Abstract
As the First-In-Human study of in situ gene therapy using an adenovirus vector carrying the human REIC (reduced expression in immortalized cell)/Dkk-3 gene (Ad-REIC), we conducted neoadjuvant intraprostatic injections in patients with high-risk localized prostate cancer undergoing radical prostatectomy (RP). Patients with recurrence probability of 35% or more within 5 years following RP, as calculated by Kattan's nomogram, were enrolled. Patients received two ultrasound-guided intratumoral injections at 2-week intervals, followed by RP 6 weeks after the second injection. After confirming the safety of the therapeutic interventions with initially planned three escalating doses of 1.0 × 1010, 1.0 × 1011 and 1.0 × 1012 viral particles (vp) in 1.0–1.2 ml (n=3, 3 and 6), an additional higher dose of 3.0 × 1012 vp in 3.6 ml (n=6) was further studied. All four DLs including the additional dose level-4 (DL-4) were feasible with no adverse events, except for grade 1 or 2 transient fever. Laboratory toxicities were grade 1 or 2 elevated aspartate transaminase/alanine transaminase (n=4). Regarding antitumor activities, cytopathic effects (tumor degeneration with cytolysis and pyknosis) and remarkable tumor-infiltrating lymphocytes in the targeted tumor areas were detected in a clear dose-dependent manner. Consequently, biochemical recurrence-free survival in DL-4 was significantly more favorable than in patient groups DL-1+2+3.
Introduction
The reduced expression in immortalized cell (REIC) gene was originally identified and cloned at the Okayama University (Okayama, Japan) and reported in 2000 as a gene whose expression is decreased through the immortalization of normal human fibroblasts.1 The sequence of the REIC gene was found to be consistent with that of the human Dkk-3 gene, a member of the Dkk family genes (hDkk-1, -2, -3 and -4). The expression of the REIC/Dkk-3 gene was found to be markedly downregulated in a broad range of human cancer cells as a tumor suppressor gene.2, 3, 4, 5, 6, 7, 8, 9 In our preclinical studies including in vivo study using immunocompetent orthotopic mouse tumor models, the therapeutic effects of Ad-REIC and its mechanisms of action have been clarified.5, 10, 11, 12, 13, 14, 15, 16, 17 The simultaneous induction of cancer-selective apoptosis and augmentation of antitumor immunity is the most characteristic feature of Ad-REIC. Ad-REIC offers a new, sophisticated way to induce selective toxicity based on different sensitivities to Ad-REIC-induced unfolded protein responses in the endoplasmic reticulum (ER) between cancer and normal cells. ER stress-induced apoptosis triggered by the activation of c-Jun N-terminal kinase is mediated in Ad-REIC-infected cancer cells but not in infected normal cells. The overproduction of interleukin-7 (IL-7) by infected normal cells including cancer-associated fibroblasts is responsible for the activation of innate immunity involving natural killer (NK) cells.18 Furthermore, secreted REIC protein with potent immunomodulatory function creates an optimal environment for the activation of host immune cells, inducing cytotoxic T lymphocytes (CTLs).14, 19 As reported previously, dendritic cells, induced by secreted REIC proteins, acquire possible cancer antigens from apoptotic cancer cells and induce tumor-associated antigen-specific CD8+ CTLs.20, 21 The resulting CTLs are expected to have a major role in systemic antitumor immunity of in situ Ad-REIC as a personalized therapeutic cancer vaccine.
To develop Ad-REIC as a new class of therapeutic cancer vaccines, the First-In-Human clinical study, a phase I/IIa study of in situ Ad-REIC gene therapy for prostate cancer (PCa), was initiated at the Okayama University from January 2011. As reported previously,22, 23 in this phase I/IIa study, two groups of patients were treated: group A consisting of patients with castration-resistant PCa (CRPC) with or without metastasis, and group B consisting of patients with high-risk, localized PCa scheduled to undergo radical prostatectomy (neoadjuvant study). Ad-REIC was injected directly into the prostate or metastatic tumor using four escalating doses of viral particles (vp), starting from 1.0 × 1010 to 3.0 × 1012 vp. In group A, direct and indirect systemic effects induced by in situ gene therapy were clearly illustrated in a case of chemotherapy-resistant advanced CRPC with bulky lymph node metastases.22 In the neoadjuvant study, patients treated with the initially planned three escalating dose levels (DLs) of 1.0 × 1010, 1.0 × 1011 and 1.0 × 1012 vp in 1.0–1.2 ml (n=3, 3 and 6) showed remarkable safety profiles of Ad-REIC without reaching the maximum-tolerated dose. Then, an additional study with a higher DL-4 of 3.0 × 1012 vp in 3.6 ml (n=6) was conducted. As of November 2014, therapeutic interventions of 18 cases in group B were completed and preliminary data on feasibility of neoadjuvant Ad-REIC gene therapy were reported in a separate brief note.23 In this communication, precise clinical results with long-term follow-up in group B were presented.
Materials and methods
Patient eligibility
Patients with clinical stage T2a–T3a adenocarcinoma of the prostate and probable candidates for RP were recruited. Kattan's nomogram score of ⩾115 (calculated 5-year recurrence-free probability of ⩽65%)24, 25 was used to select high-risk localized PCa. All participants were required to undergo needle biopsy of the prostate (at least 12 cores) to obtain tissue for pathologic analysis. A baseline chest X-ray, bone scan, computed tomography scan of the abdomen and pelvis and magnetic resonance imaging (MRI) of the pelvis and prostate were mandatory for determining the clinical stage. Patients reviewed the informed consent document and received individual counseling with a thorough discussion as to alternative treatments, including non-participation. Written informed consent was obtained from all patients.
Study design and therapy administration
The present clinical protocol was approved by the Okayama University Institutional Review Board and the Japanese Government. This protocol was an open-label, dose escalation study without the inclusion of control subjects. After getting UMIN ID (UMIN000004929) for Japanese clinical trial registration, the clinical study was initiated at the Okayama University from January 2011. Initially, three escalating DLs of 1.0 × 1010, 1.0 × 1011 and 1.0 × 1012 vp in 1.0–1.2 ml were planned. Patients received two ultrasound-guided intratumoral injections at 2-week intervals, followed by RP 6 weeks after the second injection. Based on MRI findings and biopsy mapping, one track injection to the most prominent cancer area was conducted. The injection was carried out by a newly developed injection machine driven by an air pressure (228AHBZ00005000; Nemoto Kyorindo, Tokyo, Japan) that can control injection speed and solution volume under ultrasound guidance. The safety of the therapeutic interventions with DL-1, -2 and -3 (n=3, 3 and 6) being confirmed, an additional higher dose, DL-4 of 3.0 × 1012 vp in 3.6 ml (n=6), was further studied. As for DL-4, three track injections (three times injection of 1.0 × 1012 vp in 1.2 ml) to multiple target cancer areas were conducted.
Adenovirus vector
A full-length cDNA of human REIC/Dkk-3 gene was integrated into a cosmid vector pAxCAwt and transferred into an E1/E2-deleted replication-deficient adenovirus type 5 vector with CAG (CMV early enhancer/chicken β-actin) promoter26 by the cosmid cassettes and Ad DNA-terminal protein complex (COS/TPC) method (Takara Bio, Shiga, Japan). A cGMP product of Ad5-CAG-REIC, free of replication-competent adenoviruses, was developed and supplied by a startup biotech company, Momotaro-Gene Inc. (Okayama, Japan).
Monitoring viral DNA detection and neutralizing antibody
Adenoviral DNA in blood and urine was determined by real-time PCR on day 0 (before viral injection) and on days 1, 3, 15, 17 and 56 after injection in a commercial-based laboratory (SRL, Tokyo, Japan). Neutralizing antibody titers against adenovirus were also determined on days 0, 14, 28, 56 and 84 (SRL, Tokyo, Japan).
Therapeutic evaluations
Using standard sections of RP specimens with hematoxylin and eosin (H&E) staining, final pathological diagnosis and antitumor effects mediated by Ad-REIC were determined. Terminal deoxynucleotidyl transferase dUTP nick-end labeling staining for the detection of apoptosis of cancer cells and immunohistochemical staining for the analysis of tumor-infiltrating lymphocytes were conducted in selected cases. Peripheral blood lymphocyte subsets were analyzed by multicolor flow cytometry on days 0, 1, 3, 7, 14, 15, 17, 21, 28 and 56: CD14−CD45+, CD3+CD4+CD8−, CD3+CD4−CD8+, HLA-DR/CD3, HLA-DR/CD4, HLA-DR/CD8, CD3+CD19−, CD3−CD19+ and CD3−CD19−CD16+CD56+ (SRL). NK activity, interferon-γ, tumor necrosis factor-α, IL-6 and IL-7 were also measured (SRL). Serum prostate-specific antigen (PSA) levels were analyzed before and after Ad-REIC injections by measuring on days 0, 7, 14, 21, 28 and 56. The changing rate in PSA (PSA on day 56/PSA on day 0x100) was calculated as a therapeutic parameter. After RP, PSA was measured at months 1, 2, 3 and every 3 months thereafter or as clinically indicated. Biochemical recurrence was defined as an initial PSA value exceeding 0.2 ng ml−1, followed by a subsequent confirmatory PSA value >0.2 ng ml−1. If PSA levels did not decrease to <0.2 ng ml−1 after surgery, the date of RP was defined as the date of disease recurrence.
Statistical considerations
A conventional phase I/IIa clinical study was designed with a sample size of three to six patients for each of four DLs. The maximum-tolerated dose was the dose for which the incidence of dose-limiting toxicities was lesser than 33%. Although the trial was not expected to have sufficient power to detect small differences in biomarkers, the preliminary analyses were carried out using all patients and stratified by dose. Paired t-tests or Wilcoxon's signed-rank tests were used to evaluate the changes in biomarkers. The two-way analysis of variance test was used to compare changes in biological markers between the dose groups to assess differences. Log-rank test was applied to evaluate differences in Kaplan–Meier curves representing biochemical recurrence-free survival (BRFS) after RP among four DL groups.
Results
Patient characteristics
Eighteen patients were enrolled with a median age of 65.5 (range 57–74) years. Clinical and pathological characteristics and follow-up duration after RP are shown in Table 1. As reported previously,23 there were no significant differences in patient characteristics, including Kattan's nomogram scores among groups of neoadjuvant Ad-REIC treatment (DL-1+2, DL-3 and DL-4). Most patients were regarded as a very high risk for recurrence; 83% (15/18) had a Gleason score of ⩾8 and 72% (13/18) had a Kattan's nomogram score of >130 (5-year recurrence-free probability of <5%).
Table 1. Baseline patient characteristics.
|
Vector dose level (vp) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL-1 (1 × 1010) | DL-2 (1 × 1011) |
DL-3 (1 × 1012) |
DL-4 (3 × 1012) | |||||||||||||||
|
Patient no. |
||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Age (years) | 74 | 67 | 59 | 63 | 62 | 61 | 70 | 65 | 70 | 66 | 71 | 63 | 74 | 57 | 71 | 62 | 60 | 70 |
| PSA before Tx (day 0) | 25.2 | 9.44 | 15.75 | 33.41 | 11.84 | 5.34 | 16.18 | 9.82 | 13.41 | 14.23 | 10.81 | 13.36 | 13.32 | 25.51 | 33.36 | 17.18 | 10.87 | 21.82 |
| Clinical stage | T2c | T3a | T2a | T3a | T2c | T3a | T3a | T2c | T3a | T2c | T3a | T3a | T2c | T3a | T3a | T2c | T2a | T2a |
| Gleason score (biopsy) | 4+5 | 4+5 | 4+4 | 4+4 | 5+5 | 4+5 | 4+5 | 4+4 | 4+4 | 4+4 | 4+3 | 4+3 | 4+5 | 4+4 | 4+4 | 3+4 | 4+4 | 4+5 |
| Nomogram score | 148 | 141 | 124 | 176 | 140 | 133 | 167 | 127 | 161 | 137 | 143 | 118 | 137 | 172 | 173 | 137 | 122 | 123 |
| Follow-up (months) | 60.1 | 59.2 | 59.2 | 59 | 57.8 | 57.6 | 57.1 | 55.5 | 52 | 48.5 | 46.9 | 45.2 | 42.2 | 27.5 | 27.5 | 24.2 | 18.1 | 17.7 |
Abbreviations: DL, dose level; PSA, prostate-specific antigen; vp, viral particles.
Safety and feasibility
All DLs including the additional DL-4 were feasible without reaching maximum-tolerated dose. As for adverse events, only grade 1 or grade 2 fever was observed in DL-2, -3 and -4, but was transient and treatable with antipyretics. As for laboratory toxicities, only grade 1 or grade 2 elevated aspartate transaminase/alanine transaminase was observed in 4 out of 18 cases; grade 1 was in 2 of DL-3 and grade 2 was in 2 of DL-4 (Table 2). Neither intraoperative nor postoperative complications related to neoadjuvant Ad-REIC were observed, illustrating remarkable safety profiles of in situ Ad-REIC treatment. Changes in serum-neutralizing antibody titer against adenovirus are shown in Table 3. Dose-dependent response and boosting response to the second injection were not clearly observed. Neutralizing antibody response seemed to be in no relationship with clinical outcome including adverse events of in situ Ad-REIC treatment.
Table 2. Adverse events.
|
Vector dose level (vp) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL-1 (1 × 1010) | DL-2 (1 × 1011) |
DL-3 (1 × 1012) |
DL-4 (3 × 1012) | |||||||||||||||
|
Patient no. |
||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Fever | — | — | — | — | Grade 1 | Grade 1 | Grade 2 | Grade 1 | Grade 2 | — | Grade 1 | Grade 2 | Grade 2 | Grade 2 | Grade 2 | Grade 2 | Grade 2 | Grade 2 |
| TA elevation | — | — | — | — | — | — | — | Grade 1 | — | Grade 1 | — | — | Grade 2 | — | — | — | Grade 2 | — |
| Others (possibly related) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; DL, dose level; TA, transaminases (alanine transaminase and aspartate transaminase); vp, viral particles.
Table 3. Changes in serum-neutralizing antibody titer against adenovirus.
| Day 0 | Day 14 | Day 28 | Day 56 | Day 84 | |
|---|---|---|---|---|---|
| DL-1 | |||||
| 1 | <4 | <4 | 32 | 8 | 32 |
| 2 | 16 | 16 | 8 | 4 | 16 |
| 3 | 16 | 512 | 512 | 1024 | 1024 |
| DL-2 | |||||
| 4 | 4 | 16 | 64 | 128 | 128 |
| 5 | 16 | 64 | 128 | 32 | 128 |
| 6 | 32 | 512 | 256 | 1024 | 512 |
| DL-3 | |||||
| 7 | <4 | 64 | 128 | 128 | 32 |
| 8 | 128 | 256 | 512 | 256 | 256 |
| 9 | 32 | 256 | 256 | 256 | 512 |
| 10 | 4 | 4 | 4 | 8 | 16 |
| 11 | 64 | 32 | 32 | 32 | 64 |
| 12 | <4 | 16 | 32 | 64 | 64 |
| DL-4 | |||||
| 13 | <4 | 128 | 128 | 128 | 128 |
| 14 | 4 | 32 | 64 | 32 | 64 |
| 15 | 16 | 32 | 128 | 128 | 64 |
| 16 | 16 | 32 | 64 | 1024 | 512 |
| 17 | 4 | 64 | 128 | 128 | 128 |
| 18 | 16 | 128 | 64 | 128 | 128 |
Abbreviation: DL, dose level.
The risk of dissemination of a viral vector into the environment from the treated patient, a phenomenon called shedding, is a major safety concern, especially in Japan, where the Cartagena law is applied. Quantitative kinetic analysis of adenoviral DNA in blood and in urine by real-time PCR was conducted (Tables 4A and 4B). No adenoviral DNA in blood was detected in any of the samples studied, except for one sample on day 15, one day after the second injection, from no. 18 in DL-4. Adenoviral DNA in urine was detected on days 1 and 15 in 17 out of 36 samples, one day after the injection, in a dose-dependent manner, but not detected on days 3 and 17 in most samples (31/34). No. 7 of DL-3 showed enigmatic positive result before and after injections.
Table 4A. Quantitative kinetic analysis of adenoviral DNA in blood by real-time PCR.
| Day 0 | Day 1 | Day 3 | Day 15 | Day 17 | Day 56 | ||
|---|---|---|---|---|---|---|---|
| Adenoviral DNA quantity in blood (copies per ml) | |||||||
| DL-1 | |||||||
| 1 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 2 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 3 | ND* | ND* | ND* | ND* | ND* | ND* | |
| DL-2 | |||||||
| 4 | ND* | ND* | ND* | ND* | — | ND* | |
| 5 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 6 | ND* | ND* | ND* | ND* | ND* | ND* | |
| DL-3 | |||||||
| 7 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 8 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 9 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 10 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 11 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 12 | ND* | ND* | ND* | ND* | — | ND* | |
| DL-4 | |||||||
| 13 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 14 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 15 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 16 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 17 | ND* | ND* | ND* | ND* | ND* | ND* | |
| 18 | ND* | ND* | ND* | 2.6 × 102 | ND* | ND* | |
Abbreviations: DL, dose level; ND, not detected; —, not measured.
*ND: <1.0 × 102 copies per ml.
Table 4B. Quantitative kinetic analysis of adenoviral DNA in urine by real-time PCR.
| Day 0 | Day 1 | Day 3 | Day 15 | Day 17 | Day 56 | |
|---|---|---|---|---|---|---|
| Adenoviral DNA quantity in urine (copies per ml) | ||||||
| DL-1 | ||||||
| 1 | ND* | ND* | ND* | ND* | ND* | ND* |
| 2 | ND* | ND* | ND* | ND* | ND* | ND* |
| 3 | ND* | ND* | ND* | ND* | ND* | ND* |
| DL-2 | ||||||
| 4 | ND* | ND* | ND* | ND* | — | ND* |
| 5 | ND* | ND* | ND* | ND* | ND* | ND* |
| 6 | ND* | ND* | ND* | ND* | ND* | ND* |
| DL-3 | ||||||
| 7 | 2.9 × 103 | 2.8 × 103 | 5.7 × 102 | 5.5 × 103 | 7.0 × 103 | ND* |
| 8 | ND* | 1.2 × 102 | ND* | ND* | ND* | ND* |
| 9 | ND* | ND* | ND* | 7.9 × 102 | ND* | ND* |
| 10 | ND* | ND* | ND* | ND* | ND* | ND* |
| 11 | ND* | 1.2 × 102 | ND* | ND* | ND* | ND* |
| 12 | ND* | 3.7 × 102 | ND* | 1.2 × 102 | — | ND* |
| DL-4 | ||||||
| 13 | ND* | ND* | ND* | 1.2 × 103 | ND* | ND* |
| 14 | ND* | 1.1 × 102 | ND* | 2.1 × 102 | ND* | ND* |
| 15 | ND* | 2.2 × 103 | ND* | 3.2 × 102 | ND* | ND* |
| 16 | ND* | 6.5 × 103 | 1.6 × 103 | 3.7 × 104 | ND* | ND* |
| 17 | ND* | ND* | ND* | 1.5 × 104 | ND* | ND* |
| 18 | ND* | 3.5 × 103 | 6.3 × 103 | 1.7 × 102 | ND* | ND* |
Abbreviations: DL, dose level; ND, not detected; —, not measured.
*ND: <1.0 × 102 copies per ml.
Histopathological evaluation
Post-therapeutic findings and clinical outcome are summarized in Table 5. In terms of antitumor effects, the histopathological evaluation of RP specimens was found to be the most reliable form of measurement. Cytopathic effects illustrated by clear tumor degeneration with cytolysis and pyknosis and/or remarkable tumor-infiltrating lymphocytes (TILs) in significant areas of the targeted tumor regions were observed in a clear dose-dependent manner. Although no pathological effects were detected in DL-1, two out of three in DL-2 and three out of six in DL-3 showed significant cytopathic effects or TILs, evaluating as overall effects of grade 1. Three out of six in DL-3 and all six cases in DL-4 showed significant cytopathic effects together with remarkable TIL, evaluating as overall effects of grade 2 (see Figures 1, 2, 3). As demonstrated in Figure 2 from case 15 in DL-4, tumor degeneration with pyknosis and severe disturbance of the glandular structure were prominent without remarkable (Figure 4) TILs in the specimen from section no. 10 (2C), whereas remarkable TILs were detected without clear disturbance of the glandular structure in the specimen from section no. 12 (2D). Although these two different pathological responses might represent only different time course of the same antitumor effects of Ad-REIC, these two types of responses were detected distinctively in each Ad-REIC-targeted area. As demonstrated in Table 5, overall pathological effects were evaluated as grade 1 (+) or grade 2 (++), resulting in a clear dose-dependent clinical outcome.
Table 5. Post-therapeutic findings and clinical outcome.
|
Patient no. |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Pathological stage | pT3bpN1 | pT3bpN0 | pT2cpN0 | pT3bpN0 | pT3bpN0 | pT3bpN1 | pT3bpN0 | pT2cpN0 | pT3apN0 |
| Gleason score (resected specimen) | 4+5 | 4+5 | 4+3 | 4+5 | 5+4 | 4+5 | 4+5 | 4+5 | 3+4 |
| PSA before RP (day 56) | 30.13 | 9.12 | 14.94 | 22.49 | 12.34 | 4.16 | 14.52 | 8.34 | 11.32 |
| PSA changing rate (%)a | 120 | 97 | 95 | 67 | 104 | 78 | 90 | 85 | 84 |
| Evaluation of pathological effects | |||||||||
| Cytopathic effects | − | − | − | − | + | − | + | + | + |
| Lymphocytic infiltration | − | − | − | − | − | + | + | + | − |
| Overall effects | − | − | − | − | + | + | ++ | ++ | + |
| Evaluation of MRI findings | SD | SD | SD | SD | SD | SD | SD | SD | PR |
| PSA recurrence after RP (months) | − | 9.3 | − | 0 | 0 | 6 | 0 | 9.2 | 16.1 |
|
Patient no. |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Pathological stage | pT2cpN0 | pT3bpN0 | pT2cpN0 | pT2cpN0 | pT3bpN0 | pT3apN0 | pT2cpN0 | pT2cpN0 | pT2cpN0 |
| Gleason score (resected specimen) | 4+3 | 3+5 | 4+3 | 4+5 | 4+3 | 4+4 | 3+3 | 4+3 | 4+5 |
| PSA before RP (day 56) | 13 | 9.04 | 12.2 | 9.74 | 28.78 | 29.39 | 14.09 | 9.51 | 25.32 |
| PSA changing rate (%)a | 91% | 84% | 91% | 73% | 113% | 88% | 82% | 87% | 116% |
| Evaluation of pathological effects | |||||||||
| Cytopathic effects | + | + | − | + | + | + | + | + | + |
| Lymphocytic infiltration | + | − | + | + | + | + | + | + | + |
| Overall effects | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ |
| Evaluation of MRI findings | SD | SD | SD | PR | SD | PR | SD | − | SD |
| PSA recurrence after RP (months) | 22.4 | − | 0 | − | − | − | − | − | 9.2 |
Abbreviations: MRI, magnetic resonance imaging; PR, partial response; PSA, prostate-specific antigen; RP, radical prostatectomy; SD, stable disease.
PSA changing rate (%): PAS on day 56/PSA on day 0 × 100.
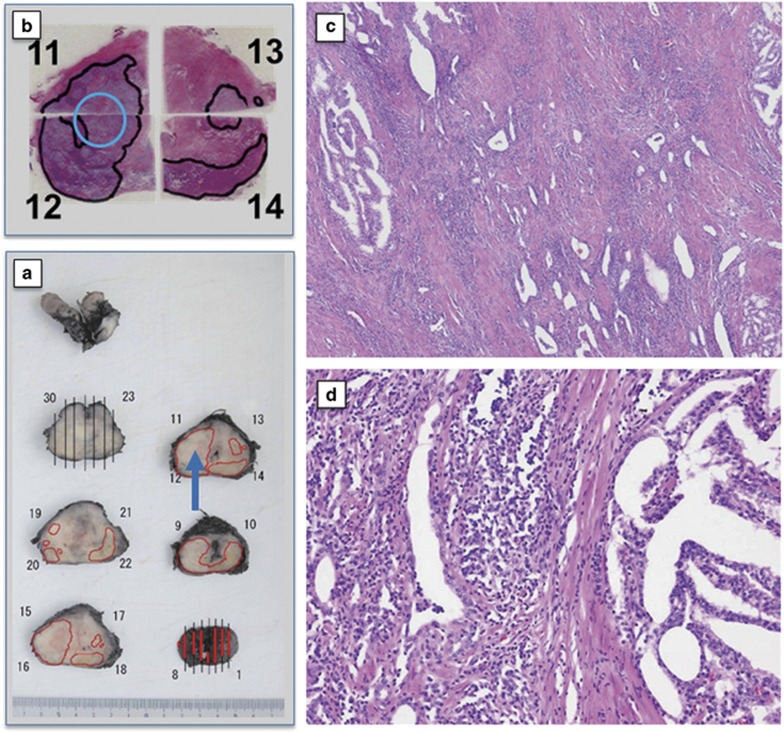
Figure 1.
Surgical specimens from case 7 in dose level-3 (DL-3) treated by one track injection with 1x1012 vp of Ad-REIC. (a) Gross appearance of radical prostatectomy (RP) specimen sliced by a standard method. Cancer distribution areas are enclosed by the red line. A blue arrow indicates the Ad-REIC-targeted area. (b) Gross appearance of hematoxylin and eosin (H&E)-stained histopathological sections. Cancer distribution areas are enclosed by the black line. A blue circle indicates the targeted area. (c) A photomicrograph of the targeted area from section no. 12, demonstrating tumor degeneration with tumor-infiltrating lymphocytes (TILs) (H&E). (d) A photomicrograph of cancer distribution area from section no. 14, demonstrating a large intact tumor area (H&E).
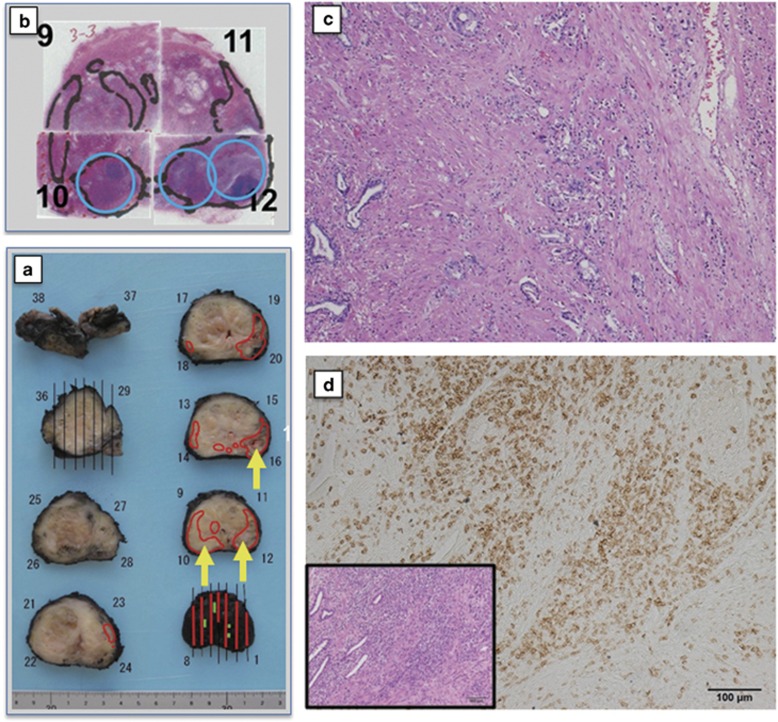
Figure 2.
Surgical specimens from case 15 in dose level-4 (DL-4) treated by three track injections with 3x1012 vp of Ad-REIC. (a) Gross appearance of radical prostatectomy (RP) specimen sliced by a standard method. Cancer distribution areas are enclosed by the red line. Three yellow arrows indicate the Ad-REIC-targeted areas. (b) Gross appearance of hematoxylin and eosin (H&E)-stained histopathological sections. Cancer distribution areas are enclosed by the black line. Three blue circles indicate the targeted areas. (c) A photomicrograph of the targeted area from section no. 10, demonstrating tumor degeneration without significant tumor-infiltrating lymphocytes (TILs) (H&E). (d) Photomicrographs of the targeted area from the inner part of section no. 12. The inset shows low magnification view of pathological effects with remarkable TILs (H&E). Immunohistochemical staining, illustrating tumor-infiltrating CD8+ lymphocytes.
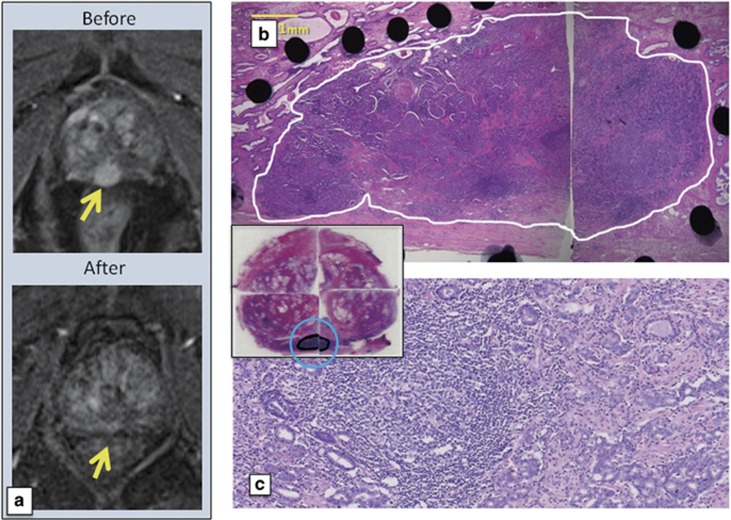
Figure 3.
Magnetic resonance imaging (MRI) and histopathological findings of case 13 in dose level-4 (DL-4) treated by three track injections with 3x1012 vp of Ad-REIC. (a) Contrast enhancement MRIs. A strong enhancement area indicated by an arrow before Ad-REIC injections disappeared after injections. (b and c) Low and high magnification photomicrographs. Whole targeted cancer area is replaced by degenerated cancer cells with remarkable tumor-infiltrating lymphocytes (TILs). The inset shows gross appearance of hematoxylin and eosin (H&E)-stained histopathological sections.
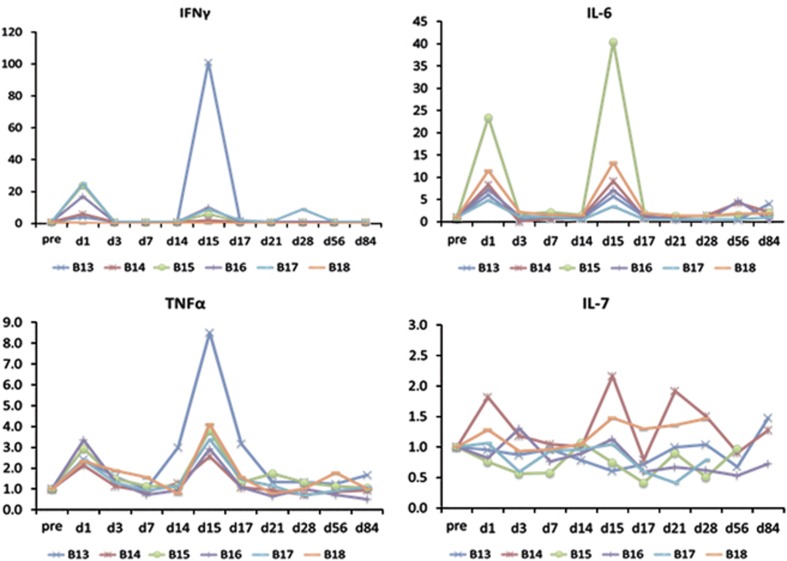
Figure 4.
Changes in cytokines related to two Ad-REIC treatments in dose level-4 (DL-4), expressed by the ratio of the pre-treatment value. Clear increases in interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) on days 1 and 15 were noted, whereas changes in IL-7 were not constant.
PSA and other biomarker response
Changes in PSA values related to two Ad-REIC treatments were quite different among DLs (see Supplementary Figure). In low DLs of DL-1 and DL-2, PSA showed no remarkable changes before and after treatments, but minor reduction (PSA on day 56/PSA on day 0) was detected in two cases of DL-2 (Table 5). Similarly, minor reduction was detected in all six cases of DL-3. Although changes in PSA values in DL-4 showed declining tendencies following transient elevation because of injections, minor elevation (PSA on day 56/PSA on day 0) was detected in two cases of DL-4. Consequently, changes in PSA values were not regarded as a reliable parameter for the evaluation of clinical outcome in the present neoadjuvant study.
Peripheral blood lymphocyte subsets were analyzed by multicolor flow cytometry on days 0, 1, 3, 7, 14, 15, 17, 21, 28 and 56 in all cases. As reported previously,23 changes in B cells, T cells, NK cells and CD4+ lymphocytes showed no tendency to increase, whereas CD8+ lymphocytes increased clearly in response to the Ad-REIC injection. The HLA-DR marker of activation was used to double label CD3+, CD4+ and CD8+ lymphocytes as a relative measure of activated T cells. HLA-DR+CD8+ (activated CTL) and HLA-DR+CD3+ (activated T) lymphocytes showed increases after Ad-REIC treatment with a tendency of dose-dependent manner. As for cytokines, no detectable changes were observed in DL-1. Interferon-γ, tumor necrosis factor-α and IL-6 increased clearly on days 1 and 15, one day after Ad-REIC injection, in a dose-dependent manner. Changes in IL-7 were not constant as compared with other cytokines (Figure 4). In any case, cytokines were not regarded as a reliable parameter for the evaluation of clinical outcome.
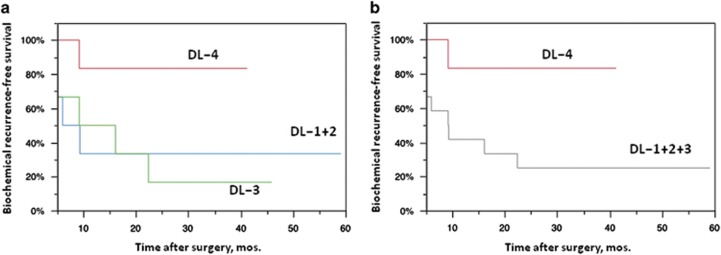
Biochemical recurrence-free survival
BRFS of each dose group was compared using the Kaplan–Meier survival analysis, demonstrating BRFS in DL-4 was significantly more favorable than in DL-1+2 plus DL-3 group patients (Figure 5). In this phase I/IIa study, one track injection to the most prominent cancer area was conducted in the initially planned DL-1+2 plus DL-3 groups, as the primary end point was to assess the safety of the in situ Ad-REIC treatment. As demonstrated in Figure 1, overall pathological effects of grade 2 was detected in the histopathological sections from blocks no. 11 and 12 of targeted tumor areas, whereas large intact tumor areas were detected in the section from block no. 14. On the other hand, three track injections to multiple target cancer areas were conducted in the DL-4 group. As illustrated in Figure 2, clear pathological effects were obtained in the sections from three targeted tumor areas. As for the case no. 13 in DL-4, three track injections were carried out on only one cancer area detected by MRI, resulting in almost complete destruction of tumor tissues with remarkable TILs (Figure 3). These pathological changes were well accorded with MRI findings and a condensed exposure of Ad-REIC seemed to be more effective. Consequently, it was obvious that multitrack injections to multiple target cancer areas with a sufficient dose of Ad-REIC were clearly effective for successful neoadjuvant therapy.
Figure 5.
Kaplan–Meier curves representing biochemical recurrence-free survival (BRFS) in patients with high-risk prostate cancer treated with neoadjuvant Ad-REIC followed by radical prostatectomy. (a) BRFS curves of three dose level groups. The differences were not significant (P=0.101, log-rank test). (b) BRFS curves of DL-1, -2 and -3 pooled group and DL-4 group. The difference was significant (P=0.034, log-rank test).
Discussion
At present more than 1500 gene therapy protocols for cancer have been conducted around the world (http://www.wiley.com/legacy/wileychi/genmed/clinical/). Interestingly, the second highest number of protocols is for PCa. The prostate is regarded as an ideal target organ for the development of cancer gene therapy. PCa is a leading internal malignancy and new therapies are needed, especially for metastatic CRPC. The prostate is easily accessible organ and biopsy and gene transfer can be easily conducted by ultrasound guidance. In addition, PSA, an extremely sensitive tumor marker, is available for clinical evaluation. More recently, a variety of clinical trials including in situ gene therapy27, 28, 29 have been conducted as a form of neoadjuvant therapy, as this approach provides a paradigm for evaluating the activity and mechanism of action of new agents with histopathological analysis using tumor tissues before and after therapy.30 In fact, histopathological analysis of RP specimens provided the most reliable parameters in the evaluation of clinical effects induced by in situ Ad-REIC treatment in the present study.
Neoadjuvant therapy is widely accepted in the treatment of patients with localized or locally advanced high-risk breast cancer and other solid cancers.31, 32, 33 In PCa, however, the beneficial effects on pathological outcomes provided by neoadjuvant androgen deprivation therapy and chemotherapy with or without androgen deprivation therapy did not link to improved disease-free survival or overall survival.34, 35, 36, 37, 38, 39 Neoadjuvant therapy with androgen deprivation therapy and chemotherapy is not currently recommended in patients with high-risk localized PCa undergoing RP. Consequently, in situ immune gene therapy has a great potential to offer a new option for neoadjuvant therapy by generating indirect, systemic antitumor effects.27, 28, 29
The ultimate aim of in situ Ad-REIC cancer gene therapy is to develop a new generation of cancer vaccines based on the concept of simultaneous induction of selective killing of cancer cells and augmentation of antitumor immunity. This kind of cancer vaccine strategy will become a leading standard in the treatment of most solid cancers with gene therapy.40 In October 2015, T-VEC (talimogene laherparepvec; an oncolytic herpes simplex type 1 virus armed with granulocyte–macrophage colony-stimulating factor)41 was approved by the Food Drug and Administration for the treatment of melanoma in patients with inoperable tumors. Similarly, Pexa-Vec (pexastimogene devacirepvec; an oncolytic vaccinia poxvirus armed with granulocyte–macrophage colony-stimulating factor) has already been successfully developed.42 In our preclinical studies,5, 11, 12, 14, 18, 20, 21 Ad-REIC presented a new, sophisticated way to induce cancer-selective apoptosis because of ER stress, providing an ideal presentation of possible cancer antigens to the host immune system. Secreted REIC protein at the tumor site also creates an optimal environment, mediating tumor-associated, antigen-specific cytotoxic T cells. In addition, the overproduction of IL-7 by cancer-associated fibroblasts activates innate immunity involving NK cells. As the induction of activated dendritic cells and antigen-specific CTLs is crucial for the development of therapeutic cancer vaccines by Ad-REIC, this process was carefully investigated in a mouse tumor model using E.G7-expressing OVA.21 The intratumoral administration of Ad-REIC-mediated robust antitumor effects with the accumulation of OVA-specific CTLs in the tumor tissues and spleen and with the upregulation of the CD86-positive dendritic cells in the tumor draining lymph nodes. In a dual tumor-bearing mouse model in the left and right back, Ad-REIC injection in one side significantly suppressed the tumor growth on both sides and significant infiltration of OVA-specific CTLs into non-injected tumor was detected. These results clearly indicate that the ER stress-induced apoptosis by Ad-REIC mediates immunogenic cancer cell death.
Recently, the concept of immunogenic cell death has been introduced with the evidence that apoptotic cancer cells induced by anthracyclines43 or ionizing irradiation44 are able to induce a potent immune response. It is now clear that ER stress has a principal role in immunogenic cell death induced by several chemotherapeutic agents and oncolytic viruses and endogenous damage-associated molecular patterns releasing from apoptotic cancer cells are responsible for promising anticancer effects including the reinitiation of immune responses suppressed by the tumor microenvironment.45, 46, 47 We are now complementing whole action mechanisms of Ad-REIC by considering roles of damage-associated molecular patterns associated with Ad-REIC treatment.
In the present neoadjuvant study, we believe that the proof of concept of simultaneous induction of selective killing of cancer cells and augmentation of antitumor immunity by Ad-REIC has been successfully established. Using RP specimens after two intratumoral Ad-REIC injections, tumor degeneration with cytolysis and pyknosis and remarkable TILs in significant areas of the targeted tumor regions were detected in a clear dose-dependent manner. As illustrated in the previous report,23 terminal deoxynucleotidyl transferase dUTP nick-end labeling staining was helpful in the detection of apoptosis of cancer cells and immunohistochemical staining revealed concurrent infiltrations of CD8+ lymphocytes and dendritic cells in the area of apoptotic cancer cells. In addition, peripheral blood CD8+ T cells and HLA-DR+CD8+ (activated CTL) increased after Ad-REIC treatment in a dose-dependent manner. Although the direct detection of tumor-associated antigen-specific CTLs was not carried out mainly because of its technical difficulties, preliminary quantitative detection of autoantibodies to cancer antigens was conducted using stocked serum samples. Recently, Futami et al.48 (Okayama University) have developed a sensitive multiplexed method for the analysis of autoantibodies to cancer antigens with chemically s-cationized full-length and water-soluble denatured proteins. Interestingly, this brand new technology using 50 cancer antigens including cancer/testis antigens revealed a clear elevation of autoantibodies to multiple antigens after one and two intratumoral injections of Ad-REIC (unpublished data), illustrating a promising evidence of Ad-REIC as personalized therapeutic cancer vaccines.
In this phase I/IIa clinical study, we used one track injection to the most prominent cancer area in the initially planned DL-1+2 plus DL-3 groups and three track injections to multiple target cancer areas in the DL-4 group. BRFS in DL-4 was significantly more favorable than in DL-1+2 plus DL-3 group patients, demonstrating an expected outcome of Ad-REIC to be a promising neoadjuvant therapy for high-risk localized PCa. To evaluate the potential activities of Ad-REIC as therapeutic cancer vaccines, we re-examined histopathological effects of non-targeted tumor areas in DL-3 but we could not obtain a clearcut evidence for indirect antitumor effects mediated by Ad-REIC-induced CTLs. At this point, it is obvious that multitrack injections to multiple target cancer areas with a sufficient dose of Ad-REIC are essential for successful neoadjuvant therapy. Although further studies are needed to determine the optimal dose, dosing interval and number of doses, the present regimen using three track injections (three times injection of 1.0 × 1012 vp in 1.2 ml) of Ad-REIC is regarded as one of the recommended options. To realize a more effective neoadjuvant therapy, a more potent, second generation of Ad-REIC using a super gene expression (SGE) system (Ad-SGE-REIC) has already been developed.49, 50, 51 The SGE system is a newly developed plasmid vector, constructed by placing three enhancers in tandem after poly A to realize extremely high expression of the targeted REIC gene. A phase I/IIa study of in situ Ad-SGE-REIC therapy in patients with localized PCa sponsored by Momotaro-Gene Inc. is now ongoing successfully in the States.
Although historically, PCa was not regarded as an immunogenic cancer, recent clinical results including the efficacy of sipuleucel-T52 for metastatic CRPC have led to a renewed interest in immunotherapy for PCa. More recently, the concept of cancer immunoediting53 and the advent of immune checkpoint inhibitors54, 55 are changing the role of immunotherapy in cancer management. Ad-REIC, a new class of therapeutic cancer vaccines, combined with immune checkpoint inhibitors will offer a new option for immunotherapy in the treatment of patients with metastatic CRPC. As for neoadjuvant therapy for high-risk localized PCa undergoing radical prostatectomy, a definitive therapeutic regimen of Ad-REIC will be proposed through prospective randomized comparative studies.
Acknowledgments
We thank Dr Sabina Mahmood, Dr Shigeru Kobayashi and Prof. Shiro Hinotsu (Okayama University Hospital, Okayama, Japan) and Hitoshi Shiomi (Momotaro-Gene Inc., Okayama, Japan) for providing valuable suggestions and help with the preparation of this manuscript. This study was supported by scientific research grants (KAKENHI: 22791473, 23390382 and 25462479) and by the Special Coordination Funds for Promoting Science and Technology (Formation of Innovation Center for Fusion of Advanced Technologies) from the Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT, FY2006-2009) and Health Labor Sciences Research Grant of Japan (FY2011-2014). A cGMP product of Ad5-CAG-REIC was supplied by Momotaro-Gene Inc.
Footnotes
Supplementary Information accompanies the paper on Cancer Gene Therapy website (http://www.nature.com/cgt)
Okayama University and Momotaro-Gene Inc., a startup biotech company from the Okayama University, holds the patents of the Ad-REIC agent and develops the agent as a cancer therapeutic medicine. HK, MW and YN demonstrated the utility of the agent and also own stocks in Momotaro-Gene Inc. HK is the Chief Scientific Officer of Momotaro-Gene Inc. Okayama University and Momotaro-Gene Inc. are working together on the development of the Ad-REIC agent. Okayama University received cGMP-grade products of Ad5-REIC from Momotaro-Gene Inc. to perform clinical studies for the treatment of cancer patients.
Supplementary Material
References
- Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem Biophys Res Commun 2000; 268: 20–24. [DOI] [PubMed] [Google Scholar]
- Nozaki I, Tsuji T, Iijima O, Ohmura Y, Andou A, Miyazaki M et al. Reduced expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int J Oncol 2001; 19: 117–121. [DOI] [PubMed] [Google Scholar]
- Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R et al. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol 2004; 171: 1314–1318. [DOI] [PubMed] [Google Scholar]
- Hsieh SY, Hsieh PS, Chiu CT, Chen WY. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene 2004; 23: 9183–9189. [DOI] [PubMed] [Google Scholar]
- Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S et al. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res 2005; 65: 9617–9622. [DOI] [PubMed] [Google Scholar]
- Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007; 28: 2459–2466. [DOI] [PubMed] [Google Scholar]
- Yang B, Du Z, Gao YT, Lou C, Zhang SG, Bai T et al. Methylation of Dickkopf-3 as a prognostic factor in cirrhosis-related hepatocellular carcinoma. World J Gastroenterol 2010; 16: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys Acta 2012; 1825: 18–28. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Asano H, Toyooka S, Tsukuda K, Soh J, Shien T et al. DNA methylation status of REIC/Dkk-3 gene in human malignancies. J Cancer Res Clin Oncol 2012; 138: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto R, Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kumon H et al. REIC/Dkk-3 as a potential gene therapeutic agent against human testicular cancer. Int J Mol Med 2007; 19: 363–368. [PubMed] [Google Scholar]
- Edamura K, Nasu Y, Takaishi M, Kobayashi T, Abarzua F, Sakaguchi M et al. Adenovirus-mediated REIC/Dkk-3 gene transfer inhibits tumor growth and metastasis in an orthotopic prostate cancer model. Cancer Gene Ther 2007; 14: 765–772. [DOI] [PubMed] [Google Scholar]
- Kashiwakura Y, Ochiai K, Watanabe M, Abarzua F, Sakaguchi M, Takaoka M et al. Down-regulation of inhibition of differentiation-1 via activation of activating transcription factor 3 and Smad regulates REIC/Dickkopf-3-induced apoptosis. Cancer Res 2008; 68: 8333–8341. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Watanabe M, Sakaguchi M, Ogasawara Y, Ochiai K, Nasu Y et al. REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther 2009; 16: 65–72. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kashiwakura Y, Huang P, Ochiai K, Futami J, Li SA et al. Immunological aspects of REIC/Dkk-3 in monocyte differentiation and tumor regression. Int J Oncol 2009; 34: 657–663. [DOI] [PubMed] [Google Scholar]
- Uchida D, Shiraha H, Kato H, Nagahara T, Iwamuro M, Kataoka J et al. Potential of denovirus-mediated REIC/Dkk-3 gene therapy for use in the treatment of pancreatic cancer. J Gastroenterol Hepatol 2014; 29: 973–983. [DOI] [PubMed] [Google Scholar]
- Shimazu Y, Kurozumi K, Ichikawa T, Fujii K, Onishi M, Ishida J et al. Integrin antagonist augments the therapeutic effect of adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma. Gene Therapy 2015; 22: 146–154. [DOI] [PubMed] [Google Scholar]
- Shien K, Tanaka N, Watanabe M, Soh J, Sakaguchi M, Matsuo K et al. Anti-cancer effects of REIC/Dkk-3-encoding adenoviral vector for the treatment of non-small cell lung cancer. PLoS One 2014; 9: e87900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M, Kataoka K, Abarzua F, Tanimoto R, Watanabe M, Murata H et al. Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7. J Biol Chem 2009; 284: 14236–14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita R, Watanabe M, Huang P, Li SA, Sakaguchi M, Kumon H et al. The cysteine-rich core domain of REIC/Dkk-3 is critical for its effect on monocyte differentiation and tumor regression. Oncol Rep 2015; 33: 2908–2914. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nasu Y, Kumon H. Adenovirus-mediated REIC/Dkk-3 gene therapy: development of an autologous cancer vaccination therapy [review]. Oncol Lett 2014; 7: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyoshi Y, Watanabe M, Eikawa S, Yamazaki C, Sadahira T, Hirata T et al. The induction of antigen-specific CTL by in situ Ad-REIC gene therapy. Gene Therapy 2016; 23: 408–414. [DOI] [PubMed] [Google Scholar]
- Kumon H, Sasaki K, Ariyoshi Y, Sadahira T, Ebara S, Hiraki T et al. Ad-REIC gene therapy: promising results in a patient with metastatic CRPC following chemotherapy. Clin Med Insights Oncol 2015; 9: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumon H, Sasaki K, Ariyoshi Y, Sadahira T, Araki M, Ebara S et al. Feasibility of neoadjuvant Ad-REIC gene therapy in patients with high-risk localized prostate cancer undergoing radical prostatectomy. Clin Transl Sci 2015; 8: 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998; 90: 766–771. [DOI] [PubMed] [Google Scholar]
- Korets R, Motamedinia P, Yeshchina O, Desai M, McKiernan JM. Accuracy of the Kattan nomogram across prostate cancer risk-groups. BJU Int 2011; 108: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K, Lin T, Hibino H, Takahashi K, Nakazaki Y, Takahashi S et al. Transduction of LacZ gene into leukemia cells using viral vectors of retrovirus and adenovirus. Leukemia 1995; 9(Suppl 1): S64–S65. [PubMed] [Google Scholar]
- van der Linden RR, Haagmans BL, Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D et al. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol 2005; 48: 153–161. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Thompson TC, Jain RK, Ayala GE, Kurosaka S, Edamura K et al. GLIPR1 tumor suppressor gene expressed by adenoviral vector as neoadjuvant intraprostatic injection for localized intermediate or high-risk prostate cancer preceding radical prostatectomy. Clin Cancer Res 2011; 17: 7174–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Martínez A, Manzanera AG, Sukin SW, Esteban-María J, González-Guerrero JF, Gomez-Guerra L et al. Intraprostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther 2013; 20: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonpavde G, Chi KN, Powles T, Sweeney CJ, Hahn N, Hutson TE et al. Neoadjuvant therapy followed by prostatectomy for clinically localized prostate cancer. Cancer 2007; 110: 2628–2639. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007; 25: 4414–4422. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Sternberg CN. Neoadjuvant systemic therapy for urological malignancies. BJU Int 2010; 106: 6–22. [DOI] [PubMed] [Google Scholar]
- Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int 2002; 90: 561–566. [DOI] [PubMed] [Google Scholar]
- Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol 2003; 170: 791–794. [DOI] [PubMed] [Google Scholar]
- Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol 2000; 38: 706–713. [DOI] [PubMed] [Google Scholar]
- Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol 2008; 180: 565–570. [DOI] [PubMed] [Google Scholar]
- Prayer-Galetti T, Sacco E, Pagano F, Gardiman M, Cisternino A, Betto G et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with non-metastatic high-risk prostate cancer. BJU Int 2007; 100: 274–280. [DOI] [PubMed] [Google Scholar]
- Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res 2005; 11: 5233–5240. [DOI] [PubMed] [Google Scholar]
- Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer 2013; 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33: 2780–2788. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Parato K, Burke J, Hwang TH, Bell JC, Kirn DH. Pexa-Vec double agent engineered vaccinia: oncolytic and active immunotherapeutic. Curr Opin Virol 2015; 13: 49–54. [DOI] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202: 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14: 1848–1850. [DOI] [PubMed] [Google Scholar]
- Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008; 15: 3–12. [DOI] [PubMed] [Google Scholar]
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 2010; 1805: 53–71. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ 2014; 21: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami J, Nonomura H, Kido M, Niidoi N, Fujieda N, Hosoi A et al. Sensitive multiplexed quantitative analysis of autoantibodies to cancer antigens with chemically S-cationized full-length and water-soluble denatured proteins. Bioconjug Chem 2015; 26: 2076–2084. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, Watanabe M, Kinoshita R, Kaku H, Ueki H, Futami J et al. ramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol Biotechnol 2014; 56: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakaguchi M, Kinoshita R, Kaku H, Ariyoshi Y, Ueki H et al. A novel gene expression system strongly enhances the anticancer effects of a REIC/Dkk-3-encoding adenoviral vector. Oncol Rep 2014; 31: 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawahara H, Shiraha H, Uchida D, Kato H, Nagahara T, Iwamuro M et al. Novel REIC/Dkk-3-encoding adenoviral vector as a promising therapeutic agent for pancreatic cancer. Cancer Gene Ther 2016; 23: 278–283. [DOI] [PubMed] [Google Scholar]
- Plosker GL. Sipuleucel-T: in metastatic castration-resistant prostate cancer. Drugs 2011; 71: 101–108. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.