Reduced lactate uptake by α-cyano-4-hydroxycinnamic acid (4-CIN), a monocarboxylate-transporter 2 (MCT2) blocker, interferes with oxidative metabolism and leads to subsequent disturbances in ion homeostasis due to decreased ATP synthesis. This was indicated by an increase in extracellular oxygen tension, accumulation of flavin adenine dinucleotide (FAD), and baseline potassium concentration and effects on evoked potentials. 4-CIN led to extracellular acidosis, which could be mimicked by addition of lactate to artificial cerebrospinal fluid. These effects are not due to activation of lactate receptors.
Keywords: 4-CIN, FAD autofluorescence, extracellular potassium, sodium and calcium concentrations, lactate shuttle, monocarboxylate transporter
Abstract
Astrocyte-derived lactate supports pathologically enhanced neuronal metabolism, but its role under physiological conditions is still a matter of debate. Here, we determined the contribution of astrocytic neuronal lactate shuttle for maintenance of ion homeostasis and energy metabolism. We tested for the effects of α-cyano-4-hydroxycinnamic acid (4-CIN), which could interfere with energy metabolism by blocking monocarboxylate-transporter 2 (MCT2)-mediated neuronal lactate uptake, on evoked potentials, stimulus-induced changes in K+, Na+, Ca2+, and oxygen concentrations as well as on changes in flavin adenine dinucleotide (FAD) autofluorescence in the hippocampal area CA3. MCT2 blockade by 4-CIN reduced synaptically evoked but not antidromic population spikes. This effect was dependent on the activation of KATP channels indicating reduced neuronal ATP synthesis. By contrast, lactate receptor activation by 3,5-dihydroxybenzoic acid (3,5-DHBA) resulted in increased antidromic and orthodromic population spikes suggesting that 4-CIN effects are not mediated by lactate accumulation and subsequent activation of lactate receptors. Recovery kinetics of all ion transients were prolonged and baseline K+ concentration became elevated by blockade of lactate uptake. Lactate contributed to oxidative metabolism as both baseline respiration and stimulus-induced changes in Po2 were decreased, while FAD fluorescence increased likely due to a reduced conversion of FAD into FADH2. These data suggest that lactate shuttle contributes to regulation of ion homeostatsis and synaptic signaling even in the presence of ample glucose.
NEW & NOTEWORTHY
Reduced lactate uptake by α-cyano-4-hydroxycinnamic acid (4-CIN), a monocarboxylate-transporter 2 (MCT2) blocker, interferes with oxidative metabolism and leads to subsequent disturbances in ion homeostasis due to decreased ATP synthesis. This was indicated by an increase in extracellular oxygen tension, accumulation of flavin adenine dinucleotide (FAD), and baseline potassium concentration and effects on evoked potentials. 4-CIN led to extracellular acidosis, which could be mimicked by addition of lactate to artificial cerebrospinal fluid. These effects are not due to activation of lactate receptors.
the brain, despite accounting for only up to 2% of body weight, utilizes about 20% of oxygen and 25% of glucose consumed by the human body (Bélanger et al. 2011). Brain function relies prominently on oxidative metabolism of glucose for the synthesis of ATP most of which are used for ion homeostasis and regulation of extracellular transmitter levels (Attwell and Laughlin 2001). During repetitive stimulation, ion changes occur in the extracellular space mostly due to K+ release and Na+ and Ca2+ influx into neurons. Such ion concentration changes can be readily measured with ion-sensitive microelectrodes and they recover usually within some ten s back to baseline involving diffusion and active as well as secondary active transport processes (Dietzel et al. 1982; Liotta et al. 2012; Lux et al. 1986). The required ATP is synthesized predominantly by oxidative energy metabolism, dependent on pyruvate supply to mitochondria. Supply to mitochondria may depend on two redundant processes. One is neuronal glucose uptake and its subsequent glycolytic breakdown into pyruvate and the other might be neuronal uptake of lactate from extracellular space by the neuronal monocarboxylate transporter 2 (MCT2) (Bélanger et al. 2011; Pellerin et al. 2007) with lactate being generated in astrocytes by glycolysis and glycogenolysis (Dringen et al. 1993; Dienel and Cruz 2015). Lactate was proposed to be released from astrocytes into the extracellular space either by hemichannels or anion channels (Karagiannis et al. 2015; Sotelo-Hitschfeld et al. 2015) or by monocarboxylate transporters MCT1 and 4, which are expressed on astrocytes (Bröer et al. 1997). Export of lactate from astrocytes and subsequent import into neurons is the basis of the astrocytic-neuronal-lactate shuttle theory (ANLS) (Pellerin and Magistretti 1994; Pellerin et al. 2007). MCT1, the most abundant form of MCT in the brain, which is also expressed in oligodendrocytes and endothelial cells, is assumed to provide lactate support for axonal survival (Fünfschilling et al. 2012).
There might be regional variation in astrocytic support to energy metabolism and ion homeostasis. For example, blocking astrocytic glycolysis in area CA1 had little effect on the kinetics of stimulus-induced rises in potassium concentration (unpublished observation) while it slowed recovery in the dentate gyrus (Xiong and Stringer 1999). On the other hand Galeffi et al. (2007) proposed a role for lactate shuttle from astrocytes to neurons by studying the effects of inhibition of neuronal lactate uptake on NADH autofluorescence signals and stimulus-induced oxygen consumption in area CA1.
Here, we determined the contribution of neuronal lactate uptake to synaptic signaling, ion homeostasis, and oxidative energy metabolism in the area CA3 by evaluating the effects of MCT2 inhibitor α-cyano-4-hydroxycinnamic acid (4-CIN) on stimulus-induced field potential and ion concentration transients, tissue Po2, as well as mitochondrial redox changes as indicated by the fluorescence of the electron carrier FAD. Area CA3 was chosen because it has a different vulnerability to hypoxia and induction of spreading depolarization than CA1, suggesting potential differences in neurometabolic coupling (Aitken et al. 1998; Gebhardt et al. 2002; Jarosch et al. 2015; Pomper et al. 2006). The area CA3, with its local recurrent synaptic connections, allows for simultaneous recording of purely presynaptic (antidromic spike) and purely postsynaptic (orthodromic spike) field potential components when stimulating Schaffer collaterals in CA1.
Reduced lactate shuttle from astrocytes to neurons may negatively interfere with ATP synthesis. On the other hand, blocking lactate uptake could increase extracellular lactate levels, leading to activation of the G-protein-coupled hydroxy carboxylic acid (HCA1) receptors, which are widely expressed in the hippocampus and cortex (Lauritzen et al. 2013). To distinguish between these possibilities, we tried to mimic the effects of 4-CIN by applying the HCA1 receptor agonist 3,5-dihydroxybenzoic acid (3,5-DHBA).
MATERIALS AND METHODS
Slice preparation and maintenance.
We performed our experiments on 47 male Wistar rats (weighing between 250 and 300 g) obtained from Janviers Labs (Le Genest-Saint-Isle, France), which were euthanized in accordance with the Helsinki declaration and institutional guidelines (as approved by the State Office of Health and Social Affairs Berlin Lageso, T0096/02) and the animal welfare regulations of Charité. After 5–7 days of accommodation in groups of two with food ad libitum and a 12-h light on light off cycle animals were decapitated under deep anesthesia with isoflurane (3% vol/vol) and laughing gas (70% N2O, 30% O2); the brain was rapidly removed from the calvarium and transferred to cold articial cerebral spinal fluid (aCSF) for slicing. The aCSF solution contained the following (in mM): 129 NaCl, 21 NaHCO3, 10 glucose, 3 KCl, 1.25 NaH2PO4, 1.6 CaCl2, and 1.8 MgCl2. The pH and the osmolarity of the solution were maintained at 7.4 and 295–305 mosM, respectively. Horizontal hippocampal slices (400-μm thick) were prepared with a Leica VT 1200 S vibratome (Wetzlar, Germany) and kept in an interface chamber with continuous aCSF perfusion equilibrated with 95% O2-5% CO2 at 34 - 36°C. Flow rate was 2 ml/min under interface and 8 ml/min under submerged recording conditions. The slices were left to recover for 2 h before starting the experiments. Experimental design followed the Arrive recommendations and recommendation for the 3 R strategies to minimize the use of experimental animals.
Electrophysiology and oxygen recordings.
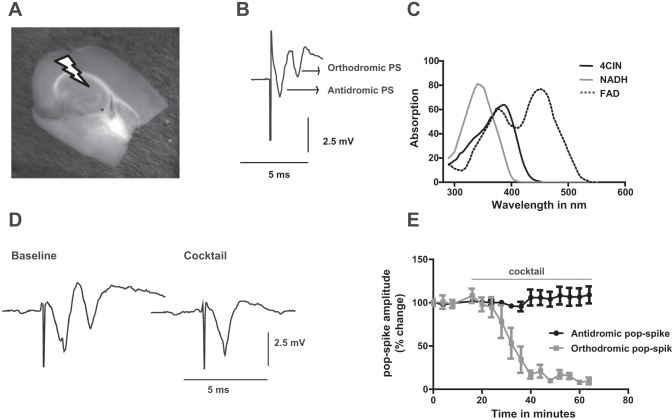
DC-coupled field potential and extracellular ion concentrations were measured using double-barreled ion sensitive microelectrodes, built as previously reported (Liotta et al. 2012). The reference side of the electrodes was filled with 154 mM NaCl while the ion-sensitive side was filled with 100 mM KCl, 100 mM CaCl2, or 150 mM NaCl to get K+-, Ca2+-, and Na+-sensitive electrodes, respectively. Ionophore cocktails (Potassium Ionophore I 60031, Calcium Ionophore I 21048, and Sodium Ionophore II 71178; all from Fluka, Buchs, Switzerland) were used to tip fill the ion selective barrels. Before each experiment the electrodes were tested using a 10-fold calibration solution (3/30 mM for KCl, 0.3/3 mM for CaCl2, and 15/150 mM for NaCl) pH microelectrodes were constructed using Hydrogen Ionophore II, Cocktail A (Fluka, Buchs, Switzerland) and backfilled with a buffer solution of pH 7.0 containing the following (mM): 500 KCl, 64.7 NaH2PO4, and 85.3 Na2HPO4. All microelectrodes were calibrated with NaCl solutions containing a mixture of KH2PO4 and Na2HPO4 to yield pH values between 6.8 and 7.8 (osmolarity: 230 mosM). We recorded from CA3 pyramidal layer while repetitively stimulating Schaffer collateral fibers antidromically with 0.1-ms pulses for 2 s with 20-Hz stimulus trains every 4 min (with an intensity evoking 80% of maximal field potential response using a bipolar stimulatin electrode with platinum wire tips separated by 100 to 150 μm; Fig. 1). Stimulation intensity to evoke 80% maximal response varied between 1.5 and 3.8 V depending on stimulation electrode properties and slice characteristic as well as distance to CA3. This stimulation protocol has been chosen as it does not induce significant synaptic potentiation or depression (Liotta et al. 2012) under control conditions. The stimulus-induced signals in area CA3 consisted of an initial antidromically propagated population spike (PS1) followed by a field postsynaptic potential and a second population spike (PS2) due to synaptic activation in area CA3 (Fig. 1B). The extracellular concentration of the ions (in mM) was calculated using a modified Nernst equation.
Fig. 1.
A: photograph of a horizontal entorhinal cortex-hippocampus slice in the interface chamber. Arrow indicates position of stimulation electrode in CA1 stratum radiatum used to activate Schaffer collateral fibers. Position of recording electrode at CA3 pyramidal layer indicated by an asterisk. B: sample trace of field potential responses. The 1st component stands for antidromic population spike (PS) while the 2nd one represents orthodromic population spike. C: α-cyano-4-hydroxycinnamic acid (4-CIN) absorption spectra. The absorption peak at 360 nm overlaps with the absorption peak of NADH but not with flavin adenine dinucleotide (FAD) (450 nm; D) sample traces of field potential responses during baseline condition and application of the cocktail which blocks the secondary orthodromic populatipn spike. E: time course of changes in antidromic and orthodromic population spikes during cocktail application. Note: the cocktail diminished the orthodromic response while it does not have any effect on antidromic population spike.
where ΔV = voltage difference measured for a monovalent cation X at room temperature equals ∼56 and at body temperature +61 mV (R = gas constant, T = absolute temperature, Z = valence of the ion, and F = Faraday constant). To adapt to individually slightly varying ion sensitivities the ΔV for a 10-fold change in a given ion concentration was used with 2.3 RT/zF replaced by the measured slope of the electrode. Oxygen response was measured using the Clark-style oxygen sensor microelectrodes (tip: 10 μm; Unisense, Aarhus, Denmark). The electrode was polarized for 2 h and calibrated in aCSF solution saturated with 0, 50, and 95% O2, respectively. The signal of the ion-sensitive electrodes and the polarographic amplifier (Chemical Microsensor II; Diamond General Development) were simultaneously digitalized and stored on computer disk. Absorption spectra of 4-CIN was investigated with a spectrophotometer NanoDrop (Thermo Scientific, Berlin, Germany).
Fluorescence recordings.
FADH2 shuttles electrons from glycolysis and the tricarboxylic acid cycle (TCA) to electron transport chain, playing a central role in cellular energy metabolism. As FAD (oxidized form) is fluorescent; it displays a stimulation-induced biphasic mirror image to the biphasic NAD(P)H autofluorescence changes (Rösner et al. 2013). FAD fluorescence represents preferentially mitochondrial redox changes (Kunz et al. 1994) whereas NAD(P)H fluorescence (including fluorescence of NADH and NADPH) originate from both mitochondria and cytosol (Schuchmann et al. 2001). For recording of FAD, we used custom built equipment with light-emitting diode (LED; Prior, Cambridge, UK) and photomultiplier tube (PMT; Seefelder Mebtechnik, Seefeld, Germany) as previously described (Rösner et al. 2013). FAD was excited at 460 ± 10 nm (Blue LED). LEDs were controlled by short pulses with 5 ms applied with a frequency of 5 Hz to minimize photobleaching (Rösner et al. 2016). Absorption of 4-CIN (200 μM), FAD (1.15 mM), and NADH (1.8 mM, all in aqueous solutions) was measured using a Nanodrop 1000 (Thermo Scientific).
Pharmacology.
To study the effect of lactate shuttle on stimulation-induced extracellular ion changes, respiration, and mitochondrial redox potential, we applied the MCT2 inhibitor 4-CIN (200 μM; Sigma-Aldrich). Application times were in most cases 20 min, which results in incomplete equilibration of the slice with the agent of choice as determined with ion and drug selective electrodes (Müller et al. 1988). Thus the applied concentration is not expected to act on glutamate receptors and mitochondrial pyruvate uptake (Nagase et al. 2014). The effect of 4-CIN could be either metabolic due to ATP depletion or mediated by the HCA1 receptor, following extracellular lactate accumulation (Lauritzen et al. 2013). Depletion of ATP can lead to opening of KATP channels. Hence, we coadministered 4-CIN with glibenclamide (50 μM; Sigma-Aldrich), a blocker of ATP-sensitive potassium channels, to counteract ATP depletion effects of 4-CIN on field potential responses (Mourre et al. 1989). To exclude receptor-mediated effects, we compared the effects of HCA1 receptor agonist 3,5-hydroxybenzoic acid, (1.5 mM; Sigma-Aldrich) with the effects of 4-CIN. Based on the findings of Liotta et al. (2012), we investigated the role of lactate shuttle into axonal compartment by inhibiting neurotransmitter release and postsynaptic receptors with a cocktail of drugs consisting of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; Sigma-Aldrich), (2R)-amino-5-phosphonopentanoate (APV; Sigma-Aldrich), (RS)-α-methyl-4-carboxyphenylglycine (MCPG; Tocris Biosciences), bicuculline methiodide (Sigma-Aldrich), and nickel (Sigma-Aldrich) with the concentration of 50 μM , 50 μM, 150 μM, 5 μM, and 2 mM, respectively.
Data analysis.
The last three stimulus-induced responses made during baseline recording and during drug application were used for statistical analysis. Field potential responses to CA1 stratum radiatum stimulation were composed of an initially antidromically propagating and a subsequent orthodromic population spike. They were used to monitor the effects of 4-CIN on synaptic transmission. We assessed amplitude, first and second half decay time (T1/2, T2/2) for K+, Na+, and Ca2+ signals. First half decay time stands for the time required for the signal to decay from its peak to half maximal amplitude while second half decay time represents the time taken for the signal to return from half recovery to baseline. Electrophysiological and fluorescent data were stored on computer disk using CED 1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK). The sampling rate was 10 and 1 kHz for field potential and ion and oxygen concentration recordings, respectively. Regarding group size with an n of 9 or 10 the statistical power was close to 0.8 as computed with G*Power program (University of Düsseldorf). In cases where significance was very high, we accepted also lower group sizes of seven. Data are presented as mean ± SE or in most figures as box plots where the median, the first and third quartile, and maxima and minima of the distribution are displayed Statistical analysis was done using Microsoft Excel and Graphipad prism. Drug effects were evaluated using paired t-test for data with normal distribution; Wilcoxon signed rank test was used for non-normal distribution.
RESULTS
4-CIN reduces synaptic transmission in the associational network of area CA3.
Electrical stimulation of CA1 stratum radiatum evoked both orthodromic and antidromic action potentials in CA3 due to activation of Schaffer collaterals and the local recurrent axonal network. Anti- and orthodromic population spikes could be distinguished by their latency, the first being the antidromically propagated population spike due to activation of the Schaffer collaterals, whereas the second population spike, on top of a positive field potential transient, was synaptically mediated due to recurrent excitatory interaction between pyramidal cells and pyramidal cells and interneurons (Fig. 2A). This interaction could be completely prevented by application of the AMPA-kainate receptor blocker CNQX 50 μM, the NMDA receptor blocker APV (50 μM), and the GABA A blocker bicuculline (5 μM) and by application of the metabotropic glutamate receptor blocker MCPG (150 μM) confirming the synaptic nature of the secondary response whereas the antidromically propagating PS1 remained intact (Fig. 1, D and E).
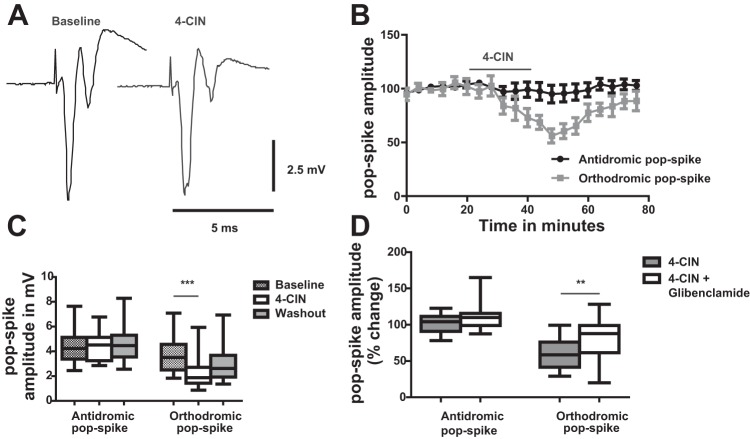
Fig. 2.
Effect of 4-CIN on stimulus-induced field potential changes in stratum pyramidale of CA3. A: effect of 4-CIN on stimulus-induced field potential in CA3. Stimulus duration was 0.1 ms, stimulus adjusted to 80% of that required to induce maximal responses. Note: no major effect of 4-CIN on the antidromically propagated population spike while the synaptically mediated secondary orthodromic population spike is reduced indicating an effect on synaptic transmission. B: time course of changes in antidromic (black) and orthodromic (grey) population spikes during 4-CIN application. C: statistical analysis of effects of 4-CIN on amplitude of antidromically and orthodromically propagating population spike. Note: 4-CIN significantly reduced orthodromic population spike. D: glibenclamide (50 μM) partly rescued the effect of 4-CIN on orthodromic population spikes. **P < 0.01, ***P < 0.001.
Perfusion of the MCT2 blocker 4-CIN (200 μM for 20 min) did not alter the antidromic population spike while it significantly and reversibly decreased the orthodromic population spike from 3.7 ± 1.3 to 2.3 ± 1.3 mV (Fig. 2, B and C; n = 10 slices, 5 rats, P < 0.0001) similar to the effect of 4-CIN observed in area CA1 by Galeffi et al. (2007).
We hypothesized that the effect of 4-CIN is due to reduced intracellular ATP levels and that the reduction of the synaptically mediated population spikes was due to activation of KATP channels. We therefore applied 4-CIN in combination with glibenclamide (50 μM), which is a well-characterized blocker of KATP channels. Application of glibenclamide partially reversed the 4-CIN effect on synaptically mediated potentials in CA3. While 4-CIN reduced orthodromically activated population spikes by 38.3%, coadministration of glibenclamide reduced the effect to 13.4% (Fig. 2D; n = 6 slices, 3 rats) suggesting that blockade of lactate uptake could impair ATP synthesis up to a level where KATP channels become activated. Combined application of 4-CIN with glibenclamide did not significantly alter antidromically propagating PS1.
4-CIN prolongs the equilibration time course of stimulation-induced changes in extracellular potassium concentration.
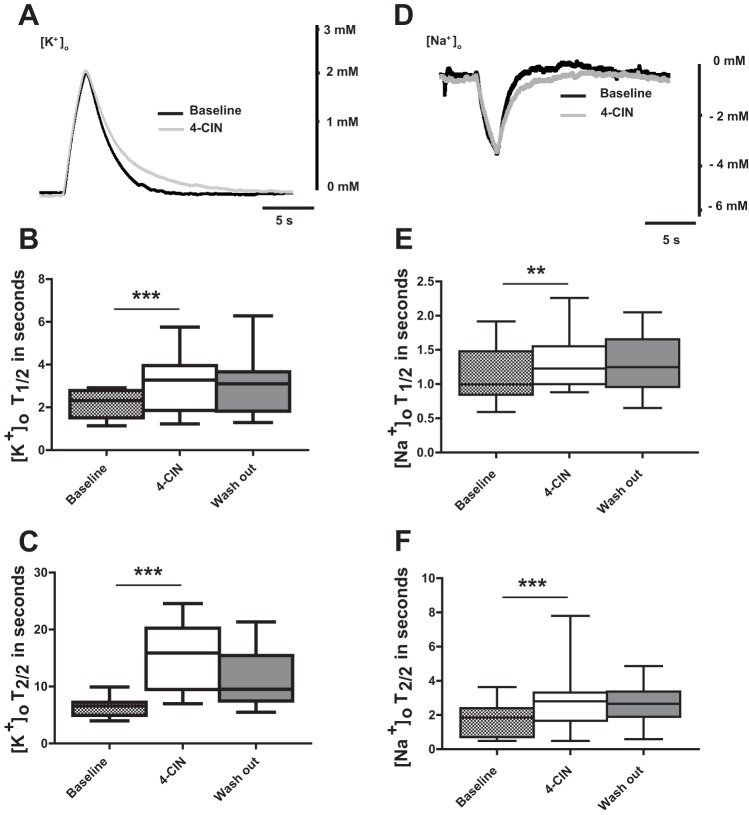
Stimulus intensity of the repetitive stimulation (20 Hz for 2 s) was adjusted to induce moderate changes in ion concentrations similar to those which can be observed also under physiological conditions (Heinemann et al. 1990). Extracellular potassium concentration ([K+]o) reached a peak of 1.7 ± 0.4 mM (n = 7; 5 rats) at the end of stimulation. After termination of stimulation, [K+]o recovered to baseline occasionally followed by an undershoot (Heinemann and Lux 1975). The decay from stimulus-induced peak [K+]o is due to diffusional equilibration (Lux and Neher 1973), astrocytic potassium buffering (Dietzel et al. 1982; Jauch et al. 2002; Orkand et al. 1966), and reuptake of released K+ by the Na+-K+- ATPase (Krnjević and Morris 1974). Therefore, recovery kinetics are strongly influenced by availability of ATP (Heinemann and Lux 1975). The MCT2 inhibitor 4-CIN did not affect peak levels in [K+]o. However, the recovery kinetics were significantly altered. The first half decay time was prolonged from 2.2 ± 0.6 to 3.2 ± 1.4 s (Fig. 3, A and C; n = 7 slices; P < 0.001) and second decay half time from 6.3 ± 1.4 to 15.3 ± 5.6 s. (Fig. 3, A and D; n = 7 slices; P < 0.001).
Fig. 3.
Effects of 4-CIN on stimulus-induced extracellular potassium and sodium concentration changes in CA3 pyramidal layer of the rat hippocampus. A: sample recordings of changes in potassium concentration-induced by 2-s/20-Hz stimulus trains applied to stratum radiatum of area CA1 before and during application of 4-CIN. Note: the prolongation of half decay time during 4-CIN application. B: statistical analysis of effects of 4-CIN on 1st half decay time of extracellular K+ concentration ([K+]o). Note: 4-CIN resulted in prolongation of first half decay time and the effect persisted during wash out. C: statistical analysis of effects of 4-CIN and on 2nd half decay time of [K+]o. Note: prolongation of 2nd half decay time with application of 4-CIN. D: sample recordings of stimulation-induced extracellular sodium concentration changes before and during 4-CIN application. Note: the prolongation of sodium half recovery time during 4-CIN application E: statistical analysis of effects of 4-CIN on 1st half recovery time of [Na+]o. Note: 4-CIN prolongs first half decay time (F) and 2nd half decay time. T1/2 and T2/2 stand for the 1st and the 2nd half decay/recovery times of [K+]o and [Na+]o, respectively. **P < 0.01, ***P < 0.001.
Remarkably, 4-CIN caused a reversible rise in baseline [K+]o with a mean percentage change of 13.4 ± 4.5% to near 3.5 mM similar to the effects of the Na+-K+-ATPase inhibitor ouabain (Xiong and Stringer 2000) indicating that proper Na+-K+-ATPase activity is necessary to keep extracellular potassium concentration low even in the absence of electrical stimulation.
4-CIN prolongs the equilibration time course of stimulation-induced changes in extracellular sodium concentration.
Considering the potential role of the Na+-K+-ATPase on recovery of transmembrane Na+ gradients, it was of interest to look for the effects of 4-CIN on the kinetics of stimulation-induced changes in extracellular sodium concentration ([Na+]o). The trough of [Na+]o changes was reduced from −5.9 ± 1.9 to −5.3 ± 1.9 mM (Fig. 3B; n = 8, 5 rats, P < 0.05) during 4-CIN application. The recovery time from the decrease in [Na+]o was prolonged as was the recovery time for changes in [K+]o. The first half-time of recovery changed from 1.2 ± 0.4 to 1.3 ± 0.4 s (Fig. 3, B and E; n = 8, 5 rats, P = 0.01). The second half time of recovery increased from 1.7 ± 0.9 to 2.7 ± 1.6 s (Fig. 3, B and F; n = 8, 5 rats, P < 0.001).
Effect of 4-CIN on extracellular calcium concentration changes.
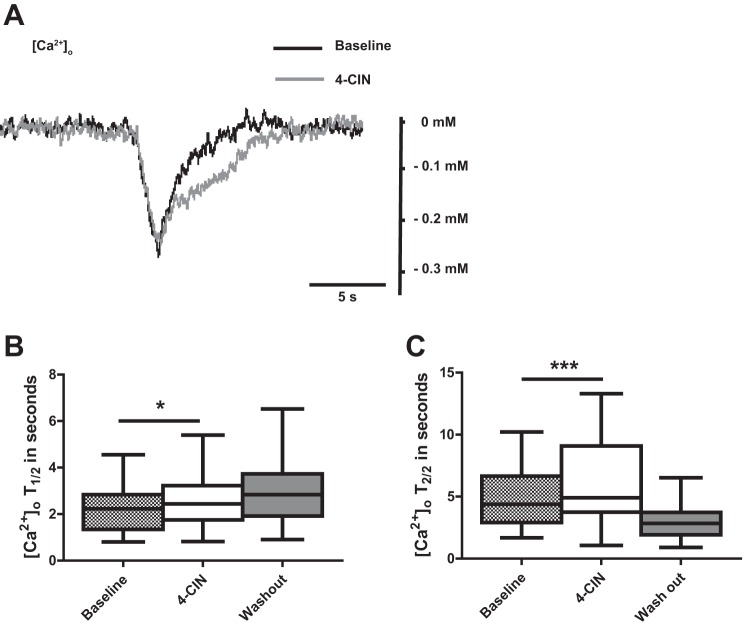
Intracellular [Ca2+] is maintained at nanomolar levels by highly effective extrusion mechanisms via pumps and exchangers, sequestration by Ca2+ binding proteins and HCO3−, as well as buffering by endoplasmic reticulum and mitochondria. As many of these processes, directly or indirectly, depend on ATP availability, we evaluated if lactate metabolism contributes to extracellular Ca2+ homeostasis (Berridge et al. 2003). Stimulus-induced decreases in [Ca2+]o were relatively small (−0.2 ± 0.1) but clearly distinguishable (Fig. 4A). 4-CIN had no effect on the amplitude, but it prolonged first half recovery time from 2.2 ± 0.9 to 2.6 ± 1.1 s (Fig. 4, A and B; n = 9, 6 rats, P < 0.05) whereas the second half recovery time increased from 4.9 ± 2.6 to 6.1 ± 3.3 (Fig. 4, A and C; n = 9, 6 rats, P < 0.001).
Fig. 4.
The effect of monocarboxylate-transporter 2 (MCT2) blocker 4-CIN on stimulation-induced calcium concentration changes. A: sample traces of stimulation-induced calcium transients during application of 4-CIN. Note: prolongation of calcium half recovery time during 4-CIN application. B: statistical analysis of effects of 4-CIN on 1st half recovery time of [Ca2+]o. Note: 4-CIN-induced prolongation of 1st and (C) 2nd half decay time. [Ca2+]o T1/2 and [Ca2+]o T2/2 stands for first and second half recovery time, respectively. *P < 0.05, ***P < 0.001.
Effect of 4-CIN on energy metabolism.
In the absence of blood flow, tissue oxygen concentration correlates with changes in oxidative metabolism. Increased neuronal activity has been shown to increase oxygen consumption (Huchzermeyer et al. 2008; Jarosch et al. 2015; Liotta et al. 2012). We monitored the effect of 4-CIN on baseline respiration and stimulation-induced changes in tissue Po2.
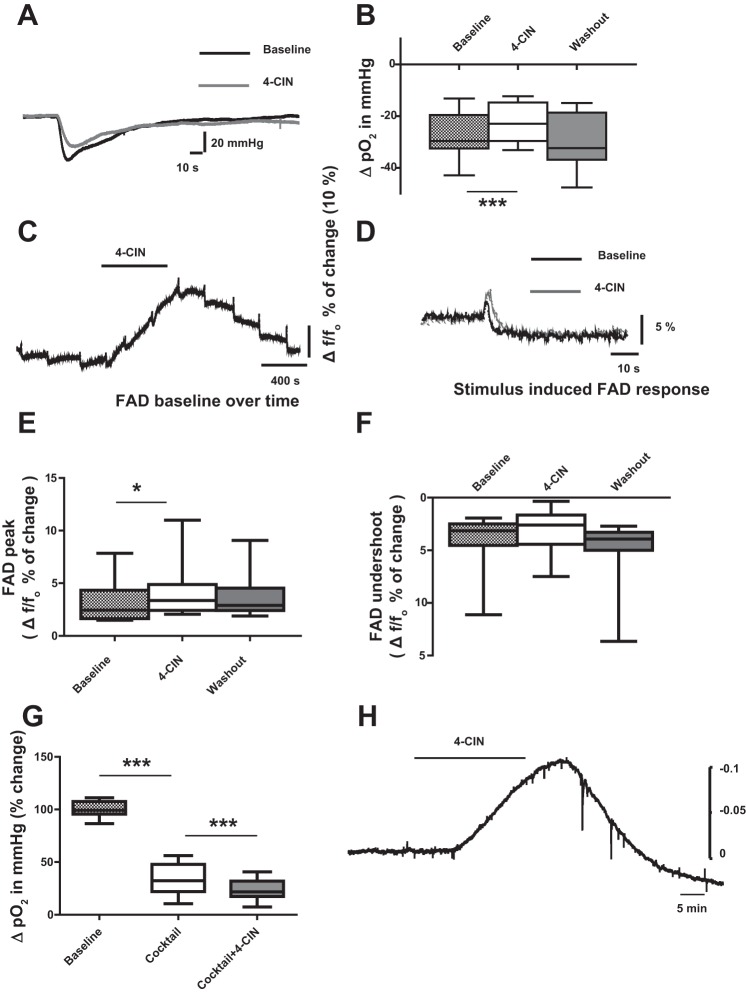
Similar to the findings of Galeffi and his colleagues in area CA1 (Galeffi et al. 2007), 4-CIN reduced stimulation-induced changes in tissue Po2 (ΔPo2) by 20% from −26.7 ± 7.9 to −22.4 ± 7.5 mmHg, (Fig. 5, A and B; n = 7, 3 rats,P < 0.0001), indicating direct contribution of intrinsic lactate to oxidative metabolism. In addition, baseline Po2 increased by 12.7% (64 mmHg), suggesting reduced oxygen consumption.
Fig. 5.
Effects of 4-CIN on partial oxygen tension (Po2) and FAD autofluorescence recorded in CA3 stratum pyramidale. A: sample traces of changes in stimulus-induced tissue oxygen tension before and during 4-CIN application. Note: reduction of change in stimulus-induced tissue oxygen tension. B: statistical analysis of effects of 4-CIN on ΔPo2. Note: ΔPo2 decreased during 4-CIN application and the effect was reversed during wash out. C: sample traces of stimulation-induced biphasic FAD/FADH2 response during 4-CIN perfusion. Note: the increase in FAD autofluorescence baseline during 4-CIN application. D: sample recordings of FAD autofluorescence are depicted before and during application of 4-CIN. E: statistical analysis of effects of 4-CIN on FAD fluorescence changes. The FAD peak significantly increased (F) while the undershoot was slightly reduced during 4-CIN application. G: statistical analysis of effects of 4-CIN on ΔPo2 associated with presynaptic action potentials. Presynaptic components of the stimulus-induced oxygen consumption was isolated by pharmacological blockade of postsynaptic glutamate and GABA receptors and presynaptic transmitters release. ΔPo2 decreased during co-application of the cocktail and 4-CIN. H: extracellular pH recording sample trace. Measurement of extracellular H+ ions was done using pH sensitive electrode; hence one observes an upward deflection with acidosis. Note: 4-CIN induced a decrease in extracellular pH. *P < 0.05, ***P < 0.001.
We have previously shown that isolated activation of axons after blockage of presynaptic transmitter release and postsynaptic receptor activation results in a strongly reduced oxygen consumption (Liotta et al. 2012). However, such stimuli still led to reductions in oxygen tension and in the biphasic NAD(P)H signals suggesting that axons are also dependent on oxidative phosphorylation. Hence, we checked for the effects of 4-CIN on stimulus-induced oxygen tension change due to activation of axons after isolating axonal response from synaptic signals by application of a cocktail of drugs as stated in materials and methods, in addition by preventing presynaptic Ca2+ influx and postsynaptic activation of glutamate and GABA receptors. We observed reduction in ΔPo2 with the application of the cocktail from 34.8 ± 8.8 to 12.2 ± 6.8 mmHg (decrease by 65.9% relative to baseline), which further got reduced to 8.7 ± 4.3 mmHg with 4-CIN (decrease by 75.6% relative to baseline; Fig. 5G; n = 7, 4 rats P < 0.0001), substantiating a role for lactate for axonal energy metabolism.
While changes in Po2 follow alterations in intracellular metabolism with a small lag presumably due to dead space around the electrode, monitoring the fluorescence of FAD gives an immediate insight into energy metabolism-dependent redox potential changes. In stimulus-induced biphasic FAD signals, an early rise in fluorescence indicates oxidation of FADH2 to FAD while the subsequent long-lasting undershoot represents reduction of FAD (Ivanov et al. 2014; Rösner et al. 2013). In the presence of 4-CIN, there was a massive shift in baseline FAD fluorescence towards increased oxidation comparable to the redox shifts observed upon changing Po2 from 20 to 95% (Fig. 5C; Huchzermeyer et al. 2008). The FAD peak of the stimulus-induced biphasic change was significantly increased from 3.5 ± 2.2 to 4.9 ± 3.8 (Fig. 5, D and E; n = 9, 5 rats, P < 0.05) in the presence of 4-CIN while the undershoot of stimulus-induced biphasic FAD fluorescence transients were slightly decreased from −4.4 ± 2.9 to −3.5 ± 2.5%, not reaching significance (Fig. 5, D and F; n = 9, 5 rats, P = 0.09).
ATP-depletion-dependent restriction of Na+-K+ ATPase activity has been shown to be involved in the generation of spreading depolarization, characterized by large negative potential shifts and rises in [K+]o to levels exceeding 20 mM (Herreras and Somjen 1993; Pomper et al. 2006). However, we never observed spontaneous spreading depolarizations with application of 200 μM 4-CIN. Repetitive stimulation following prolonged (40 min) 4-CIN exposure-induced spreading depolarization in 4 out of 16 slices. Increasing 4-CIN to 500 μM led to induction of spreading depolarization in one out of five slices, suggesting that restriction of lactate-dependent ATP synthesis is largely compensated in the presence of ample glucose.
Energy metabolism-independent effects of lactate transport blockade.
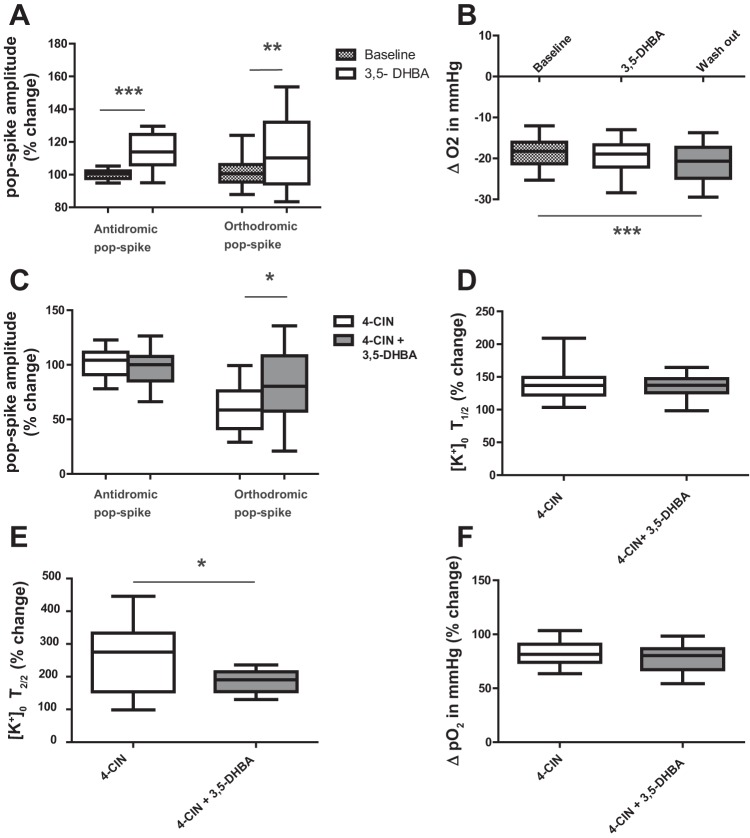
Lactate might exert metabolism-independent effects by acting on the G-protein-coupled lactate receptor HCA1, whose activation has been suggested to modulate neuronal excitability (Tang et al. 2014). We therefore set out to investigate whether the lactate receptor agonist 3,5-DHBA would mimic the effects of 4-CIN. Application of 3,5-DHBA alone caused a small increase in population spike amplitudes which is exactly the opposite of what we observed during 4-CIN application (Fig. 6A). The antidromic population spike increased by 14.6% (n = 5, 3 rats, P < 0.001) while orthodromic population spikes increased by 18.3% (Fig. 6A; n = 5, 3 rats, P < 0.01). Unlike 4-CIN, 3,5-DHBA increased stimulation-induced changes in tissue Po2 (Fig. 6B). We found that 4-CIN combined with the 3,5-DHBA did not alter the amplitude of the antidromic population spike while the reduction of the orthodromic population spike observed with 4-CIN alone was improved, when 4-CIN was combined with 3,5-DHBA (Fig. 6C; from 69 ± 3.8 to 80 ± 6.9%, n = 7,4 rats, P < 0.05). The first half decay time change was not different in both groups (Fig. 6D; n = 6,4 rats, P < 0.001) while second half decay time changed from 254 to 187% (Fig. 6E, n = 6,4 rats, P < 0.001). However, the decay kinetic in the presence of 4-CIN and 3,5-DHBA is still slower than under control conditions. As expected from the results of Po2 recordings in the presence of 3,5-DHBA, the effects of 4-CIN on Po2 were not significantly different from the effects of 4-CIN plus 3,5 DHBA (Fig. 6F). Thus we could exclude that HCA1 receptors are responsible for the effects of 4-CIN, which we had observed on field potential changes and ionic signals.
Fig. 6.
The lactate receptor agonist, 3,5-dihydroxybenzoic acid (3,5-DHBA) did not reverse effects of 4-CIN. 3,5 DHBA applied alone increased the amplitude of both antidromic (A) and orthodromic (B) population spike and the stimulus-induced tissue oxygen responses increased slowly reaching significance at the beginning of wash out. C: stimulation-induced field potential responses compared between 4-CIN alone and 4-CIN applied with 3,5 DHBA. Note: 3,5-DHBA partly reversed the effect of 4-CIN on orthodromic population spike. Prolongation of 1st (D) and 2nd (E) half decay time of stimulation-induced potassium concentration change persisted with co-administration of 4-CIN and 3,5-DHBA. F: the 4-CIN-induced decrease in ΔPo2 was unaffected by combined application of 4-CIN plus 3,5-DHBA. [K+]o T1/2 and T2/2 stand for extracellular potassium concentration first and second half decay time, respectively. *P < 0.05, **P < 0.01, ***P < 0.001.
As MCT2 catalyzes the transport of protonated lactate, blocking of MCT2 might lead to changes in extracellular pH that would finally interfere with neuronal activity as well. In a subsequent set of experiments we investigated the effects of 4-CIN on extracellular pH. Indeed we observed an extracellular acidosis with application of 4-CIN by 0.093 ± 0.034 pH units (n = 8; 3 rats). Mimicking this pH change by adjusting bicarbonate concentration did not have any significant effect on evoked potentials (data not shown). We hypothetized that the acidosis might be due to accumulation of lactate in the extracellular space. We therefore determined the amount of lactic acid required to shift the pH by 0.1 units in acidic direction and found that this could be accomplished by addition of 6 mM lactic acid into the carbogenated aCSF.
DISCUSSION
Here, we present evidence for the first time that in spite of the unlimited availability of glucose, astrocytic lactate supply contributes to restoration of ion gradients following moderate neuronal activation in brain slices. Indeed, the elevations of potassium concentration observed in the present study might be sufficient to induce astrocytic lactate release in line with a recent report (Sotelo-Hitschfeld et al. 2015). The recovery kinetics of stimulus-induced K+, Na+, and Ca2+ concentration changes were significantly prolonged due to blockade of the neuronal MCT2 by 4-CIN. Orthodromic but not antidromic population spikes were decreased by 4-CIN, at least partially due to activation of KATP channels. The aforementioned effects were mediated by a shortage of ATP rather than a direct effect on the lactate HCA1 receptor as HCA1 agonists led to effects which were opposite to those of 4-CIN. Indeed the decreased respiration and mitochondrial redox changes upon 4-CIN application indicated a significant contribution of lactate uptake to energy metabolism in spite of ample supply of glucose.
Effects of lactate uptake blockade on synaptic signaling.
Similarly to previous findings in nucleus tractus solitarii neurons and in hippocampal area CA1 (Nagase et al. 2014; Galeffi et al. 2007) synaptic signaling is reduced following reduced lactate uptake via MCT2. The 4-CIN effect on evoked potentials could be explained by a decrease in cytosolic ATP concentration leading to activation of KATP channels. This conclusion is supported by the observed effect of glibenclamide. In addition to activation of KATP channels, decreased ATP synthesis could also directly impair synaptic signaling by interfering with transmitter recycling and vesicle packaging. With respect to this, it is of importance that 4-CIN depressed the changes in Po2 associated with purely presynaptic axonal activation. In high concentrations, 4-CIN might interfere with pyruvate uptake into mitochondria. However, introduction of 4-CIN into the intracellular space by patch electrodes had no effect on postsynaptic potentials suggesting MCT2 as the main target for 4-CIN (Nagase et al. 2014). Moreover, 4-CIN bath applied for 20 min would never fully equilibrate the slices in interface chambers and thus it is very unlikely that there is enough accumulation of 4-CIN intracellularly to block pyruvate import into mitochondria. Alternatively, the 4-CIN effects on synaptic transmission could be mediated by activation of HCA1 receptors. Blockade of lactate uptake into neurons is presumably associated with extracellular lactate accumulation However, the decreased efficacy of synaptic signaling was not due to extracellular lactate accumulation as activation of these receptors by 3,5-DHBA did not mimic the effects of 4-CIN and indeed augmented antidromic and orthodromic population spikes rather than reducing them as was observed during application of 4-CIN. The fact that in presence of 4-CIN pH was shifted by 0.1 units is compatible with an extracellular lactate accumulation by 6 mM since addition of 6 mM lactic acid to the aCSF shifted the pH in this solution by the same amount.
Contribution of neuronal lactate uptake to maintenance of ion homeostasis.
Application of 4-CIN led to an increase in baseline [K+]o, suggesting that baseline Na+-K+-ATPase activity relies on lactate-dependent ATP synthesis. In spite of the initial decrease in orthodromic population spikes by 4-CIN, the maximum amplitude of stimulus-induced [K+]o changes was not significantly altered during repetitive stimulation suggesting that 4-CIN had little effect on extracellular K+ accumulation. If 4-CIN would diminish glial uptake either by spatial buffering of K+ or by KCl couptake, then the rise in K+ would be enlarged. This was indeed found when extracellular K+ accumulation during repetitive stimulation or during extracellular K+ accumulation were studied in the presence of low levels of Ba2+, which lead to depolarization of astrocytes and blockage of astrocytic Kir 4.1 channels (Gabriel et al. 1998; Jauch et al. 2002). An effect of 4-CIN on astrocytic membranes is also not expected as K+ buffering requires practically no energy and also KCl uptake is largely energy independent driven by Cl− and K+ leak channels. In fact blockers of such channels by disodium 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS) had no effect on K+ accumulation in the extracellular space (Jauch et al. 2002). Recovery of [K+]o to baseline is mediated by different processes: diffusion from release sites, facilitated diffusion due to glial K+ buffering, and transmembrane transport via the Na+-K+-ATPase and via glial KCl cotransport (Heinemann and Lux 1975; Lux et al. 1986; Hertz et al. 2015). Similar to changes in [K+]o, [Na+]o recovery to baseline was prolonged. The same applied to stimulus-induced Ca2+ concentration changes. The simplest explanation is a restriction of lactate-dependent ATP production leading to limited Na+-K+-ATPase activity. The common denominator is the inadequate synthesis of ATP caused by substrate limitation. Kinetics of stimulus-induced changes in potassium concentration have also been investigated in presence of blockers of astrocytic glycolysis in the dentate gyrus. In those experiments recovery kinetics of K+ signals were also delayed and compared with the effects of reducing efficacy of the Na+-K+-ATPase (Xiong and Stringer 1999). Our findings here and those of Galeffi et al. (2007) would suggest that neuronal glucose uptake and lactate uptake complement each other and provide for some redundancy to guarantee neuronal survival in conditions where one or the other system fails. Interestingly, the amplitude of K+ transients were about half of that for Na+ concentration, which is hard to explain if we assume a 1/1 exchange of K+ against Na+ during neuronal excitation (Alle et al. 2009). The discrepancy in K+ amplitudes to Na+ amplitudes then suggests that glial K+ buffering is very effective for limiting rises in local [K+]o. K+ buffering reduces the availability of K+ ions for exchange transport against Na+ and thereby contributes to the K+ undershoot usually observed after a stimulation- or activity-dependent rise in [K+]o (Heinemann and Lux 1975). Also, recovery kinetics of stimulus-induced changes in [K+]o and [Na+]o were different. This might be explained when the transport of Cl− is taken into account. Restoration of Cl− gradients across the plasma membrane are driven by K+-dependent cotransport with release of K+ from neurons to get Cl− out of cells (for review, see Antonio et al. 2016 ).This would slow the recovery of [K+]o. The prolonged recovery of stimulation-induced changes in [K+]o, [Na+]o, and [Ca2+]o could be due to activation of HCA1 receptor, in addition to the deprivation of lactate derived ATP and subsequent disturbance in Na+-K+-ATPase activity. However, in our model the activation of HCA1 receptors does not seem to contribute to the effects, as application of 3,5-DHBA did not augment the 4-CIN-dependent slowing of recovery kinetics. The effects of 4-CIN on baseline Po2 and stimulus-induced Po2 changes were also not significantly different with or without 3,5-DHBA.
Effects of reduced lactate uptake on oxidative energy metabolism and redox potential.
If deceleration of recovery in [K+]o, [Na+]o, and [Ca2+]o changes is due to ATP shortage, 4-CIN has to affect oxidative energy metabolism. Indeed, baseline Po2 increased in the presence of 4-CIN indicating decreased cellular respiration. This effect was not mediated by direct blockade of the mitochondrial energy metabolism as 4-CIN at the respective concentration did not influence mitochondrial pyruvate uptake (Nagase et al. 2014). Also, stimulus-induced Po2 transients decreased significantly. Assuming undisturbed neurometabolic coupling, unaltered peak [K+]o, [Na+]o, and [Ca2+]o changes should induce the same enhancement of the energy metabolism. As this is not the case, our results indicate that lactate represents an energy substrate for the brain slices, which could not be fully compensated for by enhanced glucose uptake and metabolism. This is in line with the findings that surplus additional lactate intensifies carbachol-induced gamma oscillations (Schneider et al. 2015).
The most striking effect of 4-CIN on the redox potential was the increase in FAD fluorescence. As lactate is an important source of pyruvate for neuronal oxidative metabolism, reduction of lactate uptake decreases the delivery of reducing equivalents from the Krebs cycle in general. In addition, restricted availability of NADH-linked substrates could facilitate the electron flux via complex II likely contributing to enhanced FADH2 oxidation (Kunz et al. 1994). Indeed, the oxidative peak of the biphasic FAD fluorescence transients was also markedly enhanced by 4-CIN, in spite of the depression of stimulus-induced changes in Po2. Unfortunately, the effects of 4-CIN on another redox couple, NAD+/NADH could not be assessed due to high 4-CIN-induced fluorescence extinction at the wavelengths necessary to excite NAD(P)H. This limitation should be taken into account in future studies when discussing the results of NAD(P)H imaging in the presence of 4-CIN (Galeffi et al. 2007). If lactate serves as the main energy source for maintenance of transmembrane ionic gradients, one would expect that 4-CIN will facilitate induction of spreading depolarizations. Our recordings were done in CA3 where induction of spreading depolarization by high K+ is notoriously difficult and only possible when oxygen supply or Na+-K+-ATPase activity is reduced (Haglund and Schwartzkroin 1990; Pomper et al. 2006). However, we observed spreading depolarizations only with increased 4-CIN concentration or prolonged exposure. Thus neuronal glucose uptake provides sufficient substrate to keep up with the metabolic demand of moderate neuronal activity even in the absence of astrocytic lactate as an energy source.
In conclusion, we found evidence that in the brain slice preparation astrocytic lactate is an important substrate for neuronal energy metabolism involved in the maintenance of ion gradients even under basal conditions and following moderate neuronal activation. Moreover, it is also partially responsible for ATP support of synaptic signaling. Lactate likely plays a similar role in vivo since activity-dependent fast astrocytic release of lactate has been described in the somatosensory cortex of mice (Sotelo-Hitschfeld et al. 2015). Energy metabolism of brain slices maintained in vitro might largely differ from the in vivo situation since in the absence of capillary blood flow, high oxygen, and glucose concentration is necessary to keep proper neuronal activity (Huchzermeyer et al. 2008). Oxidative metabolism of lactate in neurons would be in line with the observation that no net reducing shift occurs in astrocytes during enhanced glycolysis (Dienel and Hertz 2001). Nevertheless, an extremely high lactate concentration has to be applied to support neuronal activity in the absence of glucose in the brain slice preparation (Galow et al. 2014). This might be due to the fact that only certain components of synaptic signaling rely on lactate-derived ATP. Indeed postsynaptic components were more prominently influenced by blockade of lactate uptake than presynaptic action potentials. However, the release from oligodendrocytes through MCT-1-dependent export might play an important role on maintenance of axonal function (Lee et al. 2012).
The role of lactate as a reserve energy substrate has been emphasized with respect to hypoglycemic conditions or under pathologically enhanced neuronal activity. Lactate has been suggested to be protective as a glucose sparing metabolite in brain trauma (Bouzat et al. 2014). Recently, endothelial MCT-1 has been shown to be downregulated in temporal lobe epilepsy in resected human hippocampus tissue and chronic epileptic animal models, which gives a hint that it might play a role in epileptogenesis and development of drug refractory epilepsy (Lauritzen et al. 2011).
GRANTS
This work was supported by European Union Grant FP7 D esire (Grant Agreement 602531-1) and Deutsche Forschungsgemeinschaft (DFG) Grant He1128/18-1 (to U. Heinemann) and DFG Grant Ko3814/1-1 (to R. Kovács). U. Heinemann and his laboratory were supported by EXC Neurocure (EXC 257).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.A.A., J.R., R.K., and U.H. conception and design of research; E.A.A., J.R., A.L., and R.K. performed experiments; E.A.A. and A.L. analyzed data; E.A.A. and A.L. prepared figures; E.A.A., J.R., A.L., R.K., and U.H. approved final version of manuscript; R.K. and U.H. interpreted results of experiments; R.K. and U.H. edited and revised manuscript; U.H. drafted manuscript.
ACKNOWLEDGMENTS
We thank the Clinical Scientist Program of Charite for support (to A. Liotta) and Dipl Biol Tanja Specowius for technical assistance.
REFERENCES
- Aitken PG, Tombaugh GC, Turner DA, Somjen GG. Similar propagation of SD and hypoxic SD-like depolarization in rat hippocampus recorded optically and electrically. J Neurophysiol 80: 1514–1521, 1998. [DOI] [PubMed] [Google Scholar]
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science 325: 1405–1408, 2009. [DOI] [PubMed] [Google Scholar]
- Antonio LL, Anderson ML, Angamo EA, Gabriel S, Klaft ZJ, Liotta A, Salar S, Sandow N, Heinemann U. In vitro seizure like events and changes in ionic concentration. J Neurosci Methods 260: 33–44, 2016. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21: 1133–1145, 2001. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14: 724–738, 2011. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- Bouzat P, Sala N, Suys T, Zerlauth JB, Marques-Vidal P, Feihl F, Bloch J, Messerer M, Levivier M, Meuli R, Magistretti PJ, Oddo M. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med 40: 412–421, 2014. [DOI] [PubMed] [Google Scholar]
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem 272: 30096–30102, 1997. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Contributions of glycogen to astrocytic energetics during brain activation. Metab Brain Dis 30: 281–298, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res 66: 824–838, 2001. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, Lux HD. Stimulus-induced changes in extracellular Na+ and Cl− concentration in relation to changes in the size of the extracellular space. Exp Brain Res 46: 73–84, 1982. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623: 208–214, 1993. [DOI] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485: 517–521, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S, Kivi A, Kovacs R, Lehmann TN, Lanksch WR, Meencke HJ, Heinemann U. Effects of barium on stimulus-induced changes in [K+]o and field potentials in dentate gyrus and area CA1 of human epileptic hippocampus. Neurosci Lett 249: 91–94, 1998. [DOI] [PubMed] [Google Scholar]
- Galeffi F, Foster KA, Sadgrove MP, Beaver CJ, Turner DA. Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J Neurochem 103: 2449–2461, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galow LV, Schneider J, Lewen A, Ta TT, Papageorgiou IE, Kann O. Energy substrates that fuel fast neuronal network oscillations. Front Neurosci 8: 398, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Körner R, and Heinemann U. Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino acid carrier 1. J Cereb Blood Flow Metab 22: 569–575, 2002. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Schwartzkroin PA. Role of Na-K pump potassium regulation and IPSPs in seizures and spreading depression in immature rabbit hippocampal slices. J Neurophysiol 63: 225–239, 1990. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Lux HD. Undershoots following stimulus-induced rises of extracellular potassium concentration in cerebral cortex of cat. Brain Res 93: 63–76, 1975. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Schaible HG, and Schmidt RF. Changes in extracellular potassium concentration in cat spinal cord in response to innocuous and noxious stimulation of legs with healthy and inflamed knee joints. Exp Brain Res 79: 283–292, 1990. [DOI] [PubMed] [Google Scholar]
- Herreras O, Somjen GG. Analysis of potential shifts associated with recurrent spreading depression and prolonged unstable spreading depression induced by microdialysis of elevated K+ in hippocampus of anesthetized rats. Brain Res 610: 283–294, 1993. [DOI] [PubMed] [Google Scholar]
- Hertz L, Song D, Xu J, Peng L, Gibbs ME. Role of the astrocytic Na(+), K(+)-ATPase in K(+) homeostasis in brain: K(+) uptake, signaling pathways and substrate utilization. Neurochem Res 40: 2505–2516, 2015. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer C, Albus K, Gabriel HJ, Otáhal J, Taubenberger N, Heinemann U, Kovács R, Kann O. Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in Po2 and concomitant changes in mitochondrial redox state. J Neurosci 28: 1153–1162, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Malkov AE, Waseem T, Mukhtarov M, Buldakova S, Gubkina O, Zilberter M, Zilberter Y. Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices. J Cereb Blood Flow Metab 34: 397–407, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch MS. Gebhardt C, Fano S, Huchzermeyer C, Ul Haq R, Behrens CJ, Heinemann U. Early adenosine release contributes to hypoxia-induced disruption of stimulus-induced sharp wave-ripple complexes in rat hippocampal area CA3. Eur J Neurosci 42: 1808–1817, 2015. [DOI] [PubMed] [Google Scholar]
- Jauch R, Windmüller O, Lehmann TN, Heinemann U, Gabriel S. Effects of barium, furosemide, ouabaine and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) on ionophoretically-induced changes in extracellular potassium concentration in hippocampal slices from rats and from patients with epilepsy. Brain Res 925: 18–27, 2002. [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Sylantyev S, Hadjihambi A, Hosford PS, Kasparov S, Gourine AV. Hemichannel-mediated release of lactate. J Cereb Blood Flow Metab 36: 1202–1211, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K, Morris ME. Extracellular accumulation of K+ evoked by activity of primary afferent fibers in the cuneate nucleus and dorsal horn of cats. Can J Physiol Pharmacol 52: 852–871, 1974. [DOI] [PubMed] [Google Scholar]
- Kunz W, Gellerich FN, Schild L. Contribution to control of mitochondrial oxidative phosphorylation by supplement of reducing equivalents. Biochem Med Metab Biol 52: 65–75, 1994. [DOI] [PubMed] [Google Scholar]
- Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis 41: 577–584, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24: 2784–2795, 2013. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487: 443–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta A, Rösner J, Huchzermeyer C, Wojtowicz A, Kann O, Schmitz D, Heinemann U, Kovács R. Energy demand of synaptic transmission at the hippocampal Schaffer-collateral synapse. J Cereb Blood Flow Metab 32: 2076–2083, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux HD, Heinemann, and Dietzel U, I, 1986. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol 44: 619–639, 1986. [PubMed] [Google Scholar]
- Lux HD, Neher E. The equilibration time course of (K+)0 in cat cortex. Exp Brain Res 17: 190–205, 1973. [DOI] [PubMed] [Google Scholar]
- Mourre C, Ben Ari Y, Bernardi H, Fosset M, Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res 486: 159–164, 1989. [DOI] [PubMed] [Google Scholar]
- Müller W, Misgeld U, Heinemann U. Carbachol effects on hippocampal neurons in vitro: dependence on the rate of rise of carbachol tissue concentration. Exp Brain Res 72: 287–298, 1988. [DOI] [PubMed] [Google Scholar]
- Nagase M, Takahashi Y, Watabe AM, Kubo Y, Kato F. On-site energy supply at synapses through monocarboxylate transporters maintains excitatory synaptic transmission. J Neurosci 34: 2605–2617, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, and Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 788–806, 1966. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55: 1251–1262, 2007. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91: 10625–10629, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomper JK, Haack S, Petzold GC, Buchheim K, Gabriel S, Hoffmann U, Heinemann U. Repetitive spreading depression-like events result in cell damage in juvenile hippocampal slice cultures maintained in normoxia. J Neurophysiol 95: 355–368, 2006. [DOI] [PubMed] [Google Scholar]
- Rösner J, Liotta A, Schmitz D, Heinemann U, Kovács R. A LED-based method for monitoring NAD(P)H and FAD fluorescence in cell cultures and brain slices. J Neurosci Methods 212: 222–227, 2013. [DOI] [PubMed] [Google Scholar]
- Rösner J, Liotta A, Angamo EA, Spies C, Heinemann U, Kovács R. Minimizing photodecomposition of flavin adenine dinucleotide fluorescence by the use of pulsed LEDs. J Microsc 2016 Jul 1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347: 1362–1367, 2015. [DOI] [PubMed] [Google Scholar]
- Schneider J, Lewen A, Ta TT, Galow LV, Isola R, Papageorgiou IE, Kann O. A reliable model for gamma oscillations in hippocampal tissue. J Neurosci Res 93: 1067–1078, 2015. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Kovacs R, Kann O, Heinemann U, Buchheim K. Monitoring NAD(P)H autofluorescence to assess mitochondrial metabolic functions in rat hippocampal-entorhinal cortex slices. Brain Res Brain Res Protoc 7: 267–276, 2001. [DOI] [PubMed] [Google Scholar]
- Sotelo-Hitschfeld, Niemeyer MI, Mächler P, Ruminot I, Lerchundi R, Wyss MT, Stobart J, Fernández-Moncada I, Valdebenito R, Garrido-Gerter P, Contreras-Baeza Y, Schneider BL, Aebischer P, Lengacher S, San Martín A, Le Douce J, Bonvento G, Magistretti PJ, Sepúlveda FV, Weber B, Barros LF. Channel-mediated lactate release by K+-stimulated astrocytes. J Neurosci 35: 4168–4178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Comm 5: 3284, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZQ, Stringer JL. Astrocytic regulation of the recovery of extracellular potassium after seizures in vivo. Eur J Neurosci 11: 1677–1684, 1999. [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Stringer JL. Sodium pump activity, not glial spatial buffering, clears potassium after epileptiform activity induced in the dentate gyrus. J Neurophysiol 83: 1443–1451, 2000. [DOI] [PubMed] [Google Scholar]