Abstract
Objective
Metformin reduces cancer incidence and improves overall survival in diabetic patients. In preclinical studies, metformin decreases endometrial cancer (EC) cell growth by activation of AMPK/mTOR inhibition. We sought to determine the effects of metformin on serum/tumor biomarkers in women with EC.
Methods
In this prospective trial, newly diagnosed EC patients underwent pre-treatment blood draw/endometrial biopsy, were administered oral metformin 850 mg daily for ≥7 days, and underwent post-treatment blood draw/definitive surgery. Pre- and post- serum analyses were performed. Tumor samples were evaluated for changes in AMPK, PI3K/AKT pathway, proliferation, and apoptosis by immunohistochemistry.
Results
Twenty patients completed the trial. Median age and BMI were 57 years (range: 27–67) and 34.5 kg/m2 (range: 21.9–50.0). Median duration of metformin was 9.5 days (range: 7–24). A majority of women had endometrioid adenocarcinomas (90%) and were early stage (85%). After metformin, there were significant decreases in serum IGF-1 (p=0.046), omentin (p=0.007), insulin (p=0.012), C-peptide (p=0.018), and leptin (p=0.0035). Compared to baseline, posttreatment tissue showed decreased phospho-AKT in 18/20 patients (90%, p=0.0002), decreased phospho-S6rp in 14/20 patients (70%, p=0.057), and decreased phospho-p44/42MAPK in 15/18 patients (83.3%, p=0.0038). There was no difference in Ki67, phospho-ACC, or caspase 3. Changes did not correlate with BMI, grade, or KRAS mutation.
Conclusion
In this prospective window of opportunity study, we demonstrated that relevant serum and molecular changes occur in patients with newly diagnosed EC after a short course of metformin. Ongoing clinical trials will help determine the appropriate role for metformin in the treatment of women with EC.
Introduction
Metformin is one of the most widely prescribed oral hypoglycemic agents used to treat type 2 diabetes (1). Over the last decade, a number of studies have suggested therapeutic potential for metformin in the prevention and treatment of cancer. This hypothesis was supported by the observation that metformin has the potential to reduce the incidence of cancer in diabetic patients taking metformin compared to other hypoglycemia agents (2). In an observational cohort study of diabetics, incident cancer was diagnosed among 7.3% of 4,085 metformin users compared with 11.6% of 4,085 comparators (p<0.001) (3). Since then, multiple publications have suggested that taking metformin may benefit patients with pancreatic, breast, colorectal, ovarian, and endometrial cancer (4–7).
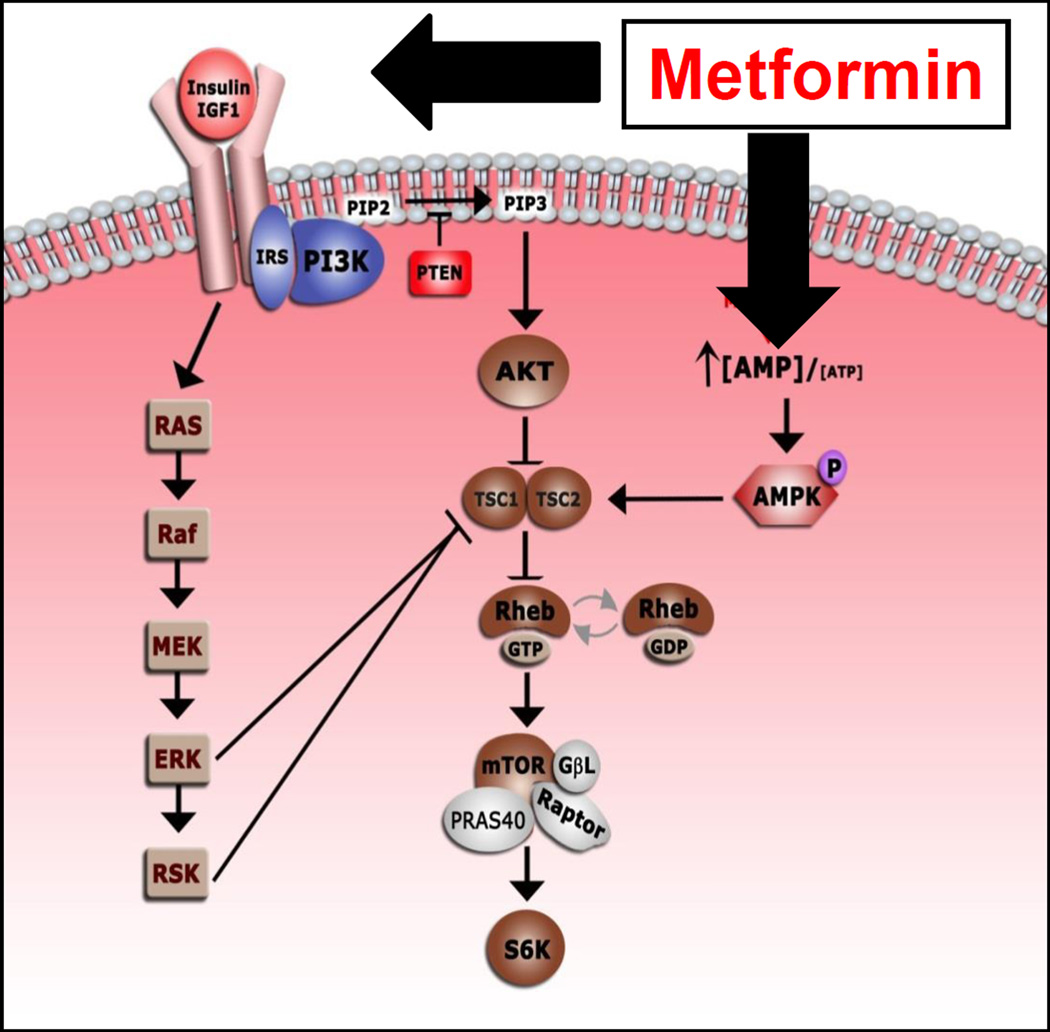
Metformin is thought to have both a direct and indirect effect on cell growth and metabolism (Figure 1). In the direct model, metformin activates AMPK, which results in phosphorylation of tuberous sclerosis 2 protein. This inhibits mTOR signaling which ultimately inhibits cell growth. Metformin also acts indirectly by increasing insulin sensitivity, increasing uptake of glucose in the cell, and subsequently decreasing circulating levels of insulin. Both insulin and IGF-1 are known factors that promote cell growth, thus, decreasing insulin would have a negative effect on cell proliferation.
Figure 1.
The direct and indirect downstream effects of metformin.
Both diabetes and insulin resistance are risk factors for endometrial cancer (8, 9). Preclinical data have shown that increasing doses of metformin were associated with a decrease in cell proliferation in several endometrial cancer cell lines (10, 11). Based on these data, the objective of our study was to determine the effects of oral metformin on the endometrial cancer cells in women with newly diagnosed endometrioid endometrial cancer. We hypothesized that treatment with oral metformin would decrease endometrial cancer cell growth by activation of AMPK and inhibition of MTOR.
Materials and Methods
After approval from the Institutional Review Board at MD Anderson Cancer Center and Lyndon Baines Johnson Hospital (LBJ), patients with newly diagnosed endometrial cancer who were candidates for definitive surgery were approached. Patients were eligible if they were a surgical candidate and had (1) histologically confirmed endometrioid adenocarcinoma, any grade, or a mixed tumor with at least an endometrioid component, (2) documented non-fasting plasma glucose level of ≤ 125 mg/dL or a fasting plasma glucose level of ≤ 125 mg/dL, (3) a creatinine clearance > 60 cc/min documented by the Cockcroft Gault formula, and (4) serum bilirubin < 2.5 mg/dL. Exclusion criteria included (1) known history of diabetes or currently taking any hypoglycemic agents, (2) use of metformin or an mTOR inhibitor in the previous 2 years, (3) prior cytotoxic or biologic therapy for endometrial cancer, or (4) any contraindication to metformin.
Once informed consent was obtained, the pre-treatment evaluation included (1) anthropometric testing including height, weight, waist circumference, and blood pressure, (2) fasting blood and urine collection, and (3) an office endometrial biopsy. Part of the tissue was flash frozen and the remaining tissue was formalin-fixed and paraffin embedded. A hematoxylin and eosin stain was performed to confirm presence of tumor tissue.
Once the baseline testing was completed, patients were started on metformin 850 mg by mouth daily for a minimum of 7 days and maximum of 30 days prior to scheduled surgery. If a patient was scheduled for CT imaging, the metformin dose was held for 48 hours after the procedure. Patients continued metformin until the day before surgery. Patients completed a drug administration and toxicity diary. Patients could be withdrawn from the study if they had > grade 3 nausea and/or diarrhea that could not be managed with medications.
Post-treatment testing was obtained on the day of surgery. This included a fasting blood and urine sample collected in the operating room after the induction of anesthesia. Care was taken to ensure that no dextrose was given prior to obtaining the samples. Post-treatment endometrial tissue was obtained from the hysterectomy specimen once it was removed from the patient and the pathologist performed the gross evaluation. Tissue samples were flash-frozen and paraffin embedded using the same technique as the pre-treatment samples.
Serum marker analysis
Serum glucose level was detected with QuantiChrom™ Glucose assay kit (BioAssay Systems, Hayward, CA). Serum markers were detected using multiplex assay from Millipore (Billerica, MA). Specifically, C-peptide, insulin, and leptin were measured with Milliplex MAP Kit Human Metabolic Hormone Magnetic Bead Panel; serum adiponectin was tested with Milliplex MAP Kit Human Adipokine Magnetic Bead Panel; IGF-1 was tested with Milliplex MAP Kit Human IGF-1 Magnetic Bead Panel. Serum omentin was measured with Human Omentin-1 ELISA kit (BioVendor, Candler, NC). All samples were measured in triplicate.
Immunohistochemistry
Paraffin-embedded sections of endometrial tumor tissue were cut at 4 µm thickness, de-paraffinized with xylene, and rehydrated using graded ethanol. Antigen retrieval using citrate buffer (PH 6.0) was performed on Lab Vision PT module (Thermo Scientific, Pittsburgh, PA). Endogenous peroxidase activity was inactivated using 3% hydrogen peroxide. After blocking with 10% horse serum, the sections were incubated in primary antibody against Phospho-S6 Ribosomal Protein (Ser235/236) (pS6p, 1:75), Phospho-Akt (Ser473) (pAkt, 1:50), Cleaved Caspase-3 (Asp175) (1:300), Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (pErk1/2, 1:400), Phospho-Acetyl-CoA Carboxylase (Ser79) (pACC, 1:50), Phosphatase and tensin homologue deleted on chromosome ten) (PTEN, 1:125) (Cell Signaling, Danvers, MA), and Ki67 (1:100, BD Pharmigen, San Jose, CA) followed by the incubation with biotinylated anti rabbit or anti mouse IgG and streptavidin-HRP (Dako, Carpinteria, CA). Diaminobenzidine solution was applied to visualize the complex and sections were counterstained with Mayer’s hematoxylin.
All staining was processed on Thermo Scientific Autostainer (Thermo Scientific, Pittsburgh, PA). To evaluate differential expression levels between pre- and post-treatment samples, the following scoring systems were used: for phospho-ACC, intensity of endometrial tumor cell cytoplasmic and membranous staining (negative, 2+, 3+) was reported; for PTEN, tumor tissue staining intensity of 2+ or above was reported as positive, otherwise was reported as negative; phospho-Akt and phospho-S6P were reported as percentage of endometrial tumor cell with staining intensity 3+. Proliferation was evaluated by Ki67 staining, scored as percentage of endometrial tumor cells with positive nuclear staining. Caspase-3 and MAPK scoring were reported as percentage of positively stained endometrial tumor cells.
KRAS mutation detection
Microdissection and DNA extraction
Approximately 1,000 endometrial tumor cells were dissected from formalin-fixed paraffin-embedded endometrial tumor tissue under stereomicroscope visualization using fine needles (25G5/8). DNA was extracted using 25 µL of Pico Pure DNA Extraction solution (Applied Biolsystems, Carlsbad, CA) containing proteinase K and incubated at 65°C for 24 hours. Subsequently, proteinase K was inactivated by heating samples at 95°C for 10 minutes.
KRAS mutation analysis
Mutations in codon 12 and 13, and exons 3, 4, 5 of KRAS were explored. DNA fragments were PCR amplified using following primers: KRAS exon 2 (for codon 12 and codon 13): KRAS-2(+): TAAGGCCTGCTGAAAATGACTG, KRAS-2(−): ATTACTGGTGCAGGACCA; KRAS exon 3: KRAS-3(+): CTGTGTTTCTCCCTTCTC, KRAS-3(−): CATGGCATTAGCAAAGACTC; KRAS exon 4: KRAS-4(+): GGTGTAGTGGAAACTAGG, KRAS-4(−): GCAATGCCCTCTCAAGAG; and KRAS exon 5: KRAS-5(+): CTTGCACATGGCTTTCCCAG, KRAS-5(−): GTTGCCACCTTGTTACC. DNA extracted from ~100 tumor cells was used for PCR amplification. Amplification was performed in 20 µL volume containing 1 µL DNA, 0.8 µL of each primer (10µM), 2 µL 10XPCR buffer, 0.2 µL Taq DNA Polymerase (Sigma-Aldrich, St. Louis, MO) and 15 µL DNase-free water. DNA was amplified for 38 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, followed by 10-minute extension at 72°C. PCR products were validated by DNA gel electrophoresis, and sequenced with Sanger Based DNA sequencing at MD Anderson Sequencing and Microarray Facility. All sequence variants were confirmed by sequencing from both directions.
Statistical analysis
Summary statistics were used to describe demographic characteristics. For the serum markers, a paired t-test with a 2-sided significance level of 0.05 was used to test whether the mean change in expression between pre- and post-treatment samples was statistically significant. For the tissue biomarkers, slides were scored independently by 2 investigators including a gynecologic pathologist (RBB) and categorized by intensity and percent positive tumor cells. A change of at least 20% positive cells was thought to be meaningful for analysis. An exact binomial test with a one-sided significance level of 0.05 was used to test whether the percent of patients with a decrease or increase (as appropriate) in biomarker expression from pre- to post-treatment was more than 50%. Logistic regression models were then used to determine if any of the significant changes in the biomarkers were associated with either obesity or histologic grade.
Results
Between October, 2010 and June, 2013 twenty-one patients were enrolled in the study. One patient was non-compliant with the metformin due to dizziness and was deemed non-evaluable. The remaining 20 cases tolerated therapy without side effects and were included in the analysis. The demographic characteristics for the patients are described in Table 1. Median age was 56.5 years (range 27 – 67). Median body mass index (BMI) was 34.5 kg/m2 (range 21.9 – 50.0). Median duration of metformin treatment was 9.5 days (range 7 to 24). Grade 1 and 2 endometrioid histology were the most common comprising 35% and 45%, respectively. A majority of patients were ultimately diagnosed with early stage (IA, IB) disease (17/20, 85%).
Table 1.
Demographic Characteristics
| N = 20 | |
|---|---|
| Median Age | 56.5 years (27 – 67) |
| Median BMI | 34.5 kg/m2 (21.9 – 50.0) |
| Median waist circumference | 109 cm (80 – 156) |
| Duration of treatment | 9.5 days (7 – 24) |
| Preoperative Histology | |
| Grade 1 | 7 (35%) |
| Grade 2 | 9 (45%) |
| Grade 3 | 4 (20%) |
| Stage | |
| IA | 14 (70%) |
| IB | 3 (15%) |
| IIIC | 2 (10%) |
| IV | 1 (5%) |
Table 2 includes the mean serum marker measurements for both pre- and post-treatment samples. After treatment with oral metformin, mean serum glucose increased from 91 mg/dL to 111 mg/dL (p=0.002). Serum IGF-1, omentin, insulin, C-peptide, and leptin all significantly decreased (p = 0.004 to 0.046). Mean adiponectin was unchanged between pre- and post-treatment samples.
Table 2.
Serum changes between pre- and post-treatment samples
| Serum marker |
Median (pre) |
Median (post) |
Median Change (post-pre) |
P-value* |
|---|---|---|---|---|
| Glucose | 90.1 | 101.4 | 18.8 | 0.001 |
| IGF-1 | 76,736.9 | 65,903.1 | −9,657.8 | 0.058 |
| Omentin | 372.7 | 318.8 | −50.2 | 0.012 |
| Insulin | 617.0 | 392.8 | −154.4 | 0.012 |
| C-peptide | 2,392.0 | 1,927.9 | −536.5 | 0.024 |
| Leptin | 30,487.2 | 25,493.4 | −4,863.5 | 0.003 |
| Adiponectin | 15.3 | 13.9 | −0.7 | 0.123 |
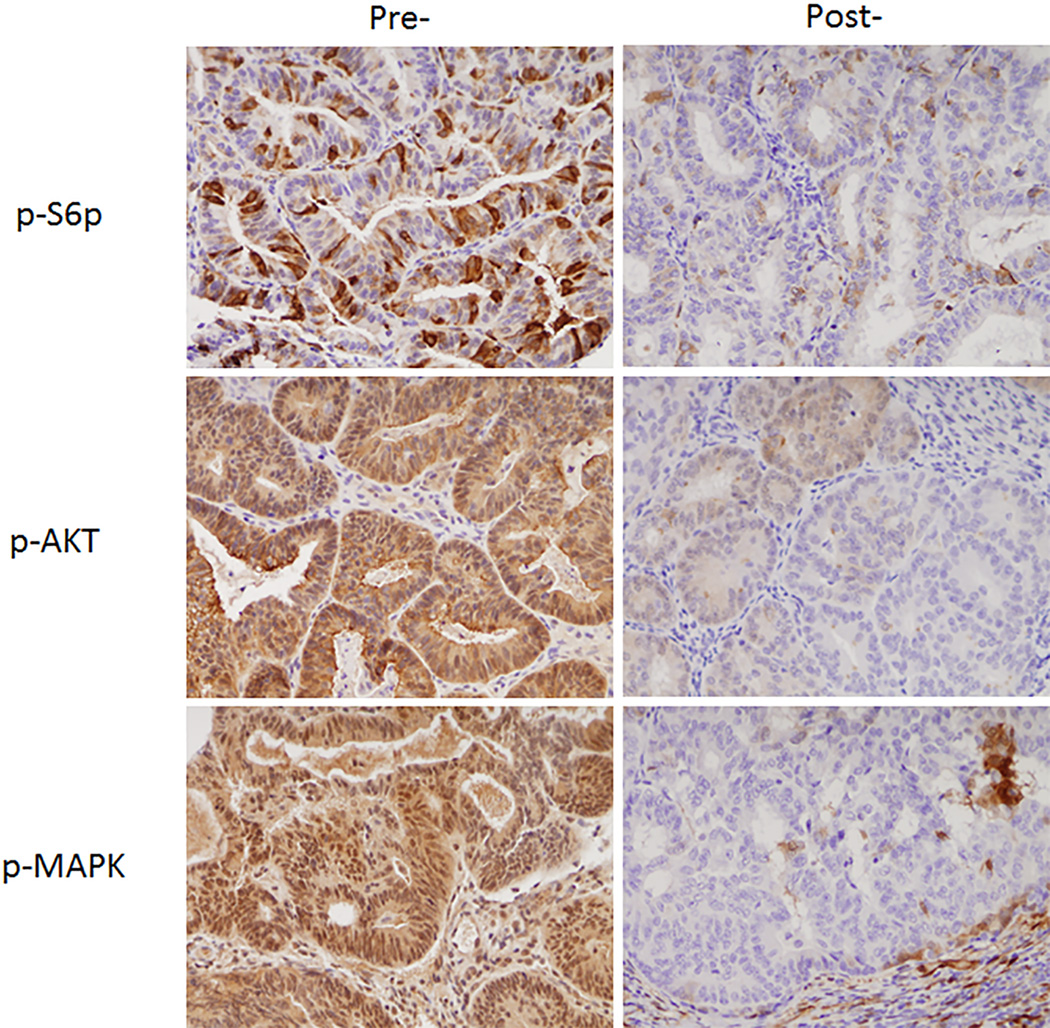
Table 3 summarizes the changes in protein expression between pre- and post-treatment tissue biopsies. Direct activation of AMPK would lead to phosphorylation and an increase in phospho-ACC. There was no significant increase from pre- and post-treatment expression of phospho-ACC (p=0.999). For phospho-S6rp, MAPK, and phospho-AKT, we would expect to see a decrease in expression after treatment with metformin. There was a decrease in phosphor-S6rp in 70% of cases, however, this was not significant (p=0.057). There was a significant decrease in MAPK expression (83%, p=0.004) and phospho-AKT expression (90%, P<0.001) between pre-and post-treatment samples. Examples of the decrease in p-S6p, phospho-AKT and MAPK immunohistochemical staining are shown in Figure 2. Finally, there was no difference in cell proliferation as measured by Ki67 (p=0.942) or apoptosis as measured by caspase 3 (p=0.979) between pre- and post-treatment samples in a majority of cases.
Table 3.
Changes in Immunohistochemical staining pre- and post-treatment
| Biomarker | Increase | No Change | Decrease | P-value |
|---|---|---|---|---|
| Phospho-ACC (activation of AMPK) |
2 (10%) | 13 (65%) | 5 (25%) | 0.979 |
| Phospho-S6rp | 1 (5%) | 5 (25%) | 14 (70%) | 0.057 |
| MAPK | 1 (5.6%) | 2 (11.1%) | 15 (83.3%) | 0.004 |
| Phospho-AKT | 1 (5%) | 1 (5%) | 18 (90%) | 0.002 |
| Ki67 (cell proliferation) | 1 (5%) | 13 (65%) | 6 (30%) | 0.942 |
| Caspase 3 (apoptosis) | 3 (15%) | 12 (60%) | 5 (25%) | 0.979 |
Figure 2.
Examples of the immunohistochemical changes in p-S6p, phosphor-AKT and MAPK in pre- and post-treatment tissue samples.
Logistic regression models were then used to determine if the statistically significant changes in the pre- and post-treatment samples were related to other factors such as obesity or tumor grade. These models revealed that there was no association between a decrease in phospho-AKT expression and BMI (p=0.60) or tumor grade (p=0.99). Similarly, there was no association between decreased MAPK expression and BMI (p=0.79). However, lower grade tumors were more likely to have a decrease in MAPK expression (p=0.001).
KRAS mutational analysis was performed on pre-treatment tissue samples. Only 2 patients (10%) were noted to have mutations in KRAS. There was no association between KRAS mutational status and changes in serum or tissue biomarkers.
Discussion
In this window of opportunity study, we showed that a short course of oral metformin results in both serum and molecular changes in the endometrial tissue of women with newly diagnosed endometrial cancer. Serum levels of IGF-1, omentin, insulin, C-peptide, and leptin all significantly decreased between pre- and post-treatment samples. In addition, phosphorylation of AKT and MAPK were significantly decreased in endometrial cancer cells after oral metformin.
Metformin has both indirect and direct effects on cell growth and metabolism. In order to better understand the anti- cancer mechanism by which metformin, we sought to evaluate both the direct and indirect effects of metformin by assessing relevant biomarkers for each of these pathways (Figure 1). Based on our findings, the indirect effects of metformin on cell growth are seen in both the serum and the tissue. As expected, we found that circulating insulin and IGF-1 were significantly decreased in the post-treatment samples. Laskov et al. also found a significant decrease in fasting plasma insulin, IGF-1 and IGFBP-1 in a similar study where patients were treated with a median of 36 days of metformin 500 mg three times daily prior to definitive surgery for endometrial cancer (12). Schuler et al. described significant differences in lipid metabolism markers among newly diagnosed endometrial cancer patients who were treated with metformin 850mg daily prior to surgery (13). In regard to the downstream tissue marker changes that would result from decreases in circulating insulin and IGF-1, we would expect a decrease in phospho-AKT and MAPK, both of which were significantly decreased in the post-treatment samples. These findings have also been shown by other authors (13).
In the direct model, metformin inhibits mitochondrial adensine-5’-triphosphate (ATP) synthesis, resulting in activation of LKB1/AMPK pathway and, ultimately, inhibition of mTOR signaling. Direct activation of AMPK would lead to the direct phosphorylation of phospho-ACC and a decrease in phospho-S6rp (downstream from mTOR). In our study, only 10% of cases showed an increase in phospho-ACC after metformin treatment. While there was a decrease in phospho-S6rp in 70% of cases, this finding may also be related to the decrease in phospho-AKT (indirect effect). It is possible that statistically significant changes in phospho-S6rp could be present with higher doses or longer metformin exposure, as was found in the Laskov and Schuler studies which showed a clear reduction in pS6 (12, 13).
Finally, we looked at markers of cell proliferation (Ki67) and apoptosis (Caspase 3) to determine if these downstream factors could be affected after a short course of oral metformin. We did not see a significant effect on cell proliferation or apoptosis. While other authors have shown a decrease in Ki67 after treatment with metformin in endometrial cancer patients, there are differences in methodology that may contribute to this discrepancy. In the Laskov study, patients were treated with higher doses (1500mg/day) for a longer period of time (median 36 days) (12). We chose a relatively short dosing schedule so that the usual time frame from diagnosis to surgical management was not affected. The dose of 850 mg per day was chosen as the dose of metformin generally has to be titrated up in order to prevent side effects. In addition, other groups who have highlighted a change in proliferation, have used any change in absolute expression to be meaningful. For our study, we required a change in 20% of protein expression to be defined as a significant change. We felt this would allow for variation in testing.
Several window of opportunity studies using metformin have also been conducted in women with breast cancer (14, 15). In the largest prospective randomized pre-surgical trial in women with breast cancer, there was no difference in Ki67 pre- and post-treatment with metformin (16). The changes in Ki67, however, did seem to be linked to the effect of metformin on the homeostasis model assessment (HOMA) index in these women. For example, among women whose HOMA index was greater than 2.8, Ki67 decreased by 10.5% after treatment with metformin. These findings suggest that metformin may have a different effect on tumor tissue based on other metabolic differences between patients.
The main strength of this study is the trial design and proof of feasibility. Endometrial cancer is an ideal cancer to study in the window of opportunity setting. Pre-treatment samples can be obtained easily during an office endometrial biopsy and can be compared to endometrial tissue from the hysterectomy after an agent is given for a period of time. This model allows for evaluation of the direct effects of a drug on endometrial tumor cells in a relatively short period of time. Other agents could be assessed in the same way to determine tissue effects before implementing large treatment protocols. In addition, this model could be used for other tumor types. We currently have protocols accruing and in development utilizing the window of opportunity model in both cervical and ovarian cancer.
The main limitations of this study were the small sample size and the short treatment interval. Because this was in part a feasibility study, one of our main goals was not to interrupt the standard treatment for women with newly diagnosed endometrial cancer. We did not want to delay definitive surgery by exposing patients to longer treatment with metformin. While we feel this may have limited the results of our study, we were still able to identify tissue and serum changes between pre- and post-treatment samples. Finally, it remains unclear how the effect of oral metformin on the serum and tissue will ultimately impact the outcome of our patients with endometrial cancer.
Although the data supporting the role of metformin in the treatment of endometrial cancer are growing, it is unclear at this point if there is an impact on overall survival (17). There are several ongoing clinical trials set out to answer this question. Metformin is being added to levonorgesterol-releasing intrauterine device in non-surgical patients with complex hyperplasia with atypia or endometrial cancer (NCT02035787). GOG286B is a randomized phase 3 study of paclitaxel and carboplatin +/− metformin in chemotherapy naïve patients with advanced stage or recurrent endometrial cancer (NCT02065687). In addition, at our institution we have a nearly completed Phase 2 study evaluating the effectiveness of everolimus, letrozole, and metformin in the treatment of advanced or recurrent endometrial cancer (NCT01797523). These studies should help us better understand how metformin can be incorporated into the treatment of women with endometrial cancer.
In this prospective window of opportunity study, we showed that a short course if oral metformin results in both serum and tissue biomarker changes in women with newly diagnosed endometrial cancer. Based on our findings, it appears that the effects of metformin are likely a result of decreased circulating insulin and insulin-like growth factors rather than the direct activation of AMPK. While we did not find direct effects of metformin or a significant effect on cell proliferation or apoptosis, it is possible that these cellular changes could occur after a longer exposure or a high dose of metformin. Ongoing clinical trials will help determine the appropriate role for metformin in the treatment of women with endometrial cancer.
Acknowledgments
This work was supported in part by American Cancer Society IRG (965712), Cancer Center Support Grant (NCI Grant P30 CA016672). National Institutes of Health K12 Calabresis Scholar Award (K12 CA 0088084) to SNW and the Endometrial SPORE (P50 CA098258), and Texas Business Women’s grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented as an oral abstract at the Society of Gynecologic Oncology Annual Meeting in 2013 in Los Angeles, California and at the American Society of Clinical Oncology Annual Meeting 2014 in Chicago, IL.
Disclosure: None of the authors have a conflict of interest.
References
- 1.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52(1):17–30. doi: 10.1007/s00125-008-1157-y. [DOI] [PubMed] [Google Scholar]
- 2.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clinical research ed. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes care. 2009;32(9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, et al. Metformin intake is associated with better survival in ovarian cancer: a case-control study. Cancer. 2013;119(3):555–562. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer prevention research. 2010;3(11):1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 8.Burzawa JK, Schmeler KM, Soliman PT, Meyer LA, Bevers MW, Pustilnik TL, et al. Prospective evaluation of insulin resistance among endometrial cancer patients. American journal of obstetrics and gynecology. 2011;204(4):355 e1–355 e7. doi: 10.1016/j.ajog.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16(2):276–280. doi: 10.1158/1055-9965.EPI-06-0751. [DOI] [PubMed] [Google Scholar]
- 10.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecologic oncology. 2010;116(1):92–98. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iglesias DA, Yates MS, van der Hoeven D, Rodkey TL, Zhang Q, Co NN, et al. Another surprise from Metformin: novel mechanism of action via K-Ras influences endometrial cancer response to therapy. Molecular cancer therapeutics. 2013;12(12):2847–2856. doi: 10.1158/1535-7163.MCT-13-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laskov I, Drudi L, Beauchamp MC, Yasmeen A, Ferenczy A, Pollak M, et al. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecologic oncology. 2014;134(3):607–614. doi: 10.1016/j.ygyno.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer medicine. 2015;4(2):161–173. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128(3):783–794. doi: 10.1007/s10549-011-1612-1. [DOI] [PubMed] [Google Scholar]
- 15.Niraula S, Dowling RJ, Ennis M, Chang MC, Done SJ, Hood N, et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135(3):821–830. doi: 10.1007/s10549-012-2223-1. [DOI] [PubMed] [Google Scholar]
- 16.Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30(21):2593–600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 17.Al Hilli MM, Bakkum-Gamez JN, Mariani A, Cliby WA, Mc Gree ME, Weaver AL, et al. The effect of diabetes and metformin on clinical outcomes is negligible in risk-adjusted endometrial cancer cohorts. Gynecologic oncology. 2015 doi: 10.1016/j.ygyno.2015.11.019. [DOI] [PubMed] [Google Scholar]