3–5 (NNT = non-significant), remission was not significantly superior to placebo. Like with ketamine, results were homogeneous throughout. The reason for non-ketamine NMDAR antagonists having smaller effect sizes than ketamine remains unknown. However, lower NMDAR affinity may be one of the mechanisms that also explains their reduced side effect potential. Nevertheless, both single infusion of ketamine and non-ketamine NMDAR antagonists was well tolerated, not leading to greater drop-out than placebo/pseudo-placebo.
The magnitude as well as speed of effect of NMDAR antagonists are remarkable. Despite long suffering during a current depressive episode lasting 45.1 ± 49.0 months that was not relieved by 6.0 ± 1.1 treatment trials, NMDAR antagonism promptly and dramatically improved depressive symptoms. Effect sizes for symptom reduction were much higher for ketamine (−0.38 to −1.00) and similar for non-ketamine NMDAR antagonists (−0.37) in patients with treatment-resistant depression compared with first-line antidepressants in acute, non-refractory depression (−0.31) (Turner et al. 2008), although effect sizes are lower when patients with milder depression are included due to greater placebo response (Kirsch et al. 2008). Effect sizes for response with ketamine (NNT = 2–5) and non-ketamine NMDAR antagonists (NNT = 4) (Melander et al. 2008) also compare very favorably to antidepressants in non-refractory depression (NNT = 7) and to second-generation antipsychotic augmentation of patients with suboptimal response to antidepressants (NNT = 7–10) (Spielmans et al. 2013).
The transient efficacy lasting 1 week post-infusion have stimulated multi-infusion studies, which have yielded encouraging results. Repeated ketamine infusions resulted in significant antidepressant effect with an extended median time to recurrence of depressive symptoms in a 4-week open-label study (Aan het Rot et al. 2010), 18-day open-label study (Murrough et al. 2013b) and 12-month, naturalistic three-patient case series (Szymkowicz et al. 2013). Furthermore, a placebo-controlled RCT (n = 152) comparing three infusions of 100 or 150 mg AZD6765 within the first week with placebo showed superior antidepressant effects starting at week 2 and lasting until week 5 (Sanacora et al. 2014).
Treatment resistance occurs in approximately 15–20% of depressed patients (Rush et al. 2006). If safe, using a fast-acting antidepressant for non-refractory depression that could speed up response and remission while the first-line antidepressant unfolds its efficacy, as shown recently (Hu et al. 2016), would be an important treatment option. Such strategy could be used during emergency room visits to shorten or, even, prevent admissions. A related question includes whether patients will be able to maintain the response if standard antidepressants are started concurrently in non-refractory depression, or if repeated NMDAR antagonist doses would be necessary.
Despite these highly favorable results, several important questions remain (Aan Het Rot et al. 2012; Martinowich et al. 2013): (i) can NMDAR antagonists be developed that have similarly large effect sizes as ketamine?; (ii) can NMDAR antagonists without the potential for neurotoxicity be developed, enabling safe repeated/chronic administration?; (iii) how long would the repeated administration interval have to be?; (iv) what is the optimal dose/dose range?; (v) what non-intravenous administration routes can be developed?; (vi) to what degree can we generalize results to elderly and pediatric populations?; (vii) what clinical or biological markers predict NMDAR antagonist response?; (viii) are NMDAR antagonists useful anti-suicidal treatments?; (ix) are there any acute/chronic cognitive side effects of NMDAR antagonists?; (x) are NMDAR antagonists helpful for other psychiatric disorders?; and (xi) are NMDAR antagonists effective in monotherapy or as add-on treatment in non-refractory depressed patients?
Several limitations of this meta-analysis deserve mentioning. First, six studies applied a cross-over design. Clearly, parallel-group trials are needed; yet, at least, the one parallel-group ketamine study (Murrough et al. 2013a) showed very similar effects as the cross-over studies. Second, we grouped three different non-ketamine NMDAR antagonists together that have different mechanisms and that were studied to find optimal doses. Thus, findings may be a conservative estimate for some or all of the non-ketamine NMDAR antagonists. Further, although fewer non-ketamine NMDAR antagonists studies reported outcomes at the same time point as ketamine studies, RCTs were larger with one and a half times as many participants (n = 354). Moreover, effect sizes in non-ketamine NMDAR antagonist studies were homogeneous and approximately two- to four-fold lower than those observed after ketamine infusion. Third, the number of studies and patients was still limited, and assessment time points differed across studies. Therefore, some effect sizes were based on one study, especially for non-ketamine NMDAR antagonists. Nevertheless, study results were homogeneous, suggesting similar results even with a larger database. Finally, significant sedative, euphoric or dissociative effects of ketamine could have unblinded patients and/or raters. In fact, a recent post-hoc analysis suggested that higher dissociation ratings were associated with greater antidepressant efficacy of ketamine (Luckenbaugh et al. 2014). While this result could have bolstered concerns about functional unblinding, it was interpreted as a lead toward a mechanisms of ketamine’s efficacy. This interpretation is supported by our meta-analysis. Dissociative symptoms and BPRS scores were significantly higher with ketamine at 40–60 min, but BPRS scores became significantly lower at day 3, and antidepressant effects lasted until days 5–7. Moreover, non-ketamine NMDAR antagonists, not causing any psychogenic effects, also had antidepressant effects, supporting the NMDA hypothesis of depression. Finally, in the midazolam-controlled study, midazolam sub-anesthetic doses that could also have unblinded treatment did not diminish ketamine’s effect sizes. However, considering that such unblinding effects of ketamine could have influenced the results, we have used the score of ‘unclear’ in the risk-of-bias assessment table for studies not using midazolam as the control.
In conclusion, results from this meta-analysis indicate that single-dose intravenous ketamine and, less so, non-ketamine NMDAR antagonists are effective in rapidly reducing depressive symptoms in patients with unresponsive/refractory MDD and BD. While these findings are highly encouraging and important for patients, clinicians, researchers and drug developers, several questions outlined above call for the conduct of sufficiently large, effectively blinded, parallel-group RCTs with single-dose and repeated-dose ketamine and, ideally, additional NMDAR antagonists.
Supplementary Material
Acknowledgments
Funding for this study was supported in part by The Zucker Hillside Hospital Mental Advanced Center for Intervention and Services Research for the Study of Schizophrenia (P30MH090590) from the NIMH, Bethesda, MD. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291716000064
Declaration of Interest
T.K. has received consultant fees from Sumitomo Dainippon, Novartis, Otsuka and Taisho and has received speaker’s honoraria from Abbvie, Banyu, Eli Lilly, Dainippon Sumitomo, Janssen, Mochida, Novartis, Otsuka Pfizer and Shionogi. He has received grant support from the Byoutaitaisyakenkyukai Fellowship (Fellowship of Astellas Foundation of Research on Metabolic Disorders), Eli Lilly Fellowship for Clinical Psychopharmacology, Research Group for Schizophrenia Japan, Dainippon-Sumitomo, Mochida and Otsuka.
J.M.C. has nothing to disclose.
K.H. is an employee of Sumitomo Dainippon Pharma, Japan.
C.A.Z. is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. C.A.Z. has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government.
J.M.K. has been a consultant to Alkermes, Amgen, Astra-Zeneca, Janssen, Pfizer, Eli Lilly, Bristol-Myers Squibb, Dainippon Sumitomo/Sepracor/Sunovion, Johnson & Johnson, Otsuka, Pierre Fabre, Vanda, Proteus, Takeda, Targacept, IntraCellular Therapies, Merck, Lundbeck, Novartis, Roche, Rules Based Medicine, Sunovion and has received honoraria for lectures from Otsuka, Eli Lilly, Esai, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck and Janssen. He is a shareholder of Vanguard Research Group and MedAvante. He has received grant support from the NIMH.
M.B. has received grant/research support from The Stanley Medical Research Institute, NARSAD, Deutsche Forschungsgemeinschaft, European Commission (FP7), American Foundation for Suicide Prevention, Bundesministerium für Bildung und Forschung (BMBF). He is a consultant for Alkermes, AstraZeneca, BristolMyers Squibb, Ferrer Internacional, Janssen, Lilly, Lundbeck, Otsuka, Servier, Takeda, and has received speaker honoraria from AstraZeneca, BristolMyers Squibb, GlaxoSmithKline, Lilly, Lundbeck, Otsuka and Pfizer.
C.U.C. has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Actelion, Alexza; Alkermes, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forum, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, NIMH, Janssen/J&J, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Takeda, Teva and Vanda. He has received grant support from BMS, Feinstein Institute for Medical Research, Janssen/J&J, NIMH, Novo Nordisk A/S and Otsuka.
References
- Aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biological Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biological Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) Journal of Traumatic Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Burch RM, Singla N, Parulan C, Burgdorf J. GLYX-13, an NMDA receptor glycine site functional partial agonist, does not elicit psychotomimetic side effects in normal human volunteers at doses expected to be therapeutic in treatment-resistant major depressive disorder. The 50th Annual Meeting of the National Clinical Drug Evaluation Unit (NCDEU); Boca Raton, FL. 14–17 June 2010.2010. [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Therapeutic Advances in Psychopharmacology. 2014;4:75–99. doi: 10.1177/2045125313507739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covvey JR, Crawford AN, Lowe DK. Intravenous ketamine for treatment-resistant major depressive disorder. Annals of Pharmacotherapy. 2012;46:117–123. doi: 10.1345/aph.1Q371. [DOI] [PubMed] [Google Scholar]
- Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Human Psychopharmacology. 2015;30:152–163. doi: 10.1002/hup.2475. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of General Psychiatry. 2010a;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. Journal of Clinical Psychiatry. 2010b;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology. 2014;231:3663–3676. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Cochrane Collaboration; London, UK: 2011. [Accessed February 2012]. ( http://www.cochrane.org/training/cochrane-handbook) [Google Scholar]
- Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, Ungvari GS, Correll CU, Chiu HF, Xue Y, Tian TF, Wu AS, Ma X, Wang G. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychological Medicine. 2016;46:623–635. doi: 10.1017/S0033291715002159. [DOI] [PubMed] [Google Scholar]
- Kasper S, Spadone C, Verpillat P, Angst J. Onset of action of escitalopram compared with other antidepressants: results of a pooled analysis. International Clinical Psychopharmacology. 2006;21:105–110. doi: 10.1097/01.yic.0000194375.42589.c3. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Medicine. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S, Loo CK. Pilot dose-response trial of i.v. ketamine in treatment-resistant depression. World Journal of Biological Psychiatry. 2014;15:579–584. doi: 10.3109/15622975.2014.922697. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. International Journal of Neuropsychopharmacology. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? Journal of Affective Disorders. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA., Jr Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. Journal of Clinical Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Jimenez DV, Zarate CA, Jr, Manji HK. Rapid antidepressant effects: moving right along. Molecular Psychiatry. 2013;18:856–863. doi: 10.1038/mp.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs. 2012;26:189–204. doi: 10.2165/11599770-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychological Medicine. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- Melander H, Salmonson T, Abadie E, van Zwieten-Boot B. A regulatory Apologia –a review of placebo-controlled studies in regulatory submissions of new-generation antidepressants. European Neuropsychopharmacology. 2008;18:623–627. doi: 10.1016/j.euroneuro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Larsen H. NR1 and NR2 subunit contributions to N-methyl-D-aspartate receptor channel blocker pharmacology. Journal of Pharmacology and Experimental Therapeutics. 1997;280:614–620. [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry: the Journal of Mental Science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. American Journal of Psychiatry. 2013a;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biological Psychiatry. 2013b;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. American Journal of Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:790–812. [Google Scholar]
- Pacchiarotti I, Bond DJ, Baldessarini RJ, Nolen WA, Grunze H, Licht RW, Post RM, Berk M, Goodwin GM, Sachs GS, Tondo L, Findling RL, Youngstrom EA, Tohen M, Undurraga J, González-Pinto A, Goldberg JF, Yildiz A, Altshuler LL, Calabrese JR, Mitchell PB, Thase ME, Koukopoulos A, Colom F, Frye MA, Malhi GS, Fountoulakis KN, Vázquez G, Perlis RH, Ketter TA, Cassidy F, Akiskal H, Azorin JM, Valentí M, Mazzei DH, Lafer B, Kato T, Mazzarini L, Martínez-Aran A, Parker G, Souery D, Ozerdem A, McElroy SL, Girardi P, Bauer M, Yatham LN, Zarate CA, Nierenberg AA, Birmaher B, Kanba S, El-Mallakh RS, Serretti A, Rihmer Z, Young AH, Kotzalidis GD, MacQueen GM, Bowden CL, Ghaemi SN, Lopez-Jaramillo C, Rybakowski J, Ha K, Perugi G, Kasper S, Amsterdam JD, Hirschfeld RM, Kapczinski F, Vieta E. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. American Journal of Psychiatry. 2013;170:1249–1262. doi: 10.1176/appi.ajp.2013.13020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM GLYX-13 Clinical Study Group. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. Journal of Psychiatric Practice. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. Journal of Clinical Psychopharmacology. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, Soleimani L, Charney DS, Foulkes AL, Mathew SJ. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depression and Anxiety. 2014;31:335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, Thase ME. Effectiveness of adjunctive antidepressant treatment for bipolar depression. New England Journal of Medicine. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, Quirk MC. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Molecular Psychiatry. 2014;19:978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Fedgchin M, Daly E, De Boer P, Cooper K, Lim P, Pinter C, Murrough J, Sanacora G, Shelton G, Kurian B, Winokur A, Fava M, Manji H, Drevets W, Van Nueten L. A double-blind, randomized, placebo-controlled, parallel group, dose frequency study of intravenous ketamine in patients with treatment-resistant depression. Society of Biological Psychiatry 69th Annual Scientific Meeting; New York, USA. 8–10 May 2014; 2014. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinology Letters. 2013;34:287–293. [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Medicine. 2013;10:e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowicz SM, Finnegan N, Dale RM. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. Journal of Affective Disorders. 2013;147:416–420. doi: 10.1016/j.jad.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressant: a critical overview. Pharmacological Reviews. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- Vieta E, Locklear J, Günther O, Ekman M, Miltenburger C, Chatterton ML, Aström M, Paulsson B. Treatment options for bipolar depression: a systematic review of randomized, controlled trials. Journal of Clinical Psychopharmacology. 2010;30:579–590. doi: 10.1097/JCP.0b013e3181f15849. [DOI] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D project results: a comprehensive review of findings. Current Psychiatry Reports. 2007;9:449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biological Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biological Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.