Abstract
OBJECTIVE
The comparative effectiveness of the 2 treatment options—surgical clipping and endovascular coiling—for unruptured cerebral aneurysms remains an issue of debate and has not been studied in clinical trials. The authors investigated the association between treatment method for unruptured cerebral aneurysms and outcomes in elderly patients.
METHODS
The authors performed a cohort study of 100% of Medicare fee-for-service claims data for elderly patients who had treatment for unruptured cerebral aneurysms between 2007 and 2012. To control for measured confounding, the authors used propensity score conditioning and inverse probability weighting with mixed effects to account for clustering at the level of the hospital referral region (HRR). An instrumental variable (regional rates of coiling) analysis was used to control for unmeasured confounding and to create pseudo-randomization on the treatment method.
RESULTS
During the study period, 8705 patients underwent treatment for unruptured cerebral aneurysms and met the study inclusion criteria. Of these patients, 2585 (29.7%) had surgical clipping and 6120 (70.3%) had endovascular coiling. Instrumental variable analysis demonstrated no difference between coiling and clipping in 1-year postoperative mortality (OR 1.25, 95% CI 0.68–2.31) or 90-day readmission rate (OR 1.04, 95% CI 0.66–1.62). However, clipping was associated with a greater likelihood of discharge to rehabilitation (OR 6.39, 95% CI 3.85–10.59) and 3.6 days longer length of stay (LOS; 95% CI 2.90–4.71). The same associations were present in propensity score–adjusted and inverse probability– weighted models.
CONCLUSIONS
In a cohort of Medicare patients, there was no difference in mortality and the readmission rate between clipping and coiling of unruptured cerebral aneurysms. Clipping was associated with a higher rate of discharge to a rehabilitation facility and a longer LOS.
Keywords: cerebral aneurysms, clipping, coiling, instrumental variable, Medicare, vascular disorders
Cerebral aneurysms are a common cause of intracranial hemorrhage, stroke, and death.3,8 Two treatment options are used in current practice.3,25 Surgical clipping involves a craniotomy and clip placement on the blood vessel to exclude the weakened area, whereas endovascular coiling is a minimally invasive angiographic technique in which aneurysm obliteration is achieved from within the blood vessel.3,8 Since the publication of the International Subarachnoid Aneurysm Trial (ISAT),25 which focused on ruptured aneurysms, there has been a paradigm shift in the treatment of cerebral aneurysms with an increased focus on coiling as the preferred intervention for patients with subarachnoid hemorrhage.3,30 This trend has been paralleled by similar practices for unruptured cerebral aneurysms,3 despite the lack of randomized studies in this population.
However, lessons learned in trials on ruptured cerebral aneurysms may not necessarily apply to patients presenting with intact cerebral aneurysms.3 The lack of cerebral edema in patients with the latter has been used as an argument supporting the equivalence of the 2 aforementioned techniques for elective cases.3 In addition, treatment efficacy in the context of carefully controlled clinical trials often does not translate into real-world effectiveness. However, since surgeons have readily adopted new endovascular techniques, enthusiasm for a trial studying unruptured aneurysms has been limited. Prior national retrospective studies1,2,4–7,9,11,12,15–18,20,21,24,27–29,32,33,37,38 have inconclusive results and methodological limitations, with most authors failing to appropriately adjust for measured or unmeasured confounders.
Here, we performed a national cohort study of Medicare patients with unruptured cerebral aneurysms, investigating the comparative effectiveness of clipping and coiling. The association between the treatment used and 1-year mortality, 90-day readmission, length of hospital stay, and discharge to a rehabilitation facility was examined. We used a battery of approaches to control for measured confounding, including regression adjustment, propensity score adjustment, and inverse probability weighting (IPW), as well as mixed effects methods to control for clustering at the hospital referral region (HRR) level. To control for unmeasured confounding, we used an instrumental variable (IV) approach, creating pseudo-randomization on the treatment method.
Methods
Data and Cohort Creation
The Dartmouth Committee for the Protection of Human Subjects approved this study. Data were anonymized and de-identified prior to use; therefore, no informed consent was required. We used 100% of the Medicare Denominator File and corresponding Medicare inpatient and outpatient claims, Parts A and B, for 2007–2012 (Medicare Provider Analysis and Review [MEDPAR], Carrier and Outpatient Claims) to select patients with an unruptured cerebral aneurysm diagnosis. Aneurysm patients were identified based on 1 or more inpatient or outpatient diagnoses (ICD-9-CM code 437.3) between 2007 and 2012. For cohort inclusion, patients were required to be 1) continuously enrolled in fee-for-service (FFS) Medicare Parts A and B for 12 months before the index diagnosis and 2) an age of 65 years or older at the time of the index diagnosis.
Intervention
We used ICD-9-CM codes to identify patients with unruptured cerebral aneurysms (ICD-9-CM code 437.3) who had clipping (code 39.51) or coiling (code 39.52 [should also have a code 88.41 and no 39.51 during the same hospitalization], 39.72, 39.75, 39.76, 39.79) between 2007 and 2012. For patients with multiple interventions, only the first was included in the final cohort.
Outcome Variables
The primary outcomes were 1-year and 30-day postprocedure mortality. Secondary outcomes were length of stay (LOS) during the initial hospitalization, rate of discharge to a short- or long-term care facility, and rate of 90-day postdischarge readmission.
Covariates
Age categories (65–69, 70–74, 75–79, 80–84, 85–99 years) were created, as well as 5 ethnicity and race categories (Asian, black, Hispanic, Native American, and other, with white being the excluded variable). The enrollee’s Zone Improvement Plan (ZIP) code was used to match to 2010 census data on income and poverty. We included the ZIP-level poverty rate separately from the income variable to reflect the differing distribution of income within the ZIP code.
Comorbidities diagnosed (in more than 2 outpatient and/or 1 inpatient encounter) at any time in the 12-month look back (before the intervention), for which outcomes were adjusted (Supplemental Table 1), included hypertension, myocardial infarction, cardiac arrhythmia, congestive heart failure, hyperlipidemia, coagulopathy, hypertension, ischemic stroke, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), other pulmonary disease, diabetes, obesity, alcohol abuse, malignancy, and dementia.
Each facility was identified with one of the 306 HRRs in the US as used by The Dartmouth Atlas of Health Care. An HRR is a region served by a hospital or group of hospitals that offers cardiovascular and neurosurgical procedures so that each HRR includes at least 1 tertiary care hospital. All ZIP codes in the US were assigned to an HRR on the basis of the migration patterns of hospital use among the elderly population. The coiling rate in each HRR was calculated by dividing the number of coiling procedures in an HRR by the number of total interventions for unruptured cerebral aneurysms in the same location and time period.
Statistical Analysis
To compare outcomes between coiling and clipping therapies, we used several methods to address measured confounding, 2 of which are based on propensities. To derive the propensity of clipping versus coiling, we developed a prediction model using logistic regression based on the covariates described above. To compare death at 30 days, death at 1 year, 90-day readmission, and discharge to a rehabilitation facility between coiling and clipping, we employed multivariable logistic regression, logistic regression with adjustment (stratification) by quantiles (we chose the number of quantiles as 20, the cube root of the sample size) of the propensity score, and IPW logistic regression. These models included the patient’s HRR as a random effects variable to control for clustering. As part of the sensitivity analysis, we examined these associations using a linear probability model, as well as a Poisson regression (instead of a logistic model). This did not change the significance of the results and thus is not presented further. For length of stay, we employed the corresponding versions of multiple linear regression models. For the initial calculations, outliers were not excluded since the distribution of observations was similar in the 2 groups. In a sensitivity analysis, we repeated this approach after logarithmic transformation of the LOS (which minimizes the impact of outliers) and after excluding patients with the top 5% LOS. The results were similar and are therefore not reported further.
To overcome confounding due to covariates not captured with these analyses (mainly the nonrandom selection of patients for either treatment or the size and location of aneurysms and the experience of the operator), we utilized an instrumental variable analysis.14 This analysis uses the differences in practice patterns across regions to simulate the structure of a randomized trial in an observational setting and has been used in several similar investigations. 26,35,36 The use of coiling varies widely across HRRs. Patients tend to seek care for unruptured aneurysms close to their residence. Someone who lives in an HRR in which coiling is primarily offered is more likely to receive this treatment. The IV approach depends on the assumption that HRR coiling rates affect outcomes only by promoting the use of coiling (exclusion restriction criterion), while they are otherwise unrelated to unmeasured risk factors affecting outcome. Hospital referral region coiling rates were not correlated with average predicted mortality within an HRR based on known confounders (r = 0.02, p > 0.10), suggesting a case mix balance between HRRs. A practical rule34 for employing an instrument is that the F-statistic (or chi-square for a binary exposure) for the association between the instrument and the treatment exceeds 10. This value was 941 in our study when using HRR coiling rates as an instrument for coiling. In our sensitivity analysis, we used the differential distance of the patient’s residence to facilities preferentially offering clipping versus coiling. Although the results were qualitatively the same, this second IV approach had a minimal ability to discriminate between treatments and thus resulted in high variance; therefore, it was not used further.
We subsequently calculated the odds ratio for the association between clipping and outcome by using a logistic regression model with an IV analysis in a moments-based approach, as previously described in the literature.13,19,31 The HRR coiling rate was used as an instrument for coiling, and we also adjusted for all other covariates listed above. For linear outcomes, we used a multiple linear regression model with an IV analysis, sometimes referred to as a “2-stage least squares approach.”
Finally, we plotted the survival of our cohort using a Kaplan-Meier estimator stratified for treatment technique, as well as an IPW-adjusted Kaplan-Meier.22 For the mortality outcomes, we modeled the dependence of time to death on the treatment of unruptured cerebral aneurysms by using a Cox proportional-hazards ratio analysis. Patients were censored on death and disenrollment from FFS Medicare. This model included all of the covariates listed above. We additionally used propensity score stratification and IPW to improve adjustment for known confounders and IV analysis to adjust for unknown confounders.23
Given that 6120 patients underwent coiling and 2585 underwent clipping, we had an 80% power to detect a difference in mortality as small as 1.3% at an α-level of 0.05. Patients with missing data (3% of poverty and income) were excluded from further analysis. All probability values were the result of 2-sided tests. The SAS version 9.4 (SAS Institute Inc.) and the 64-bit version of R.2.12.2 (R Foundation for Statistical Computing) were used for statistical analysis.
Results
Patient Characteristics
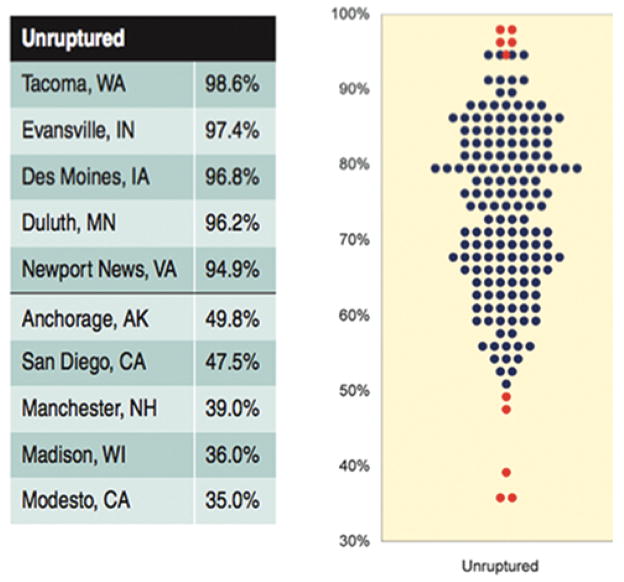
From 2007 to 2012, 8705 Medicare patients underwent treatment for unruptured cerebral aneurysms and met the study inclusion criteria. Of these patients, 2585 (29.7%) underwent surgical clipping and 6120 (70.3%) underwent endovascular coiling. The respective distribution of exposure variables between the 2 treatment methods is listed in Table 1. Figure 1 demonstrates the distribution of coiling rates per HRR.
TABLE 1.
Summary of patient characteristics*
| Parameter | Clipping | Coiling | Z Value |
|---|---|---|---|
| No. of patients | 2585 | 6120 | |
| Mean age in yrs (SD) | 70.5 (4.0) | 72.7 (5.5) | –2.3 |
| Male sex | 625 (24.2%) | 1630 (26.6%) | –18.6 |
| African American race | 169 (6.5%) | 431 (7%) | –0.8 |
| Income in 2012 US$ (SD)† | $46,900 ($17,000) | $46,000 ($17,100) | –2.3 |
| Poverty† | 233 (9%) | 483 (7.9%) | –3.7 |
| Comorbidities‡ | |||
| Hypertension | 1354 (52.4%) | 2356 (38.5%) | –0.5 |
| Hyperlipidemia | 531 (20.5%) | 1339 (21.9%) | –2.3 |
| COPD | 76 (2.9%) | 156 (2.5%) | 1.1 |
| Myocardial infarction | 328 (12.7%) | 983 (16.1%) | –3.9 |
| Cardiac arrhythmia | 139 (5.4%) | 535 (8.7%) | –5.3 |
| Coagulopathy | 19 (0.7%) | 65 (1.1%) | –1.4 |
| Renal insufficiency | 66 (2.6%) | 258 (4.2%) | –3.7 |
| Congestive heart failure | 67 (2.6%) | 309 (5.0%) | –5.1 |
| Pulmonary disease§ | 75 (2.9%) | 147 (2.4%) | 1.4 |
| Obesity | 19 (0.7%) | 46 (0.8%) | –0.01 |
| Alcohol abuse | ¶ | 14 (0.2%) | 1.3 |
| Dementia | 17 (0.6%) | 82 (1.3%) | –2.7 |
| Ischemic stroke | 263 (10.2%) | 754 (12.3%) | –2.8 |
| Diabetes | 332 (12.8%) | 918 (15.0%) | –2.5 |
| Peripheral vascular disease | 265 (10.2%) | 923 (15.1%) | –5.9 |
| Malignancy | 162 (6.3%) | 484 (7.9%) | –2.6 |
COPD = chronic obstructive pulmonary disease; SD = standard deviation.
Values represent crude numbers.
The enrollee’s ZIP code was used to match to 2010 census data on income and poverty.
Based on 12-month look back before the date of the procedure.
Non-COPD.
Output suppressed to comply with the reporting rules of Medicare, which do not allow printing of output involving less than 11 patients.
FIG. 1.
Percent of Medicare beneficiaries treated for ruptured cerebral aneurysms using coiling (2007–2012). Each dot represents 1 HRR; the higher the dot, the higher the percentage. Each blue dot represents the percent of Medicare beneficiaries treated for unruptured cerebral aneurysms with coiling in 1 of 306 HRRs in the US. Red dots indicate the regions with the 5 lowest and 5 highest rates, whose names are listed to the left. Reproduced from Bekelis K. Variation in the Care of Surgical Conditions: A Dartmouth Atlas of Health Care Series. Lebanon, NH, 2014. Published with permission. Figure is available in color online only.
Mortality
Overall, 152 deaths (5.9%) were recorded (Table 2) in the 1st year after clipping and 465 (7.6%) after coiling. As demonstrated in Table 3, clipping was associated with decreased 1-year mortality (OR 0.76, 95% CI 0.63–0.92) in the unadjusted analysis. However, adjusting for confounders with a multivariable logistic regression model revealed a lack of association between clipping and 1-year mortality (OR 1.05, 95% CI 0.86–1.29), which persisted after propensity score adjustment (OR 1.04, 95% CI 0.86–1.28) and IPW (OR 0.98, 95% CI 0.84–1.13). There was no association between treatment and mortality when using an IV analysis (OR 1.26, 95% CI 0.68–2.31). Similar associations were identified for 30-day postoperative mortality.
TABLE 2.
Outcomes*
| Outcome | Clipping | Coiling | p Value |
|---|---|---|---|
| 30-day mortality | 59 (2.3%) | 162 (2.6%) | 0.38 |
| 1-yr mortality | 152 (5.9%) | 465 (7.6%) | 0.005 |
| 90-day readmission | 605 (23.4%) | 1460 (23.8%) | 0.76 |
| Discharge to short- or long-term care facilities | 500 (19.3%) | 308 (5.0%) | <0.0001 |
| LOS in days (SD) | 7.3 (6.8) | 3.7 (5.5) | <0.0001 |
Values represent crude numbers.
TABLE 3.
Correlation between clipping and primary outcome measures
| Model | 1-Yr Mortality*
|
30-Day Mortality*
|
Mortality†
|
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | HR (95% CI) | p Value | |
| Crude | 0.76 (0.63–0.92) | 0.005 | 0.86 (0.64–1.17) | 0.338 | 0.77 (0.64–0.93) | 0.005 |
|
| ||||||
| Multivariable regression‡ | 1.05 (0.86–1.29) | 0.630 | 1.26 (0.91–1.74) | 0.156 | 1.05 (0.87–1.27) | 0.621 |
|
| ||||||
| Propensity score adjustment‡ | 1.04 (0.86–1.28) | 0.654 | 1.28 (0.93–1.76) | 0.135 | 1.05 (0.87–1.28) | 0.602 |
|
| ||||||
| IPW‡ | 0.98 (0.84–1.13) | 0.739 | 1.18 (0.93–1.50) | 0.174 | 0.99 (0.87–1.14) | 0.960 |
|
| ||||||
| IV analysis§ | 1.26 (0.68–2.31) | 0.461 | 1.65 (0.68–3.96) | 0.260 | 1.28 (0.58–2.85) | 0.538 |
Analyses based on logistic regression.
Time to event; analyses based on a Cox proportional-hazards model (limited to 1-year follow-up).
Mixed effects; includes patient’s HRR as a random effect variable.
Hospital referral region coiling rate (fraction of coiling of total procedures performed) was used as an instrument of choice of treatment.
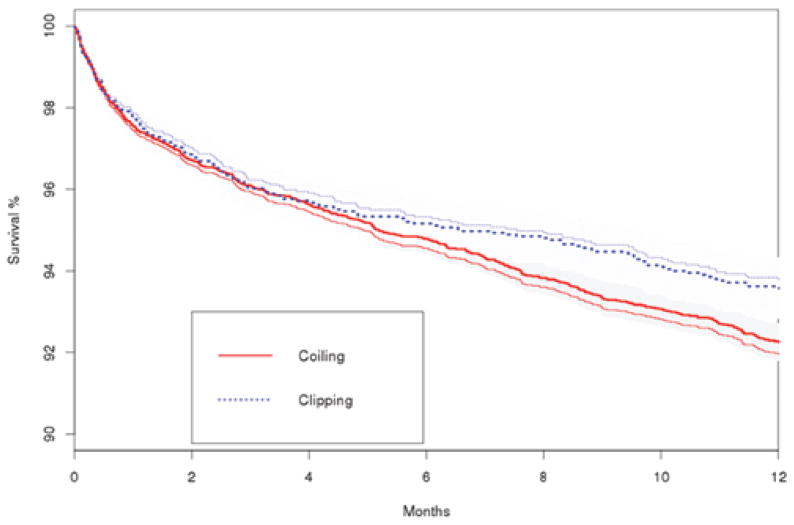
Additionally, we did not demonstrate an association between treatment method and mortality in time-to-event analyses using a multivariable Cox proportional-hazards method (Table 3; HR 1.05, 95% CI 0.87–1.27), propensity score–adjusted Cox model (HR 1.05, 95% CI 0.87–1.28), IPW Cox model (HR 0.99, 95% CI 0.87–1.14), or Cox model with IV analysis (HR 1.28, 95% CI 0.58–2.85). Figure 2 demonstrates a Kaplan-Meier plot of survival during follow-up after clipping or coiling of unruptured cerebral aneurysms.
FIG. 2.
Kaplan-Meier estimates of survival for patients with unruptured cerebral aneurysms after surgical clipping or endovascular coiling. Both unadjusted (less-bold lines) and adjusted (bold lines) estimates are presented. Shaded areas represent 95% confidence intervals. Adjustment was performed with an IPW logistic regression model. Figure is available in color online only.
Length of Stay
The average LOS was 7.3 days (SD 6.8) for patients undergoing clipping and 3.7 days (SD 5.5) days for those undergoing coiling. As demonstrated in Table 4, clipping was associated with a longer LOS in comparison with coiling (adjusted difference [AD] 3.63, 95% CI 3.36–3.91) in the crude analysis. Multiple linear regression analysis confirmed this association (AD 4.02, 95% CI 3.73–4.30). This relationship persisted after propensity score stratification (AD 4.02, 95% CI 3.74–4.31) and IPW (3.96, 95% CI 3.66–4.26). These results were confirmed in an IV analysis (AD 3.80, 95% CI 2.90–4.71).
TABLE 4.
Correlation between clipping and secondary outcome measures
| Model | Unfavorable Discharge*
|
LOS in Days†
|
90-Day Readmission*
|
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | Beta (95% CI) | p Value | OR (95% CI) | p Value | |
| Crude | 4.61 (3.95–5.36) | <0.0001 | 3.63 (3.36–3.91) | <0.0001 | 0.98 (0.88–1.09) | 0.723 |
|
| ||||||
| Multivariable regression‡ | 5.20 (4.41–6.13) | <0.0001 | 4.02 (3.73–4.30) | <0.0001 | 1.06 (0.95–1.19) | 0.289 |
|
| ||||||
| Propensity score adjustment‡ | 5.26 (4.46–6.20) | <0.0001 | 4.02 (3.74–4.31) | <0.0001 | 1.06 (0.94–1.19) | 0.323 |
|
| ||||||
| IPW‡ | 5.34 (4.75–6.00) | <0.0001 | 3.96 (3.66–4.26) | <0.0001 | 1.04 (0.95–1.13) | 0.419 |
|
| ||||||
| IV analysis§ | 6.39 (3.88–10.52) | <0.0001 | 3.80 (2.90–4.71) | <0.0001 | 1.17 (0.82–1.66) | 0.381 |
Analyses based on logistic regression.
Analyses based on linear regression; point estimates are beta coefficients.
Mixed effects; includes patient’s HRR as a random effect variable.
HRR coiling rate (fraction of coiling of total procedures performed) was used as an instrument of coiling.
Discharge to Short- or Long-Term Care Facility
Five hundred patients (19.3%) were discharged to a short- or long-term care facility after clipping and 308 (5.0%) after coiling. As demonstrated in Table 4, clipping was associated with higher rates of discharge to a facility in comparison with coiling (OR 4.61, 95% CI 3.95–5.36) in the unadjusted analysis. A multivariable logistic regression model confirmed this association (OR 5.20, 95% CI 4.41– 6.13). This relationship persisted after propensity score stratification (OR 5.26, 95% CI 4.46–6.20) and IPW (OR, 5.34, 95% CI 4.75–6.00). These results were confirmed in an IV analysis (OR 6.39, 95% CI 3.88–10.52).
90-Day Readmission
Six hundred five readmissions (23.4%) were recorded in the immediate 90-day postdischarge period after clipping and 1460 (23.8%) after coiling. As demonstrated in Table 4, clipping was not associated with a lower rate of 90-day readmission in comparison with coiling (OR 0.98, 95% CI 0.88–1.09) in the crude analysis. Multivariable logistic regression modeling confirmed this (OR 1.06, 95% CI 0.95–1.19), and the lack of association persisted after propensity score stratification (OR 1.06, 95% CI 0.94–1.19) and IPW (OR 1.04, 95% CI 0.95–1.13). These results were confirmed in an IV analysis (OR 1.17, 95% CI 0.82–1.66).
Discussion
Among Medicare patients undergoing treatment for unruptured cerebral aneurysms, we did not identify an association between surgical clipping or endovascular coiling and increased 1-year mortality or 90-day readmission. Clipping, a more invasive procedure, was associated with a longer LOS and a higher rate of discharge to short- or long-term care facilities. These results were consistent across techniques to control for measured and unmeasured confounders. In recent years, the pendulum has swung dramatically in favor of coiling for unruptured aneurysms. However, the comparative effectiveness of coiling and clipping in this population remains an issue of debate,10 resulting in striking regional variation.3 Elective coiling rates range from 35% in Modesto, California, to 98.6% in Tacoma, Washington.3
Prior investigations1,2,6,7,11,12,16,18,20,24,29,37 have demonstrated conflicting results regarding the outcomes of elective clipping and coiling. Regional analyses in California16 did not demonstrate a survival benefit for either treatment, whereas a study in New York State37 showed that coiling resulted in lower in-hospital mortality. On a national level, Brinjikji et al.,6 in a study of the Nationwide Inpatient Sample, have demonstrated that coiling in patients over 50 years old was associated with a decreased in-hospital mortality and rate of unfavorable discharge in comparison with those for clipping, in a multivariable setting. Using the same database and methodology, another author group1 confirmed these results. In elderly patients, Qureshi et al.29 studied a 5% Medicare sample and failed to demonstrate a difference in outcomes for the treatment of unruptured aneurysms. However, their sample size was significantly limited for a population-level analysis, with few patients per center, resulting in restricted explanatory power. The lack of adjustment for clustering and rigorous control for measured and unmeasured confounders (especially the fact that patients were nonrandomly selected for either treatment) significantly limits the interpretation of the results of these investigations.
In another study, McDonald et al.24 used a commercial database to assess the comparative effectiveness of the 2 techniques. These authors were unable to identify a survival benefit from either treatment during the initial hospitalization, although they did demonstrate an association between clipping and an unfavorable discharge. They employed propensity score matching to balance the covariates among treatment groups. However, participation in this database was voluntary; therefore, it is likely that hospitals incentivized to achieve higher quality standards would be overrepresented. This self-selection introduces significant unmeasured confounding, which the authors did not account for.
Our study purposefully addresses many of these methodological limitations. First, we created a cohort of almost all elderly patients in the US, giving a true picture of national practice. Second, we used advanced observational techniques to control for confounding. Propensity score stratification and IPW were used to adjust our analyses for known confounders. The possibility of clustering, which can bias the results of multicenter national studies, was accounted for by using mixed effects methods. Most importantly, an IV analysis was used to control for unknown confounders and simulate the effects of randomization on treatment. Results were consistent across techniques, supporting the validity of the observed associations. In contrast to all prior studies,1,2,6,7,11,12,16,18,20,24,29,37 which lacked long-term survival results, we modeled our primary outcome as 1-year mortality to mimic the end point of the ISAT.25 To identify the longitudinal effect of the treatment of unruptured aneurysms on outcomes, we also used time-to-event analyses. Overall, clipping and coiling were performed with similar short- and long-term mortality and readmission rates when considering equivalent patients through adjusted analyses. Aneurysm size and location were part of the treatment decision; however, the use of an IV technique is expected to balance differences in such unmeasured confounders.
Therefore, one could argue that the next step should be a randomized trial. While this is certainly a reasonable approach, our power analysis and the size of our study have demonstrated that such a trial, attempting to identify a small mortality difference between the 2 techniques, would be impractically large. Alternatively, this question can be answered by the creation of large, long-term registries, and such efforts are underway (http://www.neuropoint.org/NPA%20N2QOD.aspx). Another consideration is the use of mortality as an end point, which may not be the most suitable given the very small apparent difference between clipping and coiling. Quality of life outcome measures (such as the modified Rankin Scale), neurocognitive outcomes, or patient satisfaction metrics could be used instead in future prospective investigations. The association of clipping with a higher rate of discharge to rehabilitative care facilities and a longer LOS is probably secondary to the invasiveness of the open surgical procedure or the possibility of an increased incidence of stroke or neurological deficit. Increased pain and slower mobilization, which are expected after this intervention rather than after the percutaneous option of coiling, may lead to more secondary medical complications in this elderly cohort.
Our study has several limitations common to administrative databases. First, this is an observational study, and there is still a possibility of residual confounding. We used multiple techniques (propensity score stratification, IPW, HRR random effects, IV analysis), yielding consistent results to account for known and unknown confounders. To the extent that the HRR coiling rate is a good instrument, the possibility of residual confounding is small. Our first stage F-statistic was consistent with a strong instrument,34 and it is unlikely that the regional rate of coiling will be associated with procedural mortality in any other way than the choice of treatment. Second, coding inaccuracies can affect our estimates. In addition, the use of a 12-month look-back period may have missed some comorbidities. However, coding for procedures is rarely inaccurate given that it is a revenue generator and is under scrutiny by payers. We elected to include only the initial intervention because it would not be possible to differentiate through this database which patients had multiple interventions for the same aneurysms and which had them for different aneurysms. Therefore, we cannot study the potential effect of multiple interventions (which are more common in endovascularly treated patients) on long-term outcomes.
Third, claims data do not provide metrics on the postoperative neurological status of patients (that is, modified Rankin Scale score), chronic pain, or quality of life. Therefore, we cannot analyze the difference between clipping and coiling in regard to these measures. It is possible that elderly patients need rehabilitation after clipping more often than after endovascular treatments, but their long-term functional outcome could ultimately be the same if measured at a later time point. Alternatively, it is possible that patients undergoing craniotomy are more likely to be carefully assessed for rehabilitation needs, whereas endovascular patients are routinely discharged quickly without adequately assessing their discharge needs. Fourth, findings among this older American population may not be generalizable to younger or otherwise dissimilar populations. Fifth, we have no information on aneurysm size and location, which can affect surgical outcomes; however, the use of an IV analysis is expected to simulate a randomized trial and control for such unknown confounders. Sixth, although there is no reliable way to link readmissions to the primary procedure (and therefore we cannot comment on that), these metrics are tracked by payers and regulators. Seventh, causal inference is hard to establish based on observational data, even when using an IV analysis.14
Conclusions
Treatment options for unruptured cerebral aneurysms and their impact on outcomes remain issues of debate. We found little difference in 1-year survival between patients undergoing elective coiling or clipping of unruptured cerebral aneurysms, although surgical clipping was associated with a higher rate of discharge to rehabilitation facilities and a longer length of stay. Future comparative effectiveness studies probably need to be based on prospective registries using quality outcome metrics when determining which treatment option is best.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Aging (No. PO1-AG19783), the National Institutes of Health Common Fund (No. U01-AG046830), and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (No. UL1TR001086 to Dartmouth Clinical and Translational Science Institute).
ABBREVIATIONS
- AD
adjusted difference
- FFS
fee-for-service
- HRR
hospital referral region
- IPW
inverse probability weighting
- ISAT
International Subarachnoid Aneurysm Trial
- IV
instrumental variable
- LOS
length of stay
- ZIP
Zone Improvement Plan
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. The funders had no role in the design or execution of the study.
Supplemental material is available with the online version of the article.
Supplemental Table 1 and Supplementary Methods. http://thejns.org/doi/suppl/10.3171/2016.1.JNS152028.
Author Contributions
Conception and design: Bekelis. Acquisition of data: Bekelis, Gottlieb, Su, O’Malley, MacKenzie. Analysis and interpretation of data: Bekelis, Gottlieb, Su, O’Malley, Labropoulos, Goodney, MacKenzie. Drafting the article: Bekelis. Critically revising the article: Gottlieb, Su, O’Malley, Labropoulos, Goodney, Lawton, MacKenzie. Reviewed submitted version of manuscript: Bekelis, Labropoulos, Lawton, MacKenzie. Approved the final version of the manuscript on behalf of all authors: Bekelis. Statistical analysis: Bekelis, Gottlieb, Su, O’Malley, MacKenzie. Administrative/technical/material support: Labropoulos, MacKenzie. Study supervision: Bekelis, Labropoulos, Goodney, Lawton, MacKenzie.
References
- 1.Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, et al. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke. 2010;41:1471–1476. doi: 10.1161/STROKEAHA.110.580647. [DOI] [PubMed] [Google Scholar]
- 2.Barker FG, II, Amin-Hanjani S, Butler WE, Hoh BL, Rabinov JD, Pryor JC, et al. Age-dependent differences in short-term outcome after surgical or endovascular treatment of unruptured intracranial aneurysms in the United States, 1996– 2000. Neurosurgery. 2004;54:18–30. doi: 10.1227/01.neu.0000097195.48840.c4. [DOI] [PubMed] [Google Scholar]
- 3.Bekelis K. Variation in the care of surgical conditions: cerebral aneurysms. In: Goodney RP, Dzebisashvili N, Goodman DC, et al., editors. Variations in the Care of Surgical Conditions: A Dartmouth Atlas of Health Care Series. Lebanon, NH: Dartmouth Institute for Health Policy and Clinical Practice; 2014. [PubMed] [Google Scholar]
- 4.Blackburn SL, Abdelazim AM, Cutler AB, Brookins KT, Fargen KM, Hoh BL, et al. Endovascular and surgical treatment of unruptured MCA aneurysms: meta-analysis and review of the literature. Stroke Res Treat. 2014;2014:348147. doi: 10.1155/2014/348147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brilstra EH, Rinkel GJ, van der Graaf Y, Sluzewski M, Groen RJ, Lo RT, et al. Quality of life after treatment of unruptured intracranial aneurysms by neurosurgical clipping or by embolisation with coils. A prospective, observational study. Cerebrovasc Dis. 2004;17:44–52. doi: 10.1159/000073897. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Rabinstein AA, Lanzino G, Kallmes DF, Cloft HJ. Effect of age on outcomes of treatment of unruptured cerebral aneurysms: a study of the National Inpatient Sample 2001–2008. Stroke. 2011;42:1320–1324. doi: 10.1161/STROKEAHA.110.607986. [DOI] [PubMed] [Google Scholar]
- 7.Brinjikji W, Rabinstein AA, Lanzino G, Kallmes DF, Cloft HJ. Patient outcomes are better for unruptured cerebral aneurysms treated at centers that preferentially treat with endovascular coiling: a study of the National Inpatient Sample 2001–2007. AJNR Am J Neuroradiol. 2011;32:1065–1070. doi: 10.3174/ajnr.A2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939. doi: 10.1056/NEJMra052760. [DOI] [PubMed] [Google Scholar]
- 9.Choi SW, Ahn JS, Park JC, Kwon H, Kwun BD, Kim CJ. Surgical treatment of unruptured intracranial middle cerebral artery aneurysms: angiographic and clinical outcomes in 143 aneurysms. J Cerebrovasc Endovasc Neurosurg. 2012;14:289– 294. doi: 10.7461/jcen.2012.14.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darsaut TE, Estrade L, Jamali S, Bojanowski MW, Chagnon M, Raymond J. Uncertainty and agreement in the management of unruptured intracranial aneurysms. J Neurosurg. 2014;120:618–623. doi: 10.3171/2013.11.JNS131366. [DOI] [PubMed] [Google Scholar]
- 11.Duan Y, Blackham K, Nelson J, Selman W, Bambakidis N. Analysis of short-term total hospital costs and current primary cost drivers of coiling versus clipping for unruptured intracranial aneurysms. J Neurointerv Surg. 2015;7:614–618. doi: 10.1136/neurintsurg-2014-011249. [DOI] [PubMed] [Google Scholar]
- 12.Fargen KM, Rahman M, Neal D, Hoh BL. Prevalence of patient safety indicators and hospital-acquired conditions in those treated for unruptured cerebral aneurysms: establishing standard performance measures using the Nationwide Inpatient Sample database. J Neurosurg. 2013;119:966–973. doi: 10.3171/2013.5.JNS122378. [DOI] [PubMed] [Google Scholar]
- 13.Foster EM. Instrumental variables for logistic regression: an illustration. Soc Sci Res. 1997;26:487–504. [Google Scholar]
- 14.Garabedian LF, Chu P, Toh S, Zaslavsky AM, Soumerai SB. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161:131–138. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
- 15.Gerlach R, Beck J, Setzer M, Vatter H, Berkefeld J, Du Mesnil de Rochemont R, et al. Treatment related morbidity of unruptured intracranial aneurysms: results of a prospective single centre series with an interdisciplinary approach over a 6 year period (1999–2005) J Neurol Neurosurg Psychiatry. 2007;78:864–871. doi: 10.1136/jnnp.2006.106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonda DD, Khalessi AA, McCutcheon BA, Marcus LP, Noorbakhsh A, Chen CC, et al. Long-term follow-up of unruptured intracranial aneurysms repaired in California. J Neurosurg. 2014;120:1349–1357. doi: 10.3171/2014.3.JNS131159. [DOI] [PubMed] [Google Scholar]
- 17.Hoh BL, Chi YY, Dermott MA, Lipori PJ, Lewis SB. The effect of coiling versus clipping of ruptured and unruptured cerebral aneurysms on length of stay, hospital cost, hospital reimbursement, and surgeon reimbursement at the university of Florida. Neurosurgery. 2009;64:614–621. doi: 10.1227/01.NEU.0000340784.75352.A4. [DOI] [PubMed] [Google Scholar]
- 18.Hoh BL, Chi YY, Lawson MF, Mocco J, Barker FG., II Length of stay and total hospital charges of clipping versus coiling for ruptured and unruptured adult cerebral aneurysms in the Nationwide Inpatient Sample database 2002 to 2006. Stroke. 2010;41:337–342. doi: 10.1161/STROKEAHA.109.569269. [DOI] [PubMed] [Google Scholar]
- 19.Johnston K, Gustafson P, Levy AR, Grootendorst P. Use of instrumental variables in the analysis of generalized linear models in the presence of unmeasured confounding with applications to epidemiological research. Stat Med. 2008;27:1539– 1556. doi: 10.1002/sim.3036. [DOI] [PubMed] [Google Scholar]
- 20.Lawson MF, Neal DW, Mocco J, Hoh BL. Rationale for treating unruptured intracranial aneurysms: actuarial analysis of natural history risk versus treatment risk for coiling or clipping based on 14,050 patients in the Nationwide Inpatient Sample database. World Neurosurg. 2013;79:472–478. doi: 10.1016/j.wneu.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Lee T, Baytion M, Sciacca R, Mohr JP, Pile-Spellman J. Aggregate analysis of the literature for unruptured intracranial aneurysm treatment. AJNR Am J Neuroradiol. 2005;26:1902– 1908. [PMC free article] [PubMed] [Google Scholar]
- 22.MacKenzie TA, Brown JR, Likosky DS, Wu Y, Grunkemeier GL. Review of case-mix corrected survival curves. Ann Thorac Surg. 2012;93:1416–1425. doi: 10.1016/j.athoracsur.2011.12.094. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie TA, Tosteson TD, Morden NE, Stukel TA, O’Malley AJ. Using instrumental variables to estimate a Cox’s proportional hazards regression subject to additive confounding. Health Serv Outcomes Res Methodol. 2014;14:54–68. doi: 10.1007/s10742-014-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald JS, McDonald RJ, Fan J, Kallmes DF, Lanzino G, Cloft HJ. Comparative effectiveness of unruptured cerebral aneurysm therapies: propensity score analysis of clipping versus coiling. Stroke. 2013;44:988–994. doi: 10.1161/STROKEAHA.111.000196. [DOI] [PubMed] [Google Scholar]
- 25.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 26.Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311:2508–2517. doi: 10.1001/jama.2014.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Kim YI, Lim YC. Clinical outcomes of treatment for intracranial aneurysm in elderly patients. J Cerebrovasc Endovasc Neurosurg. 2014;16:193–199. doi: 10.7461/jcen.2014.16.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyysalo L, Luostarinen T, Keski-Nisula L, Öhman J. Long-term excess mortality of patients with treated and untreated unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2013;84:888–892. doi: 10.1136/jnnp-2012-303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qureshi AI, Chaudhry SA, Tekle WG, Suri MF. Comparison of long-term outcomes associated with endovascular treatment vs surgical treatment among Medicare beneficiaries with unruptured intracranial aneurysms. Neurosurgery. 2014;75:380–387. doi: 10.1227/NEU.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AI, Vazquez G, Tariq N, Suri MF, Lakshminarayan K, Lanzino G. Impact of International Subarachnoid Aneurysm Trial results on treatment of ruptured intracranial aneurysms in the United States. Clinical article. J Neurosurg. 2011;114:834–841. doi: 10.3171/2010.6.JNS091486. [DOI] [PubMed] [Google Scholar]
- 31.Rassen JA, Schneeweiss S, Glynn RJ, Mittleman MA, Brookhart MA. Instrumental variable analysis for estimation of treatment effects with dichotomous outcomes. Am J Epidemiol. 2009;169:273–284. doi: 10.1093/aje/kwn299. [DOI] [PubMed] [Google Scholar]
- 32.Sharma M, Brown B, Madhugiri V, Cuellar-Saenz H, Sonig A, Ambekar S, et al. Unruptured intracranial aneurysms: comparison of perioperative complications, discharge disposition, outcome, and effect of calcification, between clipping and coiling: a single institution experience. Neurol India. 2013;61:270–276. doi: 10.4103/0028-3886.115067. [DOI] [PubMed] [Google Scholar]
- 33.Smith MJ, Sanborn MR, Lewis DJ, Faught RW, Vakhshori V, Stein SC. Elderly patients with intracranial aneurysms have higher quality of life after coil embolization: a decision analysis. J Neurointerv Surg. 2015;7:898–904. doi: 10.1136/neurintsurg-2014-011394. [DOI] [PubMed] [Google Scholar]
- 34.Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65:557–586. [Google Scholar]
- 35.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xian Y, Holloway RG, Chan PS, Noyes K, Shah MN, Ting HH, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373– 380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zacharia BE, Ducruet AF, Hickman ZL, Grobelny BT, Badjatia N, Mayer SA, et al. Technological advances in the management of unruptured intracranial aneurysms fail to improve outcome in New York state. Stroke. 2011;42:2844–2849. doi: 10.1161/STROKEAHA.111.619767. [DOI] [PubMed] [Google Scholar]
- 38.Zijlstra IA, Verbaan D, Majoie CB, Vandertop P, van den Berg R. Coiling and clipping of middle cerebral artery aneurysms: a systematic review on clinical and imaging outcome. J Neurointerv Surg. 2016;8:24–29. doi: 10.1136/neurintsurg-2014-011478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.