Abstract
Superoxide radical is involved in numerous physiological and pathophysiological processes. Tetrathiatriarylmethyl (TAM) radicals are knows to react with superoxide allowing measurement of superoxide production in biological media. We report the synthesis of a Nitro conjugated TAM radical showing a rate constant of 7 × 105 M−1s−1 which is two order of magnitude higher than other TAMs allowing high sensitivity measurement of superoxide
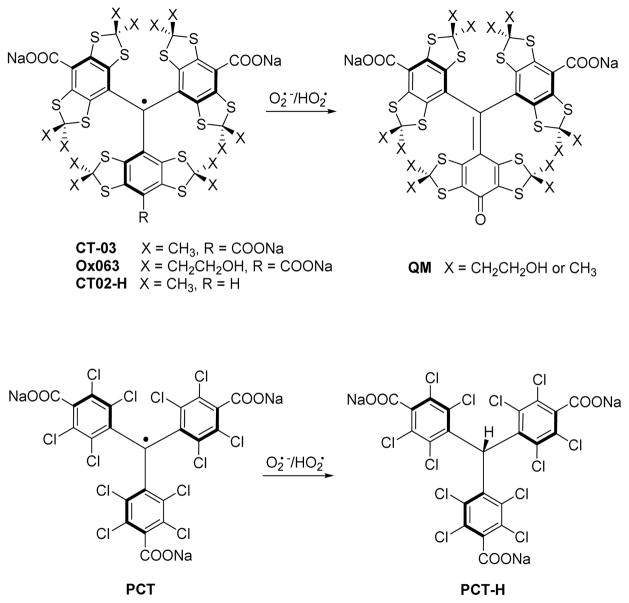
Superoxide radical formed by one electron reduction of molecular oxygen is one of the most biologically significant reactive oxygen species (ROS) involved in numerous physiological and pathophysiological processes. Trityl radicals have been recognized for their ability to react with superoxide allowing the assessment of superoxide production by electron paramagnetic resonance (EPR) or spectrophotometry in a biological media.[1] The chemical mechanism involved depends on the family of trityl radical used. Superoxide radical can add to a tetrathiatriarylmethyl radical (TAM) like CT-03 or Ox063 (Figure 1) in its para-position triggering the decarboxylation and leading to an EPR silent quinone-methide (QM) product.[2] This reaction occurs with a decay of the EPR signal. The apparent second order rate constants for Ox063 and CT-03 were reported to be about 3 × 103 M−1s−1 at physiological pH (7.4).[1, 3] The CT-02H (see Figure 1) derivative of TAM family where a carboxylic acid have been substituted by an hydrogen atom shows an increased reactivity toward superoxide with the rate constant equals to 1.7 × 104 M−1s−1.[3] In this molecule the superoxide radical reacts at the para-position substituted by the hydrogen. The increase reactivity can be explained by a lower steric hindrance and a lower electrostatic repulsion between the superoxide and the carboxylate anion.
Figure 1.
Structure and reactivity of trityl radicals with superoxide.
The reactivity of superoxide with a perchlorinated trityl (PCT) involved a reduction of the radical by superoxide leading to an EPR silent perchlorotriarylmethane. This reaction have been reported to be selective to superoxide as the radical exhibits high stability toward other reducing agents such as ascorbic acid. The selectivity could be explain by the steric hindrance of the paramagnetic center only accessible to small reducing agent. The bimolecular rate constant of this reaction was measured to be equal to 8.3 × 108 M−1s−1 making the superoxide measurement by EPR using PCT highly sensitive.
However, the intrinsic linewidth of a PCT radical is 500 mG[4] while it is less than 100 mG for a TAM radicals and less than 50 mG when the hydrogen nuclei of the methyl groups are substituted with deuterium nuclei.[5] Narrow linewidth provides a higher sensitivity of detection (the intensity is inversely proportional to the square of the linewidth) and is of primary importance for in vivo EPR oximetry.[6, 7] Moreover, no in vivo application of water soluble PCT radicals have been reported yet while TAM radicals have been extensively used in vivo and are considered to be remarkably nontoxic. In this work we explore an opportunity to combine the redox properties of the PCT radical with the narrow linewidth of TAM radical by introducing electron-withdrawing group in TAM structure which should increase the reduction potential and make a tetrathiatriarylmethyl radical reducible by the superoxide.
Materials and methods
Chemicals
Hypoxanthine (HX), xanthine oxidase (XO), diethylenetriaminepentaacetic acid (DTPA), Cu-Zn superoxide dismutase (SOD) and all solvents were purchased from Sigma-Aldrich. Potassium hexachloroiridate (IV) was purchased from Combi-Block. 15N-labeled sodium nitrite was purchased from Cambridge isotope. HyperSep C18 columns were purchased from Thermo Scientific.
Synthesis
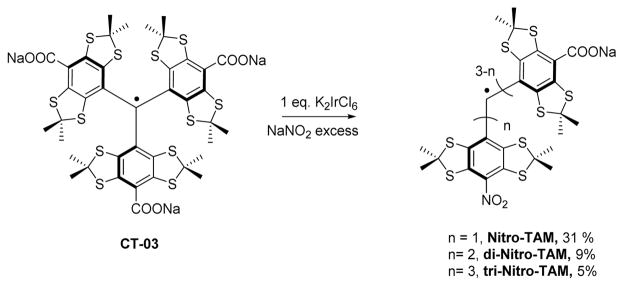
CT-03 was synthesized according to previously reported procedure[8]. Nitro-TAM (see Scheme 1) was synthesized by dissolving CT03 sodium salt (100 mg, 0.09 mmol, 1eq) in 50 mL of water, K2IrCl6 (46.3 mg, 0.09mmol, 1eq) was added and the solution was stirred for 10 min. NaNO2 (62 mg, 0.9 mmol, 10 eq) was added in one portion. The solution turned red and was stirred for 1h after that, aqueous HCl was added (10 mL, 1M) and compound extracted with ethyl acetate (3 × 10 mL). The combined organic layers were dried over MgSO4 and the solvent removed under reduced pressure. The crude residue was purified on HyperSep C18 column (10g/75 mL) using a gradient of elution from water:acetonitrile (20:80) containing 0.1 % of trifluoroacetic acid (TFA) to acetonitrile containing 0.1 % of TFA. The Nitro-TAM was isolated in 31 % yield. 42 % of CT-03 was recovered. 15N labeled mono-nitro TAM (nTAM) was synthesized using the same procedure using 15N-labeled sodium nitrite.
Scheme 1.
Synthesis of Nitro-TAM
Cyclic Voltammetry
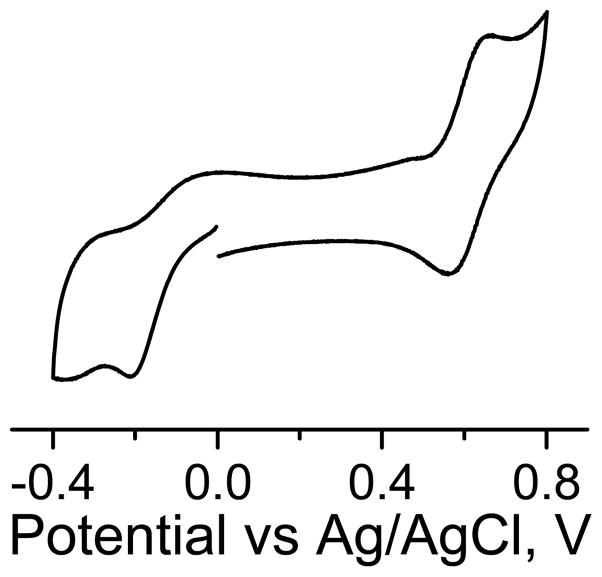
The cyclic voltammograms was recorded using a computer controlled Metrohm Autolab PGSTAT128N Potentiostat. 5 mL of 500 μM of nTAM in 0.1M phosphate buffer (PB), pH = 7.4, was place in the electroanalytical cell. Oxygen was removed by bubbling argon for 10 min. A glassy carbon electrode was used as working electrode, a platinum wire as counter electrode and an Ag/AgCl 3M NaCl as reference electrode. Scan rate was 0.2V/s. Starting potential 0 V, lower potential −0.4 V, upper potential +0.8V.
HPLC
All chromatograms were recorded on a Waters Alliance e2695 system equipped with a 2998 PDA detector using an XBridge 4.6 mm × 50 mm, 2.5 μM. Solvent A was water, solvent B acetonitrile and Solvent C water containing 1% of TFA. The condition of elution were as following: flow rate = 1.5 mL/min, column temperature = 40°C, t=0min 90%A/10%B/10%C, t=5min 0%A/90%B,10%C, t=6min 0%A/100%B/0%C. Run time = 8 min, UV detection from 200 nm to 800 nm.
EPR spectroscopy
All spectra were recorded on a Bruker X-Band EMX EPR spectrometer, the samples were loaded in glass capillaries 50 μL. For spectra recorded in deoxygenated solution, the samples were bubbled with argon gas for 15 min before loading into capillaries. Typical EPR parameters were as following: sweep width, 2.5 G; power, 0.2mW; modulation frequency, 10 KHz; modulation amplitude, 0.05G; conversion time, 20.48 ms; time constant, 40.96 ms; resolution, 1024 pt.
UV spectroscopy
UV-Vis spectra were recorded on Cary 50 UV-Vis spectrometer.
Determination of rate constant with superoxide
Estimation of bimolecular rate constant of 15N-substituted Nitro-TAM (nTAM) reduction by superoxide was determined by standard competition approach using superoxide dismutase as competitor. In this experiment superoxide is generated by xanthine oxidase with the rate V(XO) and is consumed in the competing reactions of superoxide ( ) with nTAM and superoxide dismutase (bimolecular rate constants, k and kSOD, correspondingly):
| (1) |
In the steady-state conditions, the rate of superoxide-induced nTAM reduction measured in the absence of SOD, V(no SOD), is equal to the rate of superoxide generation, V(XO), therefore:
| (2) |
| (3) |
| (4) |
where V(SOD) – the rate of nTAM reduction at given SOD concentration.
The linear fitting of the experimental data on inhibition of superoxide-induced nTAM reduction by SOD using equation (4) and assuming the value of kSOD of 2·109 M−1s−1 [9]yields the value of bimolecular constant, k.
Results and discussion
Synthesis
Recently Decross et al. reported an elegant way to synthesize asymmetrical TAM radicals through the substitution of one carboxylic acid of CT-03 by various –N, -S and –P nucleophiles.[10] This reaction involved the formation of the CT-03 cation by a water soluble iridium (IV) complex. The addition of the nucleophile triggers the decarboxylation leading to the mono-functionalized TAM. However, when sodium nitrite was used, a mixture of mono-, di- and tri-nitro TAM was observed by HPLC-MS (Scheme 1).[10] Using an excess of oxidant, the non-water soluble tri-nitro compound was isolated. We hypothesized that the mono-nitro TAM should be an excellent candidate for being efficient superoxide trap due to the expected increase of the reduction potential in the presence of highly electron- withdrawing group and high aqueous solublity provided by the presence of two carboxyl groups in the structure
The CT-03 was synthesized as previously reported and subsequently reacted with one equivalent of K2IrCl6 to procure quantitatively the trityl cation in water. Then, an excess of sodium nitrite was added.
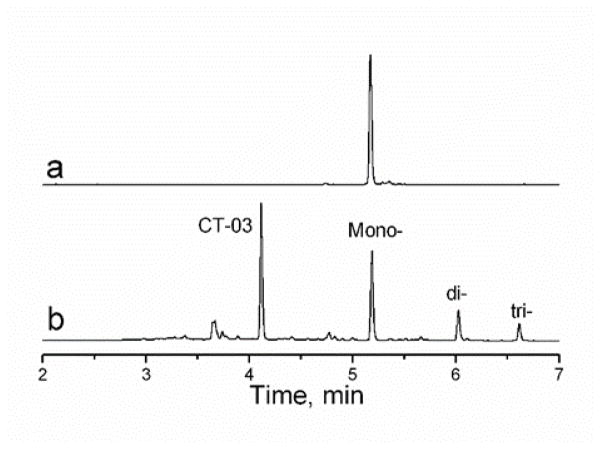
The reverse phase HPLC chromatogram of the reaction crude showed a mixture of CT-03, mono-, di- and tri-nitro TAM in agreement with the literature (Figure 2, b). Purification on C18 column allowed isolation of the mono nitro-TAM (Nitro-TAM) in 31% yield. The purity was evaluated to 95% by HPLC (Figure 2, a).
Figure 2.
Reverse phase HPLC chromatograms. a) Purified mono-nitro-TAM (Nitro-TAM).. b) Reaction crude showing the mixture of CT-03, mono-, di- and tri-nitro substituted TAM
Physicochemical characterization
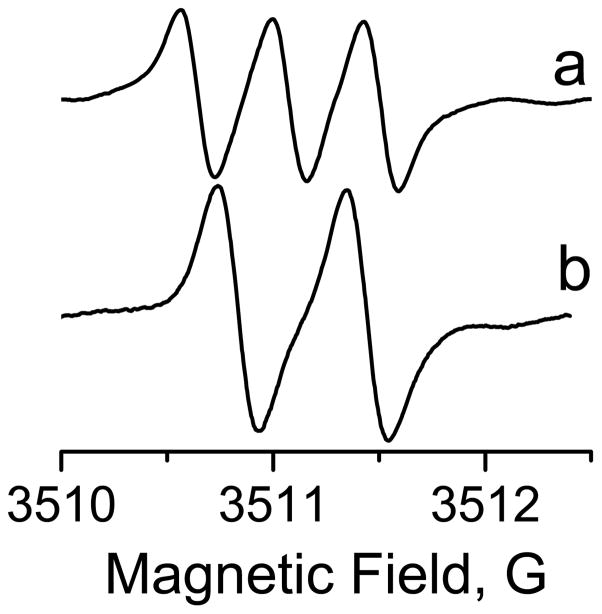
The radical was stable in phosphate buffer pH = 7.4 as no degradation was observed after 24h at room temperature. The X-Band EPR spectrum was recorded in phosphate buffer (0.1 M, pH = 7.4). According to the chemical structure, the spectrum exhibits a triplet (1:1:1) pattern as a result of the interaction of the odd electron with the 14N nucleus of the nitro moiety (SN14=1) With the hyperfine splitting constant (hfc), aN, equals to 440 mG (Figure 3a). In order to simplify the spectrum and increase the signal to noise ratio the 15N labeled mono-nitro TAM (nTAM) was synthesized using the same procedure. As the 15N nucleus possesses a spin of SN15=1/2, the spectrum of nTAM exhibits a well resolved doublet with aN =610 mG. The ratio of hfs for 15N- and 14N-substituted mono-nitro-TAMs of 1.4 is in a good agreement with the ratio of the gyromagnetic ratios of the two nitrogen isotopes.
Figure 3.
X-band EPR spectra of 14N (a) and 15N (b) labeled Nitro-TAMs (100 μM) in 0.1 M phosphate buffer, pH 7.4, under normal air pressure.
The EPR spectrum of nTAM recorded under deoxygenated solution shows a linewidth of 120 mG. The linewidth increases linearly with the concentration of oxygen due to Heisenberg spin exchange-induced line broadening to reach 200 mG under normal air condition (pO2=160 mmHg) allowing the measurement of dissolved molecular oxygen with a sensitivity of about 1 mmHg (80 mG broadening per 160 mmHg of pO2). Note that PCT radical shows only 40 mG line broadening when compared in deoxygenated and normal air conditions. This could be explained by a larger contribution of an oxygen-insensitive Gaussian component in the EPR line shape of the PCT radical compared with that for nTAM.
The effect of electron-withdrawing nitro group on the redox potential was measured using cyclic votammetry (Figure 4). The cyclic votrammogram shows a reduction of the nTAM presumably to the trityl anion at a half-wave potential of −0.147 V. This results supports an expected effect of the nitro group on redox properties of TAM as it increases the reduction potential by 0.495 V compared with the CT-03. The PCT shows a redox half-wave a-0.320 V (Table 1). This comparison shows that the nTAM can be reduced more easily than the PCT and therefore should be reduced by superoxide anion (reduction potential, −0.523 versus Ag/AgCl). On positive potentials, the nTAM is oxidized to the cation with a half wave of +0.615 V. As expected the nitro group makes the oxidation of the radical more difficult. Redox potentials of nTAM, CT-03 and PCT are summarized in the Table 1.
Figure 4.
Cyclic voltammogram of 1mM nTAM in phosphate buffer, pH = 7.4, scan rate = 0.2 V/s.
Table 1.
Half-wave redox potential for CT-03, PCT and nTAM
| Compound | Solvent | E1/2 oxa | E1/2 reda |
|---|---|---|---|
| CT-03[11] | PBS, pH=7.4 | +0.434 | −0.642 |
|
|
|||
| PCT[4] | PBS, pH=7.4 | / | −0.320 |
|
|
|||
| nTAM | PB, pH = 7.4 | +0.615 | −0.147 |
|
|
|||
Calculated according to E1/2 = (Epa + Epc)/2
Reactivity with superoxide
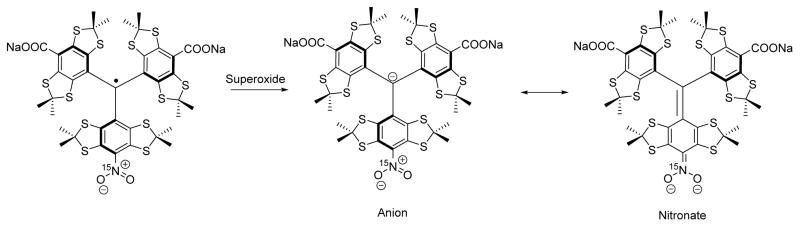
In order to assess the reactivity of the mono-nitro-TAM derivatives toward superoxide, nTAM was incubated in hypoxanthine / xanthine oxidase superoxide generating system. The reaction was followed by EPR and spectrophotometrically. Upon the reaction, appearance of a peak around 610 nm in the UV-Vis spectrum was observed, (Figure 5), which might result from the formation of an nTAM anion (Scheme 2). No UV-Vis spectral change was observed after 2h at room temperature An absorption peak at 640 nm was previously reported for the TAM anion.[12]
Figure 5.
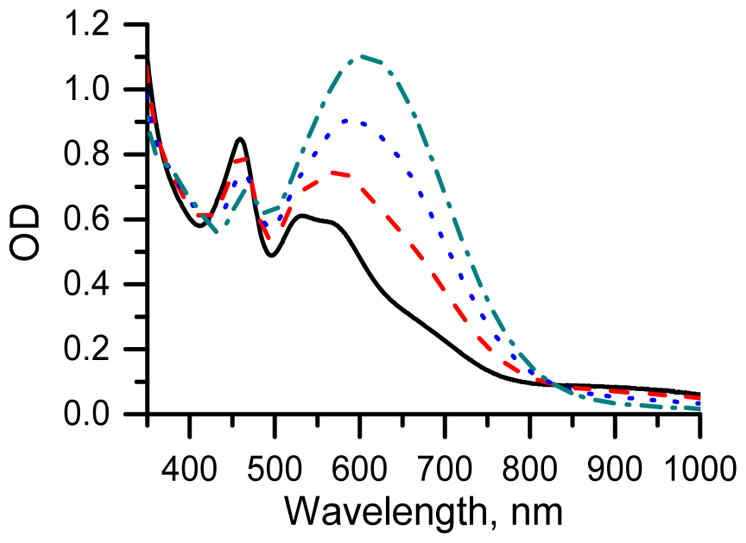
The UV-Vis spectra of 100 μM nTAM in the presence of HX/XO superoxide generating system ([HX] =0.25 mM, [XO] =10 mU/ml) in 0.1 M phosphate buffer solution, pH = 7.4 (dotted lines corresponds to different incubation times of 6, 13 and 25 minutes). Solid line represents the UV-Vis spectrum of nTAM in the absence of HX/XO.
Scheme 2.
Postulated formation of the trityl anion in resonance with its nitronate form.
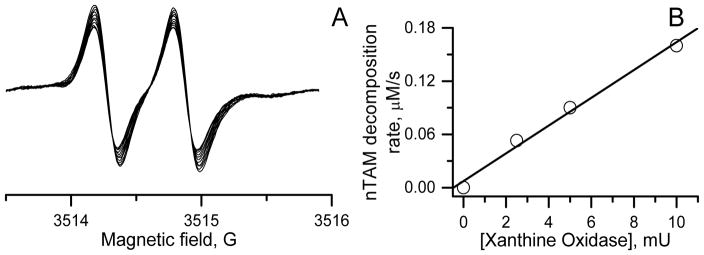
EPR measurements in HX/XO superoxide generating system (Figure 6) show decay of the signal indicating that that nTAM is converted to an EPR-silent compound.
Figure 6.
A. The time evolution of the EPR spectra of 100 μM nTAM in superoxide generating system that contains 0.25 mM of hypoxanthine and 10 mU/ml of xanthine oxidase in 0.1 M phosphate buffer. B. The dependence of rate of nTAM EPR signal decay on concentration of xanthine oxidase.
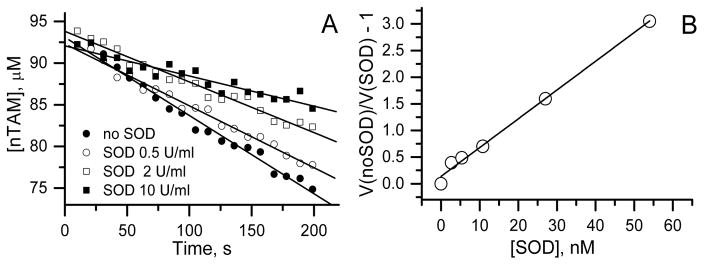
The second order rate constant measured using superoxide dismutase as competitor (see material and method for the details) has been found to be equal to 7 × 105 M−1s−1which is two order of magnitude higher than previously reported rate constants for the TAM radicals (Figure 7)
Figure 7.
A. The kinetics of EPR signal decay of nTAM radical upon superoxide generation in HX/XO system ([HX] =0.25 mM, [XO] =10 mU/ml) in the presence of different SOD concentrations. B. The dependence of relative efficiency of SOD-induced inhibition of nTAM reduction by superoxide on SOD concentration. Linear fit yields the bimolecular rate constant of nTAM reduction by superoxide equal to 7 × 105 M−1s−1. See Materials and Method Section for the details.
The high rate constant of the reaction with the superoxide and high aqueous solubility makes the nTAM useful probe for superoxide detection in biological systems.
Acknowledgments
This work was partially supported by NIH (USA) grants: EB016096, CA194013, CA192064, U54GM104942 and F.S.R Université catholique de Louvain Fellowship awarded to B.D. The WVCTSI is acknowledged for start-up to V.V.K, A.A.B The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Kutala VK, Parinandi NL, Zweier JL, Kuppusamy P. Reaction of superoxide with trityl radical: implications for the determination of superoxide by spectrophotometry. Arch Biochem Biophys. 2004;424:81–88. doi: 10.1016/j.abb.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher J-L. Oxidation of tris-(p-carboxyltetrathiaaryl)methyl radical EPR probes: evidence for their oxidative decarboxylation and molecular origin of their specific ability to react with O2[radical dot] Chem Commun. 2009:1416–1418. doi: 10.1039/b819259f. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Song Y, De Pascali F, Liu X, Villamena FA, Zweier JL. Tetrathiatriarylmethyl radical with a single aromatic hydrogen as a highly sensitive and specific superoxide probe. Free Rad Biol Med. 2012;53:2081–2091. doi: 10.1016/j.freeradbiomed.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutala VK, Villamena FA, Ilangovan G, Maspoch D, Roques N, Veciana J, Rovira C, Kuppusamy P. Reactivity of Superoxide Anion Radical with a Perchlorotriphenylmethyl (Trityl) Radical. J Phys Chem B. 2008;112:158–167. doi: 10.1021/jp076656x. [DOI] [PubMed] [Google Scholar]
- 5.Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV. Fourier Transform EPR Spectroscopy of Trityl Radicals for Multifunctional Assessment of Chemical Microenvironment. Ang Chem Int Ed. 2014;53:2735–2738. doi: 10.1002/anie.201310841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhimitruka I, Bobko AA, Eubank TD, Komarov DA, Khramtsov VV. Phosphonated Trityl Probes for Concurrent in Vivo Tissue Oxygen and pH Monitoring Using Electron Paramagnetic Resonance-Based Techniques. J Amer Chem Soc. 2013;135:5904–5910. doi: 10.1021/ja401572r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elas M, Magwood JM, Butler B, Li C, Wardak R, DeVries R, Barth ED, Epel B, Rubinstein S, Pelizzari CA, Weichselbaum RR, Halpern HJ. EPR Oxygen Images Predict Tumor Control by a 50% Tumor Control Radiation Dose. Cancer Res. 2013;73:5328–5335. doi: 10.1158/0008-5472.CAN-13-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driesschaert B, Levêque P, Gallez B, Marchand-Brynaert J. Tetrathiatriarylmethyl Radicals Conjugated to an RGD-Peptidomimetic. Eur J Org Chem. 2014;2014:8077–8084. [Google Scholar]
- 9.Pacher P, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Phys Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decroos C, Prange T, Mansuy D, Boucher JL, Li Y. Unprecedented ipso aromatic nucleophilic substitution upon oxidative decarboxylation of tris(p-carboxyltetrathiaaryl)methyl (TAM) radicals: a new access to diversely substituted TAM radicals. Chem Commun. 2011;47:4805–4807. doi: 10.1039/c1cc10426h. [DOI] [PubMed] [Google Scholar]
- 11.Driesschaert B, Levêque P, Gallez B, Marchand-Brynaert J. RGD-conjugated triarylmethyl radical as probe for electron paramagnetic imaging. Tet Lett. 2013;54:5924–5926. [Google Scholar]
- 12.Decroos C, Balland V, Boucher J-L, Bertho G, Xu-Li Y, Mansuy D. Toward Stable Electron Paramagnetic Resonance Oximetry Probes: Synthesis, Characterization, and Metabolic Evaluation of New Ester Derivatives of a Tris-(para-carboxyltetrathiaaryl)methyl (TAM) Radical. Chem Res Toxicol. 2013;26:1561–1569. doi: 10.1021/tx400250a. [DOI] [PubMed] [Google Scholar]