Dense corticothalamic projections modulate thalamic sensory responses in the rat vibrissa system as cortex selectively changes thalamic whisker responses depending upon the direction preference of thalamic and cortical neurons. We argue that this is best explained as a circuit-level “disinhibition” within the thalamo-cortico-thalamic loop. This study thus provides insight into how the rat somatic sensory system can filter incoming information to enhance selected inputs and suppress others when extracting information from the environment with its whiskers.
Keywords: corticothalamic projections, response timing, ventral posterior medial thalamus, whiskers, rat barrel cortex
Abstract
Cortex actively modulates the responses of thalamic relay neurons through corticothalamic (CT) projections. Here we investigated the temporal precision of CT modulation on sensory responses of relay neurons in rat ventral posterior medial thalamus (VPM) to direction-specific whisker stimuli. CT feedback levels were either augmented by cortical electrical microstimulation or depressed by cortical application of muscimol, a potent agonist of γ-aminobutyric acid A-type (GABAA) receptors. To evaluate the temporal specificity of CT influence, we compared the early (3–10 ms after stimulus onset) and late (10–100 ms) response components of VPM single units to whisker deflections in preferred or nonpreferred directions before and after altering CT feedback levels under urethane anesthesia. The data showed that cortical feedback most strongly affected the late responses of single VPM units to whisker stimulation. That is, cortical stimulation consistently increased the late responses of VPM units in the corresponding (homologous) barreloids to the stimulus direction preferred by neurons in the cortical locus stimulated. However, cortical stimulation could either increase or decrease the early response, depending on whether or not cortical and thalamic loci were tuned to the same direction. Such bidirectional regulation of the early and late VPM responses is consistent with a mechanism of circuit-level disinhibition in vivo. The results support the theory that CT feedback on thalamic sensory responses is mediated by a time-dependent shift of the excitation-inhibition balance in the thalamo-cortico-thalamic loop, such as would occur during sensory feature integration, plasticity, and learning in the awake state.
NEW & NOTEWORTHY
Dense corticothalamic projections modulate thalamic sensory responses in the rat vibrissa system as cortex selectively changes thalamic whisker responses depending upon the direction preference of thalamic and cortical neurons. We argue that this is best explained as a circuit-level “disinhibition” within the thalamo-cortico-thalamic loop. This study thus provides insight into how the rat somatic sensory system can filter incoming information to enhance selected inputs and suppress others when extracting information from the environment with its whiskers.
cortex and thalamus are reciprocally interconnected through bottom-up thalamocortical (TC) and top-down corticothalamic (CT) projections. CT projections are anatomically abundant and outnumber the TC projections by an order of magnitude (Deschenes et al. 1998; Guillery 1969; Liu et al. 1995; Sherman and Koch 1986). The CT projection accordingly has been found to have an intricate functional influence on TC relay neurons. CT projections have been described as a “modulatory” input to the thalamus (Bartlett and Smith 2002; Kao and Coulter 1997; Landisman and Connors 2007; Turner and Salt 1998), in contrast to the ascending “driving” input from sensory receptor surfaces such as the whiskers, retina, or cochlea (Sherman and Guillery 1998). In addition, the highly topographic organization of CT projections (Deschenes et al. 1998) provides an anatomical substrate for a high-resolution influence on thalamic relay neurons. In the auditory system, primary auditory cortex has an “egocentric” effect on the frequency tuning of TC neurons in bat medial geniculate nucleus (MGN), in that cortex can shift the tonotopic best frequency map in MGN toward the frequency preferred by stimulated CT neurons (Suga and Ma 2003). Visual system CT feedback arising from the primary visual cortex can modulate the firing mode (Denman and Contreras 2015; Sillito and Jones 2002; Wang et al. 2006), firing synchrony (Contreras et al. 1996), spatial summation (Andolina et al. 2013; Jones et al. 2015), and response timing (Andolina et al. 2007) of relay neurons in the lateral geniculate nucleus (LGN). Similarly, CT feedback from the rat barrel field cortex selectively enhances thalamic responses toward stimuli the cortex “prefers.” That is, CT feedback can alter the whisker tuning (Temereanca and Simons 2004) and angular (directional) tuning preferences of ventral posterior medial thalamus (VPM) neurons (Li and Ebner 2007).
The thalamo-cortico-thalamic loop is dynamically interactive in awake, behaving animals as sensory information and exploratory responses are continuously updated. The loop is subjected to short-term synaptic plasticity, neuromodulation, and excitation-inhibition (E-I) balance changes. Because of the dynamic nature of this loop, knowledge of the time dependence of CT modulation is of great importance for a better understanding of the underlying mechanisms of active and selective CT modulation of thalamic responses. CT feedback actively modulates the firing rate (poststimulus response magnitude profile over time) of TC neurons, which supports the proposed role in selective attention (Briggs and Usrey 2011; Casagrande et al. 2005). However, when the thalamic spikes are augmented or suppressed by CT feedback and how these changes are integrated within the spike train of cortical neurons is less well understood. Timing becomes particularly important under a sparse coding regime: for example, in rodent barrel cortex, where each whisker stimulus produces one spike on average (O'Connor et al. 2010) and coding may rely heavily on the timing of TC spikes (Bruno and Sakmann 2006). Available evidence suggests two possible scenarios. In the first case, CT feedback primarily modulates the early (short latency) thalamic response because the rapid lemniscus excitation and strong inhibition from the thalamic reticular nucleus (TRN) could result in a narrow time window of opportunity for VPM neurons to fire (Castro-Alamancos 2002a; Cox et al. 1997; Gentet and Ulrich 2003; Hirata et al. 2009; Kao and Coulter 1997; Lam and Sherman 2010; Landisman and Connors 2007; Turner and Salt 1998; von Krosigk et al. 1999). The modulation of short-latency responses is largely supported by slice experiments and also could occur in vivo. Alternatively, the dynamic nature of the thalamo-cortico-thalamic loop predicts a time-dependent, accumulative modulation of thalamic responses produced by shifting the E-I balance in TC neurons toward excitation (i.e., disinhibition: Castro-Alamancos 2002a; Castro-Alamancos and Calcagnotto 1999; Crandall et al. 2015; Gabernet et al. 2005; Kao and Coulter 1997; Lam and Sherman 2010; Landisman and Connors 2007; Mease et al. 2014; Turner and Salt 1998; von Krosigk et al. 1999; see discussion for details). Thus the cortical influence could affect both early and late (long latency) components of thalamic responses and has been shown in brain slices to have a dominant effect in the longer-latency responses (Crandall et al. 2015; also see discussion). These mechanisms would result in completely different impacts on spike timing-dependent processes in cortex, but the time dependence of CT influences has just begun to be appreciated (Crandall et al. 2015; Mease et al. 2014).
In this study we directly compared the effects of manipulating cortical activity levels on early and late responses in single VPM units after direction-preferred whisker deflections in urethane-anesthetized rats. We found that CT feedback affected both the early and late responses of VPM units but preferentially the late response. Strikingly, CT feedback could suppress the early response but simultaneously increase the late responses of the same VPM neuron. The concurrently bidirectional modulation is most consistent with the idea that CT feedback modulates the responsiveness of VPM cells through a shift in a time-sensitive, circuit-level E-I balance. Our results support the theory that CT modulation of thalamic relay neurons is primarily mediated by E-I balance shifts resulting from ongoing dynamic activity fluctuations within the thalamo-cortico-thalamic loop.
MATERIALS AND METHODS
Experimental procedures similar to those used to collect the data for this report have been published elsewhere (Li and Ebner 2006, 2007) so they are briefly summarized here. Adult Long-Evans rats (2–3 mo old, both sexes; n = 57) were anesthetized with urethane (1.5 g/kg, 30% aqueous solution ip) and subjected to microelectrode recordings in the left VPM and left (ipsilateral) barrel cortex. All experimental procedures were approved by the Vanderbilt Animal Use and Care Committee and were in compliance with National Institutes of Health guidelines for animal use.
Whisker stimulation.
Ramp-and-hold deflections (50 m/s onset velocity with 3-ms duration delivered at 1 stimulus/s, various amplitudes; see below) were applied to the principal whisker (PW) and immediately row-adjacent surround whiskers (SWs) at ∼10 mm past the guard hairs with a custom piezoelectric device. To map the angular tuning properties, groups of 50 stimuli were delivered to whiskers of interest in one of four cardinal (up, down, front, or back) directions in a pseudorandom order.
Surgery and electrophysiology.
Rats were surgically anesthetized before two circular craniotomies were made in the skull to lower microelectrodes into the left thalamus and the left barrel cortex base on respective coordinates (Li and Ebner 2007). Extracellular single-unit recording was simultaneously conducted with carbon fiber microelectrodes (Armstrong-James and Millar 1979) under urethane anesthesia from one cell in each location (Fig. 1A). Single units were recorded and digitized at 20 kHz with a CED 1401 Plus processor (Cambridge Electronic Design, Cambridge, UK) and stored on a PC (Dell, Round Rock, TX). Poststimulus time histograms (PSTHs) were constructed from spikes occurring between 3 and 100 ms poststimulus time and displayed online for receptive field (RF) and angular tuning mapping.
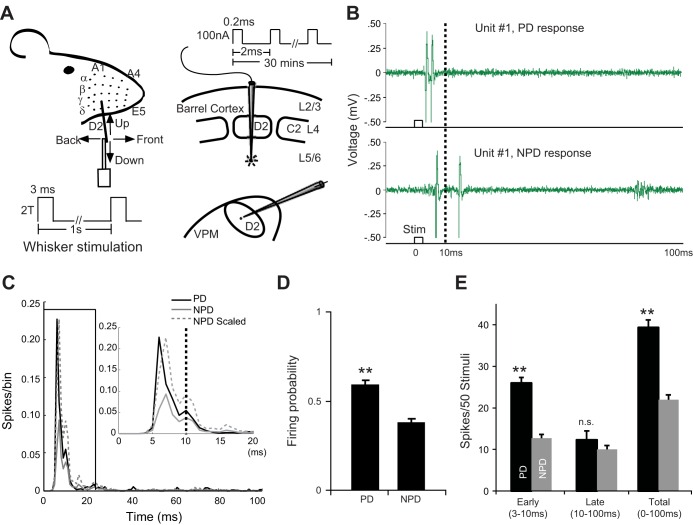
Fig. 1.
A: experimental design for cortical electrical microstimulation. Paired recordings of single-unit activity were conducted in VPM thalamus and ipsilateral barrel cortex. Angular tuning properties of single VPM and cortical units were mapped with deflections of a single PW for homologous cells in both locations. Stimuli were presented in 4 cardinal directions before and after electrical microstimulation of cortical L6. See materials and methods for details. B: example control trials in VPM unit 1 in response to PW deflections in the preferred direction (PD, top) and 1 of the 3 nonpreferred directions (NPD, bottom). Note the difference in the temporal structure between PD and NPD responses. C: population PSTHs (n = 32) of PD (black) and average NPD (gray solid line) responses of VPM units during control recording. Gray dashed line, scaled NPD response. Inset: expanded view of the boxed 0–20 ms data. D: firing probability of PD and NPD responses during control recording. E: average early, late, and total (3–100 ms) PD and NPD responses during control recording. Note that PD and NPD responses were mainly distinguished by the early (3–10 ms) but not late (10–100 ms) response. Error bars show SE. **P < 0.01. n.s., Not significant.
For VPM units, the RF first was tested manually and then mapped by delivering 50 stimuli (300-μm amplitude in caudal-rostral direction) to the PW (defined here as the whisker evoking the strongest response) and SWs (other whiskers producing a lesser response at longer latency). Spike duration (width peak to trough) was measured, and all VPM units were regular spiking units. Angular tuning curves of all VPM units were mapped by moving the PW in one of the four cardinal directions. The thalamic “preferred direction” (PD) was defined as the direction evoking the strongest response in a thalamic neuron. All three other directions were then named “nonpreferred directions” (NPDs). Next, the response threshold for PD deflections was determined by the minimal amplitude of whisker deflections still evoking ≥3 spikes/50 stimuli in the same poststimulus bin (Armstrong-James and Fox 1987). In the subsequent recording, all thalamic data were acquired with deflections at 2 times the threshold (2T) intensity. In our experiments the amplitude of 2T deflections ranged from 50 μm to 360 μm, with a median of 160 μm. We adopted this strategy to avoid VPM response saturation and leave open the possibility of capturing subtle up or down changes in the magnitude of thalamic responses.
After the RF of the VPM units was mapped, penetrations were made in the barrel field cortex with the cortical recording/stimulating microelectrode perpendicular to the cortical surface: the electrode was moved until we identified a homologous (e.g., D2 barrel column for VPM units in D2 barreloid) barrel column or a nonhomologous, immediately adjacent barrel column (e.g., D2 VPM units but D1 or D3 barrel column). This localization was facilitated by the roughly regular two-dimensional topography of the barrel cortex. Angular tuning curves of cortical units were mapped along the penetration in L2/3, 4, 5, and 6 with 300-μm PW deflections. In cases in which L6 responses were weak or absent, the angular tuning properties of L6 sites were extrapolated by those of responsive units above L6 in the penetration. To distinguish from the thalamic PD, preferred directions of cortical units were denoted as PD′. Accordingly cortical non-preferred directions were labeled as NPD′. For simplification, if both thalamic and cortical recording sites were tuned to the same direction it would be denoted as PD = PD′. Responsiveness of VPM units was periodically recorded before and after cortical manipulation. Data acquired before cortical manipulation (typically for 1–2 h) were used as controls and compared with recordings made after cortical manipulation.
Animal status was continuously monitored during the experiment by the respiration rate, corneal reflex, hindlimb withdrawal reflex, and voluntary whisker or body movements. Supplements of anesthetic (10% of initial dose) were given as needed to maintain an anesthesia level of stage III-3 (Friedberg et al. 1999) as stable as possible. To minimize possible confounding residual effects induced by cortical microstimulation or inactivation, only one VPM recording was conducted per animal. All experiments were terminated ≤14 h after the induction of anesthesia, to ensure that data were collected before the cortex showed signs of changes in responsiveness from initial state.
Cortical microstimulation.
A train of current pulses (100 nA, 0.2-ms duration, at 500 stimuli/s, tip positive) was delivered to L6 (1,500 μm below the pial surface, corresponding to L6a) through the cortical microelectrode for 30 min. This stimulation protocol has been shown to reversibly change the responsiveness of TC neurons in bats and rats (Li and Ebner 2007; Yan and Suga 1998; Zhang and Suga 2000). Reversibility of the stimulation effect suggested that no detectable injury was caused by the stimulation. Comparison of the multiunit activity data at the L6 simulation sites recorded with the same stimulating microelectrodes was done before and after the stimulation, and no abnormal neuronal discharge was found as a result of the stimulation.
Muscimol inactivation.
Focal application of muscimol was used to suppress cortical activity (Li et al. 2005). A small piece of porous material (Gelfoam) soaked with muscimol solution (10 mM in sterile saline, prepared just before the experiment) was applied to the dura to cover the entire surface of exposed barrel cortex without any direct contact with cortex. Drops of muscimol solution were supplemented periodically to keep the Gelfoam saturated. Again, only one application was made in each animal because of the possibility that irreversible alterations in excitability might be induced over time by release from the cortical suppression that could skew the data from subsequent trials.
Data analysis.
Responses of units were analyzed by batteries of 50 trials for each direction and averaged across units with common characteristics. Spontaneous discharge was calculated from the data 50 ms before the stimulus onset and subtracted from the evoked response. Stability of angular tuning properties during control recording was required for all VPM units before being included in the final database. Units showing drift in their angular tuning curves (3/57) were excluded. VPM responses during control recordings were averaged and inspected for temporal structure under our recording conditions. In this study the VPM response was divided into early (3–10 ms) and late (10–100 ms) response epochs (Fig. 1) and the magnitudes of the early, late, and total (3–100 ms) responses of VPM units were compared by whisker (PW vs. SW), deflection direction (PD vs. NPD), and experiment condition (control vs. manipulation).
To gain insights with a millisecond resolution, the average VPM response was compared for the sign of the effect of cortical manipulation, millisecond by millisecond (see Figs. 3, 5, and 7). An increase of response by cortical manipulation in a 1-ms bin was assigned a value of +1 and denoted as facilitation; −1 was assigned to a response decrease in 1-ms bins, meaning depression; 0 was assigned for no change. This comparison was conducted by deflection direction (PD vs. NPD) and experiment condition (control vs. manipulation). To extract an additional parameter of whether and how spiking pattern of VPM neurons was affected by cortical manipulation, interspike interval (ISI) of VPM responses was measured on a trial-by-trial basis and normalized by the magnitude of the corresponding control response. Firing probability, which was defined as the percentage of trials that fired at least one spike during the 3–100 ms poststimulus epoch, was also compared for each VPM unit. t-Test was used to assay statistical significance unless otherwise specified.
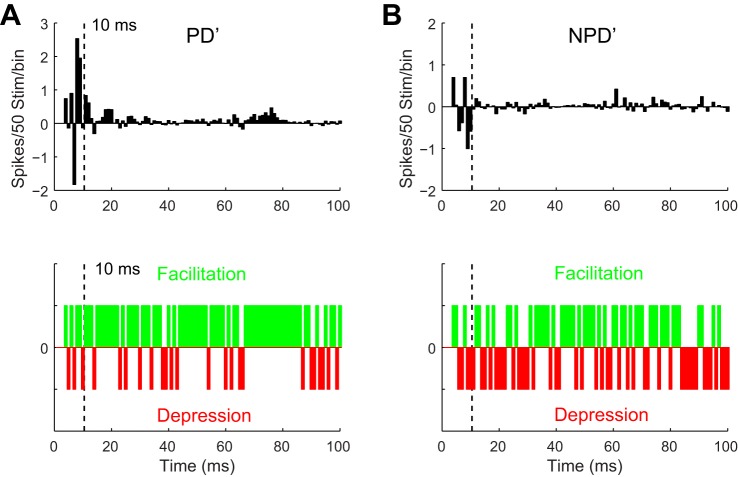
Fig. 3.
Cortical stimulation had a facilitatory effect on the late responses of homologous VPM units (n = 21). A: millisecond by millisecond VPM response difference to PD′ after CT stimulation (top), which reflected a profound facilitatory effect (green bins, difference > 0, meaning response increase after stimulation; bottom) in the majority of 1-ms bins rather than depression (red bins, difference < 0). B: for NPD′ responses, facilitation or depression of VPM responses occurred within individual 1-ms bins at chance levels.
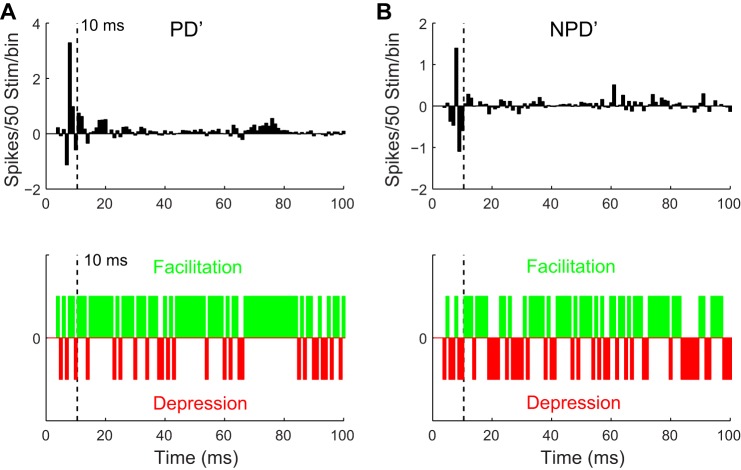
Fig. 5.
Cortical stimulation strongly facilitated the late response of homologous VPM units tuned to directions different from the cortex. A: millisecond by millisecond VPM response difference to PD′ after CT stimulation (top), showing individual 1-ms bins within the late response had a greater chance of being facilitated (green bins, meaning difference > 0) than depressed (red bins, difference < 0) (bottom). B: equivalent data for NPD′ responses, facilitation or depression of VPM responses within individual 1-ms bins occurred at a chance level.
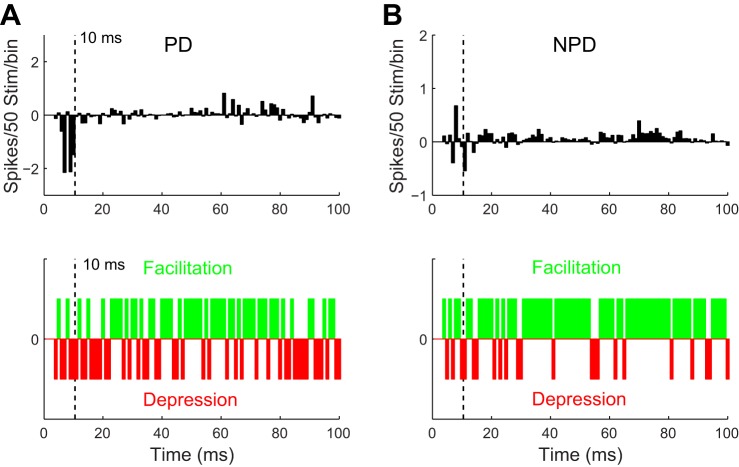
Fig. 7.
Cortical stimulation strongly facilitated the late NPD response of homologous VPM units tuned to directions different from the cortex. A: millisecond by millisecond VPM response difference to PD stimuli after CT stimulation (top). Individual 1-ms bins within the late response tended to have a greater chance to get facilitated (green bins, meaning difference > 0) than depressed (red bins, difference < 0) (bottom), but the facilitation was unable to compensate for the substantial reduction in the early response. B: NPD responses, on the other hand, had a prominent facilitation in the late response, due to 1 NPD being the PD′.
Histology.
At the end of the experiment, locations of the VPM units recorded and CT stimulated were marked with electrolytic lesions by passing a DC current (2 μA for 10 s) in VPM and L6 loci through the microelectrodes in VPM and barrel cortex, respectively. The rat was then overdosed with urethane and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer solution. The brain was postfixed overnight then transferred into 10%, 20%, and 30% sucrose. The brain was sectioned at 50 μm, and sections were stained for cytochrome oxidase activity to reconstruct the lesion (recording) sites (Wong-Riley and Welt 1980). Only experiments in which both VPM and cortical recording sites could be unambiguously identified were analyzed.
RESULTS
In the present study 52 pairs of single units were recorded: one electrode was in VPM thalamus and the other in the ipsilateral barrel cortex of 57 rats. Angular (directional) tuning preferences were mapped and compared for each neuronal pair before and after manipulation of CT feedback. Thirty-two thalamic units showed stable baseline angular tuning curves and were subsequently verified histologically to be in VPM, and only these units were included in our final analyses. These VPM units were sampled from VPM barreloids representing the whisker array row B–E and arc 1–4. Under our recording conditions, 31 of 32 VPM units were spontaneously silent but highly angularly tuned to PW deflections (Fig. 1, B–E). The control data set showed that VPM units had a higher firing probability to stimuli in their PD compared with NPD (PD vs. NPD: 0.58 ± 0.02 vs. 0.38 ± 0.02, mean ± SE, same below, P < 0.01), and the response magnitude to PD was 73% higher than that of the average NPD response (39.7 ± 2.1 spikes/50 stimuli vs. 22.9 ± 1.3, P << 0.01, 2-tailed t-test), indicating that PD deflections drove VPM neurons more reliably than NPD (Fig. 1, C–E). These results were consistent with previous studies in rat VPM (Armstrong-James and Callahan 1991; Castro-Alamancos 2002b; Diamond et al. 1992a; Ito 1988; Li and Ebner 2006; Nicolelis et al. 1993; Simons and Carvell 1989; Timofeeva et al. 2003; Waite 1973). Typical VPM units responded to sets of 50 PW deflections with an early sharp peak followed by a variable longer-latency tail: 67% of PD spikes were early spikes (within 3–10 ms poststimulus time, 26.5 ± 1.5) at a modal response latency of 6 ms. For average NPD response, only 57% of NPD spikes were early spikes (13.0 ± 0.9) and the modal response latency was delayed by 2 ms. We found that the early response was highly angularly tuned and distinguished PD consistently from NPD responses (PD vs. NPD: 26.5 ± 1.5 vs. 13.0 ± 0.9, P < 0.01). This finding is consistent with previous reports showing that VPM receives a fast and strong lemniscal input (Ito 1988; Simons and Carvell 1989; Waite 1973). No significant difference was found in the late response between PD and NPD (13.2 ± 2.1 vs. 9.9 ± 1.0, P = 0.08), suggesting that most of the late VPM response is not driven by the ascending lemniscal pathway. On the basis of these features, VPM responses were divided into early (3–10 ms) and late (10–100 ms) poststimulus response epochs for further analysis.
To investigate the temporal specificity of CT feedback on VPM responses, we first pooled all homologous cortex/VPM units (n = 21) and focused on the changes in their early and late responses with and without CT stimulation to the direction CT neurons preferred (PD′; Table 1, see Figs. 2 and 3). The data showed that CT stimulation modulated both early and late TC responses as summarized in Table 1. We next determined whether the effect of CT stimulation on early and late responses depends on the angular tuning coherence between VPM units recorded and cortical loci stimulated; we investigated the CT influence on early and late responses in a subgroup of homologous cortex/VPM units, but PD ≠ PD′ (n = 17). The rationale for this comparison is that if CT feedback acted through disinhibition, facilitatory effects should be observed on late responses, even in those VPM units that were not originally tuned to PD′. The data confirmed our hypothesis by showing a significant increase in late responses (see Figs. 4 and 5). As a control, we reanalyzed the same 17 VPM units, but by their original thalamic PD, to search for any systematic or coherent CT modulation to the thalamic PD. The data showed a bidirectional modulation by CT activation, strongly supporting the disinhibition hypothesis (see Figs. 6 and 7). In addition, we also analyzed the homologous VPM units cotuned with the cortical sites (PD = PD′, n = 4; see Fig. 8) and compared the CT stimulation results above with those VPM units when the CT feedback was suppressed by muscimol application (n = 5, see Fig. 9).
Table 1.
Grouping of VPM units by spatial and angular tuning properties with the cortex
| Angularly Matched (PD = PD′) | Angularly Mismatched (PD ≠ PD′) | Total | |
|---|---|---|---|
| VPM-L6 aligned (homologous) | PD: Early ↑*, Late ↑* | PD′: Early ↑n.s., Late ↑** | PD′: Early ↑n.s., Late ↑** |
| NPD: Early ↓n.s., Late ↓n.s. (n = 4, Fig. 8)4 | NPD′: Early ↓n.s., Late ↑n.s. (n = 17, Figs. 4 and 5)2 | NPD′: Early ↓n.s., Late ↑n.s. (n = 21, Figs. 2 and 3)1 | |
| PD: Early ↓*, Late ↑n.s. | |||
| NPD: Early ↑n.s., Late ↑* (n = 17, Figs. 6 and 7)3 | |||
| VPM-L6 misaligned (nonhomologous) | N/A | PD: Early ↓*, Late ↓n.s. | 6 |
| NPD: Early ↓n.s., Late ↓n.s. | |||
| (n = 6)5 | |||
| L6 suppression | PD: Early ↓**, Late ↑n.s. | ||
| NPD: Early ↓**, Late ↓n.s. (n = 5, Fig. 9)6 | |||
| Total | 4 | 23 | 32 |
↑, Response magnitude increase; ↓, response magnitude decrease.
Stimulating cortical L6 significantly increased the late response of homologous VPM units to the direction preferred by cortex (PD′).
In these homologous VPM units but tuned differently from cortex, L6 stimulation also significantly increased the late response to cortical PD′, even though these units were originally tuned to a different thalamic PD.
At the same time, however, L6 stimulation significantly decreased the early response of the above VPM units to thalamic PD but significantly increased average late response to 3 NPDs (which contains the cortical PD).
In homologous VPM units cotuned with cortex, L6 stimulation increased both the early and late responses to thalamic PD (which is also the cortical PD′).
In nonhomologous VPM units, L6 stimulation had a suppressive effect by significantly reducing the early response to thalamic PD.
Consistent with previous studies, suppressing cortical feedback by focal muscimol application significantly decreased the early response (and only the early response) to all directions.
P < 0.05;
P < 0.01. n.s., Not significant.
Fig. 2.
CT feedback increased both the early and late responses of VPM units in homologous barreloids (n = 21). A: average PSTHs of PD′ response in 2 example VPM units with matching PDs (top, VPM unit 3, PD = PD′) or mismatching PDs (bottom, VPM unit 9, PD ≠ PD′) with the cortical unit. Note the increase of the late response after cortical stimulation. B: CT activation increased the firing probability of homologous VPM units to PD′ but not NPD′ directions. C: CT feedback increased both the early and late PD′ responses of homologous VPM units. Left: raster plots of a proportion of trials of VPM units in response to PD′ before (top) and after (bottom) CT activation. Both early and late VPM responses to PD′ increased after CT activation. D: CT activation had no significant effect on either early or late NPD′ responses. Note the different y-scale of the raster plots. **P < 0.01.
Fig. 4.
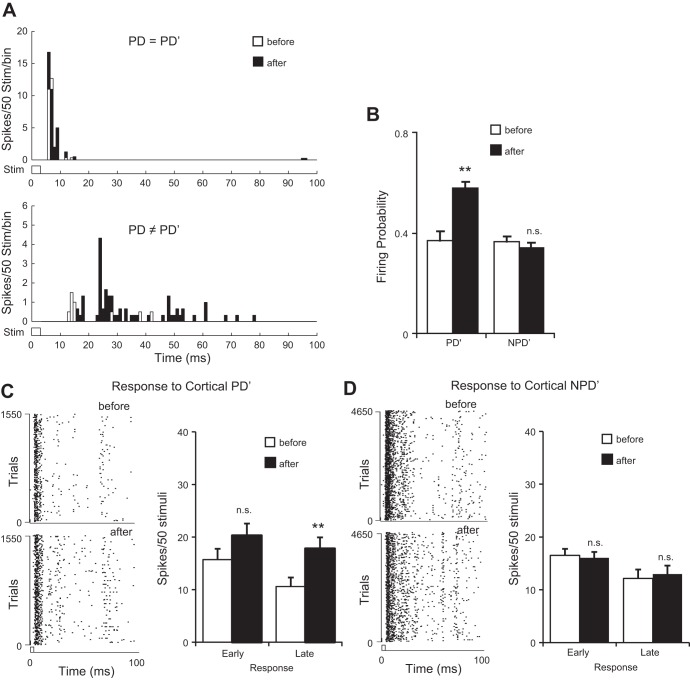
CT feedback selectively increased the late responses of homologous VPM units tuned to directions different from the cortex (PD ≠ PD′, n = 17). A: CT activation increased the firing probability of homologous VPM units to PD′ but not NPD′ directions. B: CT feedback significantly increased the late but not early PD′ responses of in these VPM units. Left: raster plots of a proportion of trials in response to PD′ before (top) and after (bottom) CT activation. Only late responses significantly increased after CT activation. C: CT activation had no significant effects on either the early or late NPD′ responses. **P < 0.01.
Fig. 6.
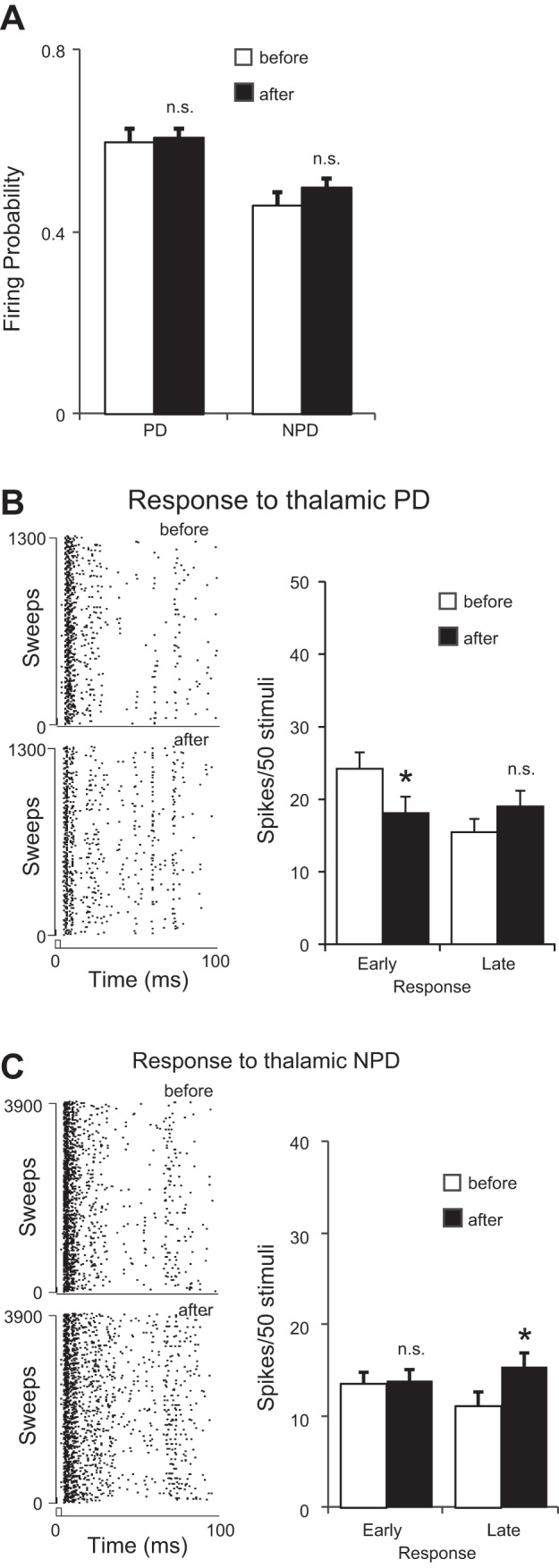
Interactions between cortical and thalamic preferred directions of stimulation (PD ≠ PD′). A: cortical stimulation had no overall effects on the firing probability to either thalamic PD or NPD stimuli. B, left: raster plots of a set of PD responses before (top) and after (bottom) cortical stimulation. Right: response latency analysis of cortical stimulation effects on PD responses. Note that the early response was significantly reduced. C: cortical stimulation significantly increased the late but not early NPD responses. *P < 0.05.
Fig. 8.
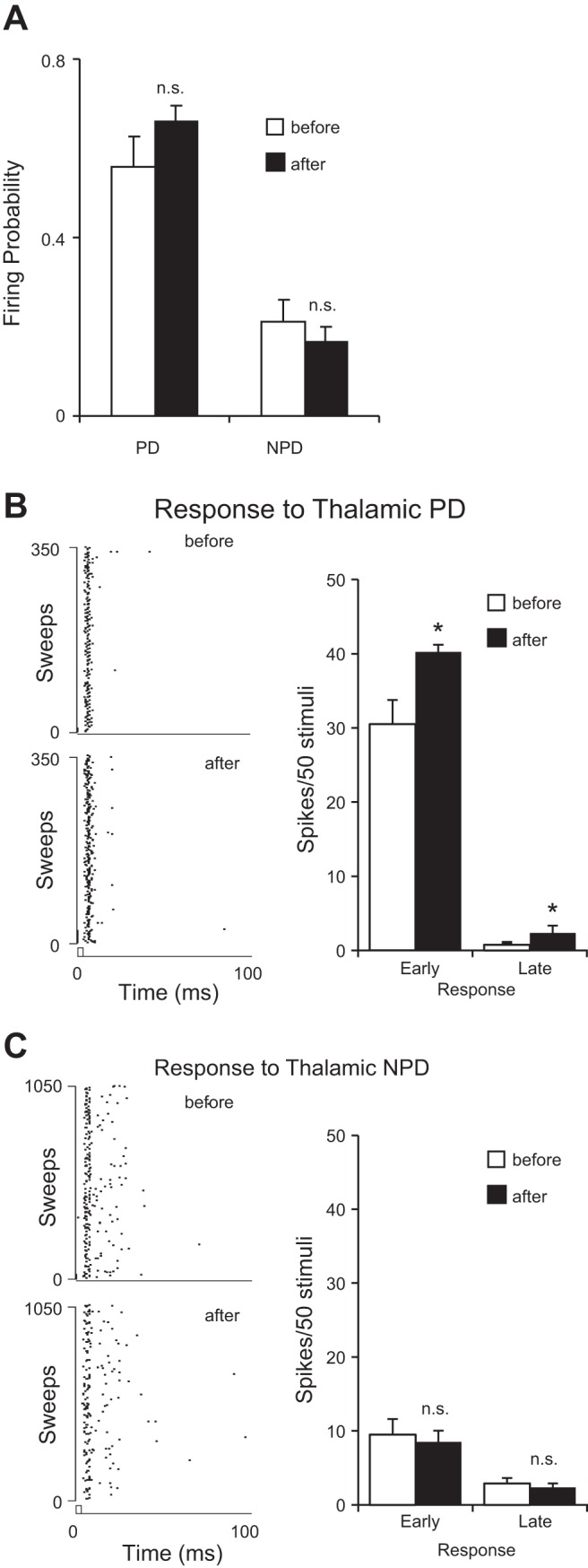
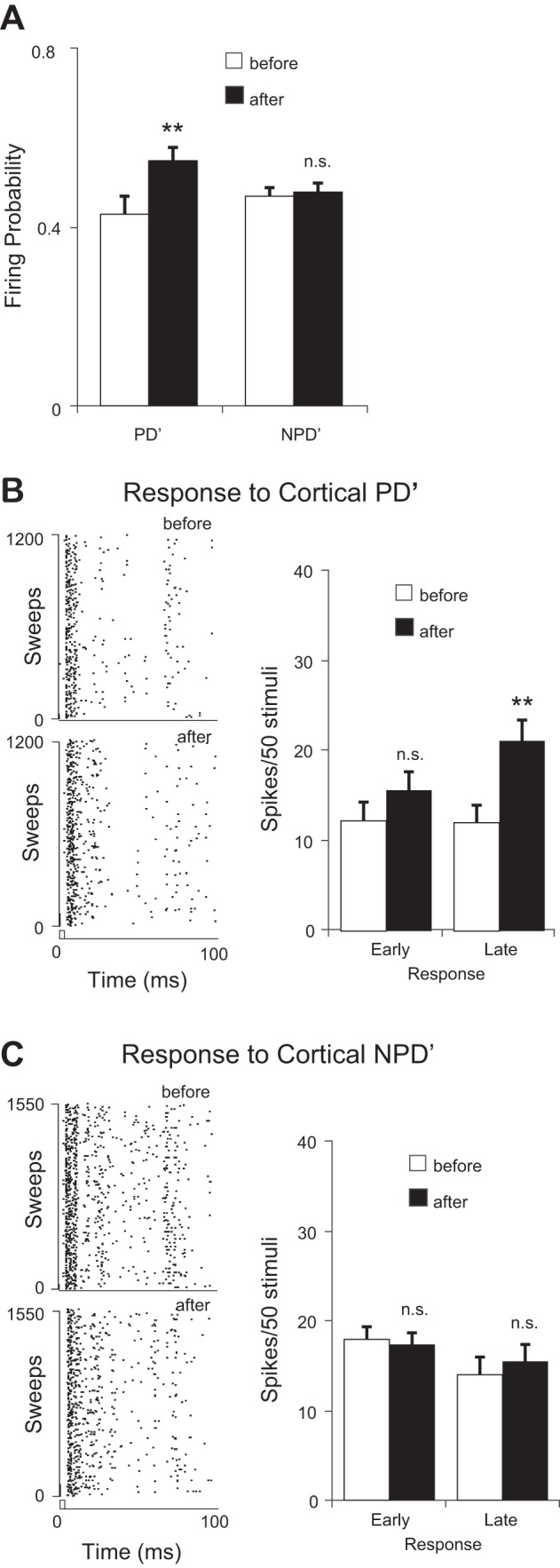
Effect of cortical feedback on the response to PD stimuli in homologous VPM neurons cotuned with cortex (PD = PD′, n = 4). A: cortical stimulation did not change the firing probability of VPM neurons to either PD or NPD stimuli. B, left: raster plots of a set of PD responses before (top) and after (bottom) cortical stimulation. Right: latency analysis of cortical stimulation effects on PD responses. Note that both the early and late responses significantly increased after cortical stimulation. C: cortical stimulation has no significant impact on NPD responses. *P < 0.05.
Fig. 9.
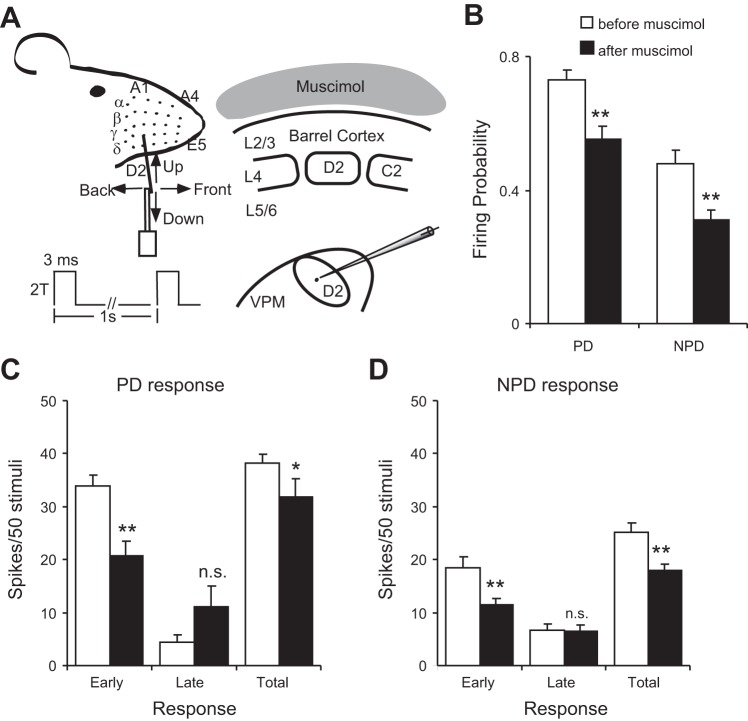
Suppressing cortical feedback decreases responses of VPM neurons to whisker stimuli. A: experimental design. Cortical suppression was achieved with muscimol applied to the cortical surface: see materials and methods for details. B: focal application of muscimol decreased thalamic firing probability following both PD and NPD stimuli. C: latency analysis of the PD response. The total response decrease is produced by a significant decrease in the short-latency component. D: latency analysis of NPD response showing a decrease in short-latency and total response after cortical suppression. *P < 0.05; **P < 0.01.
CT activation increased both early and late responses in homologous VPM TC neurons.
Previous results showed that cortex can “redirect” the spatial and angular tuning properties of VPM neurons having the same PW toward the angular preference of the cortical neurons (Li and Ebner 2007; Temereanca and Simons 2004). To examine how CT feedback affected the temporal structure of VPM responses, we first analyzed how CT activation modulated the early and late VPM responses to PD′ (the direction CT neurons preferred) by pooling all homologous VPM units (n = 21). These data showed that the CT projections modulated both early and late TC responses under our recording conditions (Fig. 2A) and are consistent with what is predicted by the disinhibition hypothesis. CT activation increased the firing probability of VPM units in response to PD′ stimuli (0.37 ± 0.04 vs. 0.58 ± 0.02, P < 0.01), but not NPD′ stimuli (0.36 ± 0.02 vs. 0.34 ± 0.02, P = 0.49), confirming that after CT activation VPM neurons fired more reliably to the direction preferred by cortex (Fig. 2B). The early VPM response to CT PD′ increased by 29% (before vs. after: 15.7 ± 2.1 vs. 20.3 ± 2.3, P = 0.08) and the late response by 68% (10.6 ± 1.7 vs. 17.8 ± 2.1, P < 0.01; Fig. 2C), indicating that CT activation had a stronger influence on the late TC response. For NPD′ responses, CT activation insignificantly affected the early (16.5 ± 1.3 vs. 16.0 ± 1.1, P = 0.44), late (12.1 ± 1.7 vs. 13.1 ± 1.6, P = 0.36; Fig. 2D), and total (28.6 ± 2.1 vs. 29.1 ± 1.9, P = 0.42, data not shown) response to 3 NPD′ directions, confirming that CT effect is selective to the direction cortex prefers. We also analyzed whether cortex influenced the spiking pattern of VPM neurons by comparing the ISI of VPM response before and after CT activation. We found that the median ISI insignificantly decreased from 5.2 ms to 3.6 ms after CT activation (P = 0.35, Wilcoxon test), but the ISI distribution of TC response to CT PD′ was significantly shifted by CT activation [P < 0.01, Kolmogorov-Smirnov (K-S) test]. No significant changes were found in the NPD′ responses for either ISI distribution (P = 0.25, K-S test) or median ISI (14.5 vs. 13.5 ms, P = 0.9, Wilcoxon test) of NPD′ response after CT activation.
To achieve better temporal resolution, we compared the average PSTH change in each 1-ms poststimulus bin (see materials and methods). The comparison indicated the late response increase was prominent in late (>10 ms) poststimulus bins (Fig. 2A and Fig. 3A). Responses (3–100 ms) to cortical PD′ per 1-ms bin had a much greater chance to be facilitated than depressed (facilitation vs. depression: 73/97 vs. 24/97, P < 0.01, binomial test) after cortical stimulation. Chances of facilitation were higher for the late response than the early response (early vs. late: 4/7 vs. 70/90; Fig. 3A). However, for NPD′ response there was no clear sign of change by cortical influence at any time (facilitation vs. depression: 50/97 vs. 47/97, P < 0.08, binomial test; Fig. 3B). Taken together, these data confirmed that CT activation increased both the early and late VPM responses, but the late response was more strongly affected. The increase in the early VPM response after CT activation suggested that CT feedback could modulate the excitability and response timing of VPM neurons, which is consistent with a direct CT excitation on VPM neurons. Increase of the late response, on the other hand, suggested that CT modulation may exploit short-term synaptic dynamics to alter the E-I balance in VPM neurons, which may also affect the early response, and modulate sensory information processing in VPM.
To further elucidate mechanisms underlying CT modulation, we compared the CT influence on early and late responses in homologous VPM units in which PD ≠ PD′ (n = 17) because the effect of CT activation on early and late responses may depend on whether VPM units are tuned coherently with the cortical locus stimulated (Li and Ebner 2007). We reasoned that if the CT modulation was produced through disinhibition we should observe the same effect on the late response, even though the VPM unit was not originally tuned to PD′. Our analysis showed that the modulation was only significant in the late not the early responses (Fig. 4). CT activation significantly increased the firing probability of VPM neurons to PD′ deflections (0.43 ± 0.04 vs. 0.55 ± 0.03, P < 0.01; Fig. 4A), indicating that CT feedback caused VPM units to fire more reliably to PD′ stimuli even though the thalamic neurons originally preferred a different stimulus direction. More importantly, after CT stimulation the late PD′ response was significantly increased (12.0 ± 1.9 vs. 21.1 ± 2.3, P < 0.01) but not the early response (12.2 ± 2.1 vs. 15.6 ± 2.0, P = 0.13; Fig. 4B). Consistently, significant changes were found in both ISI distribution (P < 0.01, K-S test) and median ISI of the response to PD′ stimuli (12.5 vs. 25.1 ms, P < 0.01, Wilcoxon test).
VPM responses to the average of three NPD′ deflections, however, showed no significant changes in either firing probability (0.47 ± 0.02 vs. 0.48 ± 0.02, P = 0.37) or early (17.7 ± 1.4 vs. 17.3 ± 1.3, P = 0.39), late (13.9 ± 2.0 vs. 15.3 ± 1.9, P = 0.29), or total (31.6 ± 2.4 vs. 32.6 ± 2.2, P = 0.39, Fig. 4, A and C) responses. ISI distribution (P = 0.33, K–S test) and median ISI (15.4 vs. 15.1 ms, P = 0.83, Wilcoxon test) were not affected. Consistent with these results, the average PSTH showed a prominent facilitation in response to deflections in cortical PD′ after cortical stimulation (facilitation vs. suppression: 72/97 vs. 25/97, P < 0.01, binomial test; Fig. 5A). The early response to NPD′ did not show a clear indication of facilitation (2/7 vs. 5/7, P < 0.17), which was present in the late response (53/90 vs. 37/90, P < 0.02; Fig. 5B). These results confirmed that the CT projection preferentially affected the late VPM responses and were consistent with the idea of a circuit-level disinhibition mediated by CT feedback.
To test whether CT modulation was more coherent with responses to the thalamic PD, we reanalyzed the 17 VPM units sorted by their original thalamic PD. To our surprise, we found that cortical stimulation significantly decreased the early thalamic PD response (24.2 ± 2.2 vs. 18.0 ± 2.2, P < 0.03) but had no significant effect on the late component (15.5 ± 1.6 vs. 19.0 ± 1.9, P = 0.10) or on firing probability (0.61 ± 0.03 vs. 0.60 ± 0.02, P = 0.34) of responses to PD deflections (Fig. 6, A and B). A decrease in the early PD response confirmed that CT activation increased the TRN inhibition onto VPM cells, consistent with the idea that cortical feedback may bidirectionally modulate the excitability of VPM neurons. Data from NPD responses showed a simultaneous facilitatory effect, which was temporally restricted to the late response. Cortical stimulation did not change the early NPD response (13.6 ± 1.3 vs. 14.0 ± 1.2, P = 0.83) but significantly increased the late response (11.2 ± 1.5 vs. 15.5 ± 1.5, P < 0.02) and firing probability (0.46 ± 0.03 vs. 0.50 ± 0.02, P < 0.04) of NPD responses (Fig. 4, A and C). Inspection of the response change by 1-ms bins did not reveal any significant facilitatory effect in PD response (facilitation vs. suppression: 52/97 vs. 45/97, P = 0.06, binomial test; Fig. 7A).
The late PD response appeared to be facilitated (facilitation vs. suppression: 50/90 vs. 40/90, P < 0.05) but was apparently not strong enough to compensate for the suppression in early PD responses. However, for the thalamic NPD response, which by default contained the cortical PD′, a clear facilitation was found in the late response (facilitation vs. suppression: 71/90 vs. 19/90, P < 0.01). The early response showed no direction of change (3/7 vs. 4/7, P = 0.27), confirming previous findings that cortex preferentially modulated the late response in VPM units (Fig. 7B). These results provided direct support for the disinhibition theory: it is not the suppression or facilitation alone but the shift of E-I balance by CT stimulation that exceeded the TRN inhibition and mediated the response changes observed in the thalamus.
The ISI distribution in PD responses was significantly altered (P < 0.05, K-S test) and median ISI was significantly reduced (4.1 vs. 2.9 ms, P < 0.01, Wilcoxon test) by cortical stimulation. ISI distribution (P < 0.01, K-S test) and median ISI (4.3 vs. 11.8, P < 0.01, Wilcoxon test) of NPD response were also significantly changed. Analyzing all homologous VPM units (n = 21) by their PD had the same trend (data not shown). These results clearly showed that cortex did not simply alter the overall excitability of VPM neurons but rather changed the E-I balance in a stimulus direction-dependent manner.
We also analyzed the homologous VPM units tuned to the same direction as the cortical locus (PD = PD′, n = 4): in these cases, CT activation did not increase the firing probability (0.57 ± 0.07 vs. 0.65 ± 0.04, P = 0.46; Fig. 8A). However, both the early (30.5 ± 3.3 vs. 40.2 ± 2.2, P < 0.03) and late (0.75 ± 0.49 vs. 2.3 ± 0.8, P < 0.05) responses to PD deflections significantly increased their response magnitude after CT stimulation (Fig. 8B). No significant changes were found in either the ISI distribution (P = 0.96, K-S test) or median ISI (2.7 vs. 2.7 ms, P = 0.7, Wilcoxon test). We further analyzed responses to NPD stimuli and found that CT activation had no significant effect on either early (11.4 ± 2.3 vs. 10.5 ± 1.8, P = 0.36) or late (4.4 ± 0.8 vs. 3.8 ± 0.7, P = 0.28) responses. Similarly, no significant changes were found in either firing probability (0.21 ± 0.05 vs. 0.16 ± 0.03, P = 0.42), ISI distribution (P = 0.99, K-S test), or median ISI (6.5 vs. 5.1 ms, P = 0.36, Wilcoxon test) of responses to NPD deflections (Fig. 8C). These results could be affected by the small sample size.
Six units were found histologically within VPM but were nonhomologous (different PWs) from the CT stimulation sites. They also were tuned to different directions, thus nonhomologous and PD ≠ PD′. These VPM units showed strong suppression of the early responses to PD deflections (data not shown), which would be predicted from previous reports (Li and Ebner 2007; Temereanca and Simons 2004).
Inactivating cortex decreased short-latency responses of VPM neurons.
Previous studies concluded that removal of CT feedback by cortical cooling depresses the responsiveness of certain thalamic neurons (Diamond et al. 1992b; Yuan et al. 1985, 1986). Because the CT influence interacts dynamically with VPM and TRN, we studied whether suppression of CT activity would produce any shift of the E-I balance in a manner reversing the effects of what occurred after cortical microstimulation. Because precise, local inactivation of CT neurons within single barrel columns is technically difficult to prove, we used pharmacological inactivation with muscimol, a potent GABAA receptor agonist, to silence the feedback from the entire barrel cortex as a first approximation (Fig. 9A). Latency analyses were conducted in histologically verified VPM units (n = 5) before and after muscimol inactivation. Effectiveness of this method of muscimol application has been documented previously (Li et al. 2005; Li and Ebner 2006). Thirty minutes of muscimol application significantly suppressed the early PD response (33.9 ± 2.2 vs. 20.7 ± 2.8, P < 0.01), suggesting a tonic excitatory influence of CT projections on VPM neurons, consistent with previous findings (Diamond et al. 1992b; Yuan et al. 1985, 1986). Surprisingly, however, the late response to PD deflections showed an increase (4.4 ± 1.4 vs. 11.2 ± 3.9, P = 0.06), suggesting that a disinhibition prevailed after TRN lost its CT excitatory drive. Firing probability to PD deflections (0.73 ± 0.03 vs. 0.55 ± 0.04, P < 0.05) and the total PD response magnitude (38.2 ± 1.6 vs. 31.9 ± 3.5, P < 0.05) showed a modest decrease. These results suggest that CT suppression resulted in a greater reduction of TRN inhibition onto TC neurons than the effect of the decrease in direct CT excitation, resulting in a delayed disinhibition. This reduction in late inhibition increased the late response in VPM TC neurons. VPM neurons produced fewer action potentials after CT inactivation, but when firing they tended to fire more late spikes, which is consistent with the idea that CT projections have a strong excitatory drive to VPM and TRN but the excitatory drive to TRN is stronger than that to VPM under many conditions. This effect is specific to PD deflections, because late responses to NPD stimuli were unaffected (6.6 ± 1.2 vs. 6.4 ± 1.1, P = 0.46) while the early NPD response also was significantly decreased (18.5 ± 1.9 vs. 11.5 ± 1.3, P < 0.01; Fig. 9). No significant change was found in ISI distribution or median ISI in either PD or NPD responses after muscimol application (data not shown). In summary, inactivation of cortical feedback caused a disinhibition in late PD responses but suppressed the overall responses of VPM neurons. Because muscimol application removed the entire excitatory CT input to TRN neurons, more spatially localized (e.g., within a single barrel column) methods for cortical inactivation are needed to further sort out circuit suppression mechanisms.
DISCUSSION
Our data show that cortical feedback modulates the responses of rat VPM neurons to whisker stimulations in a time-specific (both early and late response epochs) and angular tuning-dependent manner. Cortical inputs affect both early and late responses but have a more prominent effect on the late responses. A striking finding is that CT feedback could modulate the responsiveness of VPM cells through a time-dependent shift in circuit-level E-I balance. This is based on the key observation that CT feedback produced simultaneous but opposite modulation of the early and late responses in VPM neurons, as we reason below: 1) The decrease of early responses means that TRN inhibition onto VPM neurons was enhanced after cortical stimulation. This is because there is no evidence showing that ascending lemniscal excitatory drive to VPM would be suppressed by stimulating cortex; thus the reduction of early VPM response must be caused by the increase of TRN inhibition. 2) At the same time, the late response of these VPM neurons increased, meaning that excitation ramps up along the time and overruns inhibition, which is disinhibition by definition. It remains possible that changes in intrinsic excitability may also contribute to the observation, but we do not think it can account for the entire bidirectional change in response magnitude because there is no known mechanism that allows the intrinsic excitability decreases just for ∼10 ms and then increases afterwards. A circuit-level disinhibition is the most likely and more parsimonious interpretation, and it has good consistency with previously published studies: recently it has been shown in mouse thalamocortical brain slices that stimulating CT axons at ∼10 Hz increases VPM spikes in both early and late latency epochs in response to stimulations of the ascending lemniscal fibers (Crandall et al. 2015), which is highly consistent with our in vivo findings in which direction of whisker deflection is an added parameter. To our knowledge, the present study provides the first in vivo evidence showing that CT projections regulate the late response of thalamic relay neurons. The long duration effects of CT feedback may have a profound impact on the temporal integration of sensory inputs to cortical neurons, through which coincidence detection can be performed in barrel cortex. The finding could have further implications in cross-whisker communication, feature detection, and even long-term synaptic modifications. Although it currently remains technically challenging to reliably measure the E-I ratio in projection neurons, we hope future studies can directly compare the E-I ratio in VPM neurons before and after manipulating CT feedback to gain more mechanistic insights.
The topography of the CT projections correlates with the precision of CT influence on tuning properties in thalamic relay neurons, which has been well demonstrated in auditory, somatosensory, and visual systems in multiple species (Andolina et al. 2007; Denman and Contreras 2015; Li and Ebner 2007; Olsen et al. 2012; Sillito and Jones 2002; Suga and Ma 2003; Temereanca and Simons 2004; Wang et al. 2006; Yan and Suga 1996). To better analyze the spatial correspondence between the cortical and thalamic sites, in the present study we chose a classical method of restricted loci electrical microstimulation, instead of a more recent technique of optogenetics (Mease et al. 2014), to manipulate the cortical feedback. Our decision is based upon the fact that 1) optogenetics tools are largely available in mice, but a species difference may exist between rats and mice, given the fact that no systematic study of CT modulation on whisker tuning has been done in mice yet, and 2) it is technically challenging to spatially restrict the activation of opsins within a single barrel column with currently available methods of delivery, such as viral injection. Also, optogenetics requires electrophysiological verification. Therefore, in the present study electrical microstimulation with a microelectrode was selected to manipulate cortical activity levels in restricted loci in vivo.
A second methodology note is that this study only focused on CT projections from L6, without considering the separate contribution of corticofugal projections from L5. In rats L5 projects heavily to the brain stem with axon collaterals to the thalamus. Unlike L6 CT projections, L5 axons primarily terminate in the medial division of the posterior nucleus with only sparse terminals in VPM (Deschenes et al. 1998). L5 projections are morphologically and physiologically distinct and have been suggested to have a role in the cortico-thalamo-cortical loop, such as the communication between S1 and S2 (Theyel et al. 2010). The separate effect of L5 projections on VPM responses remains largely untested, and their separate influence was not dissected here, so they could be additional modulators of information processing in VPM.
Our results are consistent with existing knowledge of the anatomy and dynamics of the thalamo-cortico-thalamic loop. The minimal thalamo-cortico-thalamic loop consists of three nodes: excitatory TC and CT neurons with interposed TRN GABAergic cells. TRN neurons receive homologous excitatory inputs from thalamus and cortex and in turn provide inhibitory inputs exclusively to the thalamic neurons. Excitatory TC projections connect the thalamus with cortex and on route give rise to topographically localized excitatory collaterals to TRN. The excitatory CT projections reciprocally innervate both the TC nuclei and TRN, the latter being the only source of inhibitory terminals in rat VPM (Jones 1985). The connectivity pattern between TC and TRN results in ascending trigeminal activity producing excitation followed by a strong TRN inhibition of TC neurons both in vitro and in vivo (Castro-Alamancos 2002a; Castro-Alamancos and Calcagnotto 1999; Cox et al. 1997; Crandall et al. 2015; Gentet and Ulrich 2003; Hirata et al. 2009; Kao and Coulter 1997; Lam and Sherman 2010; Landisman and Connors 2007; Mease et al. 2014; Turner and Salt 1998; von Krosigk et al. 1999).
In the presence of stimuli, CT axons can conduct at high (∼2 ms) or low (>10 ms) velocity and strongly facilitate thalamic firing at high firing frequency (Bartlett and Smith 2002; Castro-Alamancos and Calcagnotto 1999; Kao and Coulter 1997; Kelly et al. 2001; Kwegyir-Afful and Simons 2009; Landisman and Connors 2007; Li et al. 2003; Swadlow 1992; Turner and Salt 1998). In brain slices, high-frequency stimulation of CT axons can directly activate TC neurons at short latency (Kao and Coulter 1997; Li et al. 2003; Turner and Salt 1998). This fast, monosynaptic CT excitation on TC cells may precede the subsequent TRN inhibition, which involves disynaptic transmission (Crandall et al. 2015; Hirata et al. 2009; Lam et al. 2010; Landisman and Connors 2007; Mease et al. 2014). Therefore, CT projections may have an overall direct excitatory influence on thalamic firing that will increase the responsiveness of TC cells within a narrow time window of opportunity before inhibition dampens the response. Because of this temporally tight coupling of CT excitation and TRN inhibition, one may predict that CT feedback could boost the early responses of TC neurons, without necessarily altering the temporal distribution (ISI) of TC action potentials, because synchronous activation of several TC terminals on single cortical neurons is required to effectively fire L4 neurons (Bruno and Sakmann 2006) and following sensory stimuli the CT feedback from L6 may increase the early thalamic response to boost the TC firing synchrony (Andolina et al. 2007; Contreras et al. 1996; Sherman 2001; Wang et al. 2006; see Fig. 8).
Recorded dynamics in the thalamo-cortico-thalamic loop, however, supports the idea that cortex can modulate both the early and late TC responses. It has been shown that CT synapses are strongly facilitated at high frequencies of activation compared with CT-TRN synapses (Crandall et al. 2015; Cruikshank et al. 2010; Gentet and Ulrich 2004; Jurgens et al. 2012; Landisman and Connors 2007). With sustained CT activation, the difference in short-term synaptic dynamics between CT, CT-TRN, and TRN-TC synapses can profoundly enhance the efficacy of excitatory CT synapses onto TC neurons (Castro-Alamancos 2002a; Castro-Alamancos and Calcagnotto 1999; Crandall et al. 2015; Kao and Coulter 1997; Landisman and Connors 2007; Mease et al. 2014; Turner and Salt 1998; von Krosigk et al. 1999). Consequently, the E-I balance in TC neurons can be shifted toward excitation and result in a time-restricted, accumulative push-pull-like “disinhibition.” As the disinhibition (as we defined here) develops, the cortical influence on the late spikes will also be amplified. These pieces of evidence, both in vivo and in vitro, together suggest that the integration of local VPM and TRN activity plus CT feedback will modulate the late responses of VPM neurons. Thus we conjecture that the increase of late spikes could have a circuit-level origin. The augmented response may spread to more temporally dispersed TC activity in which CT feedback affects both early and late TC responses over time.
The CT modulation of late VPM spikes through disinhibition may appear paradoxical but is the most likely explanation based on the connectivity pattern and synaptic dynamics in the thalamo-cortico-thalamic loop. Rat VPM neurons are reliably driven by ascending projections from the brainstem trigeminal complex and fire short-latency spikes in response to PW deflections both under anesthesia and in the awake state (Armstrong-James and Callahan 1991; Ito 1988; Simons and Carvell 1989; Waite 1973). The fast (short latency) response evokes strong TRN inhibitory feedback to VPM neurons, which can be occluded by synaptic adaptation given sufficient CT activation (Mease et al. 2014). Under our experimental conditions, CT terminals were presumably strongly facilitated by cortical microstimulation, producing a supralinear increase in CT synapse efficacy (Castro-Alamancos 2002a; Castro-Alamancos and Calcagnotto 1999; Cox et al. 1997; Crandall et al. 2015; Gentet and Ulrich 2003; Hirata et al. 2009; Kao and Coulter 1997; Lam and Sherman 2010; Landisman and Connors 2007; Li et al. 2003; Mease et al. 2014; Turner and Salt 1998; von Krosigk et al. 1999). It should be noted that CT stimulation might depress inhibitory TRN synaptic efficacy in VPM under certain conditions (Castro-Alamancos 2002a; Castro-Alamancos and Calcagnotto 1999; Crandall et al. 2015; Gabernet et al. 2005; Kao and Coulter 1997; Lam and Sherman 2010; Landisman and Connors 2007; Mease et al. 2014; Turner and Salt 1998; von Krosigk et al. 1999), resulting in an even stronger “push-pull”-like circuit behavior producing imbalances in the E-I equilibrium in TC neurons toward excitation. It has been reported that under high-frequency cortical activation protocols the CT axons can directly excite VPM neurons (Castro-Alamancos and Calcagnotto 1999; Li et al. 2003; Shin and Chapin 1990). Hence, both early and late TC responses should be augmented by CT activation under certain conditions. Another possible modulator of CT influence on TC firing could occur through recruiting frequency-dependent activation of T-type calcium channels (Deleuze et al. 2012; Jahnsen and Llinas 1984; Wang et al. 2006).
Our data are consistent with previous studies showing that CT projections can affect thalamic responses through multiple mechanisms within the L6-TRN-VPM circuitry. The CT-TRN projection has also been reported to facilitate TRN activity (Gentet and Ulrich 2004; Liu and Jones 1999). This may compensate for the synaptic depression on TRN-TC synapses and could potentially alter the TRN suppression of TC responses when a cortical site is active but also influence nonaligned thalamic sites. Consistently, local manipulation of TRN activity has been shown to change the RF size in response to lesions in TRN (Lee et al. 1994a). A strong enhancement effect on late responses has been demonstrated in rat VPM in vivo after suppression of inhibition by microdialysis of GABA receptor antagonists (Hirata et al. 2009; Lee et al. 1994b). This matches nicely with our in vivo data and further supports an important role of disinhibition in shaping VPM responses. VPM neurons receive convergent excitatory and inhibitory inputs. As demonstrated by Crandall et al., in the VPM-TRN-L6 circuitry excitation or inhibition alone has less meaning than the E-I ratio and how the E-I balance evolves over time. Excitation and inhibition may increase or decrease, but excitation increases more (or decreases less) than inhibition and this would constitute a circuit-level “disinhibition.”
The disinhibitory CT modulation suggests an overall CT excitatory tone. Even though the CT projections are anatomically abundant, the L6 neurons are not highly spontaneously active in anesthetized animals (Innocenti and Manzoni 1972; Swadlow 1989; Swadlow and Hicks 1996). From published data, we estimate that effective CT modulation may require bursting activity and/or sustained tonic firing (>3–6 Hz) in L6 (Crandall et al. 2015; Cruikshank et al. 2010; Jurgens et al. 2012; Kao and Coulter 1997; Landisman and Connors 2007; Mease et al. 2014; Turner and Salt 1998). It is not fully understood when CT neurons are active and how cortex modulates thalamic response through CT projections in awake animals. Fully awake, freely moving animals show several behavioral/arousal states (Fanselow et al. 2001). These behavioral states clearly affect how the information is transmitted from thalamus to cortex (Gentet et al. 2012; Krupa et al. 2004; Poulet and Petersen 2008; Sherman and Guillery 1996, 2002). In mouse visual cortex, an active (running) state is accompanied by elevated neural activity in all six layers (Neill and Stryker 2010), and this activity depends on neuromodulatory activity (e.g., norepinephrine and acetylcholine) inputs (McCormick 1992; Polack et al. 2013). Neuromodulation can be activated naturally by brain stem activation to increase “alertness,” which in turn changes the response profile of neurons in thalamus and cortex (Castro-Alamancos 2004). Available data suggest that certain L6 neurons can be active as a result of a cohort of network activity, brain states, and/or neuromodulation, especially when the animal is alert and engaged. Under these circumstances, reported CT effects could be a possible pathway mediating selective attention. The technical difficulty of selectively manipulating cortical feedback activity in freely moving animals is well known, but such data would be necessary to address the functional importance of CT system in higher-order brain functions such as attention. Studies in visual thalamus suggest that attention can modulate transmission of visual information through primate LGN (Casagrande et al. 2005). A combination of optogenetics, electrophysiology, and behavior testing is required to further elucidate the role of cortical feedback in active rats in a behavior context, such as discrimination between surfaces of different textures.
An important question is how the modifiable late spike trains participate cortical information processing. We speculate that late TC spikes may be recruited for coincidence detection in cortical neurons; depending on the transmission delay of the coactivated inputs the cortical neurons will integrate from the thalamus, within and between barrel columns, as well as other brain regions. One possibility is that the increased late thalamic response would facilitate the temporal integration of salient features, for example, through coincidence detection. As the rat moves its whiskers across a textured surface, CT feedback increases late TC spikes during the post-whisker-contact time of 10–100 ms. These spikes would be temporally additive with those from activated neighboring barrel columns onto the home neuron and nonlinearly increase its likelihood of firing, which may facilitate the event detection. Furthermore, because of the sparseness of evoked neural activity in rat S1, spike timing is critical to sensory coding/decoding, plasticity, and learning (Dan and Poo 2006; Tiesinga et al. 2008). Therefore, cortical modulation of the timing of thalamic responses could be of central importance for sensory information processing. Because the timing of L4 responses largely depends on synchronous thalamocortical activation (Bruno and Sakmann 2006; Gil et al. 1999; Stratford et al. 1996), the increase of correlated early response in thalamus could profoundly impact cortical information propagation, in addition to changing the firing mode by CT feedback (Sherman 2001; Wang et al. 2006). Moreover, changes of early or late TC responses may incur timing-dependent plasticity mechanisms, which could contribute to reshaping the cortical circuits during the repetitive trials used in learning paradigms.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-025907 and a Vanderbilt University Discovery Grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.L. and F.F.E. conception and design of research; L.L. performed experiments; L.L. analyzed data; L.L. and F.F.E. interpreted results of experiments; L.L. prepared figures; L.L. drafted manuscript; L.L. and F.F.E. edited and revised manuscript; L.L. and F.F.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Allen Institute founders, Paul G. Allen and Jody Allen, for their vision, encouragement, and support.
REFERENCES
- Andolina IM, Jones HE, Sillito AM. Effects of cortical feedback on the spatial properties of relay cells in the lateral geniculate nucleus. J Neurophysiol 109: 889–899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina IM, Jones HE, Wang W, Sillito AM. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc Natl Acad Sci USA 104: 1685–1690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Callahan CA. Thalamo-cortical processing of vibrissal information in the rat. II. Spatiotemporal convergence in the thalamic ventroposterior medial nucleus (VPm) and its relevance to generation of receptive fields of S1 cortical “barrel” neurones. J Comp Neurol 303: 211–224, 1991. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol 263: 265–281, 1987. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Millar J. Carbon fibre microelectrodes. J Neurosci Methods 1: 279–287, 1979. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience 113: 957–974, 2002. [DOI] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Corticogeniculate feedback and visual processing in the primate. J Physiol 589: 33–40, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312: 1622–1627, 2006. [DOI] [PubMed] [Google Scholar]
- Casagrande V, Sary G, Royal D, Ruiz O. On the impact of attention and motor planning on the lateral geniculate nucleus. Prog Brain Res 149: 11–29, 2005. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539: 567–578, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol 87: 946–953, 2002b. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74: 213–247, 2004. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. Presynaptic long-term potentiation in corticothalamic synapses. J Neurosci 19: 9090–9097, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274: 771–774, 1996. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc Natl Acad Sci USA 94: 8854–8859, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall SR, Cruikshank SJ, Connors BW. A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron 86: 768–782, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev 86: 1033–1048, 2006. [DOI] [PubMed] [Google Scholar]
- Deleuze C, David F, Béhuret S, Sadoc G, Shin HS, Uebele VN, Renger JJ, Lambert RC, Leresche N, Bal T. T-type calcium channels consolidate tonic action potential output of thalamic neurons to neocortex. J Neurosci 32: 12228–12236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman DJ, Contreras D. Complex effects on in vivo visual responses by specific projections from mouse cortical layer 6 to dorsal lateral geniculate nucleus. J Neurosci 35: 9265–9280, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Rev 28: 286–308, 1998. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462–476, 1992a. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Budway MJ, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J Comp Neurol 319: 66–84, 1992b. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA 98: 15330–15335, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol 81: 2243–2252, 1999. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48: 315–327, 2005. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 15: 607–612, 2012. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Ulrich D. Strong, reliable and precise synaptic connections between thalamic relay cells and neurones of the nucleus reticularis in juvenile rats. J Physiol 546: 801–811, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Ulrich D. Electrophysiological characterization of synaptic connections between layer VI cortical cells and neurons of the nucleus reticularis thalami in juvenile rats. Eur J Neurosci 19: 625–633, 2004. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23: 385–397, 1999. [DOI] [PubMed] [Google Scholar]
- Guillery RW. A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z Zellforsch Mikrosk Anat 96: 39–48, 1969. [DOI] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Influence of subcortical inhibition on barrel cortex receptive fields. J Neurophysiol 102: 437–450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Manzoni T. Response patterns of somatosensory cortical neurones to peripheral stimuli. An intracellular study. Arch Ital Biol 110: 322–347, 1972. [PubMed] [Google Scholar]
- Ito M. Response properties and topography of vibrissa-sensitive VPM neurons in the rat. J Neurophysiol 60: 1181–1197, 1988. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Voltage-dependent burst-to-tonic switching of thalamic cell activity: an in vitro study. Arch Ital Biol 122: 73–82, 1984. [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum, 1985. [Google Scholar]
- Jones HE, Andolina IM, Shipp SD, Adams DL, Cudeiro J, Salt TE, Sillito AM. Figure-ground modulation in awake primate thalamus. Proc Natl Acad Sci USA 112: 7085–7090, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens CW, Bell KA, McQuiston AR, Guido W. Optogenetic stimulation of the corticothalamic pathway affects relay cells and GABAergic neurons differently in the mouse visual thalamus. PLoS One 7: e45717, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CQ, Coulter DA. Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurons in vitro. J Neurophysiol 77: 2661–2676, 1997. [DOI] [PubMed] [Google Scholar]
- Kelly MK, Carvell GE, Hartings JA, Simons DJ. Axonal conduction properties of antidromically identified neurons in rat barrel cortex. Somatosens Mot Res 18: 202–210, 2001. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MA. Layer-specific somatosensory cortical activation during active tactile discrimination. Science 304: 1989–1992, 2004. [DOI] [PubMed] [Google Scholar]
- Kwegyir-Afful EE, Simons DJ. Subthreshold receptive field properties distinguish different classes of corticothalamic neurons in the somatosensory system. J Neurosci 29: 964–972, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb Cortex 20: 13–24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. VPM and PoM nuclei of the rat somatosensory thalamus: intrinsic neuronal properties and corticothalamic feedback. Cereb Cortex 17: 2853–2865, 2007. [DOI] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. I. Assessment of receptive field changes following thalamic reticular nucleus lesions. J Neurophysiol 71: 1702–1715, 1994a. [DOI] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. II. Differential effects of GABAA and GABAB receptor antagonists on responses of VPM neurons. J Neurophysiol 71: 1716–1726, 1994b. [DOI] [PubMed] [Google Scholar]
- Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol 90: 3429–3440, 2003. [DOI] [PubMed] [Google Scholar]
- Li L, Ebner F. Balancing bilateral sensory activity: callosal processing modulates sensory transmission through the contralateral thalamus by altering the response threshold. Exp Brain Res 172: 397–415, 2006. [DOI] [PubMed] [Google Scholar]
- Li L, Ebner FF. Cortical modulation of spatial and angular tuning maps in the rat thalamus. J Neurosci 27: 167–179, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rema V, Ebner FF. Chronic suppression of activity in barrel field cortex downregulates sensory responses in contralateral barrel field cortex. J Neurophysiol 94: 3342–3356, 2005. [DOI] [PubMed] [Google Scholar]
- Liu X, Honda C, Jones E. Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. J Comp Neurol 352: 69–91, 1995. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Predominance of corticothalamic synaptic inputs to thalamic reticular nucleus neurons in the rat. J Comp Neurol 414: 67–79, 1999. [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39: 337–388, 1992. [DOI] [PubMed] [Google Scholar]
- Mease RA, Krieger P, Groh A. Cortical control of adaptation and sensory relay mode in the thalamus. Proc Natl Acad Sci USA 111: 6798–6803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. Proc Natl Acad Sci USA 90: 2212–2216, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65: 472–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67: 1048–1061, 2010. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature 483: 47–52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16: 1331–1339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454: 881–885, 2008. [DOI] [PubMed] [Google Scholar]
- Sherman SM. A wake-up call from the thalamus. Nat Neurosci 4: 344–346, 2001. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol 76: 1367–1395, 1996. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators.” Proc Natl Acad Sci USA 95: 7121–7126, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357: 1695–1708, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res 63: 1–20, 1986. [DOI] [PubMed] [Google Scholar]
- Shin HC, Chapin JK. Mapping the effects of SI cortex stimulation on somatosensory relay neurons in the rat thalamus: direct responses and afferent modulation. Somatosens Mot Res 7: 421–434, 1990. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci 357: 1739–1752, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989. [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382: 258–261, 1996. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4: 783–794, 2003. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in S-1 vibrissa cortex of the awake rabbit: receptive fields and axonal properties. J Neurophysiol 62: 288–308, 1989. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Monitoring the excitability of neocortical efferent neurons to direct activation by extracellular current pulses. J Neurophysiol 68: 605–619, 1992. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Hicks TP. Somatosensory cortical efferent neurons of the awake rabbit: latencies to activation via supra- and subthreshold receptive fields. J Neurophysiol 75: 1753–1759, 1996. [DOI] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41: 639–651, 2004. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci 13: 84–88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga P, Fellous JM, Sejnowski TJ. Regulation of spike timing in visual cortical circuits. Nat Rev Neurosci 9: 97–107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva E, Merette C, Emond C, Lavallee P, Deschenes M. A map of angular tuning preference in thalamic barreloids. J Neurosci 23: 10717–10723, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. J Physiol 510: 829–843, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Monckton JE, Reiner PB, McCormick DA. Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus. Neuroscience 91: 7–20, 1999. [DOI] [PubMed] [Google Scholar]
- Waite PM. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol 228: 541–561, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jones HE, Andolina IM, Salt TE, Sillito AM. Functional alignment of feedback effects from visual cortex to thalamus. Nat Neurosci 9: 1330–1336, 2006. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci USA 77: 2333–2337, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Suga N. Corticofugal modulation of time-domain processing of biosonar information in bats. Science 273: 1100–1103, 1996. [DOI] [PubMed] [Google Scholar]
- Yan W, Suga N. Corticofugal modulation of the midbrain frequency map in the bat auditory system. Nat Neurosci 1: 54–58, 1998. [DOI] [PubMed] [Google Scholar]
- Yuan B, Morrow TJ, Casey KL. Responsiveness of ventrobasal thalamic neurons after suppression of S1 cortex in the anesthetized rat. J Neurosci 5: 2971–2978, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Morrow TJ, Casey KL. Corticofugal influences of S1 cortex on ventrobasal thalamic neurons in the awake rat. J Neurosci 6: 3611–3617, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bats. J Neurophysiol 84: 325–333, 2000. [DOI] [PubMed] [Google Scholar]