Abstract
The formation of inner ear sensory epithelia is believed to occur in two steps, specification of sensory competent (prosensory) regions followed by determination of specific cell-types, such as hair cells (HCs) and supporting cells. However, studies in which the HC determination factor Atoh1 was ectopically expressed in non-prosensory regions indicated that expression of Atoh1 alone is sufficient to induce HC formation suggesting that prosensory formation may not be a prerequisite for HC development. To test this hypothesis, interactions between Sox2 and Atoh1, which are required for prosensory and HC formation respectively, were examined. Forced expression of Atoh1 in non-prosensory cells resulted in transient expression of Sox2 prior to HC formation, suggesting that expression of Sox2 is required for formation of ectopic HCs. Moreover, Atoh1 overexpression failed to induce HC formation in Sox2 mutants, confirming that Sox2 is required for prosensory competence. To determine whether expression of Sox2 alone is sufficient to induce prosensory identity, Sox2 was transiently activated in a manner that mimicked endogenous expression. Following transient Sox2 activation, non-prosensory cells developed as HCs, a result that was never observed in response to persistent expression of Sox2. These results, suggest a dual role for Sox2 in inner ear formation. Initially, Sox2 is required to specify prosensory competence, but subsequent down-regulation of Sox2 must occur to allow Atoh1 expression, most likely through a direct interaction with the Atoh1 promoter. These results implicate Sox2-mediated changes in prosensory cells as an essential step in their ability to develop as HCs.
Keywords: Sox2, Atoh1, hair cell competence, hair cell induction, cell fate
INTRODUCTION
In vertebrates, sounds are perceived through the stimulation of mechanosensory hair cells located within the cochlear duct of the inner ear. Virtually all of the cell types within the cochlear duct are derived from cells initially located in the otocyst, a placodally-derived structure located adjacent to the hindbrain (Adam et al., 1998; Morsli et al., 1998; Riccomagno et al., 2002). At the most superficial level, otocyst cells can be considered to adopt one of three primary fates, neurons of the statoacoustic (VIIIth) nerve, prosensory cells that will go on to form the mechanosensory hair cells and associated supporting cells, or other non-sensory cells. Recent studies have begun to identify some of the signaling pathways that specify the neuronal and prosensory lineages, as well as some of the factors that direct cells towards a hair cell fate. In particular, the high-mobility-group (HMG) transcription factor Sox2 is required for prosensory formation while the basic helix-loop-helix molecule Atoh1 acts as a specification factor for the hair cell fate (Bermingham et al., 1999; Kiernan et al., 2005; Woods et al., 2004).
Sox2 belongs to the B1 family of Sox proteins which have been shown to be involved in various developmental processes including determination of cell fate and differentiation (Bylund et al., 2003; Dabdoub et al., 2008; Graham et al., 2003). As a transcription factor, Sox2 binds directly to DNA and can act as either a transcriptional activator or repressor depending on context (Liu et al., 2013; Uchikawa et al., 1999). In the developing inner ear, Sox2 is initially expressed in the otic progenitor cells that will develop as both the prosensory and neuronal cell lineages (Dabdoub et al., 2008; Kiernan et al., 2005; Puligilla et al., 2010). Loss of Sox2 expression in the otocyst leads to a complete loss of prosensory cells and consequently both hair cells and supporting cells (Kiernan et al., 2005). In addition, all known markers of the prosensory domain are absent in Sox2 mutants, demonstrating a key role for Sox2 in prosensory formation. In contrast with Sox2, Atoh1 expression does not begin until prosensory cells have become post-mitotic and is thought only to directly regulate the formation of cells as hair cells (Woods et al., 2004; Zheng and Gao, 2000). Forced expression of Atoh1 is sufficient to induce hair cell formation both within the prosensory domain and in adjacent non-sensory cells (Kelly et al., 2012; Woods et al., 2004; Zheng and Gao, 2000) and although ectopic hair cells will induce neighboring cells to develop as supporting cells, Atoh1 expression is not directly required for supporting cell formation (Woods et al., 2004).
A key component of the prosensory hypothesis is the idea that only specific regions of the otocyst, the prosensory regions, become competent to develop as hair cells and supporting cells. Therefore, within the context of this hypothesis, factors that specify the prosensory region should do so by fundamentally changing the developmental competence of cells within those regions. However, the observation that ectopic expression of Atoh1 can lead to hair cell formation in regions of the inner ear that do not normally contain hair cells and are therefore not thought to contain prosensory cells raises the possibility that uniquely competent prosensory regions do not exist. Rather, these results suggest that expression of Atoh1 may be sufficient to induce hair cell formation and that the role of Sox2 may, therefore be limited to induction or enhancement of the expression of Atoh1 in specific regions of the developing inner ear. Since, as mentioned, developing hair cells have the ability to recruit surrounding cells to develop as supporting cells, regulation of the spatial expression pattern of Atoh1 could be sufficient to regulate patterning of sensory epithelia within the inner ear. To examine these hypotheses, the experiments described here addressed the ability of Atoh1 to induce hair cell formation in the absence of Sox2 with the results providing additional insights into the role of Sox2 in formation of inner ear sensory epithelia. A better understanding of the role of Sox2 and the existence and nature of the prosensory domain, should provide valuable insights regarding inner ear formation and possible regenerative strategies.
METHODS
Mice
All animal procedures were performed according to the guidelines and regulations of the Institutional Animal Care and Use Committee at the National Institutes of Health and The Medical University of South Carolina (MUSC). The generation and genotyping of Lcc mutant mice was described previously (Kiernan et al., 2005). Briefly, Sox2-deficient cochleae were obtained by crossing Sox2Lcc/+ heterozygotes. Cochleae from wild-type CD1 (Charles River; Harlan Laboratories) or Sox2Lcc/Lcc mice were collected from timed-pregnant females at specific time points between E13 and P0.
Generation of plasmid constructs
For transient expression of Sox2, a Sox2.ERT2 fusion construct was generated by cloning Sox2 (pCIG-Sox2) and ERT2 (pCAG-ERT2) fragments in frame using primers that were designed to remove the stop codon from the Sox2 open reading frame and to introduce a stop codon at the end of ERT2 sequence. The resulting fusion was confirmed by sequencing and then inserted into the pIRES2.EGFP vector to generate Sox2ERT2.IRES.EGFP (Sox2ERT2:EGFP). For Atoh1 overexpression, we used pIRES2.EGFP.Atoh1 and pIRES2.EGFP.Atoh1ER as described previously (Puligilla et al., 2010; Woods et al., 2004). The expression vector, pCIG.Sox2.EGFP was used for continuous expression of Sox2.
Cochlear organ cultures and electroporation
Cochlear explant cultures were prepared and electroporated as described previously (Haque et al., 2015). Briefly, timed pregnant CD1 mice were anesthetized deeply by CO2 inhalation and then euthanized. Cochleae were dissected from staged embryos and the sensory epithelium was exposed. For electroporation, cochleae were placed in a 10 μl drop of water containing 2 μg/μl of plasmid DNA and transfected using a square wave electroporator, CUY-21 (BEX, Protech International, Inc.). Following electroporation, the explants were oriented “sensory epithelium up” onto matrigel-coated MatTek dishes (MatTek Corporation) and maintained in culture medium containing DMEM, 10% fetal bovine serum, 0.2% N2, and .001% Ciprofloxacin. Explants were maintained at 37°C in the presence of 5% CO2. NIH3T3 cells were maintained as previously described and were transfected using lipofectamine 2000 (Invitrogen). 4-hydroxytamoxifen (Sigma) was used to induce activation of the Sox2.ERT2 fusion protein in both cell lines and cochlear explants.
Immunostaining
Cochlear explants were fixed in 4% paraformaldehyde for 10 min, washed in PBS, permeabilized with 0.5% Tween-20 and then blocked with 10% serum. Samples were then incubated overnight in primary antibodies at 4° C with rocking followed by extensive rinsing. Binding of primary antibodies was detected using Alexa Fluor-conjugated secondary antibodies (Molecular Probes). The following antibodies were used: Sox2 (Chemicon; Santa Cruz), Myo6, Myo7a (Proteus Biosciences), Jagged1 (Santa Cruz), Prox1 (Chemicon), Phalloidin (Molecular Probes), and Atoh1 (Driver et al., 2013). Confocal z-stack images were obtained using a Zeiss LSM 510 confocal microscope and subsequently processed using Adobe Photoshop.
Chromatin Immunoprecipitation (ChIP) assay
ChIP was performed using the ChIP kit (Pierce) according to the manufacturer's protocol. Briefly, protein was isolated from around 65 cochleae at E14.5 and then cross-linked to DNA by addition of formaldehyde. Chromatin was sheared by sonication to 100 to 200 bp fragments and the soluble chromatin fraction was collected. 10% of the resulting supernatant was used for input. Equivalent concentrations of either anti-IgG (negative control) or anti-Sox2 were added and incubated according to the protocol. Purified eluted DNA fragments were subsequently amplified by PCR. Primer sequences will be provided upon request.
RESULTS
Ectopic overexpression of Atoh1 induces Sox2 expression
Forced expression of Atoh1 in non-prosensory regions of embryonic or early postnatal cochleae leads to the formation of ectopic hair cells. In many cases, cells that form ectopic hair cells in response to forced expression of Atoh1 are not located in Sox2-positive regions of the inner ear at the time of transfection, suggesting that expression of Atoh1 alone is sufficient to induce hair cell formation. To examine this hypothesis further, non-sensory cells located within Kolliker's organ, an embryonic/early postnatal structure located medial to the developing organ of Corti, were forced to express Atoh1 by electroporation. Transfected cells were subsequently analyzed at different post-transfection time points for the expression of both hair cell-specific markers and Sox2. As previously reported, by 96 hours (hrs) following transfection, many Atoh1-expressing cells were positive for the hair cell differentiation markers Myosin 6 (Myo6) or Myosin 7a (Myo7a). At the same time point, transfected cells were also negative for Sox2. However, when similarly transfected cells were fixed only 17 hrs following transfection, antibody-labeling indicated that 92% of EGFP-positive cells were positive for Sox2 (Figure 1A-D). In addition, transfected cells were also positive for Jagged1, an additional marker of prosensory identity (Figure 1I-K). In contrast, none of the EGFP-positive cells expressed Myo6 after 17 hrs. Explants that were maintained for 36 hrs following electroporation contained large numbers of EGFP-positive, Myo6-positive, Sox2-negative cells (Figure 1E-H). These results indicate that Atoh1-transfection leads to a relatively rapid, but transient, expression of Sox2 as part of the hair cell induction process. In contrast, non-sensory cells transfected with an empty vector containing EGFP alone (control.EGFP) were neither positive for the prosensory marker, Sox2 nor the hair cell marker, Myo6 (Figure 1I-L). It is important to note that some cells within KO appeared to be positive for Sox2 and/or Myo6, despite no obvious expression of EGFP, suggesting a possible non-autonomous role for Atoh1. To examine this result further, the gain for detection of expression of EGFP was increased in order to visualize cells with low levels of transfection or expression of the transgene. Upon re-examination, virtually all KO cells positive for Sox2 and/or Myo6 were also positive for expression of EGFP, suggesting that effects of Atoh1 were primarily, or entirely autonomous. Overall, these data suggest that Atoh1-transfected cells pass through a “prosensory” phase prior to differentiation as hair cells and are consistent with Atoh1 overexpressing cells following an in vivo developmental program in which cells transiently express Sox2 prior to differentiating as hair cells.
Figure 1. Atoh1 induces ectopic expression of Sox2 in cochlear non-sensory cells.
Cochlear non-sensory cells located in Kolliker's organ were transfected with Atoh1.EGFP at E13 and incubated for 17 hrs (A-D) or 36 hrs (E-F) in vitro prior to fixation. Analysis of electroporated cells (A, green) by immunolabeling for Sox2 (B, red) and Myo6 (C, blue) demonstrates that most transfected cells are positive for Sox2 but negative for Myo6 at 17 hrs after transfection (arrows in D). Total number of explants (n) = 5; total number of transfected cells (t) = 81/86. In contrast, at the 36-hour time point, many transfected cells are negative for Sox2 (E,F) but positive for Myo6 (G, blue; arrows in H) suggesting that down-regulation of Sox2 coincides with hair cell differentiation (n= 5; t = 93/94 cells were negative for Sox2 and 65/68 cells were positive for Myo7a). Similarly, Atoh1.EGFP transfected cells are also positive for another prosensory marker, Jagged1 at 17-hour time point (n = 3 explants; t = 17/21). (I-K; arrows in K). Cells transfected with a control vector expressing only EGFP were negative for both Sox2 and Myo6 at both the 17 (L,M) and 36 (N,O) -hour time points (n = 3; t = 32/32). Scale bar: A-H (in H), 20 μm; I-K (in K), 20 μm; L-O (in O), 20 μm.
Sox2 is required for Atoh1-induced hair cell formation
The results presented above indicate that Atoh1-induced ectopic hair cells transiently express Sox2 prior to hair cell formation. To determine whether Sox2 expression is necessary for hair cell formation, cochlear cells from control and Sox2Lcc/Lcc mutants were transfected with an Atoh1-expression vector at two developmental time points, E13 and P0. Atoh1-expression in Kolliker's organ cells in control cochleae at E13 resulted in a 96% induction rate for hair cell formation based on expression of Myo6 (Figure 2A-C). Transfection of Atoh1 in control explants at P0 was still sufficient to induce hair cell formation; however, the efficiency of induction was reduced to 58% (Table 1). The basis for the decrease in efficiency is unclear, but is consistent with recent results showing a progressive decrease and eventual loss in the ability of ectopic Atoh1 to induce a hair cell fate in Atoh1 transgenic mice (Kelly et al., 2012; Liu et al., 2012).
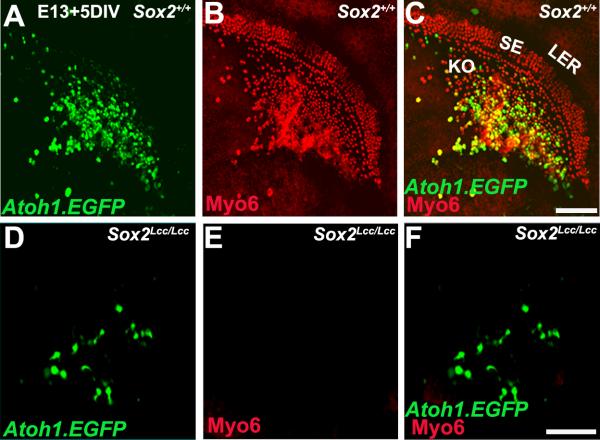
Figure 2. Sox2 is required for hair cell induction by Atoh1.
Cochlear explants established from WT (Sox2+/+) (A-C) or Sox2-deficient (Sox2Lcc/Lcc) (D-F) mice at E13 and transfected with Atoh1.EGFP (green). After 6 DIV explants were fixed and analyzed for expression of the hair cell marker Myo6 (red). In WT cochleae, transfection of Kolliker's organ cells (KO in C) induces broad expression of Atoh1 (A, green) and the formation of multiple ectopic hair cells (B, red). Note the presence of the endogenous hair cell population within the sensory epithelium (SE in C). Many of the cells transfected with the Atoh1 construct appear yellow indicating that they are developing as hair cells. As has been reported before, ectopic hair cells that did not appear to express the transgene were also observed (red cells in KO in C). Whether these cells are weakly transfected or represent paracrine induction of hair cells has not been determined. However, examination of individual cells at increased gain indicated that most ectopic hair cells do express EGFP at some level. In contrast with WT, none of the transfected cells in Sox2Lcc/Lcc cochleae were positive for Myo6 (D-F). The endogenous population of hair cells is absent, as previously reported. LER, lesser epithelial ridge. Scale bar: A-C (in C), 20 μm; D-F (in F), 20 μm.
| Genotype | Myo6/7a positive | |
|---|---|---|
| E13.5 | P0 | |
| Sox2WT | n=3, t=79/84 (96%) | n=4, t=47/82 (58%) |
| Sox2Lcc/Lcc | n=4, t=0/33 | n=3, t=0/28 |
n= total no: of explants, t = total no: of transfected cells
In contrast with control cochleae, transfection of Atoh1.EGFP into Kolliker's organ cells in Sox2Lcc/Lcc cochleae at either E13 or P0 failed to induce expression of either Myo6 or Myo7a, suggesting that Atoh1 was insufficient to induce a hair cell fate in the absence of Sox2 (Figure 2D-F) (Table 1). Transfected cells also did not develop stereociliary bundles, as were observed on transfected cells in controls (data not shown), suggesting that defects in Atoh1-induced hair cell formation extended beyond expression of specific markers. To determine whether forced expression of Atoh1 is sufficient to induce prosensory cell identity in the absence of Sox2, expression of the prosensory markers, Jagged1 and Cdkn1, were examined in Sox2Lcc/Lcc cochleae. Results indicated no expression of either marker (not shown), suggesting that the absence of Sox2 arrests cochlear cells prior to prosensory specification.
Transient expression of Sox2 is sufficient to induce hair cell formation
The results described above demonstrate that Sox2 expression is required for the acquisition of prosensory identity. Yet, previous work has demonstrated that prolonged strong expression of Sox2 also acts to inhibit the expression and function of Atoh1 in mammals (Dabdoub et al., 2008; Neves et al., 2012). Further, Sox2 expression is lost as prosensory cells develop as hair cells (Dabdoub et al., 2008; Hume et al., 2007). Taken together, these results suggest that transient expression of Sox2 may be sufficient to induce hair cell formation. To test this hypothesis, an inducible version of Sox2 was generated by fusing the Sox2 ORF with ERT2 sequence. This sequence was then introduced into an IRES expression vector that also generates EGFP as an independent transcript. The resulting construct (Sox2ERT2:EGFP) allows transient expression of Sox2 in cells that constitutively express EGFP.
To confirm that the construct generates an inducible version of Sox2 that is expressed in cellular cytoplasm in the absence of tamoxifen and translocated to the nucleus in the presence of tamoxifen, NIH3T3 cells were transfected with Sox2ERT2:EGFP. Sox2 protein was clearly present in EGFP-positive cells following transfection but was restricted to the cytoplasm in the absence of tamoxifen (Figure 3A-C). Following administration of tamoxifen, Sox2 was translocated to cell nuclei (Figure 3D-F). To confirm biological activity of the Sox2ERT2 fusion, the ability of Sox2 to induce expression of Prox1, a known inner ear Sox2 target (Dabdoub et al., 2008) was examined both in NIH3T3 cells (Figure 3G-I) and in cochlear explants (data not shown). Results indicated induction of Prox1 expression in both conditions following tamoxifen treatment.
Figure 3. Characterization of Sox2ERT2.EGFP construct in NIH3T3 cell lines.
NIH3T3 cells transfected with Sox2ERT2.EGFP in the absence (A-C) and presence of tamoxifen (D-I) and immunolabeled with antibodies against Sox2 (red, B,C,E,F). Sox2ERT2 transfected cells are identified based on expression of EGFP (green in A,D). NIH3T3 cells do not express Sox2 endogenously. In the absence of tamoxifen (A-C), Sox2 is localized to the cytoplasm of each cell (arrows in B). In contrast, in the presence of tamoxifen (D-F), Sox2 is translocated to the nucleus. To confirm that the Sox2ERT2 fusion protein is functional, induction of the Sox2 target, Prox1 was examined (G-I). Following transfection, induction of Sox2ERT2 was sufficient to induce Prox1 expression (red in H). In contrast, no Prox1 induction was observed in untransfected cells (not shown). Scale bar: A-C (in C) 10 μm; D-F (in F) 10 μm’; G-I (in I), 20 μm.
Next, to determine whether transient activation of Sox2 is sufficient to induce hair cell development, non-sensory cells within Kolliker's organ were transfected with either the EGFP or Sox2ERT2:EGFP construct. Individual transfected cells in explants transfected with EGFP alone or in explants transfected with Sox2ERT2:EGFP but maintained in the absence of tamoxifen never developed as hair cells. Furthermore, in the absence of tamoxifen, Sox2ERT2.EGFP transfected cells displayed a diffuse cytoplasmic distribution pattern (n=4 explants and 3T3 cell lines; t = 26 out of 27 transfected 3T3 cells). In contrast, when explants transfected with Sox2ERT2:EGFP were exposed to tamoxifen for 24 hrs followed by a 72-hr recovery period, most of the transfected cells were positive for the prosensory marker, Jagged1 (Figure 4A-C) as well as for Atoh1 (n=4 explants, t=30 out of 41 transfected explant cells were Atoh1-positive) (Figure 4D-F). In addition, the EGFP signal was observed to be translocated to the nucleus (n=4, t=41/41). However, none of the transfected cells expressed a definitive hair cell marker, such as Myo6 or Myo7a (not shown). Given the time required for normal hair cell development, we suspected that insufficient time had been allowed following the tamoxifen treatment. Therefore, additional explants were established and treated with tamoxifen for 24 hrs as described above, but were subsequently allowed to recover for 120 hrs (5 days) prior to fixation. At this recovery time point, approximately 70% of transfected cells were positive for a hair cell marker, either Myo6 or Myo7a (Figure 4G-I). Moreover, many of these cells also contained a stereociliary bundle (Figure 4J-L). As was the case for cells transfected with Atoh1, some Kolliker's organ cells that were positive for Myo7a did not appear to express EGFP, however upon re-examination at increased gain, some level of EGFP was detected in virtually all Myo7a-positive Kolliker's organ cells.
Figure 4. Transient expression of Sox2 induces hair cell formation.
(A-L) Kolliker's organ cells in a WT cochlear explant transfected with Sox2ERT2.EGFP at E13 and then treated with tamoxifen for 24 hrs beginning after 24 hrs in culture. Tamoxifen was removed after 24 hrs and explants were maintained for an additional 72 hrs prior to fixation (E13+24hr+24hrTx+72hr recovery). Transient activation of Sox2 (green) results in expression of a prosensory marker, Jag1 (A-C; arrows), and induction of Atoh1 (D-F; arrows) and Myo7a (G-I; arrows). (J-L) In addition, transfected cells also develop stereociliary bundles (arrows in K; actin in red). For panels G-L, cells were explanted at E13 and treated with tamoxifen for 24 hrs after 1DIV and maintained for an additional 120 hrs recovery time prior to fixation (E13+24hr+24hrTx+120hr). Scale bar: A-C (in C), 10 μm; D-F (in F), 20 μm; G-J (in J), 20 μm.
While approximately 96% of Kolliker's organ cells transfected with Atoh1 develop as hair cells (Table 1), only approximately 70% of cells with induced transient expression of Sox2 developed as hair cells. The molecular bases for the difference in hair cell induction efficiency is not clear but could be due to incomplete penetration of tamoxifen or possible hypomorphic activity of the Sox2ERT2 fusion protein as was suggested for a similar Atoh1ER fusion construct (Woods et al., 2004). Finally, cell-cell interactions within a group of Sox2ERT2-transfected cells could result in some of those cells being diverted from a hair cell fate and towards a supporting cell fate through activation of the Notch pathway by developing hair cells. Finally, in explants transfected with Sox2ERT2:EGFP but then treated with tamoxifen continuously for 6 DIV, no transfected cells were observed to develop as hair cells (not shown). This result is consistent with previous work using a constitutively active Sox2-expression construct (Dabdoub et al., 2008). To examine the sensitivity of transfected Kolliker's organ cells to transient expression of Sox2, explants transfected with Sox2ERT2:EGFP were treated with tamoxifen for 2 DIV followed by a 94 or 118 hr recovery period. In these explants, transfected cells also failed to induce hair cell formation (not shown). These results suggest that cellular sensitivity to Sox2 expression is fairly high with just 48 hrs of Sox2 activity potentially leading to long term inhibition of a hair cell fate.
Sox2 directly interacts with regulatory elements of Atoh1
The observation that transient activation of Sox2 leads to Atoh1-positive hair cells prompted us to determine whether Sox2 binds to regions within the Atoh1-enhancer. Previous work had demonstrated that Sox2 binds to and regulates a conserved Sox2 consensus sequence 5’-AACAAAG in a reporter construct driven by the Atoh1 1.3’-enhancer (Ahmed et al., 2012). However an interaction between Sox2 and endogenous Atoh1 had not been demonstrated in the mouse inner ear. Therefore, we performed chromatin immunoprecipitation (ChIP) assays using extracts of cochlear tissue at E14, a time point that corresponds with the onset of Atoh1 expression. Chromatin isolated from E14 cochleae was immuno-precipitated either with a control goat IgG antibody or a Sox2-specific IgG antibody and analyzed by PCR for binding to regions within the Atoh1 3’ enhancer (Figure 5A). In contrast to the control, the consensus Sox2 binding region within the Atoh1 enhancer showed preferential amplification following precipitation with anti-Sox2. Furthermore, the association was specific for this region as two other control sites were not amplified (Figure 5B; data not shown). Our results indicate a strong interaction with a consensus binding site in the Atoh1 enhancer. Taken together, these data indicate that Sox2 binds to a consensus target sequence in the Atoh1 enhancer, suggesting that Atoh1 is a direct target of Sox2 in the developing cochlea.
Figure 5. Sox2 binds to the 3’ enhancer region of Atoh1 in vivo.

(A) Schematic representation of the Atoh1 gene including enhancer elements at the 3’end of the Atoh1 ORF with the putative Sox2 transcription factor binding site AACAAAG indicated. PCR primer pairs are designed to amplify the Sox2 consensus binding site following chromatin immunoprecipitation. (B) ChIP assays using anti-Sox2 or IgG control antibodies were performed on chromatin isolated from E14.5 cochlea. The equivalent fraction of the sonicated chromatin was treated as ‘input’ DNA which is not immunoprecipitated. PCR assay was used to determine immunoprecipitated chromatin for each primer set. A band of the expected size is present in the Sox2-precipitated and the input lanes but not in the IgG-precipitated or control lanes. n = 4 separate experiments.
DISCUSSION
Our understanding of the signaling pathways that direct cells within the otocyst towards a hair cell fate has increased significantly in recent years. The demonstration that Atoh1 is both necessary and sufficient for hair cell formation (Bermingham et al., 1999; Gubbels et al., 2008; Woods et al., 2004) followed by the discovery that Sox2 acts up stream of Atoh1 to induce the formation of the prosensory population (Dabdoub et al., 2008; Kiernan et al., 2005; Neves et al., 2012) established a molecular genetic pathway that could direct otocyst-derived cells towards a hair cell fate. More recent work has demonstrated roles for the homebox protein Six1 and co-activator Eya1 in the induction of Sox2 expression and for the Notch pathway genes, Jag1 and Rbpj in the maintenance of Sox2 expression (Ahmed et al., 2012; Kiernan et al., 2006; Yamamoto et al., 2011; Zou et al., 2008). However, significant gaps regarding the exact nature of the interactions between these factors still exist. The experiments described here were intended to further define the interactions between Sox2 and Atoh1 in hair cell induction.
The demonstration that the ability of Atoh1 to induce hair cell formation in embryonic cochlear epithelial cells is lost in the absence of Sox2 is consistent with Sox2 conferring hair cell/supporting cell competence to prosensory cells. This function of Sox2 appears to be conserved across vertebrates as studies in chicken and zebrafish have drawn similar conclusions based on both gain- and loss-of-function experiments ((Millimaki et al., 2010; Neves et al., 2012; Sweet et al., 2011). From a developmental stand point, competence can be defined as the ability of a particular progenitor cell to develop into a particular cell fate or lineage. In contrast, a molecular definition of competence can be considerably harder to elucidate. Sox2 is known to confer neuronal competence primarily through the positive induction of neural progenitor factors such as Nestin and Tlx (Graham et al., 2003; Shimozaki et al., 2012). A second possible mechanism for Sox2-mediated regulation of neural competence could be through epigenetic modification of possible target genes. The results presented here suggest a similar role for Sox2 in the development of hair cells; however, the specific progenitor factors that might be regulated by Sox2 have not been identified.
Atoh1 fails to induce hair cell formation in the absence of Sox2
Recent results from both in vivo and in vitro experiments indicate that expression of Atoh1 is sufficient to induce hair cells in multiple regions of the inner ear and cochlear duct, including regions, such as the spiral ligament, that are not thought to arise from prosensory cells and therefore are unlikely to have ever expressed Sox2 (Hume et al., 2007; Kelly et al., 2012). These results suggested the possibility that expression of Sox2 might not alter the state of prosensory cells beyond modulating the expression of Atoh1. However, the demonstration here that forced expression of Atoh1 in cochleae from Sox2Lcc/Lcc mutants is insufficient to induce hair cell formation clearly suggests that Sox2 acts to change the competence of otocyst cells in terms of their ability to respond to Atoh1. The specific changes that are mediated through Sox2 expression are unknown. One possibility would be that expression of Sox2 promotes competence through the induction of expression of progenitor factors, such as Egr-1, Nestin or Tlx that are required for the specification of cell identity in the nervous system. A similar role has been described for Sox2 during neural development (Wells et al., 2011) but the specific factors that are up-regulated in prosensory cells in response to Sox2 have not been determined. Six1/Eya1 have been shown to induce hair cell formation when electroporated into Kolliker's organ cells and their early expression in prosensory regions of the otocyst are consistent with a role in competence, but promoter binding studies demonstrated a direct role in expression of Atoh1, suggesting that Six1/Eya1 might regulate hair cell formation rather than competence. The requirement for transient expression of Sox2 would also seem to be consistent with this regulatory mechanism as it could lead to induction of factors that would induce transition to a progenitor state followed by down-regulation of those same genes to allow cellular differentiation (Bani-Yaghoub et al., 2006; Bylund et al., 2003; Klajn et al., 2014).
An alternate, or possibly complementary, role for Sox2 in specification of prosensory cell competence could be modification of the epigenetic status of prosensory cells that would regulate the responsiveness to Atoh1 targets. Sox2 has been shown to modulate expression of both HDACs and DNMTs, suggesting a possible mechanistic role in epigenetic modifications (Lyssiotis et al., 2007). Consistent with this idea is the observation of a loss of the ability of Atoh1 to induce hair cells over developmental time periods, also suggesting that epigenetic modifications may play a role in regulation of Atoh1 targets (Kelly et al., 2012; Stojanova et al., 2015). Similarly, the recent generation of Atoh1 transcriptomes for cells from the developing cerebellum and spinal cord have revealed both common and unique Atoh1 targets within each tissue (Klisch et al., 2011; Krizhanovsky et al., 2006). One of the mechanisms that can account for differential expression profiles arising from the same transcription factor is heterogeneity in the ability of that transcription factor to bind to specific promoter sequences as a result of differences in epigenetic modifications including methylation and acetylation states (Berger, 2007; Bonasio et al., 2010). Unfortunately, while hair cell transcriptomes have been recently published (Beisel et al., 2007; Burns et al., 2015; Liu et al., 2014), similar information on Atoh1 targets in hair cells is not yet available.
Atoh1 is a direct target of Sox2
Consistent with previous results from both chick and mouse (Ahmed et al., 2012; Neves et al., 2012), the experiments described here demonstrate direct binding of Sox2 to the Atoh1 enhancer. This result is consistent with the hypothesis that Atoh1 is directly up-regulated by Sox2, possibly in cooperation with Six1/Eya1, even though continued Sox2 expression acts to inhibit hair cell formation. The nature of this inhibition is unclear. Similar transient patterns of expression for Sox2 have been observed in developing neuronal tissue in which Sox2 is up-regulated in order to induce a neural progenitor state but then must be down regulated for final neuronal differentiation (Bylund et al., 2003). Giraldez and colleagues (Neves et al., 2012) have suggested that this type of interaction can be described by an “incoherent feedback loop” in which Sox2 in the inner ear, would simultaneously activate both Atoh1 and an Atoh1 inhibitor. The delay required for the buildup of the inhibitor would result in a transient expression of Atoh1, such as is observed in developing hair cells. This is an intriguing model and several factors that can act as direct inhibitors of Atoh1, including members of the Hes/Hey family of bHLHs, have been shown to be expressed in developing inner ear sensory epithelia (Petrovic et al., 2015). But, a specific mutant in which the transient expression of Atoh1 in hair cells is lost has not been described yet.
In contrast with hair cells, supporting cells maintain expression of Sox2 in to adult ages. Similar results have been shown for developing glial cells (Lang et al., 2011; Walters et al., 2015) and a recent report demonstrated a role for Sox2 in the differentiation of oligodendrocytes (Hoffmann et al., 2014). These findings raise the intriguing possibility that Sox2 could also play a role in supporting cell differentiation. Deletion of Sox2 at a late embryonic stage would probably be required to address this possibility.
Finally, developing ectopic hair cells induced through electroporation of Atoh1 were shown to be transiently positive for Sox2. In light of the other data presented in this study, this result is not surprising given that molecular effects mediated by Sox2 seem to be required for hair cell formation. However, the ability of Atoh1 to induce Sox2 expression, either directly or through the induction of a “proto-hair cell phase” is unexpected. Typically members of the Class II bHLH family are thought to drive progenitor cells along a differentiative pathway that does not include expression of factors, like Sox2, that are associated with less differentiated stages in development (Bylund et al., 2003). However, an analysis of the Sox2 promoter region indicates the presence of 4 E-box consensus sequences one of which also meets the criteria for an Atoh1 E-Box Associated Motif (Akazawa et al., 1995; Helms et al., 2000), a 10 nucleotide sequence highly correlated with Atoh1-binding (Klisch et al., 2011) . Therefore, a direct regulation of Sox2 by Atoh1 may occur during inner ear development. As discussed sbove, onset of Atoh1 expression correlates with the down-regulation of Sox2 in mammalian hair cells and co-expression of both factors in KO cells has been shown to lead to mutual antagonism, while in zebrafish sox2 expression is dependent on expression of Atoh1. These results suggest a potentially complex interaction between Atoh1 and Sox2 that may change depending on cellular context. For instance, Atoh1 expression in naïve cells may induce Sox2 expression while Atoh1 expression in a cell that is already positive for Sox2 might lead to inhibition. Additional experiments examining the specific abilities of Atoh1 to directly modulate Sox2 expression are clearly required.
Finally, it is important to consider that all of the experiments described here focused on cells located in Kolliker's organ. While these cells have been considered to be non-sensory cells based on their ultimate fates as part of the inner sulcus, there is growing evidence to suggest that it may be more appropriate to consider these cells as part of the prosensory population even though they do not develop as hair cells or supporting cells. Previous studies have demonstrated that these cells possess a high degree of plasticity including the ability to develop as hair cells, support cells or neurons following forced ectopic gene expression (Gubbels et al., 2008; Kawamoto et al., 2003; Puligilla et al., 2010; Woods et al., 2004; Zheng and Gao, 2000). Moreover, at least some of these cells are actively repressed from forming ectopic sensory patches through activation of the sonic hedgehog signaling pathway (Driver et al., 2008). Lineage tracing indicates that some of the cells arise from the neurosensory lineage, suggesting that they may have expressed Sox2 at some point in their development, and recent profiling of single cochlear cells indicates a high degree of transcriptional similarity between Kolliker's organ cells and some supporting cells within the organ of Corti (Burns et al., 2015). These observations suggest that by comparison with other non-sensory regions of the inner ear, Kolliker's organ cells may possess a higher level of competence for development among the otic neurosensory lineages. However, the results of other studies have demonstrated at least some potential for sensory development in other regions of the cochlear duct. In particular, forced expression of Atoh1 or activation of the canonical wnt signaling pathway is sufficient to induce additional sensory epithelia in the lateral region of the cochlear duct (Jacques et al., 2012; Kelly et al., 2012) and deletion of Lmo4 has recently been shown to result in a duplication of the organ of Corti within the spiral prominence (Deng et al., 2014). Therefore, additional experiments are clearly required to discern both the transcriptional differences and developmental potentials that exist in different non-sensory regions of the cochlear duct.
In summary, in this study we used ectopic hair cell formation in Kolliker's organ to examine the relationship between Sox2 and Atoh1. Our results demonstrate that Sox2 is essential for Atoh1-mediated hair cell formation and also confirm the hypothesis that Sox2 acts as both an activator of Atoh1, most likely through direct binding to the Atoh1 enhancer and as an Atoh1 antagonist through, as yet undetermined, mechanisms. Moreover, the results presented here also suggest that transient expression of Sox2 is crucial for ectopic hair cell formation and suggest that a decrease in the ability to reactivate expression of Sox2 could potentially underlie the decrease in the ability of Atoh1's to induce hair cell formation in postnatal cochleae.
ACKNOWLEDGEMENTS
We thank Drs Bradley Schulte (MUSC, Charleston SC) and Norio Yamamoto (Kyoto University, Kyoto, Japan) and Doris Wu (NIDCD, NIH, Bethesda, MD) for the critical reading of this manuscript. We thank Ms. Haque & Dr. Pandey from the Puligilla Laboratory and all the members of the Kelley Laboratory for technical assistance and valuable discussions during this work. This research was supported by NIH grant K99/R00 (C.P) and funds from the Intramural Program at NIDCD (M.W.K).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Beisel KW, Rocha-Sanchez SM, Ziegenbein SJ, Morris KA, Kai C, Kawai J, Carninci P, Hayashizaki Y, Davis RL. Diversity of Ca2+-activated K+ channel transcripts in inner ear hair cells. Gene. 2007;386:11–23. doi: 10.1016/j.gene.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612–616. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun. 2015;6:8557. doi: 10.1038/ncomms9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Luo XJ, Pan L, Yang H, Xie X, Liang G, Huang L, Hu F, Kiernan AE, Gan L. LMO4 functions as a negative regulator of sensory organ formation in the mammalian cochlea. J Neurosci. 2014;34:10072–10077. doi: 10.1523/JNEUROSCI.0352-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Sillers L, Coate TM, Rose MF, Kelley MW. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev Biol. 2013;376:86–98. doi: 10.1016/j.ydbio.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque KD, Pandey AK, Kelley MW, Puligilla C. Culture of embryonic mouse cochlear explants and gene transfer by electroporation. J Vis Exp. 2015:52260. doi: 10.3791/52260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hoffmann SA, Hos D, Kuspert M, Lang RA, Lovell-Badge R, Wegner M, Reiprich S. Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development. 2014;141:39–50. doi: 10.1242/dev.098418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis AK, Kelley MW, Dabdoub A. A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32:6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klajn A, Drakulic D, Tosic M, Pavkovic Z, Schwirtlich M, Stevanovic M. SOX2 overexpression affects neural differentiation of human pluripotent NT2/D1 cells. Biochemistry (Mosc) 2014;79:1172–1182. doi: 10.1134/S0006297914110042. [DOI] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci U S A. 2011;108:3288–3293. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Soreq L, Kliminski V, Ben-Arie N. Math1 target genes are enriched with evolutionarily conserved clustered E-box binding sites. J Mol Neurosci. 2006;28:211–229. doi: 10.1385/JMN:28:2:211. [DOI] [PubMed] [Google Scholar]
- Lang H, Li M, Kilpatrick LA, Zhu J, Samuvel DJ, Krug EL, Goddard JC. Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear. J Assoc Res Otolaryngol. 2011;12:151–171. doi: 10.1007/s10162-010-0244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Pecka JL, Zhang Q, Soukup GA, Beisel KW, He DZ. Characterization of transcriptomes of cochlear inner and outer hair cells. J Neurosci. 2014;34:11085–11095. doi: 10.1523/JNEUROSCI.1690-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters' cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32:6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis CA, Walker J, Wu C, Kondo T, Schultz PG, Wu X. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2007;104:14982–14987. doi: 10.1073/pnas.0707044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic J, Galvez H, Neves J, Abello G, Giraldez F. Differential regulation of Hes/Hey genes during inner ear development. Dev Neurobiol. 2015;75:703–720. doi: 10.1002/dneu.22243. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozaki K, Zhang CL, Suh H, Denli AM, Evans RM, Gage FH. SRY-box-containing gene 2 regulation of nuclear receptor tailless (Tlx) transcription in adult neural stem cells. J Biol Chem. 2012;287:5969–5978. doi: 10.1074/jbc.M111.290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanova ZP, Kwan T, Segil N. Epigenetic regulation of Atoh1 guides hair cell development in the mammalian cochlea. Development. 2015;142:3529–3536. doi: 10.1242/dev.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol. 2011;358:113–121. doi: 10.1016/j.ydbio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchikawa M, Kamachi Y, Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev. 1999;84:103–120. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Yamashita T, Zuo J. Sox2-CreER mice are useful for fate mapping of mature, but not neonatal, cochlear supporting cells in hair cell regeneration studies. Sci Rep. 2015;5:11621. doi: 10.1038/srep11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T, Rough K, Carter DA. Transcription Mapping of Embryonic Rat Brain Reveals EGR-1 Induction in SOX2 Neural Progenitor Cells. Front Mol Neurosci. 2011;4:6. doi: 10.3389/fnmol.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol. 2011;353:367–379. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]