Summary
Streptococcus pneumoniae is a common colonizer of the human nasopharynx and a leading cause of bacterial pneumonia and otitis media, among other invasive diseases. During both colonization and invasive disease S. pneumoniae ferments host-derived carbohydrates as its primary means of generating energy. This pathogen is adept at transporting and metabolizing a wide variety of carbohydrates. We found the highly conserved PTS ManLMN contributes to growth on glucose and is also essential for growth on a variety of nonpreferred carbohydrates, suggesting it is a multisubstrate transporter. Exploration of this phenotype revealed ManLMN is required for inducing expression of downstream metabolic genes in response to carbohydrate stimuli. We further demonstrate that ManLMN’s role as a constitutively expressed transporter is likely unique and integral to pneumococcus’s strategy of carbon catabolite repression (CCR). Using a selection for suppressors, we explored how ManLMN is integrated into the CCR regulatory framework in S. pneumoniae. We identified two hypothetical small proteins and the virulence regulator SmrC as potential mediators of CCR in connection with ManLMN. Characterization of these two hypothetical proteins revealed they influence transcriptional regulation of carbohydrate transporters. We propose a model unifying these observations in which ManLMN is a versatile surveyor of available carbohydrates in S. pneumoniae.
Introduction
ManLMN, encoded by the manLMN operon is a conserved phosphoenolpyruvate-dependent phosphotransferase system (PTS) that plays a predominant role in carbohydrate metabolism and metabolic regulation in many Gram positive species, in particular low G + C species (Abranches et al., 2003). ManLMN is typically a major glucose transporter that is also capable of transporting a varying number of other carbohydrate substrates including mannose, fructose, galactose and N-acetyl glucosamine (GlcNAc) (Vadeboncoeur et al., 2000; Jahreis et al., 2008; Bidossi et al., 2012). Unlike PTS systems with limited substrate specificity, manLMN is frequently constitutively expressed, meaning its regulation is not responsive to the presence or absence of any particular carbohydrate substrate (Jahreis et al., 2008). Similar to other PTS’s of the mannose class, manLMN encodes a fused EIIAB domain protein (ManL) responsible for phosphorylation of the incoming carbohydrate, and EIIC and EIID proteins (ManMN, respectively) constituting the permease through which the carbohydrate molecule enters the cell (Zúñiga et al., 2005; Jahreis et al., 2008). This complex and ManL in particular have been implicated as a mediator of CCR not only for its role in glucose transport but also as an independent regulator. Much like EIIBAglu from Escherichia coli, ManL was previously named Enzyme III because its pleotropic effects on carbohydrate metabolism set it apart from other EIIA/EIIB proteins according to what was understood of the scope of these proteins’ functions (Vadeboncoeur and Gauthier, 1987). Inactivation of manL in Streptococcus salivarius positively and negatively affected the expression and activity of other PTS systems, caused general proteome changes and eliminated diauxic growth in some conditions (Vadeboncoeur et al., 1983; Vadeboncoeur and Pelletier, 1997). Inactivation of manL in Streptococcus mutans caused general proteome changes, and affected transcription of over 100 genes including many carbohydrate transporters (Abranches et al., 2003, 2006; Moye et al., 2014b). It was also speculated that ManL may directly influence a transcriptional regulator’s activity in S. mutans (Zeng and Burne, 2008).
Despite being conserved in all sequenced Streptococcus pneumoniae strains, relatively little is known about the function of ManLMN (encoded by SP_0282-4) in this organism. As the fitness of this human pathogen is dependent on fermentation of host derived carbohydrates, it is important to understand how carbon sources are acquired. Given the importance of ManLMN in related Streptococci we hypothesized ManLMN would play a central role in carbohydrate metabolism in S. pneumoniae. Studies in D39 (serotype 2) showed manLMN is repressed by both the global CCR regulator, catabolite protein A (CcpA) and CiaR, the response regulator of the conserved two-component system CiaRH implicated in competence, autolysis and β-lactam resistance (Mascher et al., 2003; Halfmann et al., 2007, 2011; Marx et al., 2010; Carvalho et al., 2011). In D39, inactivation of manM encoding the EIIC component, resulted in a mild growth defect in glucose, and more severely reduced growth in GlcNAc, mannose and galactose (Bidossi et al., 2012). In contrast to D39, we found ManLMN in TIGR4 (serotype 4) to be essential for growth on five nonpreferred carbohydrates, and required to induce expression of downstream metabolic genes. We show ManLMN contributes to growth on glucose as a high affinity glucose transporter, and is required for inducer exclusion of lactose. Using a selection for suppressors that relieve the dependence on ManLMN, we identified two hypothetical proteins as putative factors mediating ManLMN-dependent regulation of nonpreferred carbohydrate metabolism gene expression.
Results
ManLMN is required for growth on a variety of nonpreferred carbohydrates
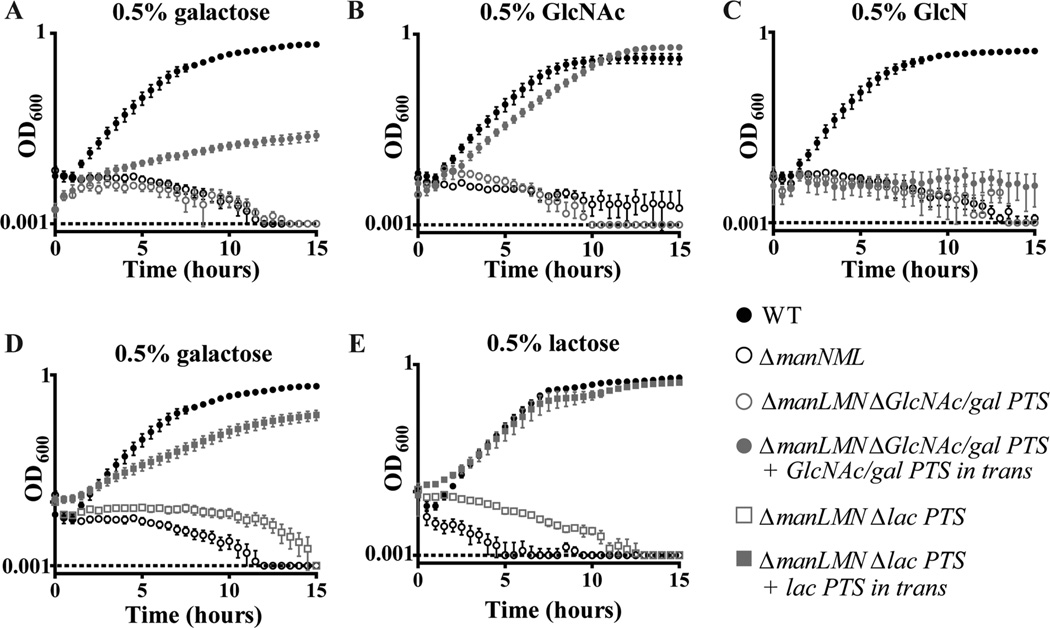
Deletion of manLMN in the TIGR4 strain of S. pneumoniae abrogated growth on the nonpreferred carbohydrates galactose, lactose, glucosamine (GlcN), GlcNAc and fructose. Growth was diminished on the nonpreferred carbohydrates mannose and raffinose, while growth on the preferred carbohydrates glucose, sucrose and on the glucose disaccharides trehalose and maltose was unaffected (Fig. 1A–H, Supporting information Fig. S1A–C). Based on homology to transporters in related species, S. pneumoniae has at least one additional putative transporter for each of these nonpreferred carbohydrates. In additional to ManLMN, there are three predicted galactose transporters (SP_0061-3; PTS, SP_0090-2; ABC and SP_0645-7; PTS), one predicted GlcN transporter (SP_1684; incomplete PTS), one predicted GlcNAc transporter (SP_0061-3; PTS) and one predicted fructose transporter (SP_0877; PTS), encoded in the TIGR4 genome. There is only one predicted lactose transporter (SP_1185-6; PTS) (Bidossi et al., 2012). We chose to analyze the contribution of three such PTS transporters to growth in these conditions. Although there are other transporters predicted to recognize these substrates, for clarity we refer to the transporters we investigated as the GlcNAc/gal PTS (SP_0061-3), the lac PTS (SP_1185-6) and the fru PTS (SP_0877). In contrast to the manLMN deletion strain, inactivation of the GlcNAc/gal, lac or fru PTS had no effect on growth in these conditions (Fig. 1B, C and F respectively). One explanation of these results is that ManLMN is required for growth in these conditions because it supplies the initial intracellular carbohydrate-inducers needed to induce expression of these other transporters and downstream metabolic enzymes.
Fig. 1.
ManLMN, but not substrate-specific PTS transporters, is required for growth on multiple carbohydrates. WT, ΔmanLMN, ΔGlcNAc/gal PTS, Δlac PTS, Δfru PTS and the manLMN complemented strain were grown in chemically defined medium (CDM) with 0.5% final concentration of one of the following carbohydrates; glucose (A), galactose (B), lactose (C), GlcN (D), GlcNAc (E), fructose (F), mannose (G) or raffinose (H). Absorbance at 600 nm was measured every 30 min over the course of 15 h of growth at 37°C. Each data point represents the average of at least six biological replicates from at least two separate days. Error bars represent the standard error of the mean (SEM).
The manLMN operon is constitutively expressed
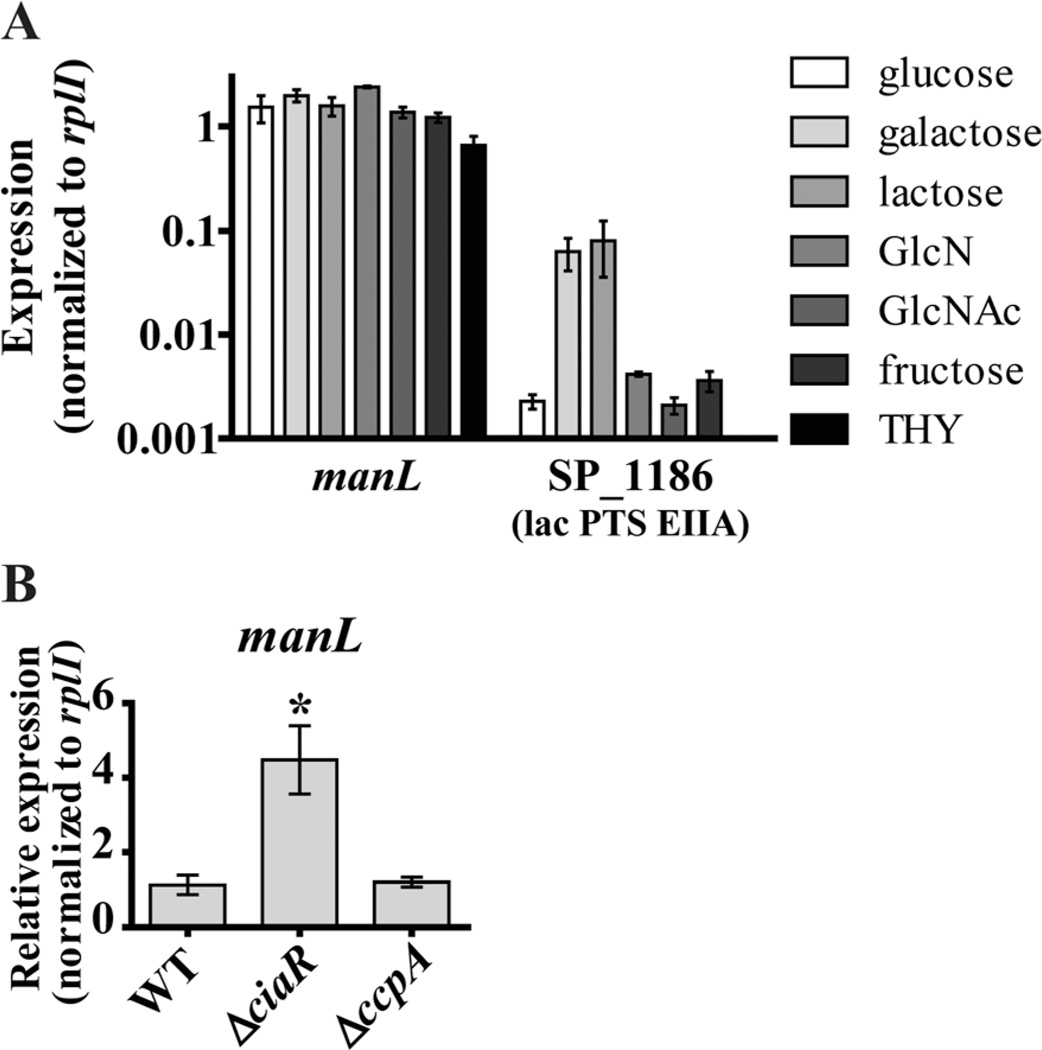
Transcriptional analysis of manL as a representative of the manLMN operon revealed this operon is constitutively expressed in a variety of preferred and nonpreferred carbohydrate conditions (Fig. 2A). This included the glucose-replete rich broth THY. Constitutive expression is in contrast to the typical regulation pattern of substrate-specific transporters, which are substantially upregulated in response to growth in their cognate carbohydrate (Fig. 2A) (Deutscher et al., 2006). Constitutive expression is consistent with our finding that ManLMN is essential for growth in these conditions.
Fig. 2.
The manLMN operon is constitutively expressed and repressed by CiaR.
A. Transcription of manL as a representative of the manLMN operon was assessed by quantitative reverse transcriptase PCR (qRT-PCR) during mid-exponential growth of WT in THY or CDM with 0.5% final concentration of glucose, galactose, lactose, GlcN, GlcNAc or fructose. Transcription of SP_1186 encoding the lac PTS EIIA was assessed as a representative limited-substrate carbohydrate transporter gene. Each bar represents the average of five biological replicates collected on at least two separate days, normalized to rplI. Error bars represent the SEM.
B. Transcription of manL in WT, ΔciaR and ΔccpA during mid-exponential growth in THY was assessed by qRT-PCR. The average of five biological replicates collected on at least two separate days, normalized to rplI and relative to the WT sample, is graphed for each. Error bars represent the SEM. * indicates P value ≤ 0.05.
In S. pneumoniae D39, manLMN is repressed by two global regulators; the conserved two-component system response regulation CiaR and the CCR regulator CcpA (Mascher et al., 2003; Halfmann et al., 2007, 2011; Marx et al., 2010; Carvalho et al., 2011). In order to determine if manLMN is similarly regulated in TIGR4, we analyzed transcription of manL in the ΔciaR and ΔccpA strains. Deletion of ciaR but not ccpA resulted in de-repression of manL in all growth media tested (Fig. 2B and Supporting information Fig. S2B and C). This difference with D39 could be due to the different growth medium used.
ManLMN is required for induction of nonpreferred carbohydrate metabolic genes
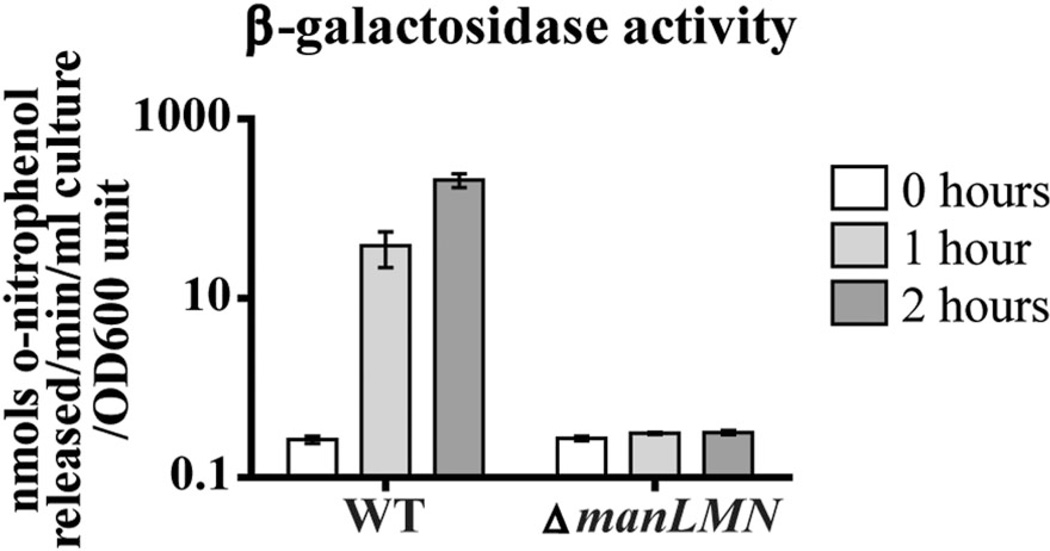
Our observation that unlike other carbohydrate transporters, manLMN is constitutively expressed and is required for growth on many carbohydrates led us to hypothesize that ManLMN is providing the carbohydrate-inducers needed to induce expression of substrate-specific transporters and downstream metabolic enzymes. According to this hypothesis, induction of galactose, lactose, GlcN, GlcNAc and fructose metabolism genes should be reduced if not abolished in ΔmanLMN. To test this we compared lactose-dependent induction of endogenous β-galactosidase (BgaA) activity in WT and ΔmanLMN. Streptococcus pneumoniae encodes multiple β-galactosidases but only BgaA recognizes o-nitrophenyl-galactopyranoside (ONPG) as a substrate (Zäahner and Hakenbeck, 2000; Kaufman and Yother, 2007; Jeong et al., 2009; Cheng et al., 2012). Unlike WT, which showed over 800-fold induction of BgaA activity two hours after introduction to lactose medium, ΔmanLMN showed no induction (Fig. 3).
Fig. 3.
ManLMN is required for induction of β-galactosidase activity. Mid-exponential cultures of WT and ΔmanLMN grown in 0.5% glucose CDM were washed and then resuspended in 0.5% lactose CDM. β-galactosidase activity was measured at the end of growth in glucose and 1 and 2 h after introduction to lactose CDM. Each bar represents the average of six biological replicates from two separate days. Error bars represent the SEM.
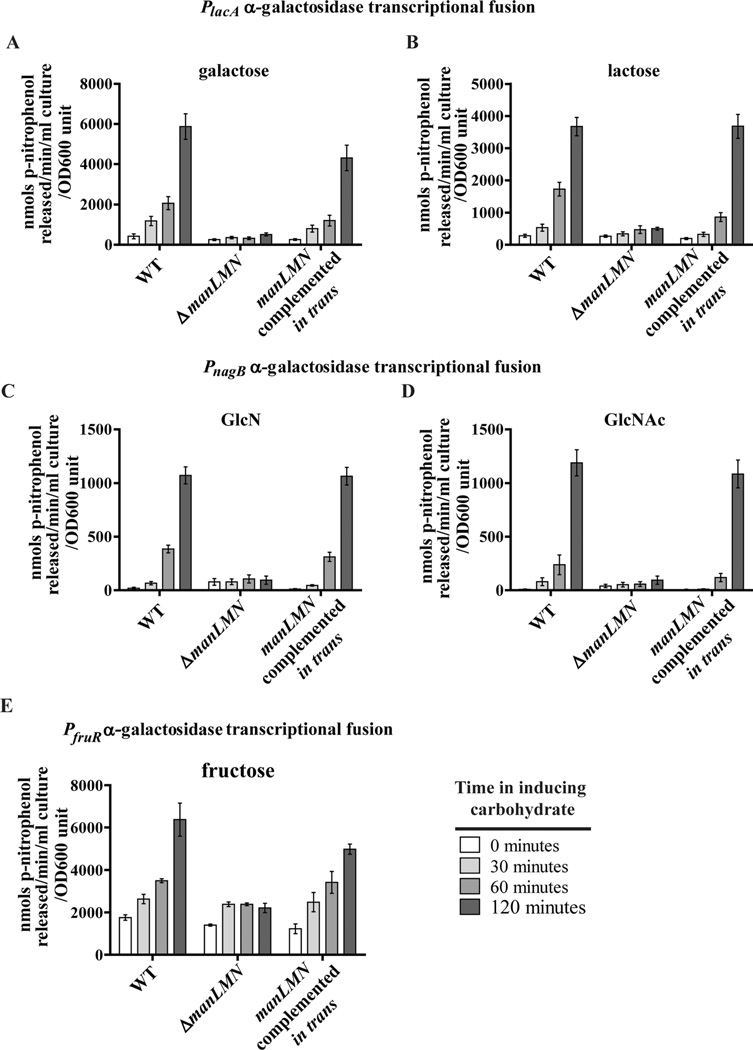
In order to determine if deleting manLMN similarly affects induction of other metabolism operons, we constructed transcriptional reporter strains in which expression of α-galactosidase (the reporter) is controlled by different carbohydrate metabolism operon promoters. As another test for loss of lactose/galactose-dependent induction, we used the tagatose-6-phosphate operon (lacABCD; SP_1190-3) promoter (PlacA). This promoter has been shown previously to be upregulated in TIGR4 during growth in galactose and lactose (Fleming et al., 2015). To test for loss of GlcN/GlcNAc-dependent induction, we used the glucosamine-6-phosphate deaminase (nagB; SP_1415) promoter (PnagB). Although NagB is uncharacterized in S. pneumoniae in a variety of other species it enables energy generation from GlcN and GlcNAc, which induce its expression (White, 1968; Bertram et al., 2011; Moye et al., 2014a). And to test for loss of fructose-dependent induction we used the fructose operon (fruRCA; SP_0875-7) promoter (PfruR). This operon closely resembles the well characterized fructose-inducible fruRCA operon in Lactococcis lactis (Barriere et al., 2005), and the encoded fructose-type PTS transporter (FruA) was shown to contribute to growth of serotype 2 S. pneumoniae in fructose medium (Bidossi et al., 2012). The genomic coordinates of the exact promoter regions used are listed in Supporting information Table S1. Using these reporter constructs we analyzed temporal induction of α-galactosidase reporter activity in response to carbohydrate inducers in the WT, ΔmanLMN and the manLMN complemented in trans strain backgrounds. In the WT background, each of these reporter constructs showed α-galactosidase activity was responsive to the predicted cognate carbohydrate(s) (Fig. 4). By two hours the PlacA reporter showed a 13-fold increase in α-galactosidase activity in response to galactose or lactose (Fig. 4A and B respectively). Deletion of manLMN completely abolished this induction. Deletion of manLMN had a similar effect on GlcN- and GlcNAc-dependent induction of α-galactosidase activity in the PnagB reporter strain. While in the WT background this construct showed 56-fold increase in α-galactosidase activity in response to GlcN and over 100-fold induction in response to GlcNAc, the ΔmanLMN strain maintained uninduced levels of α-galactosidase activity over the course of the experiment (Fig. 4C and D). These results corroborate our analysis of endogenous β-galactosidase activity and are consistent with ManLMN facilitating induction of downstream metabolism operons.
Fig. 4.
ManLMN is required for induction of galactose/lactose and GlcN/GlcNAc metabolism genes and partially required for induction of fructose metabolism genes. Carbohydrate-dependent transcriptional induction was assessed using α-galactosidase reporter strains. Strains were grown in 0.5% glucose CDM to mid-exponential phase, washed and then resuspended in CDM with 0.5% of the corresponding carbohydrate. α-galactosidase activity was measured at the end of growth in glucose and after 30, 60 and 120 min of incubation with the inducing carbohydrate. A tagatose-6-phosphate promoter reporter construct was tested for response to galactose (A) or lactose (B). A glucosamine-6-phosphate deaminase promoter reporter construct was tested for response to GlcN (C) or GlcNAc (D). A fructose operon promoter reporter construct was tested for response to fructose (E). Each bar represents the average of six biological replicates from at least two separate days. Error bars represent the SEM.
In the WT background, the PfruR reporter construct showed higher basal α-galactosidase reporter activity without fructose, and at most fourfold induction of activity in response to fructose (Fig. 4E). This induction was reduced to less than twofold by deleting manLMN, but was not completely abolished. This result in in contrast to the other transcriptional reporter strains, and suggests ManLMN contributes to induction of the fruRCA operon in conjunction with another fructose transporter. One possibility is that the higher basal level of transcription of this operon allows the fru transporter encoded by fruA to contribute to further induction by transporting fructose. For all three reporter constructs, induction was largely or completely restored by complementing manLMN expression in trans (Fig. 4A–E).
Growth of ΔmanLMN is partially rescued by deleting CcpA
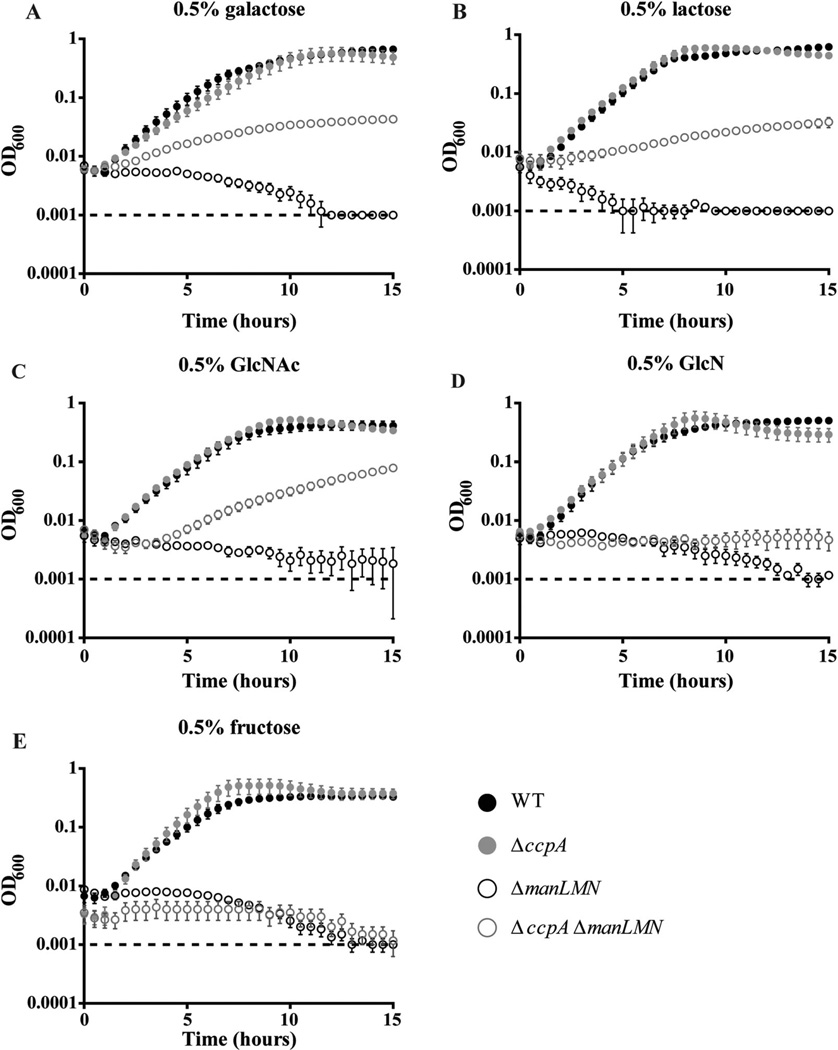
Analysis of the α-galactosidase reporter strains demonstrated that ManLMN is required for induction of certain nonpreferred carbohydrate metabolism genes. Because the β-galactosidase we monitored activity of (BgaA) is co-transcribed with a predicted galactose PTS system (Zäahner and Hakenbeck, 2000; Kaufman and Yother, 2007), our analysis of β-galactosidase activity further suggested that ManLMN may be required for induction of at least some substrate-specific carbohydrate transporters. Accordingly, we hypothesized that elevated expression of one of these carbohydrate-specific transporters should rescue growth and induction in ΔmanLMN in the corresponding carbohydrate condition. CcpA, the master transcriptional CCR regulator, is known to partially repress transcription of multiple carbohydrate transport systems (Warner and Lolkema, 2003; Lorca et al., 2005; Deutscher et al., 2006; Carvalho et al., 2011; Fleming et al., 2015), and thus we hypothesize deletion of ccpA should partially rescue growth of ΔmanLMN. To test this hypothesis, we determined the growth profile of the ΔmanLMN ΔccpA double mutant strain in the five conditions that require ManLMN (galactose, lactose, GlcN, GlcNAc and fructose). Deletion of ccpA partially rescued growth of ΔmanLMN in galactose, lactose and GlcNAc (Fig. 5A–C respectively). Due to CcpA’s known role in transcriptional repression of carbohydrate transporters, these results suggest partially de-repressed transcription of galactose, lactose and GlcNAc transporters circumvents the requirement for ManLMN for growth in these conditions. The poor growth of the ΔmanLMN ΔccpA double mutant strain in these conditions is likely due to the global mis-regulation caused by ccpA inactivation (Carvalho et al., 2011). Growth was not rescued in GlcN nor fructose (Fig. 5D and E respectively), which is inconsistent with our hypothesis. It is possible CcpA does not repress the GlcN and fructose transporter(s). As discussed, TIGR4 encodes at least one predicted fructose PTS (SP_0877) which was shown to contribute to growth on fructose in D39. Deletion of SP_1684 encoding a predicted EIIBC GlcN transporter reduced growth of D39 in GlcN medium, again suggesting other transporters exist for these carbohydrates (Bidossi et al., 2012).
Fig. 5.
Deletion of ccpA rescues growth of ΔmanLMN in select conditions. WT, ΔmanLMN, ΔccpA and ΔmanLMN ΔccpA were grown in CDM with 0.5% final concentration of one of the following carbohydrates; galactose (A), lactose (B), GlcN (C), GlcNAc (D), fructose (E). Absorbance at 600 nm was measured every 30 min over the course of 15 h of growth at 37°C. Each data point represents the average of at least six biological replicates from two separate days. Error bars represent the SEM.
To more directly test our hypothesis that expression of predicted substrate-specific carbohydrate transporters can circumvent the requirement for ManLMN, we individually deleted three PTS systems in ΔmanLMN, and expressed them in trans under the control of the manLMN operon promoter (Pman). Expression of the GlcNAc/gal PTS under the control of Pman partially restored growth of ΔmanLMN in galactose and nearly fully restored growth in GlcNAc (Fig. 6A and B respectively). Constitutive expression of this PTS was not able to rescue growth in GlcN or any other condition tested (Fig. 6C and data not shown). Similarly, expression of the lac PTS system under the control of Pman partially restored growth of ΔmanLMN in galactose and fully restored growth in lactose (Fig. 6D and E respectively), but did not affect growth in any other condition tested (data not shown). The two PTS systems we chose are known to be repressed by CcpA in TIGR4 (Fleming et al., 2015), and deletion of neither transporter in the WT background affected growth in these conditions (Fig. 1B and C). These results corroborate our interpretation of the ΔmanLMN ΔccpA strain growth, and together, support our hypothesis that defective growth of ΔmanLMN on nonpreferred carbohydrates is due to loss of ManLMN-dependent induction of specific transport and metabolic genes. In contrast, constitutive expression of the fru PTS under the control of Pman was not sufficient to rescue growth of ΔmanLMN in fructose (Supporting information Fig. S3). However, this PTS behaves differently from the GlcNAc/gal and lac PTSs in that it has a high basal level of expression (Fig. 4E) and despite having a predicted CcpA-binding site in its promoter region, ΔmanLMN growth in fructose could not be rescued by inactivating ccpA. It is unknown why the high basal level of transcription of the fructose operon is unable to promote growth of ΔmanLMN on fructose. It is possible that the encoded fructose-type PTS system (FruA) is not a fructose transporter in TIGR4.
Fig. 6.
Constitutive expression of substrate-specific PTS systems rescues growth of ΔmanLMN in select conditions. Growth of the ManLMN-GlcNAc/gal PTS double deletion strain (ΔmanLMN ΔGlcNAc/gal PTS) and its corresponding complemented strain (ΔmanLMN ΔGlcNAc/gal PTS + GlcNAc/gal PTS complemented in trans) were compared to growth of WT and ΔmanLMN in galactose (A), GlcNAc (B) and GlcN (C). Growth of the ManLMN-lac PTS double deletion strain (ΔmanLMN Δlac PTS) and its corresponding complemented strain (ΔmanLMN Δlac PTS + lac PTS complemented in trans) was compared to growth of WT and ΔmanLMN in galactose (D) and lactose (E). For all strains, optical density readings were taken every 30 min over the course of 15 h of growth at 37°C. Each data point represents the average of at least six biological replicates from at least two separate days. Error bars represent the SEM.
Constitutive expression of the lac PTS in place of ManLMN abolishes CCR of lactose metabolism
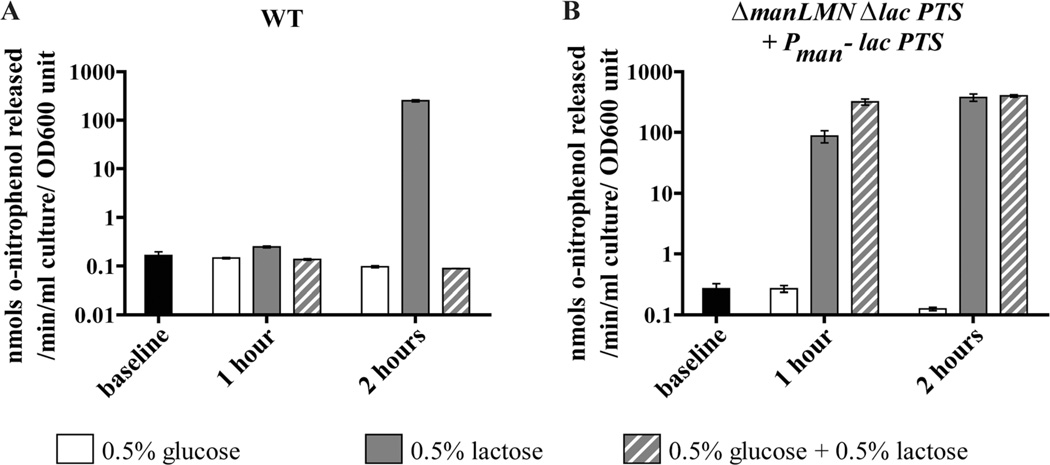
Having established that manLMN required for induction of other metabolic genes, we next wanted to determine if ManLMN serves a unique role in CCR. To address this question we asked if the strain constitutively expressing the lac PTS, ΔmanLMN Δlac PTS Pman-lac PTS, is capable of lactose inducer exclusion. We monitored the activity of the endogenous extracellular β-galactosidase (BgaA) as a reporter for lactosedependent induction. The strains were grown in rich medium (baseline activity), washed and then introduced to glucose CDM (noninducing condition), lactose CDM (inducing condition), or glucose + lactose CDM (CCR-repressing condition), for 1 or 2 h. Over time, the WT strain showed induction of β-galactosidase activity only in the inducing condition (Fig. 7A). In contrast the strain constitutively expressing the lac PTS showed equivalent levels of induction of β-galactosidase activity in both the inducing condition and the CCR condition (Fig. 7B). This result suggests that this strain is unable to restrict transport through the lac PTS in the presence of a preferred carbohydrate. Conversely, the Δlac PTS Pman-lac PTS strain which constitutively expresses the lac PTS but still has ManLMN, was able to inducer exclude lactose in the CCR-repressing condition (Supporting information Fig. S4A). These results suggest ManLMN is notable not only for its expression pattern and impact on nonpreferred carbohydrate gene expression, but also for contributing directly or indirectly to inducer exclusion.
Fig. 7.
A strain constitutively expressing the lac PTS and lacking manLMN is unable to repress β-galactosidase activity. Mid-exponential THY cultures of WT (A), and ΔmanLMN Δlac PTS + lac PTS complemented in trans (B) were washed and switched to CDM containing 0.5% glucose, 0.5% lactose or 0.5% glucose + 0.5% lactose. Samples were collected from the THY cultures (‘baseline’) as well as one and 2 h after switching to the CDM conditions. Each bar represents the average of six biological replicates collected on at least two separate days, except the 2 h glucose only condition which was only assayed three times. Error bars represent the SEM.
ManLMN supports growth in glucose
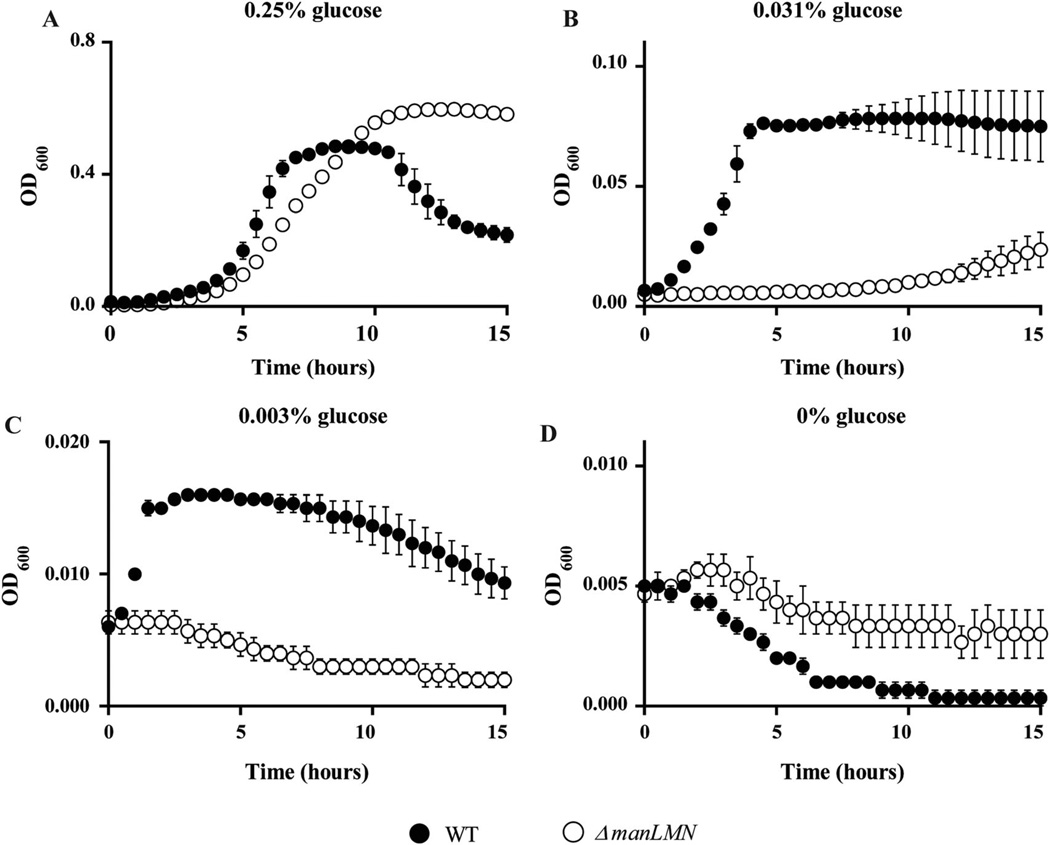
Consistent with ManLMN playing a role in CCR, the ΔmanLMN ΔccpA strain had a pronounced growth defect on glucose compared to the manLMN and ccpA single deletion strains (Supporting information Fig. S5), suggesting CcpA and ManLMN are functionally related. This result could also indicate that ManLMN is a glucose transporter, despite ΔmanLMN having no growth defect in glucose. CcpA in related species is known to activate transcription of glucose transporters (Warner and Lolkema, 2003; Lorca et al., 2005; Deutscher et al., 2006). Thus, the absence of manLMN and reduced expression of other glucose transporter(s) could have a compounded negative effect on growth of ΔmanLMN ΔccpA in glucose. We reasoned that although ΔmanLMN has no growth defect at the high concentration of glucose we routinely use (0.5%), it may exhibit a difference in growth when glucose is limiting. To test this hypothesis we compared growth of WT and ΔmanLMN in decreasing glucose concentration. The ΔmanLMN strain showed altered growth dynamics fromWT in 0.25% glucose (Fig. 8A) and failed to grow in 0.03% and below, whileWT maintained growth in concentrations as low as 0.003% (Fig. 8B and C). This result suggests that ManLMN may function as a high affinity glucose transporter.
Fig. 8.
The ΔmanLMN strain has a reduced ability to grow in limiting glucose. Growth of WT and ΔmanLMN was compared in CDM containing the following growth limiting concentrations of glucose; 0.25% (A), 0.031% (B), 0.003% (C) and 0% (D). Both strains were tested at least six times over the course of multiple days. Each data point represents the average of three biological replicates from a representative day and error bars represent the SEM from a representative experiment.
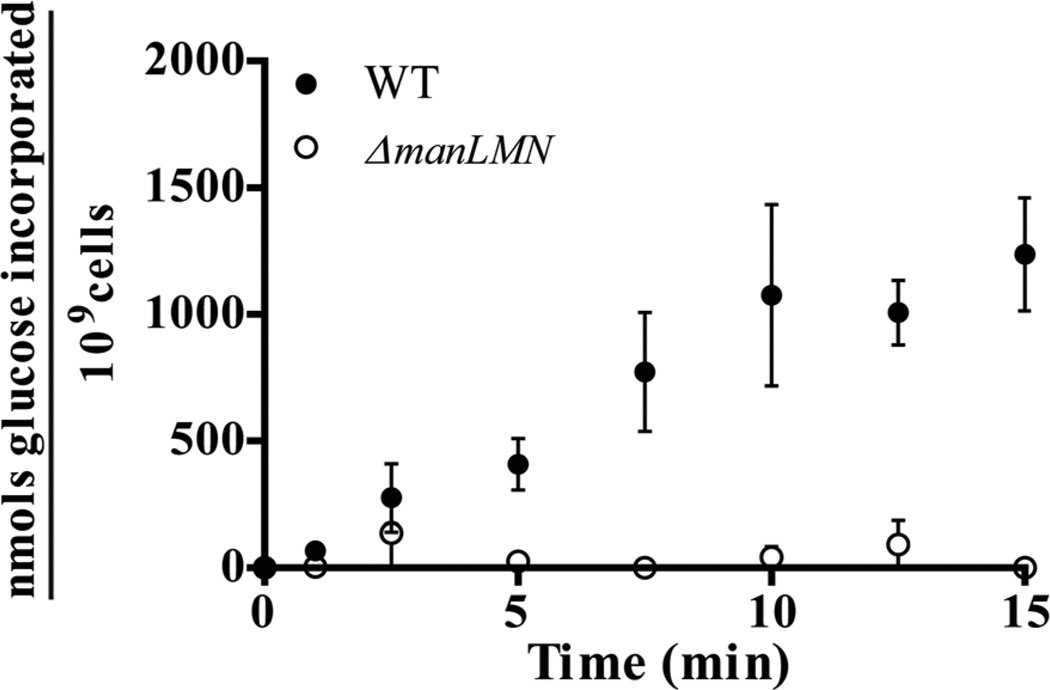
To more directly test if ManLMN is capable of transporting glucose, we compared glucose uptake by WT and ΔmanLMN using a radio-labelled carbohydrate transport assay in a glucose-limiting condition (0.007% glucose). Following growth in glucose rich medium, WT transported glucose with an appreciable rate, whereas glucose transport was undetectable in ΔmanLMN (Fig. 9). These results support the conclusion that ManLMN recognizes glucose as a substrate and has a relatively high affinity for glucose compared to the other glucose transporter(s) expressed under these conditions.
Fig. 9.
The ΔmanLMN strain is deficient at glucose transport in a glucose limiting condition. Glucose incorporation by WT (closed circles) and ΔmanLMN (open circles) was measured using a radiolabelled-carbohydrate incorporation assay. Mid-exponential phase glucose-grown cells were washed before exposure to a mixture of 400 µM unlabelled glucose plus H3-labelled glucose at 37°C. Accumulation of the H3-labelled glucose was determined in filtered samples over time according by standard liquid scintillation method. Values are reported as total nmols of carbohydrate accumulated/109 cells by accounting for the concentration of unlabelled carbohydrate and the colony forming units in each reaction. Each point is the average of five biological replicates collected on multiple days, and error bars are the standard error of the mean.
In other species, the EIIA and/or EIIB components of major glucose PTS transporters have been shown to have CCR-regulatory activities (Abranches et al., 2003; Aké et al., 2011). The manL gene encodes the dual domain EIIAB protein of the ManLMN transporter. To determine if ManL fulfills a function independently of the rest of this system, we tested if a ΔmanLMN strain having manL complemented in trans was sufficient to rescue growth in nonpreferred carbohydrates and restore induction of the α-galactosidase reporter strains. In all cases, we found ManL was insufficient to restore these phenotypes (Supporting information Fig. S6 and data not shown).
ManLMN’s high affinity for glucose may be crucial for CCR
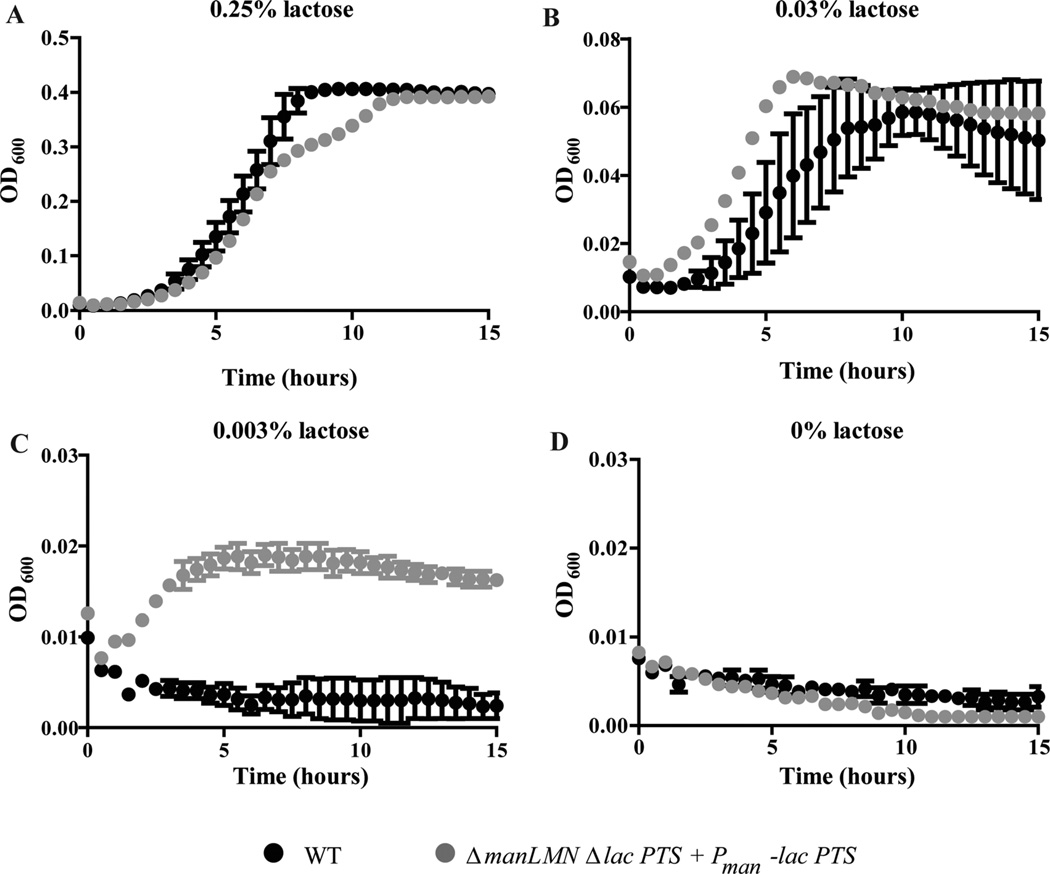
Thus far we have demonstrated that ManLMN is required for growth and induction of some metabolic genes in a variety of nonpreferred carbohydrate replete conditions. We have also shown that ManLMN is required for transport and growth in glucose-limited but not glucose-replete conditions. By constitutively expressing the lac PTS with and without ManLMN present, we showed that ManLMN is also required for lactose inducer exclusion. One model which unifies these growth, induction, transport and CCR phenotypes is that ManLMN is a multisubstrate transporter in TIGR4 as it is in related species. We hypothesize that by having a higher affinity for glucose over nonpreferred carbohydrate substrates such as lactose, ManLMN may be able to selectively transport glucose, thereby contributing to CCR. As a rudimentary way of testing this hypothesis, we compared growth of WT to the lac PTS constitutively expressed strains (ΔmanLMN Δlac PTS Pman-lac PTS, and Δlac PTS Pman-lac PTS) on decreasing concentrations of lactose. Strikingly, while WT showed a markedly decreased growth rate at 0.03% lactose and failed to grow at 0.003% lactose, the strains constitutively expressing the lac PTS were able to grow in all conditions tested except for 0% lactose (Fig. 10A–D and Supporting information Fig. S4B–E). These results suggest that constitutive expression of the lac PTS enables detection of low lactose concentrations that WT (which according to this model is relying on ManLMN) otherwise cannot detect. This result is in contrast to our finding that WT grows robustly in low glucose conditions, provided ManLMN is constitutively expressed. Although these results are consistent with a model in which ManLMN has a higher affinity for glucose than for lactose, ManLMN’s ability to transport non-glucose substrates has yet to be shown in S. pneumoniae.
Fig. 10.
The ΔmanLMN strain constitutively expressing the lac PTS has a heightened ability to grow in limiting lactose. Growth of WT and the lac PTS constitutive expressing strain (ΔmanLMN Δlac PTS + lac PTS complemented in trans) was compared in CDM containing the following growth-limiting concentrations of lactose; 0.25% (A), 0.031% (B), 0.003% (C) and 0% (D). Both strains were tested at least six times over the course of multiple days. Each data point represents the average of three biological replicates from a representative day and error bars represent the SEM from a representative experiment.
A selection for suppressors identifies factors that relieve dependence on ManLMN
We showed a ΔmanLMN strain constitutively expressing the lac PTS cannot inhibit lactose transport (inducer exclude) in the presence of glucose (Fig. 7). This phenotype did not result merely from constitutive expression of the lac PTS, but rather from constitutive expression of this PTS in the absence of ManLMN, since the Δlac PTS Pman-lac PTS strain which still has ManLMN was able to inducer exclude lactose (Supporting information Fig. S4A). Accordingly, we hypothesize that glucose transport by ManLMN has a regulatory influence on the activity of non-glucose transporters. We chose to use a selection for suppressors to address this hypothesis. By identifying mutations that relieve the necessity of ManLMN for growth on nonpreferred carbohydrates, we anticipated we would uncover factors that regulate the activity and/or expression of non-glucose transporters.
For the selection, approximately 108 cells of glucose-grown ΔmanLMN were plated on CDM agar plates with 0.5% final concentration of galactose, lactose, GlcN, GlcNAc or fructose. Suppressor colonies were picked 2–5 days after plating, colony purified and tested for their ability to grow in the selection condition. An average of six suppressors from each condition were whole genome sequenced to identify the location of mutation(s) contributing to the growth phenotype.
A complete list of the mutations identified is available in Supporting information Table S2. The vast majority of suppressor mutants sequenced had more than one polymorphism compared to its parental ΔmanLMN strain. In order to predict which mutation(s) are responsible for rescuing growth of ΔmanLMN, we compiled a list of all genes and operons that were mutated in at least two independent isolates. The location, frequency and predicted consequence of these mutations are listed in Table 1. The majority (~60%) of these reoccurring mutations are expected to activate the expression or activity of carbohydrate transporters including the GlcNAc/gal PTS and the lac PTS. The prevalence of these mutations and the ability of the corresponding isolates to grow on GlcNAc/galactose and galactose/lactose corroborates our previous results, and suggests our selection was technically sound.
Table 1.
Reoccurring suppressor mutations.
| Gene and/or operon | Genes hit | Encoded protein | Consequence of mutation |
Selection carbohydrate(s) |
|---|---|---|---|---|
| SP_0058-SP_0063* | SP_0058 | SP_0058, repressor of GlcNAc/gal PTS operon |
Ile9Arg | GlcNAc |
| Upstream of SP_0060 |
Upstream of GlcNAc/gal PTS system |
Disruption of regulation site? |
GlcNAc | |
| SP_0148-0152 | SP_0151 | ABC transporter ATP- binding domain |
Val323fs | Galactose |
| Gln97His | Fructose | |||
| SP_0152 | ABC transporter permease | Trp145* | Fructose | |
| SP_0451 | SP_0451 | Hypothetical small protein | −35 mutation (TTGACA–>TTGAAA) |
GlcNAc |
| −35 mutation (TTGACA–>TTGATA) |
GlcNAc | |||
| promoter | Start codon (ATG–> ATT) |
GlcNAc | ||
| Glu63* | GlcNAc | |||
| SP_0576-0578* | SP_0576 | LicT transcriptional anti- terminator |
Pro117Leu | GlcN |
| Tyr266Asp | GlcN | |||
| SP_0577 | β-Glucoside specific PTS system EIIABC |
Met107Ile | GlcNAc | |
| His534Tyr | GlcN (2) | |||
| His513Asn | GlcN | |||
| Ser471* | Fructose | |||
| SP_0875-0877* | SP_0877 | fru PTS system EIIABC | Phe44Ser | Fructose |
| Gly73Cys | Fructose | |||
| Gly177Val | Fructose | |||
| Ala223Asp | Fructose | |||
| Ala484Asp | Fructose | |||
| Ser442* | Fructose | |||
| SP_0927-0939* | SP_0927 | SmrC; LysR family tran- scriptional regulator |
Glu20Lys | GlcNAc, lactose (2) |
| Pro103Leu | GlcN | |||
| Ser124Leu | Lactose (2) | |||
| Ser138Ile | GlcN | |||
| Thr202Ile | Lactose | |||
| Thr245Ile | Galactose, lactose, fructose |
|||
| SP_1184-1187 | Upstream | Upstream of lac PTS sys- tem operon |
Disruption of regulation site? |
Galactose |
| SP_1185 | lac PTS system EIIBC | Val466Gly | Galactose | |
| SP_1186 | lac PTS system EIIA | Ala31Asp | Galactose | |
| Ala53Asp | Galactose | |||
| SP_1895-1898* | SP_1895 | Raffinose ABC transporter permease |
Gly48Val | Fructose |
| SP_1895 | 5’ 10 bp deletion - frameshift |
Fructose | ||
| SP_2173-2176 | SP_2173 | Lipoteichoic acid synthesis enzyme DltD |
C–>T bp change 2bp upstream of start |
Galactose (2) |
| SP_2176 | Lipoteichoic acid synthesis enzyme DltB |
Glu67* | Galactose |
This chart shows all genes and/or operons that had a suppressor mutation in at least two independent isolates. The gene number, encoded protein, the consequence of the mutation and the carbohydrate selection condition are all listed. References for gene functions are listed in Supporting information Table S2 which provides the complete list of all suppressor mutations identified.
Signifies genes and/or operons that had suppressor mutations in both ΔmanLMN backgrounds; manLMN::CHESHIRE and manLMN::spec.
Mutations in smrC, encoding the LysR-family transcriptional regulator, SmrC, were the second most common type of mutation (Table 1). SmrC is considered to be a critical virulence regulator that is highly active during infections in mice (Lau et al., 2001; Mahdi et al., 2013, 2014). Mutations in smrC arose from selection on multiple carbohydrates suggesting SmrC has a global regulatory influence on carbohydrate metabolism. We recovered suppressors with mutations mapping to both the DNA-binding and ligand-binding domains of SmrC. It is unclear if the mutations we uncovered cause a loss or gain of function, although the lack of nonsense or frame-shift mutations suggests that a complete loss of SmrC function does not answer the selection. Neither the DNA recognition sequence nor the ligand(s) recognized by SmrC’s ligand binding domain are known.
Two hypothetical small proteins identified in suppressor screen as putative carbohydrate regulators
A small number of suppressor mutations are predicted to affect the expression or function of hypothetical proteins. From the GlcNAc condition, we recovered four suppressors with mutations mapping to SP_0451, encoding a 73 amino acid hypothetical protein (Table 1 and Supporting information Fig. S7A). The −35 signal of the predicted SP_0451 promoter was made nonconsensus by two separate mutations (Supporting information Fig. S7A). These mutations likely decrease transcription from this promoter. The missense mutation affecting the start codon is expected to abolish translation, and the internal nonsense mutation will result in a truncated product (Supporting information Fig. S7A). The SP_0451 gene and putative amino acid sequence encode no conserved domains and no homologs in other species except for some closely related Streptococci. Similar to SP_0451, we uncovered a start codon mutation in SP_1473 encoding another hypothetical protein (Supporting information Table S2). The predicted product of SP_1473 is an 83 amino acid protein that is highly homologous to YnzC, encoded by the last gene of a three-gene operon present in many Gram positive species. In B. subtilis this operon is repressed by the SOS regulator, LexA (Kawai et al., 2003). The function of YnzC in B. subtilis is not known, but YneA (encoded by first gene in the operon) is involved in preventing cell division during the SOS response (Kawai et al., 2003). Neither yneA nor the second gene in this operon are conserved in S. pneumoniae. We chose to pursue characterization of the hypothetical proteins encoded by SP_0451 and SP_1473 in order to determine their roles in carbohydrate regulation.
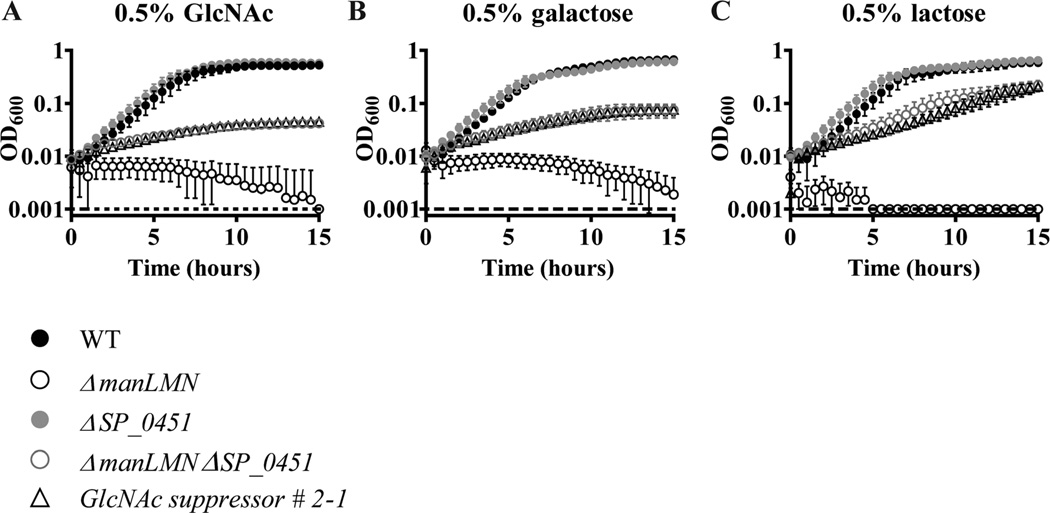
All four suppressor isolates containing a SP_0451 mutation rescued growth to a similar extent in GlcNAc medium (Supporting information Fig. S7B). This result suggests the SP_0451 mutations are responsible for the rescue phenotype. Although these suppressors were isolated in GlcNAc medium, all four showed similar abilities to rescue growth in galactose and lactose as well (Supporting information Fig. S7C and D). As the mutations occurring in this locus are expected to negatively affect expression or function of the encoded small protein, we made marked deletions of SP_0451 in the WT and ΔmanLMN backgrounds. The single deletion strain grew like WT in all conditions tested (Fig. 11A–C). As expected, deletion of SP_0451 rescued growth of ΔmanLMN in GlcNAc, galactose and lactose to a similar extent as the SP_0451 suppressor mutations (Fig. 11A–C). This result suggests the protein encoded by SP_0451 represses galactose, lactose and GlcNAc metabolism.
Fig. 11.
Inactivation of SP_0451 rescues growth of ΔmanLMN. Growth of ΔmanLMN ΔSP_0451 was assessed in CDM containing 0.5% galactose (A), GlcNAc (B) or lactose (C). WT and ΔmanLMN controls were included in all conditions. Each data point represents the average of at least four biological replicates collected on multiple days and error bars represent the SEM.
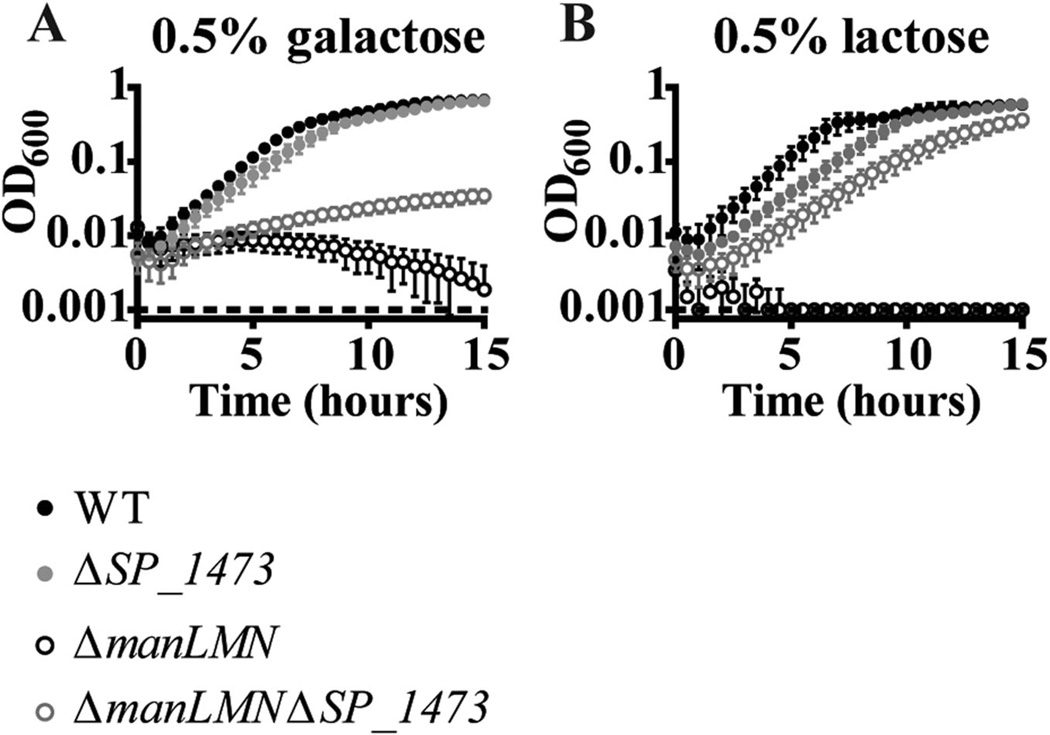
Similar results were obtained for SP_1473. The start codon suppressor mutation (galactose suppressor #1-1) was able to partially restore growth in lactose and fructose, and very mildly restored growth in GlcNAc in addition to its selection condition, galactose (Supporting information Fig. S8A–E). Deletion of SP_1473 rescued growth of ΔmanLMN in galactose and lactose media, but not in GlcNAc or fructose media (Fig. 12A and B, and data not shown). The inability to grow in GlcNAc or fructose was unexpected, but may be due to a spontaneous mutation acquired in the capsular biosynthesis gene cps4E. Mutations in Cps4E like the one we recovered (Gly125Asp) are known to result in an acapsular phenotype (Shainheit et al., 2015). We have not explored the possible connection between ManLMN, SP_1473 and capsule metabolism further.
Fig. 12.
Inactivation of SP_1473 rescues growth of ΔmanLMN. Growth of ΔmanLMN ΔSP_1473 was assessed in CDM containing 0.5% galactose (A) or lactose (B). WT and ΔmanLMN controls were included in all conditions. Each data point represents the average of at least four biological replicates collected on multiple days and error bars represent the SEM.
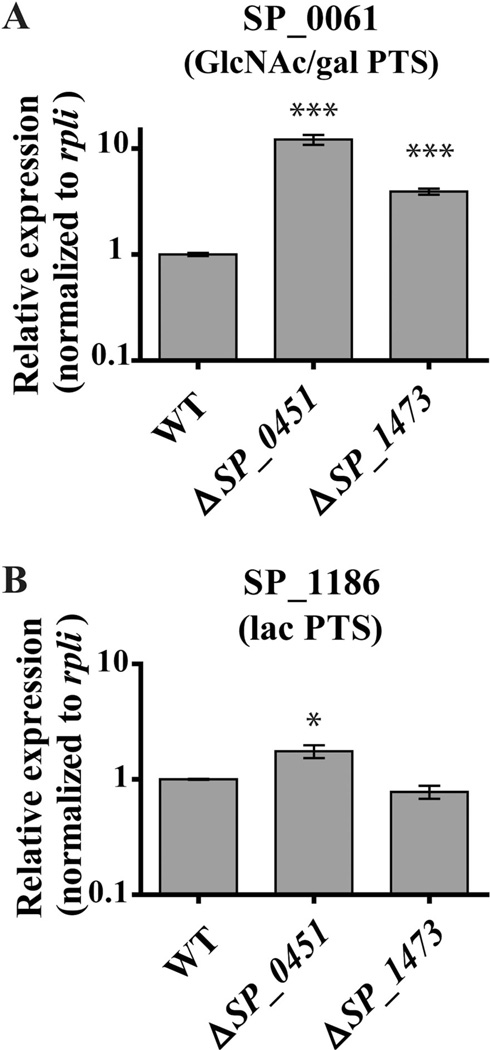
The ability of the ΔmanLMN ΔSP_0451 and ΔmanLMN ΔSP_1473 strains to grow in multiple nonpreferred carbohydrate conditions suggests SP_0451 and SP_1473 have pleiotropic effects on carbohydrate metabolism. Based on the prevalence of carbohydrate transporter mutations in our suppressor isolates, we hypothesized that the proteins encoded by SP_0451 and SP_1473 may function to diminish the expression and/or activity of non-glucose carbohydrate transporters. The effect could be transcriptional or posttranscriptional. To test the former possibility, we compared transcript levels of the GlcNAc/gal and lac PTS system genes in WT, ΔSP_0451 and ΔSP_1473 in the glucose-based rich medium, THY, which is a condition in which non-glucose transporters are robustly repressed (Kaufman and Yother, 2007; Carvalho et al., 2011; Fleming et al., 2015). Both ΔSP_0451 and ΔSP_1473 showed significant de-repression of the GlcNAc/gal PTS operon (Fig. 13A). This result corroborates our previous analysis of the ΔmanLMN ΔSP_0451 strain, and suggests that deletion of SP_0451 rescues growth of ΔmanLMN in GlcNAc and galactose by transcriptionally de-repressing the GlcNAc/gal PTS operon. Although ΔSP_1473 also resulted in de-repression of this PTS operon, the magnitude was less than that seen in the ΔSP_0451 strain, which may explain why the ΔmanLMN ΔSP_1473 strain cannot grow on GlcNAc (Supporting information Fig. S8).
Fig. 13.
PTS system expression is de-repressed in ΔSP_0451 and ΔSP_1473. Transcription of SP_0061 as a representative of the GlcNAc/gal PTS (A) and SP_1186 as a representative of the lac PTS (B) was assessed by qRT-PCR during mid-exponential growth of WT, ΔSP_0451 and ΔSP_1473 in THY. The average of at least three biological replicates normalized to rplI and relative to the WT sample, is graphed for each. Error bars represent the SEM. * indicates P value ≤ 0.05, and *** indicates P value ≤ 0.001.
Deletion of SP_0451 resulted in modest but significant de-repression (~twofold) of the lac PTS (Fig. 13B). Given that the lac PTS likely has a high affinity for lactose, it is plausible that twofold de-repression of this system is sufficient to jump-start growth in lactose medium. Deletion of SP_1473 did not affect transcription of the lac PTS (Fig. 13B). It is possible that SP_1473 represses additional PTS genes for these carbohydrates that were not examined. These results suggest that SP_0451 and SP_1473 function primarily as transcriptional repressors, although whether direct or indirect is not known.
Discussion
Numerous studies have established a strong link between carbohydrate metabolism and pneumococcal persistence and virulence (Hava and Camilli, 2002; Chapuy-Regaud et al., 2003; Hava et al., 2003; King et al., 2004; Iyer et al., 2005; Iyer and Camilli, 2007; Dalia et al., 2010; Limoli et al., 2011; Marion et al., 2011; van Opijnen and Camilli, 2012; Singh et al., 2014). In all stages of colonization and invasive disease, fermentation of host-derived carbohydrates likely represents the primary mode of energy generation for S. pneumoniae (Lamblin et al., 2001; King et al., 2006; Burnaugh et al., 2008; Yesilkaya et al., 2008, 2009; King, 2010; Buckwalter and King, 2012). All S. pneumoniae serotypes examined encode a great deal of machinery anticipated to aid in harvesting and metabolizing these carbohydrates (Tettelin et al., 2001; Bidossi et al., 2012). This includes a preponderance of predicted carbohydrate transporters (Tettelin et al., 2001; Bidossi et al., 2012). Despite encoding 29 known and predicted carbohydrate transporters, we found a single PTS, ManLMN, to be essential for growth of TIGR4 S. pneumoniae in five nonpreferred carbohydrates under the conditions tested. These observations support a model in which ManLMN acts as a constitutively expressed environmental carbohydrate surveyor, whose activity is required for subsequent induction of downstream metabolic genes and possibly other transporters. It is notable that some of these carbohydrates are among the most prevalent in host airway glycoconjugates (Lamblin et al., 2001; King et al., 2004, 2006; Monzon et al., 2006; Burnaugh et al., 2008; Buckwalter and King, 2012). This circumstance suggests that ManLMN plays a critical role in colonization and lung infection. Indeed, inactivation of manL was shown to be detrimental to nasopharyngeal colonization and lung invasive disease in mice (van Opijnen and Camilli, 2012). We demonstrate that induction of multiple operons involved in the metabolism of these carbohydrates is abolished in a ΔmanLMN strain. We further show that this dependence on ManLMN for growth can be relieved for particular carbohydrates by constitutively expressing other, cognate PTS systems encoded in the genome. Together these results suggest that ΔmanLMN’s inability to grow in these nonpreferred carbohydrate conditions is the result of failure to induce expression of required metabolic enzymes, potentially including other carbohydrate transporters.
We showed ManLMN was required for transport and growth in growth-limiting glucose concentrations, but was dispensable for growth in excess glucose. This result suggests ManLMN functions as a high affinity glucose transporter in S. pneumonaie. In contrast, a strain constitutively expressing the lac PTS in place of manLMN displayed a heightened ability to detect growth-limiting lactose concentrations compared to WT (in which manLMN is constitutively expressed). We found this strain constitutively expressing the lac PTS in the absence of ManLMN to be unable to inducer exclude lactose (evidenced by induction of β-galactosidase activity). Loss of CCR in this strain is likely not because of an inability to detect the glucose as a nonlimiting amount of glucose was used. Interestingly, constitutive expression of the lac PTS in the presence of ManLMN had minimal effect on lactose inducer exclusion, suggesting ManLMN is required for CCR of lactose metabolism. Adding to our model, we propose ManLMN serves both as a conduit for multiple carbohydrate-inducers, and a critical CCR-regulator. The relative affinity ManLMN has for each of its substrates could determine the hierarchy of most-preferred to least-preferred carbohydrate.
By selecting for suppressor mutations that relieve the dependence on ManLMN for growth on nonpreferred carbohydrates, we reasoned we could elucidate mechanisms through which ManLMN influences CCR. As expected, we recovered many suppressors with putatively activating mutations in predicted or previously characterized carbohydrate transporters or their regulators, including the GlcNAc/gal PTS, a β-glucoside PTS, the lac PTS, the fru PTS, the raffinose ABC transporter and a GntR-family regulator (SP_0058) of the GlcNAc/gal PTS operon (Fleming et al., 2015; Leprohon et al., 2015). The SP_0058-encoded repressor was previously shown to participate in CCR of this operon, suggesting our selection was suitable for identifying factors involved in CCR (Fleming et al., 2015).
We isolated four suppressors on galactose medium with mutations in or near the lac PTS operon. One of these mutations mapped to the region between the predicted promoter and the first gene of gene of this operon, potentially increasing transcription by altering a regulatory site. Interestingly, the three remaining mutations occurred within the coding regions of the lac PTS components, EIIAlac and EIICBlac. Two of these mutations are expected to result in aspartic acid substitutions of two different well conserved alanine residues in EIIAlac (Ala31Asp and Ala53Asp). We hypothesize these are gain of function mutations, however, they are not proximal to the active, phosphorylation, metal coordination or trimer stabilization sites in EIIAlac. It is possible these mutations increase transport through the lac PTS by improving the interaction of EIIAlac with the PTS-active form of HPr (HPr-His~P), or increase stability of the EIIAlac protein. The final mutation in this operon is expected to cause a glycine substitution of a conserved valine residue in the EIIB domain of EIICBlac (Val466Gly). Val466 is proximal to the EIIB phosphorylation site suggesting this mutation may improve the phosphorylation dynamics between EIICBlac and EIIAlac and/or EIICBlac and its carbohydrate substrate. It is also possible that one or more of the sites mutated in EIIAlac and EIICBlac are critical for a currently uncharacterized method of direct CCR-inhibition of this lac PTS. Overall, we hypothesize these mutations increase the activity or stability of the lac PTS.
Similar to the lac PTS, the multiple mutations we recovered in the fru PTS are anticipated to cause amino acid substitutions in all domains of the encoded multidomain fructose-type PTS, EIIABCfru. Although we could not definitively show that this system transports fructose, the fact that suppressor isolates with fru PTS mutations can grow on fructose and the frequency with which these mutations arose in fructose medium suggest that the encoded transporter is involved in fructose transport. Unlike all the other PTS systems identified in this selection, all six of the fru PTS suppressor mutations mapped to the coding region and not to classic transcriptional regulatory regions. The nature of the EIIABCfru mutations and the high basal activity in our fruRCA promoter α-galactosidase transcriptional strain favour the idea that this PTS requires a specific posttranslational modification or interaction to be active. This conclusion is further supported by our observation that constitutive expression of this transporter was not sufficient to rescue growth of ΔmanLMN in fructose medium. Fructose PTS systems in a variety of species have been shown to generate fructose-1-phosphate during transport (Wen et al., 2001; Barriere et al., 2005). Another possible explanation is that the fru PTS may generate a different form of phosphorylated fructose (fructose-1-phosphate versus fructose-6-phosphate), that what is needed to restore growth to ΔmanLMN.
Suppressor mutations identified in our selection also implicated two hypothetical small proteins in carbohydrate regulation. We showed deletion of the genes encoding these proteins partially rescued growth of ΔmanLMN in multiple carbohydrate conditions. Transcriptional analysis suggested these small proteins repress expression of carbohydrate transporters, however, these results do not fully explain the growth phenotypes of these strains. It will be interesting to explore these proteins further to determine what other genes they regulate, and the mechanism of their regulation.
PTS transporters in other species have been shown to act as carbohydrate sensors. Perhaps the most well studied example is PTSMPo in Listeria monocytogenes. PTSMPo serves as a constitutively expressed glucose sensor, and is responsible for inducing expression of manLMN, encoding the major glucose transporter in this species (Aké et al., 2011). In this system, EIIBMPo has a regulatory influence over ManR, the transcriptional activator of the manLMN operon. Our growth, transcriptional induction, transport and CCR data suggest ManLMN may play a similar but more extensive role in S. pneumoniae. ManLMN’s function as a multisubstrate transporter is widely conserved in other species. Inactivation of manLMN commonly results in upregulation of other carbohydrate transporters (Vadeboncoeur et al., 1983; Vadeboncoeur and Pelletier, 1997; Abranches et al., 2003, 2006; Moye et al., 2014a,b). Streptococcus pneumoniae is set apart by its strict dependence on this transporter for growth in a multitude of carbohydrate conditions. It remains to be seen, however, if ManLMN functions as a multisubstrate transporter in S. pneumoniae. ManLMN undoubtedly has a profound effect on nonpreferred carbohydrate metabolism whether it be due to its transport and/or regulatory activities.
There is a precedence for centralized modes of carbohydrate transport regulation in S. pneumoniae. Many serotype strains encode a number of ABC carbohydrate permeases that apparently lack an ATP-binding cassette protein (Marion et al., 2011; Bidossi et al., 2012). It was shown that a single ATP-binding cassette protein, MsmK, was capable of powering transport though multiple ABC permeases (Marion et al., 2011). ManLMN’s effect on growth and regulation in nonpreferred carbohydrate conditions is reminiscent of MsmK’s role in ABC transport. Both systems are amenable to instigating CCR of nonpreferred carbohydrate transporters. By sequestering MsmK and/or prioritizing its interaction with a particular ABC permease, nonpreferred carbohydrate transport could be restricted when needed. Similarly, we imagine ManLMN’s high affinity for glucose and its effect on other carbohydrate transporters (such as the lac PTS) will enable prioritization of preferred carbohydrate transport.
It is noteworthy that MsmK was shown to be essential for transport of sialic acid; another prevalent carbohydrate on human airway glycoconjugates. Together ManLMN and MsmK may play central roles in regulating carbon source utilization by S. pneumoniae within its natural niche, the human nasopharynx. Both the diversity and low abundance of free carbohydrates in the nasopharynx likely favour multifunctioning carbohydrate transporters that are inherently cost-saving yet able to enact wide-sweeping metabolic regulation, such as CCR.
Experimental procedures
Bacterial strains and culture conditions
Unless otherwise noted, S. pneumoniae TIGR4 (serotype 4) and isogenic mutants (Table 2) were grown at 37°C and 5% carbon dioxide in Todd Hewitt broth with 0.5% yeast extract (THY; BD Biosciences), chemically defined medium with varying carbohydrates [CDM (Kloosterman et al., 2006)] or on 5% sheep’s blood agar plates (Northeast laboratory services). All liquid culture conditions were supplemented with 300 U/ml of catalase (Worthington Biochemicals). Antibiotics were used at the following concentrations for selection of mutants: chloramphenicol (4 µg ml−1), spectinomycin (200 µg ml−1), and erythromycin (0.1 µg ml−1).
Table 2.
Strains used in the study.
| Streptococcus pneumoniae strain | Genotype | Reference |
|---|---|---|
| Wild type | TIGR4 | Laboratory strain |
| ΔmanLMN | manLMN::spec | This study |
| ΔmanLMN | manLMN::CHESHIRE | This study |
| ΔmanLMN complemented in trans |
manLMN::CHESHIRE with manLMN promoter and cod- ing region cloned into the SP_1773 locus |
This study |
| ΔmanLMN, manL complemented in trans |
manLMN::CHESHIRE with manL promoter and coding region cloned into the SP_1773 locus |
This study |
| ΔGlcNAc/gal PTS | SP_0061-3::cat | This study |
| Δlac PTS | SP_1185-6::cat | This study |
| Δfru PTS | SP_0877::cat | This study |
| ΔciaR | ciaR::spec | This study |
| ΔccpA | ccpA::cat | Laboratory strain |
| PlacA - agaA | TIGR4, lacA promoter region inserted upstream of agaA start codon, catR |
This study |
| PnagB - agaA | TIGR4, nagB promoter region inserted upstream of agaA start codon, catR |
This study |
| PfruR - agaA | TIGR4, fruRCA promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN PlacA - agaA | ΔmanLMN, lacA promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN PnagB - agaA | ΔmanLMN, nagB promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN PfruR - agaA | ΔmanLMN, fruRCA promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN complemented in trans PlacA - agaA | ΔmanLMN complemented in trans, lacA promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN complemented in trans PnagB - agaA |
ΔmanLMN complemented in trans, nagB promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN complemented in trans PfruR - agaA | ΔmanLMN complemented in trans, fruRCA promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN, manL complemented in trans PlacA - agaA |
ΔmanLMN, manL complemented in trans, lacA promoter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN, manL complemented in trans PnagB - agaA |
ΔmanLMN, manL complemented in trans, nagB pro- moter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMN, manL complemented in trans PfruR - agaA |
ΔmanLMN, manL complemented in trans, fruRCA pro- moter region inserted upstream of agaA start codon, catR |
This study |
| ΔmanLMNΔGlcNAc/gal PTS | manLMN::spec, SP_0061-3::cat | This study |
| ΔmanLMNΔlac PTS | manLMN::spec, SP_1185-6::cat | This study |
| ΔmanLMNΔfru PTS | manLMN::spec, fruA::cat | This study |
| ΔmanLMNΔGlcNAc/gal PTS, GlcNAc/gal PTS complemented in trans |
manLMN::spec, SP_0061-3::cat Pman-SP_0061-3 cloned into the SP_1773 locus |
This study |
| ΔmanLMNΔlac PTS, lac PTS complemented in trans |
manLMN::spec, SP_1185-6::cat Pman-SP_1185-5 cloned into the SP_1773 locus |
This study |
| ΔmanLMNΔfru PTS, fru PTS complemented in trans |
manLMN::spec, fruA::cat Pman-fruA cloned into the SP_1773 locus |
This study |
| ΔSP_0451 | SP_0451::spec | This study |
| ΔSP_1473 | SP_1473::spec | This study |
| ΔmanLMNΔSP_0451 | manLMN::CHESHIRE, SP_0451::spec | This study |
| ΔmanLMNΔSP_1473 | manLMN::CHESHIRE, SP_1473::spec | This study |
Generation of mutants
All strains used in this study are listed in Table 2. Marked deletion strains were created by transforming WT or other strains with a PCR product encoding the desired mutation. The manLMN unmarked deletion strain was created by natural transformation using the CHESHIRE cassette system, as described previously (Bricker and Camilli, 1999).
Complementation in trans of PTS deletions was achieved using the CHESHIRE cassette system. In all cases, the neutral locus SP_1773 was replaced with manLMN, manL only or the genes encoding one of the predicted substrate specific PTS systems (lactose; SP_1185-1186, GlcN/GlcNAc; SP_0061-0063 and or fructose; SP_0877) under the control of the manLMN promoter. Selection and curing of the CHESHIRE cassette were performed as described previously (Weng et al., 2009; Fleming et al., 2015).
The α-galactosidase transcriptional reporter strains were created by inserting the lacABCD, nagB or fruRCA promoter region directly upstream of the start of the agaA open reading frame. Each promoter construct consisted of the predicted −35, −10 and RBS elements from the operon of interest, any predicted regulator binding sites, an outward-reading chloramphenicol cassette with a bidirectional transcriptional terminator for selection of transformants, and regions of homology to the target locus.
Splicing by overlap extension (SOE) PCR was used to generate the DNA constructs for these purposes, as described previously (Horton et al., 1990). All primers used for SOE PCRS are listed in Supporting information Table S3. All mutations generated in this study were confirmed by Sanger sequencing by Eton Bioscience (http://www.etonbio.com).
Growth analysis
Mid-exponential THY cultures of each strain were washed once and back diluted to an optical density at 600 nm (OD600) of 0.01 in CDM with 0.5% of either glucose, galactose, lactose, GlcN, GlcNAc, fructose, mannose, raffinose, sucrose, maltose or trehalose, with catalase (300 U ml−1). For WT and the lac PTS complemented in trans strains, growth was also compared in a series of lactose concentrations ranging from 0.5% to 0%. OD600 was monitored in 96-well plates using a BioTek Synergy HT plate reader (BioTek Instruments), over the course of 15 h of incubation at 37°C and ambient CO2 levels without aeration. At least six biological replicates from at least two different days were assayed for each strain.
Expression analysis
Transcription of manL as a representative of the manLMN operon during mid-exponential growth was assessed by quantitative reverse transcriptase PCR (qRT-PCR). WT, ΔccpA and ΔciaR cells were grown to mid-exponential phase in THY. WT cells were additionally grown in CDM with one of the following carbohydrates; 0.5% glucose, galactose, lactose, GlcN, GlcNAc, fructose, mannose or raffinose. Two milliliter samples were stored at −80°C in RNAprotect cell reagent (Qiagen) according to the manufacturer’s instructions, for a minimum of 12 h before isolating total RNA by Trizol-chloroform extraction. RNA was further purified using the RNAeasy mini kit (Qiagen), supplemented with treatment with the TURBO DNA-free kit (Ambion, Life Technologies). No more than 1 µg of the purified RNA was used to template a single cycle reverse transcriptase reaction with the iScript cDNA Synthesis Kit (Bio-Rad). Reactions lacking reverse transcriptase were included for each sample to assess for residual genomic DNA contamination. The resulting cDNA was prepared for qPCR reactions in IQ SYBR green Supermix (Bio-Rad) with the primer sets listed in Supporting information Table S3. The MxP3005P real-time PCR system with MxPro qPCR software (Stratagene) was used to measure cycle threshold (Ct) values, which were subsequently corrected for primer efficiency differences before normalizing to the housekeeping gene, rplI. No less than five biological replicates from at least two separate days were tested for each carbohydrate condition. Transcription of SP_0061 and SP_1185 as representatives of the GlcNAc/gal and lac PTS systems respectively was determined in WT, ΔSP_0451, ΔSP_1473 during mid-exponential growth in THY as described above.
β-galactosidase assay
β-galactosidase assays were performed as previously described (Iyer et al., 2005). Briefly, midexponential cultures grown in 0.5% galactose CDM conditions were collected, washed, permeabilized and assayed for β-galactosidase activity by monitoring the cleavage of ONPG at absorbance 420 nm. Data reported represent the average of six biological replicates tested on at least two separate days. For analysis of temporal induction of β-galactosidase activity, mid-exponential phase cultures grown in THY were washed once and resuspended in CDM supplemented with 0.5% glucose, 0.5% lactose or both. Samples were collected and analyzed after 1 and 2 h of incubation at 37°C.
α-galactosidase assay of transcriptional fusion strains
Mid-exponential phase THY cultures were, washed once and resuspended in CDM with no added carbohydrate or CDM supplemented with the following; 0.5% galactose or 0.5% lactose for the PlacA, 0.5% GlcN or 0.5% GlcNAc for the PnagB, or 0.5% fructose for the PfruR transcriptional reporter strains. Following resuspension in CDM, samples were collected by centrifugation after 30, 60 and 120 min of incubation at 37°C, and resuspended in half the original volume of 100 mM sodium phosphate buffer (pH 7.5). The OD600 of each cell suspension was recorded, and the remaining volume of cells were permeabilized by incubating for 10 min at 37°C with Triton X-100 at a final concentration of 0.1% (w/v). The α-galactosidase activity in 10 µl of each lysate was assayed by monitoring absorbance at 405 nm as a measure of hydrolysis of p-nitrophenol α-d-galactopyranoside as described previously (Rosenow et al., 1999). Samples were taken from the original THY cultures to determine basal activity of each strain.
Radio-labelled carbohydrate uptake assays
Glucose incorporation was assayed generally as described previously but with the following modifications (Fleming et al., 2015). WT and ΔmanLMN were grown in THY to mid-exponential phase, washed twice and resuspended in CDM containing no added carbohydrate. Cell suspensions were kept on ice until the start of the experiment. The cells were added to a chilled flask containing an equivalent volume of 800 µM unlabelled glucose CDM with 1 µCi of d-glucose [C6-H3] (American Radiochemicals). In a final volume of 6.5 ml the final OD600 was 0.2 and the final unlabelled carbohydrate concentration was 400 µM (0.007% w/v). A time 0 sample was collected before the flask was put into a 37°C water bath to account for nonspecific association of radioactivity to the cells. Additional samples were collected at 1, 2.5, 5, 7.5,10, 12.5 and 15 min. Each sample was applied to a 0.45 µm nitrocellulose filter assembled on a vacuum apparatus, and washed twice with 1 ml of PBS (EMD Millipore). After drying, filters were mixed with 10 ml of Ecoscint H scintillation fluid (National Diagnostics), before measuring disintegrations per minute (DPMs) on a Beckman LS 6500 Scintillation System (Beckman). DPMs were converted to nmols of total carbohydrate incorporated per 109 cells using a DPM to µCi standard curve generated for d-glucose [C6-H3] and normalizing to the number of cells based on dilution plating of cell solutions. The graphed data represent the average value of five biological replicates tested on multiple days, with error bars representing the standard error of the mean.
Selection of ΔmanLMN suppressor mutants
Approximately 108 cells of CDM-glucose grown ΔmanLMN (both manLMN::CHESHIRE and manLMN::aad9 [Spectinomycin-resistance gene]) were plated on a CDM plate with 0.5% final concentration of one of the following carbohydrates; galactose, lactose, GlcN, GlcNAc or fructose. Cells were plated directly or following one wash in CDM with no added carbohydrate. Each plating scheme was performed in biological duplicate such that each carbohydrate condition comprised a total of four plates per strain. The plates were incubated at 37°C in 5% CO2 and suppressor colonies were picked after 2–5 days after plating. Colonies were picked and single colony purified on either the identical CDM carbohydrate plate condition or blood agar plates. The ability of each suppressor mutant to grow in the condition it was isolated from was confirmed by either passaging on CDM plates or liquid broth with the corresponding carbohydrate. Illumina DNA sequencing libraries were prepared for at least six suppressor isolates from each carbohydrate selection condition, using the Nextera XT DNA library prep kit (Illumina) according to the manufacturer’s instructions. Whole genome sequencing was performed by the Tufts University Core Facility (http://tucf-genomics.tufts.edu/) using an Illumina HiSeq 2500. Sequence reads from each isolate were mapped to the reference TIGR4 genome (accessension number NC_003028.3), and mutations were identified using CLC Genomic Workbench software (Version 6.8 or 8; CLC Bio, Denmark)
Supplementary Material
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Abranches J, Chen YYM, Burne RA. Characterization of Streptococcus mutans strains deficient in EIIABMan of the sugar phosphotransferase system. Appl Environ Microbiol. 2003;69:4760–4769. doi: 10.1128/AEM.69.8.4760-4769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J Bacteriol. 2006;188:3748–3756. doi: 10.1128/JB.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aké FMD, Joyet P, Deutscher J, Milohanic E. Mutational analysis of glucose transport regulation and glucose-mediated virulence gene repression in Listeria monocytogenes. Mol Microbiol. 2011;81:274–293. doi: 10.1111/j.1365-2958.2011.07692.x. [DOI] [PubMed] [Google Scholar]
- Barriere C, Veiga-da-Cunha M, Pons N, Guedon E, van Hijum SA, Kok J, et al. Fructose utilization in Lactococcus lactis as a model for low-GC gram-positive bacteria: its regulator, signal, and DNA-binding site. J Bacteriol. 2005;187:3752–3761. doi: 10.1128/JB.187.11.3752-3761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R, Rigali S, Wood N, Lulko AT, Kuipers OP, Titgemeyer F. Regulon of the N-acetylglucosamine utilization regulator NagR in Bacillus subtilis. J Bacteriol. 2011;193:3525–3536. doi: 10.1128/JB.00264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, Pozzi G, et al. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS One. 2012;7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker A, Camilli A. Transformation of a type 4 encapsulated strain of Streptococcus pneumoniae. FEMS Microbiol Lett. 1999;172:131–135. doi: 10.1111/j.1574-6968.1999.tb13460.x. [DOI] [PubMed] [Google Scholar]
- Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012;20:517–522. doi: 10.1016/j.tim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnaugh AM, Frantz LJ, King SJ. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J Bacteriol. 2008;190:221–230. doi: 10.1128/JB.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho SM, Kloosterman TG, Kuipers OP, Neves AR. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS One. 2011;6:e26707. doi: 10.1371/journal.pone.0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy-Regaud S, Ogunniyi AD, Diallo N, Huet Y, Desnottes JF, Paton JC, et al. RegR, a global LacI/GalR family regulator, modulates virulence and competence in Streptococcus pneumoniae. Infect Immun. 2003;71:2615–2625. doi: 10.1128/IAI.71.5.2615-2625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Wang L, Jiang YL, Bai XH, Chu J, Li Q, et al. Structural insights into the substrate specificity of Streptococcus pneumoniae β(1,3)-galactosidase BgaC. J Biol Chem. 2012;287:22910–22918. doi: 10.1074/jbc.M112.367128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia AB, Standish AJ, Weiser JN. Three surface exoglycosidases from Streptococcus pneumoniae NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect Immun. 2010;78:2108–2116. doi: 10.1128/IAI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming E, Lazinski DW, Camilli A. Carbon catabolite repression by seryl phosphorylated HPr is essential to Streptococcus pneumoniae in carbohydrate rich environments. Mol Microbiol. 2015;97:360–380. doi: 10.1111/mmi.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann A, Kovács M, Hakenbeck R, Brüuckner R. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol Microbiol. 2007;66:110–126. doi: 10.1111/j.1365-2958.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- Halfmann A, Schnorpfeil A, Müuller M, Marx P, Güunzler U, Hakenbeck R, Brüuckner R. Activity of the two-component regulatory system CiaRH in Streptococcus pneumoniae R6. J Mol Microbiol Biotechnol. 2011;20:96–104. doi: 10.1159/000324893. [DOI] [PubMed] [Google Scholar]
- Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–1405. [PMC free article] [PubMed] [Google Scholar]
- Hava DL, LeMieux J, Camilli A. From nose to lung: the regulation behind Streptococcus pneumoniae virulence factors. Mol Microbiol. 2003;50:1103–1110. doi: 10.1046/j.1365-2958.2003.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- Iyer R, Camilli A. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol Microbiol. 2007;66:1–13. doi: 10.1111/j.1365-2958.2007.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Baliga NS, Camilli A. Catabolite control protein a (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J Bacteriol. 2005;187:8340–8349. doi: 10.1128/JB.187.24.8340-8349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahreis K, Pimentel-Schmitt EF, Brüuckner R, Titgemeyer F. Ins and outs of glucose transport systems in eubacteria. FEMS Microbiol Rev. 2008;32:891–907. doi: 10.1111/j.1574-6976.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Kwon O, Lee YM, Oh DB, Lee JM, Kim S, et al. Characterization of the Streptococcus pneumoniae BgaC protein as a novel surface beta-galactosidase with specific hydrolysis activity for the Galbeta1-3GlcNAc moiety of oligosaccharides. J Bacteriol. 2009;191:3011–3023. doi: 10.1128/JB.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman GE, Yother J. CcpA-dependent and-independent control of beta-galactosidase expression in Streptococcus pneumoniae occurs via regulation of an upstream phosphotransferase system-encoding operon. J Bacteriol. 2007;189:5183–5192. doi: 10.1128/JB.00449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Moriya S, Ogasawara N. Identification of a protein, YneA, responsible for cell division suppression during the SOS response in Bacillus subtilis. Mol Microbiol. 2003;47:1113–1122. doi: 10.1046/j.1365-2958.2003.03360.x. [DOI] [PubMed] [Google Scholar]
- King SJ. Pneumococcal modification of host sugars: a major contributor to colonization of the human airway? Mol Oral Microbiol. 2010;25:15–24. doi: 10.1111/j.2041-1014.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- King SJ, Hippe KR, Gould JM, Bae D, Peterson S, Cline RT, et al. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol Microbiol. 2004;54:159–171. doi: 10.1111/j.1365-2958.2004.04252.x. [DOI] [PubMed] [Google Scholar]
- King SJ, Hippe KR, Weiser JN. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol. 2006;59:961–974. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman TG, Bijlsma JJE, Kok J, Kuipers OP. To have neighbour’s fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiology. 2006;152:351–359. doi: 10.1099/mic.0.28521-0. [DOI] [PubMed] [Google Scholar]
- Lamblin G, Degroote S, Perini JM, Delmotte P, Scharfman A, Davril M, et al. Human airway mucin glycosylation: a combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconj J. 2001;18:661–684. doi: 10.1023/a:1020867221861. [DOI] [PubMed] [Google Scholar]
- Lau GW, Haataja S, Lonetto M, Kensit SE, Marra A, Bryant AP, et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol Microbiol. 2001;40:555–571. doi: 10.1046/j.1365-2958.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- Leprohon P, Gingras H, Ouennane S, Moineau S, Ouellette M. A genomic approach to understand interactions between Streptococcus pneumoniae and its bacteriophages. BMC Genomics. 2015;16:972. doi: 10.1186/s12864-015-2134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli DH, Sladek JA, Fuller LA, Singh AK, King SJ. BgaA acts as an adhesin to mediate attachment of some pneumococcal strains to human epithelial cells. Microbiology. 2011;157:2369–2381. doi: 10.1099/mic.0.045609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca G, Chung Y, Barabote R. Catabolite repression and activation in Bacillus subtilis : dependency on CcpA, HPr, and HprK. J Bacteriol. 2005;187:7826–7839. doi: 10.1128/JB.187.22.7826-7839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi LK, Ebrahimie E, Adelson DL, Paton JC, Ogunniyi AD. A transcription factor contributes to pathogenesis and virulence in Streptococcus pneumoniae. PLoS One. 2013;8:e70862. doi: 10.1371/journal.pone.0070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi LK, Deihimi T, Zamansani F, Fruzangohar M, Adelson DL, Paton JC, et al. A functional genomics catalogue of activated transcription factors during pathogenesis of pneumococcal disease. BMC Genomics. 2014;15:769. doi: 10.1186/1471-2164-15-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion C, Aten AE, Woodiga SA, King SJ. Identification of an ATPase, MsmK, which energizes multiple carbohydrate ABC transporters in Streptococcus pneumoniae. Infect Immun. 2011;79:4193–4200. doi: 10.1128/IAI.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P, Nuhn M, Kovács M, Hakenbeck R, Brüuckner R. Identification of genes for small non-coding RNAs that belong to the regulon of the two-component regulatory system CiaRH in Streptococcus. BMC Genomics. 2010;11:661. doi: 10.1186/1471-2164-11-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Zahner D, Merai M, Balmelle N, de Saizieu AB, Hakenbeck R. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. Society. 2003;185:60–70. doi: 10.1128/JB.185.1.60-70.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzon ME, Casalino-Matsuda SM, Forteza RM. Identification of glycosaminoglycans in human airway secretions. Am J Respir Cell Mol Biol. 2006;34:135–141. doi: 10.1165/rcmb.2005-0256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye ZD, Burne RA, Zeng L. Uptake and metabolism of N-acetylglucosamine and glucosamine by Streptococcus mutans. Appl Environ Microbiol. 2014a;80:5053–5067. doi: 10.1128/AEM.00820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye ZD, Zeng L, Burne RA. Modification of gene expression and virulence traits in Streptococcus mutans in response to carbohydrate availability. Appl Environ Microbiol. 2014b;80:972–985. doi: 10.1128/AEM.03579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Opijnen T, Camilli A. A fine scale phenotype-genotype virulence map of a bacterial pathogen. Genome Res. 2012;22:2541–2551. doi: 10.1101/gr.137430.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenow C, Maniar M, Trias J. Regulation of the alpha-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system. Genome Res. 1999;9:1189–1197. doi: 10.1101/gr.9.12.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainheit MG, Valentino MD, Gilmore MS, Camilli A. Mutations in pneumococcal cpsE generated via in vitro serial passaging reveal a potential mechanism of reduced encapsulation utilized by a conjunctival isolate. J Bacteriol. 2015;197:1781–1791. doi: 10.1128/JB.02602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Pluvinage B, Higgins MA, Dalia AB, Woodiga SA, Flynn M, et al. Unravelling the multiple functions of the architecturally intricate Streptococcus pneumoniae β-galactosidase, BgaA. PLoS Pathog. 2014;10:e1004364. doi: 10.1371/journal.ppat.1004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C, Gauthier L. The phosphoenolpyruvate: sugar phosphotransferase system of Streptococcus salivarius Identification of a IIIManL protein. Can J Microbiol. 1987;33:118–122. doi: 10.1139/m87-020. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C, Pelletier M. The phosphoenolpyruvate: sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 1997;19:187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C, Bourgeau G, Mayrand D, Trahan L. Control of sugar utilization in the oral bacteria Streptococcus salivarius and Streptococcus sanguis by the phosphoenolpyruvate: glucose phosphotransferase system. Arch Oral Biol. 1983;28:123–131. doi: 10.1016/0003-9969(83)90119-x. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C, Frenette M, Lortie L. Regulation of the pts operon in low G + C gram-positive bacteria. J Mol Microbiol Biotechnol. 2000;2:483–490. [PubMed] [Google Scholar]
- Warner JB, Lolkema JS. CcpA-dependent carbon catabolite repression in bacteria. Microbiol Mol Biol Rev. 2003;67:475–490. doi: 10.1128/MMBR.67.4.475-490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Browngardt C, Burne RA. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcusmutans. FEMS Microbiol Lett. 2001;205:337–342. doi: 10.1111/j.1574-6968.2001.tb10969.x. [DOI] [PubMed] [Google Scholar]
- Weng L, Biswas I, Morrison DA. A self-deleting Cre-lox-ermAM Cassette, CHESHIRE, for marker-less gene deletion in Streptococcus pneumoniae. J Microbiol Methods. 2009;79:353. doi: 10.1016/j.mimet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968;106:847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilkaya H, Manco S, Kadioglu A, Terra VS, Andrew PW. The ability to utilize mucin affects the regulation of virulence gene expression in Streptococcus pneumoniae. FEMS Microbiol Lett. 2008;278:231–235. doi: 10.1111/j.1574-6968.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Yesilkaya H, Spissu F, Carvalho SM, Terra VS, Homer KA, Benisty R, et al. Pyruvate formate lyase is required for pneumococcal fermentative metabolism and virulence. Infect Immun. 2009;77:5418–5427. doi: 10.1128/IAI.00178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zäahner D, Hakenbeck R. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J Bacteriol. 2000;182:5919–5921. doi: 10.1128/jb.182.20.5919-5921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Burne RA. Multiple sugar: phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans. Mol Microbiol. 2008;70:197–208. doi: 10.1111/j.1365-2958.2008.06403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga M, Comas I, Linaje R, Monedero V, Yebra MJ, Esteban CD, et al. Horizontal gene transfer in the molecular evolution of mannose PTS transporters. Mol Biol Evol. 2005;22:1673–1685. doi: 10.1093/molbev/msi163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.