Abstract
Several lines of evidence suggest a role for mitochondrial dysfunction in the pathophysiology of bipolar disorder (BD). The tricarboxylic acid cycle (TCA cycle) is fundamental for mitochondrial energy production and produces substrates used in oxidative phosphorylation by the mitochondrial electron transport chain. The activity of the key TCA cycle enzymes citrate synthase, malate dehydrogenase, and succinate dehydrogenase has never been evaluated in BD. In the present study, these enzymes were assayed from leukocytes of drug-naïve BD patients in a major depressive episode (n = 18) and compared to 24 age-matched healthy controls. Drug-naïve BD patients did not show differences in activities of citrate synthase (p = 0.79), malate dehydrogenase (p = 0.17), and succinate dehydrogenase (p = 0.35) compared with healthy controls. No correlation between any TCA cycle enzyme activity and severity of depressive symptoms was observed. Overall, these data suggest that the activities of the TCA cycle enzymes are not altered in major depressive episodes of recent-onset BD, which may support the concept of illness staging and neuroprogression in BD.
Keywords: Bipolar disorder, Mitochondria, Bioenergetics, Tricarboxylic acid cycle, Pathophysiology, Depression
1. Introduction
Different lines of evidence suggest that mitochondrial dysfunction plays an important role in the pathophysiology of bipolar disorder (BD) [1,2]. Previous reports showed increased oxidative stress, a measure indicative of mitochondrial dysfunction, in peripheral blood [3,4] and postmortem brains of BD patients [5].Also, a decrease in mitochondrial electron transport chain (ETC) activity was reported in postmortem BD [5]. The tricarboxylic acid cycle (TCA cycle) is critical for mitochondrial activity because it produces substrates that fuel oxidative phosphorylation in the mitochondrial ETC [6].
Some specific enzymes play an especial role in TCA cycle. The first step of TCA cycle is catalyzed by the enzyme citrate synthase and its activity has been shown to be a marker of intact mitochondria number and function [7]. Succinate dehydrogenase is the only TCA cycle enzyme directly integrated to the ETC, being a part of the ETC complex II [8]. Malate dehydrogenase is a TCA cycle enzyme that utilizes the NAD/NADH system and is responsible for malate production, which is the last step of the TCA cycle [9].
Decreases of TCA cycle enzymes activity was observed in animal models of mania [10,11]. Moreover, BD patients have shown reduced mRNA expression of the TCA cycle enzyme malate dehydrogenase in a study evaluating postmortem brains [12].
To our knowledge, no study evaluated in vivo the activity of TCA cycle enzymes in BD patients. This would be important to investigate in recent onset of BD, since it is possible that the activity of TCA cycle enzymes seem to decrease later in the course of other illnesses. This potential finding would suggest that mitochondrial dysfunction occurs as the illness progresses and support the staging of BD [4,13].
Due to the difficulty to assess directly the brain tissue, this study aimed to evaluate peripheral blood cells, which show transcription of energy production genes correlated to brain transcription [14]. Also, leukocytes have shown a role in biomarkers investigation in neuropsychiatric disorders [15] and BD [16]. The activities of the key TCA cycle enzymes citrate synthase, malate dehydrogenase, and succinate dehydrogenase were investigated in leukocytes of BD patients during an acute major depressive episode. Our hypothesis was that TCA cycle enzymes activity would be decreased in drug naïve bipolar depression vs. matched healthy controls.
2. Methods
2.1. Subjects
This study evaluated 18 drug-naïve BD outpatients during a depressive episode, 13 (72.2%) women, with a mean age of 29.1 (±4.9) years (Table 1). Five (27.8%) had a diagnosis of BD I and 13 (72.2%) BD II. Patients had a mean duration of illness of 2.7 (±1.6) years and a history of affective psychosis was present in 1 patient (5.6%). Patients had mean Hamilton Depression Scale (HAM-D) scores = 22.7 (±3.8) [17] and Young Mania Rating Scale scores = 7.5 (±6.7) [18].
Table1.
Clinical and demographic characteristics of patients with bipolar disorder (BD) patients during depressive episodes and healthy controls.
| BD (n=18) | Controls (n=24) | p | |
|---|---|---|---|
| Gender | |||
| Male/female, n (%) | 5 (27.8)/13 (72.2) | 14 (58.3)/10 (41.7) | 0.03*,a |
| Age, years | 29.1 (±4.9) | 27.6 (±6.6) | 0.44b |
| BD subtype | |||
| Subtype I/subtype II, n (%) | 5 (27.8)/13 (72.2) | ||
| Illness duration, years | 2.7 (±1.6) | ||
| History of Psychosis, n (%) | 1 (5.6) | ||
| HAM-D | 22.7 (±3.8) | ||
| YMRS | 7.5 (±6.7) |
HAM-D–Hamilton Depression Scale, YMRS–Young Mania Rating Scale.
Significantly different.
Chi-square test.
Student’s t test.
The diagnosis of BD was confirmed by the Structured Clinical Interview for Axis I DSM-IV-TR Disorders (SCID-I) [19]. Patients were recruited using media advertisement and were evaluated at the Institute of Psychiatry, University of Sao Paulo, Brazil, from August 2010 to June 2012. Inclusion criterion was depression scores ≥ 18 on the 21-item HAM-D. Exclusion criteria for BD patients were the presence of any medical disorder that could affect the central nervous system, current substance abuse or dependence, or an intellectual disability.
In the control group, 24 age-matched (within a 3-year difference of BD patients) volunteers were enrolled. As confirmed by the SCID-I, healthy volunteers (10 women, 41.7%, mean age of 27.6 ± 6.6 years) had no lifetime history of any psychiatric disorders, including substance disorders, no presence of medical disease affecting central nervous system, and no first degree-relatives with a history of mood or psychotic disorders. The present study was conducted in accordance with the 1964 Declaration of Helsinki, was approved by the local Ethics Committee and all the participants gave written informed consent before entering the study.
2.2. Blood sampling and laboratorial analysis
After an 8-h fasting, blood samples were collected from 8:00 to 10:00 AM. Samples were then centrifuged with ficoll (Sigma) at 20°C and 700 × g for 40 min; the mononuclear cells were separated using ficoll gradient to obtain leukocytes [20]. The plasma (first layer) was removed and then discarded. The leukocytes (second layer) were gently removed and then transferred to other tube, where they were washed twice with phosphate buffered saline (PBS) and centrifuged at 20°C and 700 × g for 15 min. After being centrifuged, PBS and dimethyl sulfoxide (DMSO) <0.01% were added to the samples, which were progressively frozen at −10°C for 30 min, at −20°C for 30 min, and finally stored at −80°C. The biochemical assays were ran in duplicates by a person who was blind to the source of the samples.
Regarding TCA cycle enzymes activities, the techniques are briefly described as follows: proteins dosage was performed according to the Lowry et al. [21] method. Citrate synthase activity was assayed following Shepherd and Garland method. The reaction mixture comprised 100 mM Tris, pH 8.0, 100 mMacetyl CoA, 100 mM 5,5′-di-thiobis-(2-nitrobenzoic acid), 0.1% triton X-100, and 2–4 µg supernatant protein, being started with 100 µM oxaloacetate and then monitored at 412 nm for 3 min at 25°C [22]. Malate dehydrogenase measurement was performed as described by Kitto [23]. Aliquots (of 20 mg protein) were transferred into a medium containing 10 mM rotenone, 0.2% Triton X-100, 0.15 mM NADH, and 100 mM potassium phosphate buffer, pH 7.4, at 37°C; the reaction was started by adding 0.33 mM oxaloacetate. Absorbance was monitored as described above [23]. The activity of succinate dehydrogenase was determined according to the method of Fischer et al. [24], The reaction mixture of 40 mM potassium phosphate, pH 7.4, 16 mM succinate and 8 µM 2,6-DCIP was first preincubated with 40–80 µg homogenate protein at 30°C for 20 min. Subsequently, 7 µM rotenone, 4 mM sodium azide, and 40 µM 2,6-DCIP were added, and the reaction was started by an addition of 1 mM PMS and was monitored for5 min; the reaction measured the decrease in absorbance due to the reduction of 2,6-di-chloro-indophenol (2,6-DCIP) in the presence of phenazine methosulphate (PMS) at 600 nm with 700 nm as the reference wavelength (ε = 19.1 mM−1 cm−1). The activities of TCA cycle enzymes were calculated as nmol min−1 × mg protein−1.
2.3. Statistics
Chi-square test was used to compare the gender distribution of patients vs. controls. In the comparison of patients and controls, a generalized linear model was performed to control for the difference in gender distribution between the groups. Spearman test was used to calculate the correlations of enzyme activity and depressive symptoms. Citrate synthase had one missing value due to technical issues. All statistical analyses were performed with SPSS 21.0 and the significance level was set at <0.05 (two-tailed). All data are presented as mean ± SD.
3. Results
3.1. Patients with BD and controls showed no difference in TCA cycle enzymes
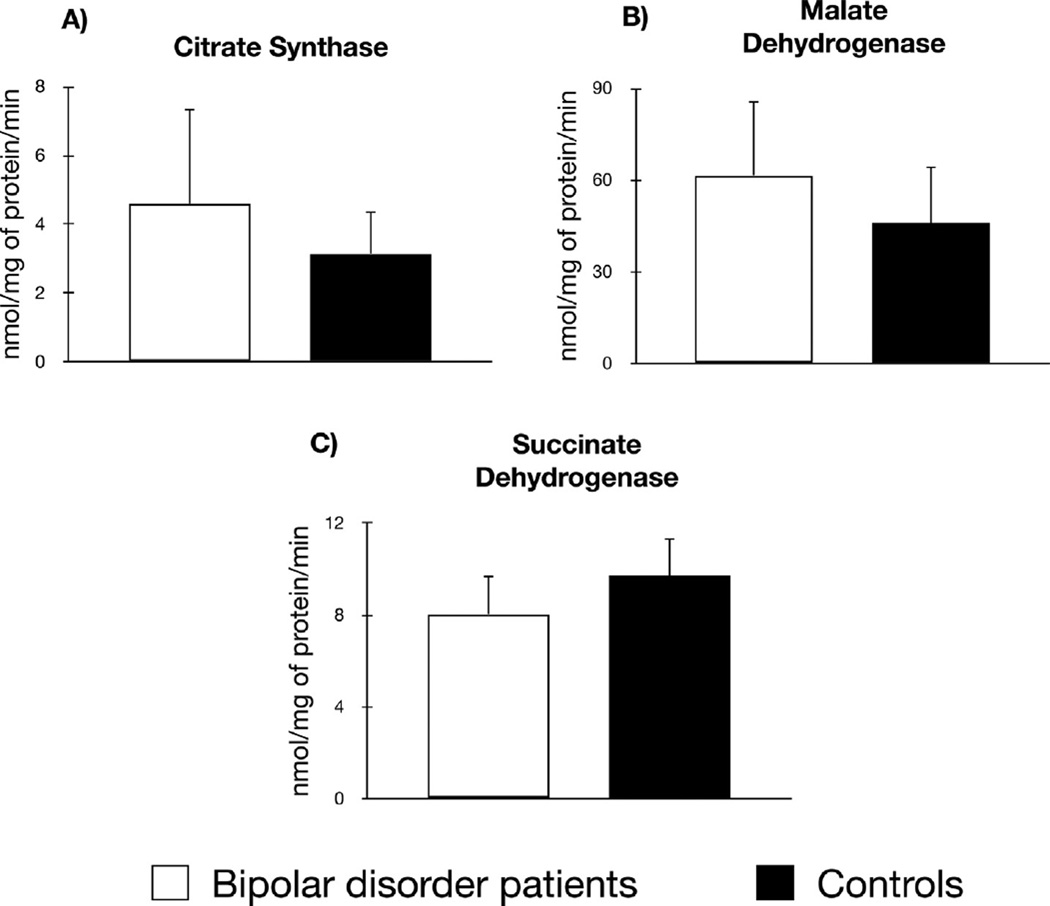
In comparison to healthy controls, patients with BD did not show different activities of citrate synthase (BD = 4.57 ± 11.41 nmol mg/min protein vs. controls = 3.15 ± 6.07, p = 0.79), malate dehydrogenase (BD = 61.65 ± 101.86 nmol mg/min protein vs. controls = 46.08 ± 88.75, p = 0.17), and succinate dehydrogenase (BD = 8.05 ± 7.29 nmol mg/min protein vs. controls = 9.71 ± 8.05, p = 0.35) (Fig. 1).
Fig. 1.
Activities of the tricarboxylic acid cycle enzymes in 18 patients with bipolar disorder during a depressive episode (white bars) compared to 24 healthy controls (black bars). In omparison to healthy controls, patients with BD did not show different activities of citrate synthase (p = 0.79), malate dehydrogenase (p = 0.17), and succinate dehydrogenase (p = 0.35).
No correlation of depressive symptoms (measured by HAM-D scores) and TCA cycle enzymes activities citrate synthase (ρ = 0.35, p = 0.15), malate dehydrogenase (ρ = 0.45, p = 0.06), and succinate dehydrogenase (ρ = 0.33, p = 0.19) was observed. Also, no significant correlations of TCA cycle enzymes activities and age or length of history were observed (data not shown).
4. Discussion
To the best of our knowledge, this is the first study evaluating the TCA cycle enzymes in BD. The present study found no alteration in the activities of TCA cycle enzymes citrate synthase, malate dehydrogenase, and succinate dehydrogenase in drug-naïve patients with BD depression compared to healthy controls.
The sample analyzed in the present study included patients with recent-onset BD (in average less than 3 years of history), who may have been spared from the biological alterations observed in cases with longer duration of illness or medication exposure [13,25]. Perhaps in recent-onset BD, compensatory mechanisms prevent the alteration of mitochondrial TCA cycle enzymes, which would be in line with the minimal changes in mitochondrial DNA content [26], mitochondrial electron transport chain activity [27], and oxidative stress [4] found in the same sample by our group. However, it is also possible that the alterations in the TCA cycle enzymes would be present only in manic phases but not in the depressive phases has suggested by abnormalities found in TCA cycle enzymes in animal models of [28] mania [10]. Another possibility would be that only later in the illness changes in the TCA cycle enzymes would take place, which is consistent with the staging model of the illness [4, 13].
Although the present analyses were performed in leukocytes and not in brain tissue, mitochondrial TCA cycle enzyme activity in peripheral cells have shown analogy with different tissues [29]. Moreover, mitochondrial enzymes activity in leukocytes have also been widely used as a surrogate marker of brain activity in neurological disorders [15]. In addition, DNA methylation in the brain is correlated with DNA methylation in leukocytes [30]. The evidence suggests that the values observed in our study are very likely to reflect TCA cycle enzymes activities in the brain. Factors such as young age of the subjects, short length of illness, same mood phase in all BD subjects, and the drug-naïve status of the patients increase the specificity of the findings.
Overall, the present study suggests that TCA cycle enzymes may not be altered in recent-onset BD. Further studies in larger samples and in patients with longer duration of illness and in mania and/or euthymia are warranted to explore the role of the activities of TCA cycle enzymes in BD.
HIGHLIGHTS.
Mitochondria is likely to play a role in the pathophysiology of bipolar disorder (BD).
The tricarboxylic acid cycle (TCA) has a main role in mitochondrial energy production.
TCA enzymes activities were evaluated in 18 drug-naïve BD patients during depression.
Recent-onset BD patients showed no alteration in TCA activity compared to controls.
Acknowledgments
We thank Sao Paulo Research Foundation for funding our study (FAPESP, Brazil; Grant 2009/14891-9, RM-V); FAPESP had no role in the design of the study, collection, analysis of data, and decision to publish. We also thank Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS) and JNK Empreendimentos e Incorporaçõessupport to Laboratory of Neurosciences.
Abbreviations
- BD
bipolar disorder
- TCA cycle
tricarboxylic acid cycle
- ETC
electron transport chain
- HAM-D
Hamilton Depression Scale
- SCID
structured clinical interview for axis I DSM-IV-TR disorders (SCID)
- YMRS
Young Mania Rating Scale.
References
- 1.de Sousa RT, Machado-Vieira R, Zarate CA, Manji HK. Targeting mitochondrially mediated plasticity to develop improved therapeutics for bipolar disorder. Expert Opin. Ther. Targets. 2014;18:1131–1147. doi: 10.1517/14728222.2014.940893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA. Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J. Biol. Psychiatry. 2013;15:84–95. doi: 10.3109/15622975.2013.830775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V, da Silva Vargas R, Kapczinski F, Portela LV, Souza DO, Salvador M, Gentil V. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci. Lett. 2007;421:33–36. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 4.de Sousa RT, Zarate CA, Zanetti MV, Costa AC, Talib LL, Gattaz WF, Machado-Vieira R. Oxidative stress in early stage bipolar disorder and the association with response to lithium. J. Psychiatr. Res. 2014;50:36–41. doi: 10.1016/j.jpsychires.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch. Gen. Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 6.Blass JP, Brown AM. Lower activity of Krebs cycle enzymes than of electron transport in human brain: disease implications. Neurobiol. Aging. 2000;21:81. [Google Scholar]
- 7.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oyedotun KS, Lemire BD. The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. Homology modeling cofactor docking, and molecular dynamics simulation studies. J. Biol. Chem. 2004;279:9424–9431. doi: 10.1074/jbc.M311876200. [DOI] [PubMed] [Google Scholar]
- 9.Minard KI, McAlister-Henn L. Isolation, nucleotide sequence analysis, and disruption of the MDH2 gene from Saccharomyces cerevisiae: evidence for three isozymes of yeast malate dehydrogenase. Mol. Cell. Biol. 1991;11:370–380. doi: 10.1128/mcb.11.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feier G, Valvassori SS, Varela RB, Resende WR, Bavaresco DV, Morais MO, Scaini G, Andersen ML, Streck EL, Quevedo J. Lithium and valproate modulate energy metabolism in an animal model of mania induced by methamphetamine. Pharmacol. Biochem. Behav. 2013;103:589–596. doi: 10.1016/j.pbb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Valvassori SS, Calixto KV, Budni J, Resende WR, Varela RB, de Freitas KV, Gonçalves CL, Streck EL, Quevedo J. Sodium butyrate reverses the inhibition of Krebs cycle enzymes induced by amphetamine in the rat brain. J. Neural Transm. 2013;120:1737–1742. doi: 10.1007/s00702-013-1056-3. [DOI] [PubMed] [Google Scholar]
- 12.Lee BD, Walss-Bass C, Thompson PM, Dassori A, Montero PA, Medina R, Contreras S, Armas R, Ramirez M, Pereira M, Salazar R, Leach RJ, Quezada P, Raventos H, Escamilla MA. Malic enzyme 2 and susceptibility to psychosis and mania. Psychiatry Res. 2007;150(1):1–11. doi: 10.1016/j.psychres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, Conus P, Bechdolf A, Moylan S, Malhi GS. Stage managing bipolar disorder. Bipolar Disord. 2013;16:471–477. doi: 10.1111/bdi.12099. [DOI] [PubMed] [Google Scholar]
- 14.Cai C, Langfelder P, Fuller TF, Oldham MC, Luo R, van den Berg LH, Ophoff RA, Horvath S. Is human blood a good surrogate for brain tissue in transcriptional studies? BMC Genom. 2010;11:589. doi: 10.1186/1471-2164-11-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiasi P, Hosseinkhani S, Noori A, Nafissi S, Khajeh K. Mitochondrial complex I deficiency and ATP/ADP ratio in lymphocytes of amyotrophic lateral sclerosis patients. Neurol. Res. 2012;34:297–303. doi: 10.1179/1743132812Y.0000000012. [DOI] [PubMed] [Google Scholar]
- 16.Middleton FA, Pato CN, Gentile KL, McGann L, Brown AM, Trauzzi M, Diab H, Morley CP, Medeiros H, Macedo A, Azevedo MH, Pato MT. Gene expression analysis of peripheral blood leukocytes from discordant sib-pairs with schizophrenia and bipolar disorder reveals points of convergence between genetic and functional genomic approaches. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;136B:12–25. doi: 10.1002/ajmg.b.30171. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York: New York Psychiatric Institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Ed. [Google Scholar]
- 20.Noble PB, Cutts JH. Separation of blood leukocytes by Ficoll gradient. Can. Vet. J. 1967;8:110–111. [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Shepherd D, Garland PB. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem. J. 1969;114:597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitto GB. Intra-and extramitochondrial malate dehydrogenases from chicken and tuna heart. Methods Enzymol. 1969:106–116. [Google Scholar]
- 24.Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin. Chim. Acta. 1985;153:23–36. doi: 10.1016/0009-8981(85)90135-4. [DOI] [PubMed] [Google Scholar]
- 25.Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci. Biobehav. Rev. 2007;31:858–873. doi: 10.1016/j.neubiorev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.de Sousa RT, Uno M, Zanetti MV, Shinjo SM, Busatto GF, Gattaz WF, Marie SK, Machado-Vieira R. Leukocyte mitochondrial DNA copy number in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:32–35. doi: 10.1016/j.pnpbp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 27.de Sousa RT, Streck EL, Zanetti MV, Ferreira GK, Diniz BS, Brunoni AR, Busatto GF, Gattaz WF, Machado-Vieira R. Lithium increases leukocyte mitochondrial complex I activity in bipolar disorder during depressive episodes. Psychopharmacology (Berl.) 2015;232:245–250. doi: 10.1007/s00213-014-3655-6. [DOI] [PubMed] [Google Scholar]
- 28.Valvassori SS, Bavaresco DV, Budni J, Bobsin TS, Gonçalves CL, de Freitas KV, Streck EL, Quevedo J. Effects of tamoxifen on tricarboxylic acid cycle enzymes in the brain of rats submitted to an animal model of mania induced by amphetamine. Psychiatry Res. 2013 doi: 10.1016/j.psychres.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Chretien D, Rustin P, Bourgeron T, Rötig A, Saudubray JM, Munnich A. Reference charts for respiratory chain activities in human tissues. Clin. Chim. Acta. 1994;228:53–70. doi: 10.1016/0009-8981(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.Masliah E, Dumaop W, Galasko D, Desplats P. Distinctive patterns of DNA methylation associated with Parkinson disease: identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics. 2013;8:1030–1038. doi: 10.4161/epi.25865. [DOI] [PMC free article] [PubMed] [Google Scholar]