Abstract
Prior findings indicate that adolescent rats exhibit difficulty using negative occasion setters to guide behavior compared to adult rats [12]. Here, additional groups of juvenile rats were trained in the same negative occasion setting procedure to further define the development of negative occasion setting. Beginning on either postnatal day (PND) 30, 40, or 50, rats received daily training sessions in which a tone was paired with food reinforcement on some trials, while on other trials a light preceded the tone and no reinforcement was delivered. We found that rats that began training on PND 50 required 10 training sessions to discriminate between the two types of trials, consistent with prior findings with young adult rats. Interestingly, rats in the PND 30 group (pre-adolescents) also required just 10 training sessions, in stark contrast to adolescent rats that began training on PND 35 (adolescents) and required 18 sessions [12]. Rats that began training on PND 40 (adolescents) also required more sessions than the PND 30 group. These data indicate that the development of negative occasion setting is non-linear and have direct bearing on understanding the behavioral and neural substrates that underlie suboptimal behavioral control in adolescents.
Keywords: Adolescence, Inhibition, Behavior, Learning
1. Introduction
An occasion setter is a cue that provides information that resolves the ambiguity of another stimulus and modulates behavior that is directed to it [1–4]. In the case of a negative occasion setter, the cue indicates that the response to another stimulus should be withheld. In this way, negative occasion setters have direct bearing on adaptive behavior in that they indicate the conditions under which a response will not be associated with an anticipated outcome and should be inhibited. Importantly, negative occasion setting reflects a form of learning that is not readily accounted for by standard models of associative learning [e.g., 5]. Indeed, there are currently several different theories that have been proposed to explain negative occasion setting [e.g., 3, 6–10]. Moreover, only a few studies have considered the brain systems that support negative occasion setting or the ontogeny of this form of learning.
We recently began to address the latter issue by training adult and adolescent rats in a serial feature negative discrimination procedure that produces negative occasion setting [11]. Beginning on either postnatal day (PND) 35 (adolescent group) or PND 70 (adult group), rats received daily training sessions consisting of two trial types. On reinforced trials, a ‘target’ stimulus (a tone) was presented by itself and immediately followed by delivery of food reinforcement. On non-reinforced trials, a light (the ‘feature’ stimulus) was presented before the tone and on those trials no food was delivered. After 10 daily sessions, adult rats approached the food cup during presentation of the tone significantly more on trials when it was presented alone, compared to trials when it was preceded by the light [12], consistent with prior studies using normal adults rats and the same procedures [11, 13–15]. In comparison, the adolescent group required almost twice as many (~18) sessions to successfully discriminate between the two trial types [12]. Further, we demonstrated that adolescents could in fact learn the dual meaning of the tone in as few sessions as adults, but were unable to express that learning until they reached ~53 days old. Interestingly, that age corresponds to the time at which the prefrontal cortex (PFC) is thought to be fully mature in rats [16], suggesting that PFC is necessary for successful negative occasion setting. Indeed, we have also found that lesioning the PFC of adult rats impairs negative occasion setting in that rats could not discriminate between the two types of trials [14].
In the present study, we trained additional groups of juvenile rats to further investigate the ontogeny of negative occasion setting. One group began training beginning on PND 30 (i.e., pre-adolescence) [17], which is earlier than the adolescent group in our prior study. We hypothesized that if a fully mature PFC is necessary and sufficient to exhibit negative occasion setting, then the PND 30 group would require at least as many training sessions to exhibit negative occasion setting as rats that began training on PND 35 in our prior study (18 sessions) [12], suggestive of a linear development of inhibitory-related behavior and functionality of PFC [18–21]. An alternative outcome was that the PND 30 group would exhibit adult-like performance and discriminate between the two trials types after only ~10 sessions. That would be consistent with the alternate theory that certain types of behavior instead develop in a non-linear fashion. Indeed, Casey and others have posited that the differential development of top-down control systems and subcortical reward areas results in a functional imbalance that affects behavioral control specifically during adolescence [22–27]. The development of PFC lags behind the development of regions such as the nucleus accumbens (NAC) during adolescence and accordingly, activity is disproportionately higher in NAC than PFC during adolescence compared to either childhood or adulthood [23, 24, 27–31]. If this were the case, then rats that began training prior to adolescence (i.e., the PND 30 group) would be expected to be comparable to adults with regard to the number of sessions that are required to exhibit negative occasion setting. In addition, we included a group of rats that began training on PND 50 and predicted that they would exhibit discrimination after ~10 sessions, as shown previously [12] because the balance between PFC and NAC is purportedly resolved by that age. We also included a group of rats that began training on PND 40. Since this group began training as adolescents, like the PND 35 group in our prior study, we expected that they would require more sessions than the PND 50 group but fewer than the PND 35 group in our prior study. This latter prediction was made because rats in the PND 40 group would reach 53 days of age (i.e., maturation of PFC) after fewer training sessions than the PND 35 group.
2. Materials and Methods
2.1 Subjects
Male Long Evans rats (n = 36) were obtained from Harlan Laboratories (Indianapolis, IN). Rats were weaned from their dam on PND 21, and were shipped and received on the same day. Rats were housed individually with free access to water at all times during the study. Food (2014 Teklad Global 14% Protein Rodent Maintenance Diet, Harlan Laboratories) was available ad libitum up until four days prior to the first day of training. Rats were handled and weighed daily during the week prior to behavioral training and body weights were gradually reduced over a four day period to 85% of the daily weight of free-feeding age-matched control rats. All groups remained food restricted until completion of behavioral training, with supplemental rat chow provided after each daily session to maintain the target weight. The colony room was maintained on a 14:10-h light-dark cycle and monitored and cared for in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
2.2 Behavioral apparatus
Behavioral procedures took place in standard conditioning chambers (24 × 30.5 × 29 cm; Med Associates) constructed of aluminum front and back walls, clear acrylic sides and top, and grid floors. Each chamber was outfitted with a dimly illuminated food cup, recessed in the center of the front wall, a 2.8 W white panel light located 5 cm above the opening to the food cup, and a speaker located 15 cm above and to the right of the food cup, used to present the 1500 Hz, 78 dB auditory stimulus. Each chamber was enclosed in a sound-attenuating cubicle (62 × 56 × 56 cm) with an exhaust fan to provide airflow and background noise (~68 dB) and a red house-light (mounted on the ceiling) to provide background illumination. Delivery of two 45 mg grain-based food pellets (Bio-serv) served as the unconditioned stimulus. Each chamber was equipped with a pair of infrared photocells located across the entrance to the food cup to monitor entries into the cup and connected to a PC-clone computer. The cubicles also contained surveillance cameras used to monitor the rats during behavioral training.
2.3 Behavioral Procedure
At the start of every training session rats were weighed and moved in plastic transporters from the colony room to the conditioning chambers. Rats were first trained to eat from the food cup during a single 64 min session in which 45 mg food pellets were randomly delivered 16 times (average intertrial interval (ITI) of 4 min; ranging from 2.5 to 5.5 min). Subsequently, behavioral training in the negative occasion setting paradigm consisted of daily 68 min sessions with four reinforced and 12 non-reinforced trials. During reinforced trials the tone was presented for 5 sec and followed immediately by the delivery of two food pellets. Rats received a total of ~0.36 g of food pellet reinforcer per session. On non-reinforced trials, the panel light was presented for 5 sec, followed by a 5 sec empty period, and then a 5 sec presentation of the tone, after which no food was delivered. The two trial types occurred randomly during each session and the presentation order along with the inter-trial intervals varied daily (average ITI of 4 min, ranging from 2.5 to 5.5 min). Rats began training on PND 30 (n = 12), PND 40 (n = 12) or PND 50 (n = 12) and continued training for 16 daily sessions.
2.4 Data analyses
The primary variable of interest was the number of sessions that were required until rats exhibited successful discrimination between reinforced and non-reinforced presentations of the tone. To assess this, the amount of time that the infrared photobeam located across the entry of the food cup was broken during the presentation of the tone was recorded during each trial. This data was averaged across rats in each group for each trial type and training session. The difference in responding between trial types was obtained by subtracting the time spent in the food cup during the tone on non-reinforced trials from the time spent in the food cup during the tone on reinforced trials. Z-scores were calculated by dividing that result by the SEM of the difference in responding across all rats in a group. Successful discrimination between the two trial types was defined as a greater amount of time spent in the food cup during presentation of the tone on reinforced trials than during non-reinforced trials (a Z-score of at least 2.325, P < 0.01) for 3 consecutive daily sessions, as in prior studies [12, 32].
To test for potential age-related differences in overall levels of conditioned responding, the amount of time spent in the food cup during the presentation of the tone on reinforced and non-reinforced trial types was compared between the three age groups across all training sessions. Food cup behavior was then subjected to a repeated measures ANOVA with age as the between subjects factor and session and trial type as within subjects factors.
To test for potential age-related differences in baseline responding the amount of time spent with the head in the food cup was assessed during the 5 sec period immediately before any stimuli were presented (Pre-CS period). Food cup behavior during this epoch was calculated for each rat during each trial type, collapsed across sessions and subjected to a one-way multivariate ANOVA.
Finally, to test for differences in motivation, food cup responding immediately after food was delivered (Post-CS period) was assessed. This data was collapsed across sessions and subjected to a one-way ANOVA. All significant ANOVAs were succeeded with appropriate pairwise comparisons using an alpha level of 0.05. All analyses were carried out using SPSS and R statistical packages.
3. Results
3.1 Number of sessions to reach criterion for discrimination
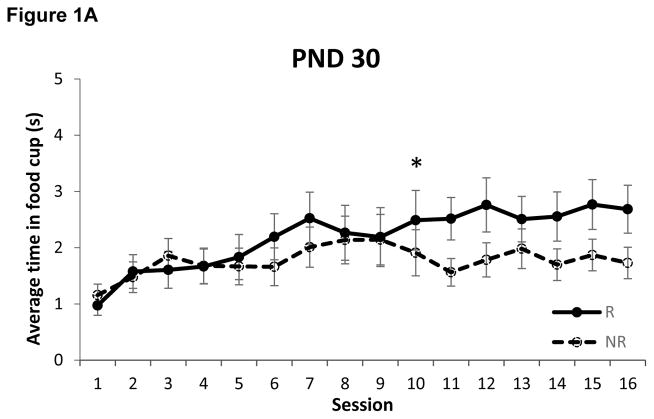
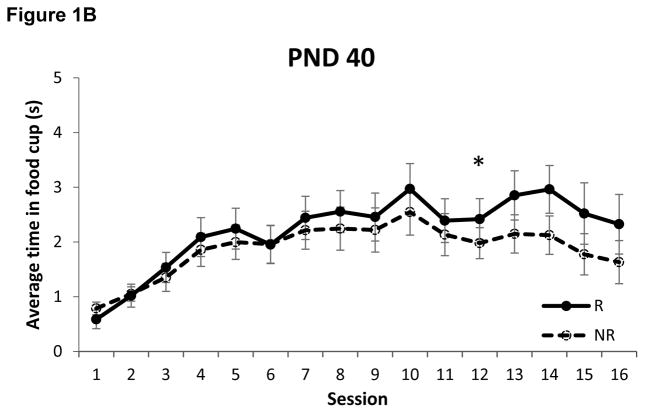
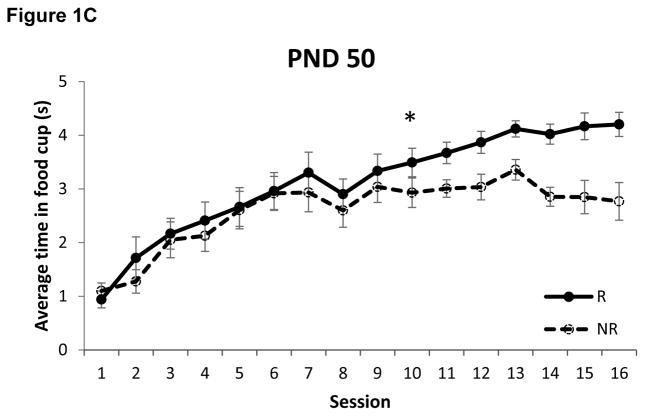
The amount of time spent with the snout inside the food cup during presentation of the tone on each of the two trial types is presented in Figure 1. Rats that began training on PND 50 required 10 sessions (Z = 3.42, p < 0.0005) to discriminate between the reinforced and non-reinforced trials, replicating our prior finding [12]. Interestingly, rats that began training on PND 30 also required just 10 sessions to exhibit negative occasion setting (Z = 2.63, p < 0.005). In comparison, adolescent rats that began training on PND 40 required 12 sessions (Z = 3.05, p < 0.005) to discriminate between the trial types.
Figure 1.
Food cup behavior during presentation of the tone on reinforced (R) and non-reinforced (NR) trials in rats that began training on postnatal day (PND) 30 (panel A), PND 40 (panel B) and PND 50 (panel C). Rats that began training on either PND 30 or PND 50 learned to discriminate between trial types on session 10, while rats that began training on PND 40 did not exhibit successful discrimination until session 12. * p < 0.01. Data are means ± SEM.
3.2 Overall conditioned responding
An analysis of time spent in the food cup during presentation of the tone revealed that responding during reinforced trials was significantly higher than during non-reinforced trials [main effect of trial type; F(1, 33) = 66.71, p < 0.001]. An interaction between trial type and session confirmed that rats are able to learn to differentiate responding based on trial type across training [F(15, 495) = 13.56, p < 0.001]. However a lack of interaction between age and trial type [F(2, 33) = 1.31, p > 0.2] as well as age, trial type and session [F(30, 495) = 1.45, p > 0.1] suggests that all rats exhibited a comparable magnitude of discrimination between trial types. A main effect of session [F(15, 495) = 23.32, p < 0.001] indicated an increase in conditioned responding across training. In addition, a significant interaction between session and age [F(30, 495) = 2.13, p < 0.005] revealed that rats that began training on PND 50 reached higher levels of responding sooner than rats that began training on either PND 40 or PND 30. This was accompanied by significantly higher overall levels of conditioned responding between subjects [F(2, 33) = 3.67, p < 0.05]. Post hoc comparisons using the Fisher LSD test revealed group PND 50 spent more time in the food cup overall than group PND 40 (p < 0.05) and group PND 30 (p < 0.05), but the latter two groups did not differ (p > 0.8).
3.3 Pre-CS Responding
The amount of food cup behavior exhibited during the 5 second epoch prior to the start of a trial (pre-CS) was very low and did not differ between the three age groups [F(4, 64) = 0.19, p > 0.9]. The average time spent in the food cup during this period on reinforced trials was (0.66 ± 0.13 s for PND 30 rats, 0.61 ± 0.07 s for PND 40 rats, and 0.63 ± 0.12 s for PND 50 rats) and on non-reinforced trials was (0.60 ± 0.11 s for PND 30 rats, 0.52 ± 0.06 s for PND 40 rats, and 0.55 ± 0.08 s for PND 50 rats).
3.4 Post-CS Responding
Responding immediately after termination of the tone on reinforced trials (coincident with food delivery) differed between age groups [F(2, 33) = 7.07, p < 0.005]. Follow-up analyses revealed this effect was due to lower levels of post-CS responding by PND 30 rats (3.8 ± 0.30 s) than PND 40 rats [4.6 ± 0.11 s; t(22) = −2.5, p < 0.03] as well as PND 50 rats [4.8 ± 0.12 s; t(22) = −3.0, p < 0.01]. However, PND 40 rats did not differ from PND 50 rats [t(22) = −1.2, p > 0.2]. Importantly, satiety is unlikely to account for differences in post-CS responding because all rats consumed all food pellets delivered in each session.
4. Discussion
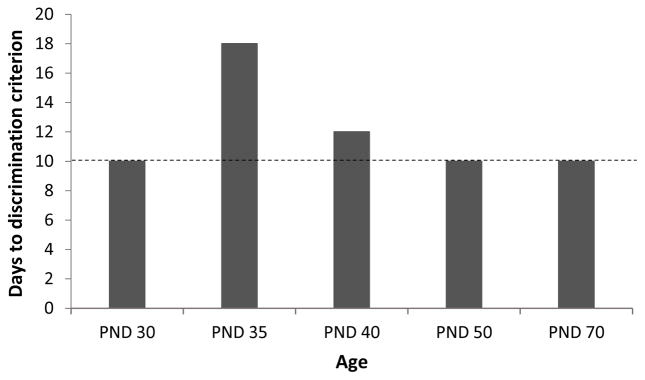
A primary goal of the present study was to determine if the development of negative occasion setting is linear or non-linear across development. To that end, juvenile rats began training in a negative occasion setting procedure either on PND 30 (pre-adolescents), PND 40 (adolescents), or PND 50 (late-adolescents/young adults). As in our prior study [12], we found that rats in the PND 50 group required 10 sessions to successfully discriminate between reinforced and non-reinforced presentations of the target cue, like fully-adult rats that started training on PND 70 [12]. Interestingly, we found that the PND 30 group likewise required only 10 sessions to discriminate, which stands in marked contrast to our prior finding that rats that began training on PND 35 required 18 sessions to successfully discriminate between the trial types [12], as illustrated in Figure 3. This demonstrates that the development of negative occasion setting is non-linear, consistent with current models that maintain that adolescence is a unique period of development compared to either pre-adolescence or adulthood [23, 24, 27].
Figure 3.
A schematic of the number of sessions required to discriminate between reinforced and non-reinforced trials in the present experiment (rats that began training on postnatal day (PND) 30, 40 or 50) as well as previously published findings (rats that began training on PND 35 or 70; [12]).
Importantly, in the present experiment, food cup behavior prior to stimulus onset was equivalent across all groups, indicating that there were no age-related differences in the motivational significance of the food cup. PND 30 rats did exhibit lower levels of food cup behavior after food was delivered (post-CS) compared to both PND 40 and PND 50 rats. However, because these rats still discriminated in 10 sessions, it is unlikely that these levels of responding reflect differences in motivation to obtain food. We did observe that conditioned responding (i.e., time spent in the food cup during the tone) reached higher levels in group PND 50 than either group PND 30 or group PND 40. However, it is unlikely that this difference contributed to the adolescent delay in withholding responding. Indeed, rats in group PND 30 learned the discrimination in the same amount of training (10 sessions) as rats in group PND 50, despite having lower levels of conditioned responding comparable to rats in group PND 40. Furthermore, no age differences were observed in the magnitude of the difference in responding during the tone on reinforced compared to non-reinforced trials. This indicates that despite differences in overall levels of conditioned responding, rats in all groups were able to discriminate between trial types to the same extent.
Contemporary research regarding adolescence has suggested that the non-linearity of adolescent behavioral phenotypes is thought to reflect an imbalance in activity in subcortical reward regions and top-down control regions that exists specifically during adolescence [21, 23, 24, 26–31, 33, 34]. In particular, there is evidence that subcortical regions, such as NAC, attain functional maturity during adolescence [19, 21, 35, 36], whereas both structural and functional maturation of PFC lags behind during this period [37–41]. In contrast, in both pre-adolescents and adults, there is a relative balance between NAC and PFC activity, with both regions being mature in adults and both regions being immature in pre-adolescents [21, 23, 24, 34, 42, 43]. Consistent with this imbalance hypothesis, the continued structural and functional development of frontostriatal circuitry in humans [28, 33] and animals [29–31] has been implicated in establishing the efficiency of cognitive control [21, 40, 44–48]. Because negative occasion setting follows a similar non-linear developmental trajectory, it may be that the difficulty using negative occasion setters in adolescence likewise may result from this imbalance. A balance in PFC/NAC activity may thus be necessary for rats to express negative occasion setting.
The present results are also consistent with fear conditioning data from mice that reveal a similar non-linear development of the ability to express contextual-fear memories. In particular, adolescent mice exhibited very little fear when returned to a context that had previously been paired with footshock, compared to either pre-adolescent or adult mice [49]. Moreover, the pre-adolescent and adult mice exhibited comparable levels of contextual fear responses [49]. These results are particularly relevant given that contextual stimuli are thought to be occasion setters in that they indicate the environmental conditions under which another stimulus will be reinforced (or not) [cf, 50]. In addition, the extinction of cued fear also develops in a non-linear fashion. Specifically, compared to either younger or older mice, adolescents exhibited attenuated extinction of freezing behavior when a discrete stimulus was no longer paired with foot-shock, [51, 52]. This pattern was associated with blunted neural responses in ventromedial prefrontal subregions in adolescent mice compared to other ages [51], consistent with evidence that extinction learning requires top-down prefrontal control of subcortical limbic regions [53–55]. Importantly, extinction involves acquiring a secondary inhibitory meaning about an initially excitatory cue. Thus, these data are also informative for negative occasion setting, in which the target has been suggested to acquire both excitatory and inhibitory associations with the unconditioned stimulus [8, 9, 56].
An additional finding in the present study was that rats that began training on PND 40 exhibited discrimination in 12 sessions. Like the PND 35 group in our prior study, these rats also began training as adolescents; thus, the purported imbalance in activity between NAC and PFC would supposedly be present in both groups. Nonetheless, the PND 40 group exhibited negative occasion setting after only 12 sessions, compared to the 18 sessions required by the PND 35 group (Figure 3). Interestingly, this is exactly what would be expected if the expression of negative occasion setting was dependent on the full maturation of PFC to offset the increase in NAC activity during this stage of development. Indeed, we found previously that rats that began training on PND 35 began to exhibit discrimination when they were 53 days old (i.e., after 18 training sessions), which corresponds to the age at which PFC is thought to reach functional maturity [16]. Because rats in the PND 40 group began training when they were 5 days older than rats in the PND 35 group, they would reach 53 days old after ~12–13 sessions. Thus, perhaps it is not surprising that rats in the PND 40 group required 12 sessions to successfully discriminate between the reinforced and non-reinforced presentations of the tone and exhibit negative occasion setting. The functional maturation of PFC at ~PND53 may also explain why the same amount of daily sessions were required for rats beginning training on either PND 50 or PND 70 (Figures 1C and 3; [12]).
The importance of PFC for negative occasion setting is supported by substantial prior research. Indeed, PFC has a well-established role in executive functioning, including inhibition [18, 57–68]. Furthermore, compared to adults, adolescents recruit more diffuse prefrontal regions when performing inhibition and executive control tasks and brain activity in regions that correlate with cognitive performance becomes more fine-tuned with age [18–21]. Importantly, our laboratory has previously shown that PFC is necessary for negative occasion setting in adults [14].
The efficient detection of environmental cues that indicate whether a response is appropriate or whether it should be inhibited is essential for adaptive behavior and behavioral control. Much research has focused on the so-called ‘stop signal’, which is a cue that is presented after a ‘go’ signal, indicating that a response should be interrupted or aborted (e.g. as in Stop-Signal Reaction Time [SSRT] tasks) [68–73]. This form of behavioral control is referred to as reactive inhibition. Conversely, proactive inhibition refers to preparing to stop an upcoming response tendency [79]. Less research has focused on types of proactive cues (i.e., negative occasion setters) that signal whether or not a response should be initiated in the first place (versus aborting one already in progress). Yet, contemporary literature has begun to emphasize the wider validity of proactive control with regard to modeling the inhibition of inappropriate behavior [79]. Furthermore, emerging evidence indicates that using occasion setters is a critical feature of inhibitory control [74–78]. Indeed, learning to use negative occasion setters is integral for resolving the ambiguity encountered in daily life, where the occurrence of an anticipated outcome depends on the context in which the cue occurs [74]. The flexible form of learning reflected by negative occasion setting is pertinent across the lifespan, but is particularly relevant during adolescence, when individuals are encountering a number of new environments and learning about the subtleties that confer which aspects of these environments are most useful in informing behavior. Paradoxically, the present data suggest that adolescents in particular may experience difficulties mediating ambiguity compared to other ages. This may contribute to a number of behavioral phenotypes that predominate during this period of development, including maladaptive tendencies such as heightened risk taking behavior compared to any other age group [25].
Another intriguing aspect of the present results is that rats in the PND 30 group were able to exhibit discrimination after 10 training session, despite the fact that this length of training partly overlaps with that of the PND 35 group, which instead required 18 sessions to discriminate [12]. One explanation for this is that the learning that occurs during first few training sessions may be particularly critical for subsequent performance. For example, perhaps differences in the way that information is encoded in the first five sessions can contribute to the later efficiency of expressing inhibition in subsequent (~10) sessions. This is consistent with previous evidence suggesting that 35 day old rats are able to learn about the contingencies presented in the negative occasion setting paradigm during as few as six sessions, despite not being able to express this learning until 18 sessions [12]. It may be that the processes required for translation of this initial learning into differential responding between trial types is influenced by age, and specifically, is disrupted during adolescence.
5. Conclusions
Together with our prior findings [12], the results of the present study indicate that the development of negative occasion setting is non-linear and confirm that adolescent rats experience difficulties using negative occasion setters to withhold a behavioral response. These findings may also provide insight into the neural substrates underlying the maturation of behavioral control. Indeed, our results are consistent with contemporary models that attribute adolescent-specific behavior to the differential development of subcortical systems implicated in incentive and emotional processing, and top-down control systems involved in behavioral control. Finally, our results have further implications for the nature of behavioral phenotypes canonical to the adolescent period. Indeed, learning about occasion setters enables an individual to categorize and retain multiple incongruent experiences with stimuli and helps to adapt expectations and responses to the present environment. Thus, difficulties inhibiting behavior may be exacerbated by an inability to use occasion setters, including contextual cues, to determine the appropriate course of action.
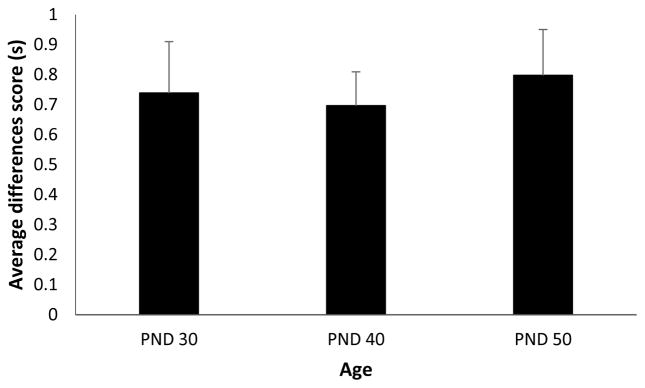
Figure 2.
Average difference in time spent in the food cup during the presentation of the tone on the two trial types for rats that began training on postnatal day (PND) 30, 40 or 50. Data are means ± SEM.
Highlights.
The ontogeny of negative occasion setting was studied in rats
Performance was comparable in preadolescent and young adult rats
Negative occasion setting develops in a non-linear fashion
Acknowledgments
The authors acknowledge the contributions of Molly Chodakewitz and Ethan Adner in generating pilot data leading to this study. This research was supported by NIH Grants F31MH107138 (HCM) and R01DA027688 (DJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pavlov IP. Conditioned reflexes. Oxford, UK: Oxford University Press; 1927. [Google Scholar]
- 2.Skinner BF. The behavior of organisms: an experimental analysis. D. Appleton-Century; NY: 1938. [Google Scholar]
- 3.Holland PC. Occasion-Setting in Pavlovian Conditioning. In: Medin DL, editor. The Psychology of Learning and Motivation. Vol. 28. San Diego: Academic Press; 1992. pp. 69–125. [Google Scholar]
- 4.Bouton ME. Learning and Behavior: A Contemporary Synthesis. Sunderland MA: Sinauer Associates; 2006. [Google Scholar]
- 5.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- 6.Rescorla RA. Conditioned inhibition and facilitation. In: Miller RR, Spear NE, editors. Information processing in animals: Conditioned inhibition. Hillsdale, NJ: Erlbaum; 1985. pp. 299–326. [Google Scholar]
- 7.Holland PC, Coldwell SE. Transfer of inhibitory stimulus control in operant feature-negative discrimination. Learning and Motivation. 1993;24:345–375. [Google Scholar]
- 8.Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. Journal of Experimental Psychology Animal Behavior Processes. 1994;20:51–65. [PubMed] [Google Scholar]
- 9.Bouton ME. Signals for whether versus when an event will occur. In: Bouton ME, Fanselow MS, editors. Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles. Washington, DC: American Psychological Association; 1997. pp. 385–409. [Google Scholar]
- 10.Bonardi C, Bartle C, Jennings D. US specificity of occasion setting: hierarchical or configural learning? Behavioural Processes. 2012;90:311–322. doi: 10.1016/j.beproc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–57. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Meyer HC, Bucci DJ. The ontogeny of learned inhibition. Learning & Memory. 2014;21:143–52. doi: 10.1101/lm.033787.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLeod JE, Potter AS, Bucci DJ. Nicotine administration enhances conditioned inhibition in rats. European Journal of Pharmacology. 2006;551:76–79. doi: 10.1016/j.ejphar.2006.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod JE, Bucci DJ. Contributions of the subregions of the medial prefrontal cortex to negative occasion setting. Behavioral Neuroscience. 2010;124:321–8. doi: 10.1037/a0019344. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod JE, Vucovich M, Bucci DJ. Differential effects of nicotinic acetylcholine receptor stimulation on negative occasion setting. Behavioral Neuroscience. 2010;124:656–661. doi: 10.1037/a0020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman L, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 2000;108(Suppl, 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–247. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 19.May JC, Delgado MR, Dahl RE, Stenger A, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 21.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casey BJ, Getz S, Galvan A. The adolescent brain and risky decisions. Developmental Reviews. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinions in Neurobiology. 2010;20:236–44. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 28.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg D, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–7. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg D, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 31.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 32.Meyer HC, Putney RB, Bucci DJ. Inhibitory learning is modulated by nicotinic acetylcholine receptors. Neuropharmacology. 2015;89:360–367. doi: 10.1016/j.neuropharm.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey BJ, Galvan A, Hare T. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–44. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, … Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 37.Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 38.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 39.Giedd J, Blumenthal J, Jeffries N, Castellanos F, Liu H, Zijdenbos A, … Rapoport J. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 40.Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, … Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 41.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 42.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 43.Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, … Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–35. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, … Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 45.Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 46.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–60. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 47.Fareri D, Martin L, Delgado M. Reward-related processing in the human brain: Developmental considerations. Development and Psychopathology. 2008;20:1191–211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- 48.Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences, USA. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rescorla RA, Durlach PJ, Grau JW. Contextual learning in Pavlovian conditioning. In: Balsam P, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. pp. 23–56. [Google Scholar]
- 51.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, … Lee FS. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences, USA. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: Effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 54.LeDoux JE. Emotion circuits in the brain. Annual Reviews in Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 55.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 56.Bouton ME, Nelson JB. Mechanisms of feature-positive and feature-negative discrimination learning in an appetitive conditioning paradigm. In: Schmajuk N, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: American Psychological Association; 1998. pp. 69–112. [Google Scholar]
- 57.Iversen SD, Mishkin M. Perseverative inference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Research. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 58.Guitton D, Buchtel H, Douglas R. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Experimental Brain Research. 1985;58:455–72. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- 59.Diamond A. The development and neural bases of memory functions as indexed by the A-not-B and delayed response tasks in human infants and infant monkeys. Annals of the New York Academy of Sciences. 1990;608:267–317. doi: 10.1111/j.1749-6632.1990.tb48900.x. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- 61.Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–71. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- 62.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Annals of the New York Academy of Sciences. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 63.Cetin T, Freudenberg F, Fuchtemeier M, Koch M. Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neuroscience Letters. 2004;370:114–117. doi: 10.1016/j.neulet.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 64.McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–87. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Cox S, Andrade A, Johnsrude I. Learning to like: a role for human orbitofrontal cortex in conditioned reward. Journal of Neuroscience. 2005;25:2733–40. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. Journal of Neuroscience. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosokawa T, Kato K, Inoue M, Mikami A. Correspondence of cue activity to reward activity in the macaque orbitofrontal cortex. Neuroscience Letters. 2005;389:121–82. doi: 10.1016/j.neulet.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 68.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–13. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 69.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 70.Potter AS, Newhouse PA. The effects of acute nicotine administration on behavioral inhibition in adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD) Psychopharmacology. 2004;176:182–94. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- 71.Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacology Biochemistry and Behavior. 2008;88:407–17. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behavioral Neuroscience. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 73.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berlin) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 74.Chatham CH, Claus ED, Kim A, Curran T, Banich MT, Munakata Y. Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. PLoS One. 2012;7:e31546. doi: 10.1371/journal.pone.0031546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munakata Y, Snyder HR, Chatham CH. Developing Cognitive Control: Three Key Transitions. Current Directions in Psychological Science. 2012;21:71–77. doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swick D, Chatham CH. Ten years of inhibition revisited. Frontiers in Human Neuroscience. 2014;8:329. doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chevalier N, Chatham CH, Munakata Y. The practice of going helps children to stop: the importance of context monitoring in inhibitory control. Journal of Experimental Psychology: General. 2014;143:959–965. doi: 10.1037/a0035868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winter W, Sheridan M. Previous reward decreases errors of commission on later ‘No-Go’ trials in children 4 to 12 years of age: evidence for a context monitoring account. Developmental Science. 2014;17:797–807. doi: 10.1111/desc.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aron AR. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]