Abstract
Novel treatment modalities are necessary for pancreatic cancer. Immunotherapy with immune checkpoint inhibition has shown effect in other solid tumors, and could have a place in pancreatic cancer treatment. Most available clinical studies on immune checkpoint inhibitors for pancreatic cancer are not yet completed and are still recruiting patients. Among the completed trials, there have been findings of a preliminary nature such as delayed disease progression and enhanced overall survival after treatment with immune checkpoint inhibitors in mono- or combination therapy. However, due to small sample sizes, major results are not yet identifiable. The present article provides a clinical overview of immune checkpoint inhibition in pancreatic cancer. PubMed, ClinicalTrials.gov and American Society of Clinical Oncology’s meeting abstracts were systematically searched for relevant clinical studies. Four articles, five abstracts and 25 clinical trials were identified and analyzed in detail.
Keywords: Pancreatic cancer, Immune checkpoint inhibitors, Clinical trials
Core tip: Immunotherapy is a rapidly expanding field within pancreatic cancer research. Here we summarize the effectiveness of immune checkpoint inhibition in the treatment of pancreatic cancer, focusing on the anti-tumor response and toxicity of drugs targeting cytotoxic T lymphocyte antigen 4, programmed cell death 1 and programmed cell death ligand 1. Based on the results from small series it appears that immune checkpoint inhibitors may be safe and effective, but still little published evidence is available to prove or disprove the clinical benefit of these drugs in patients with pancreatic cancer. Several well-designed clinical trials are ongoing and the results from these trials are eagerly awaited.
INTRODUCTION
The human immune system is an elaborative biological system of cellular interactions, structures and processes that have been evolved to protect the body from foreign substances. In diseases such as cancer, understanding the role of the immune system in disease development and progression has resulted in important insights.
Recent research attempts aimed at utilizing the immune system for cancer therapy have shown promising results in cancer elimination. It is now known that the innate and the adaptive immune system recognizes tumor-specific antigens such as neoantigens (derivatives associated with carcinogenesis mutation) and oncogenic virus derivatives, in order to act against cancers in a process referred to as tumor immune surveillance[1]. However, the tumor modifies the human immune system to avoid detection, both locally and systematically. A novel insight in addressing the challenge has been found in the concept of immune checkpoints.
An integral function of the immune system is its ability to differentiate between self and non-self. For this purpose the immune system depends on multiple “checkpoints”, which are molecules on certain immune cells that need to be activated or inactivated to start an immune reaction. Tumor cells often take advantage of these checkpoints to avoid being detected and attacked by the immune system. Checkpoint inhibitors have been investigated as a novel mode of cancer treatment[2].
Immunotherapy is a treatment modality that encompasses a wide and varied range of techniques. In cancer related cases, these often consist of the use of vaccines, cytokines and monoclonal antibodies that stimulate the human immune system in general or target specific cells. The potential benefits with immunotherapy, compared to other approaches, is its ability to detect specific tumor cells, creating a durable response and a much better survival-prognosis in cancer patients[3]. Unfortunately, it has been a therapy with little success in solid tumors, especially in pancreatic ductal adenocarcinoma (PDA)[4].
PDA is a highly aggressive malignancy, characterized by delayed diagnosis and treatment resistance[5]. At the time of clinical detection, most cancers are either locally advanced, or metastatic, i.e., ineligible for surgical resection and with a five-year survival in the single digits[6]. One of the reasons for the poor effect of treatment is the ability of PDA to evade host immune surveillance[1,7]. The tumor microenvironment of PDA is composed of a dense fibrotic stroma of extracellular matrix components and a variety of inflammatory cells[6]. On one level, there are infiltrating immunosuppressive cells, such as regulatory T cells (Tregs), myeloid-derived suppressive cells (MDSCs), tumor-associated macrophages (TAMs), transmitting cancer-inflammatory signals that hinder the immunologic cell activity of cytotoxic T cells (CTLs) and natural killer cells (NKs)[1,4]. On another level, the tumor cells avoid detection, in several ways: by the use of immunosuppressive factor secretion, such as interleukin (IL)-10, vascular endothelial growth factor, downregulation of major histocompatibility complex (MHC) class I presentation and using contact-dependent factors, such as immune checkpoint molecules, resulting in immunosuppression and tumor progression[1,4]. PDA has been considered a non-immunogenic cancer. However, four subtypes of PDA were recently reported. One subtype, containing upregulated immune networks with tumor infiltrating cells (TILs) including CD4+ and CD8+ T-cells and immune checkpoint molecule expression in its tumor microenvironment, was defined as immunogenic[8].
The existing treatment modalities, including surgical resection and conventional chemotherapies, prolong survival but fail to cure the disease. New novel treatment modalities are needed[4,6]. Immunotherapy with immune checkpoint inhibition has shown effect in other solid tumors. It could also have a place in PDA treatment. This review aims to discuss the current development status of and future challenges in utilizing immune checkpoint inhibitors for PDA, with focus on cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1), three immune checkpoints with current clinical information.
LITERATURE SEARCH
A systematic literature search on immune checkpoint therapy of pancreatic ductal adenocarcinoma was conducted. Results of articles, abstracts and clinical trials from the United States National Library of Medicine’s PubMed database, from American Society of Clinical Oncology’s (ASCO) Gastrointestinal Cancers Symposiums and from the United States National Institutes of Health Clinical Trials were obtained. The search was conducted in March 2016. Articles were found through the mesh-term “Carcinoma, Pancreatic Ductal”, combined with “immune checkpoint therapy”; the mesh-term “Pancreatic Neoplasms”, combined with “immune checkpoint therapy”, “Ipilimumab”, “CTLA 4”, “PD-L1”, “PD-1”; the mesh-term “Carcinoma, Pancreatic Ductal/therapy” combined with the mesh-term “Carcinoma, Pancreatic Ductal/immunology”; the term “pancreatic neoplasm” and “pancreas cancer” were both combined with “Immune therapy”; the term “pancreatic cancer” was combined with “Ipilimumab”, “anti PDL1”, “anti PD1”, “anti CTLA4” and further restricted to studies based on humans and to articles published in English. The Boolean operator “AND” was used throughout the whole search. Additional articles were retrieved by manually cross-checking bibliographies of relevant articles. The search strategy is shown in Figure 1. Abstracts from Multidisciplinary Treatment, Translational Research of Cancer of the Pancreas, Small Bowel and Hepatobiliary Tract from the 2009-2016 Gastrointestinal Cancer Symposium were studied. Ongoing or completed clinical trials based on the question formulation were found through the condition: “Pancreatic Neoplasms”. Identified duplicates of the same study, when searched in the different databases, were removed. One reviewer (Johansson H) conducted the study selection and data extraction. A second reviewer (Ansari D) independently checked data for omissions or inaccuracies. Extracted information for each study included study characteristics, intervention, comparison, outcome and adverse events. The data was tabulated and narratively synthesized.
Figure 1.
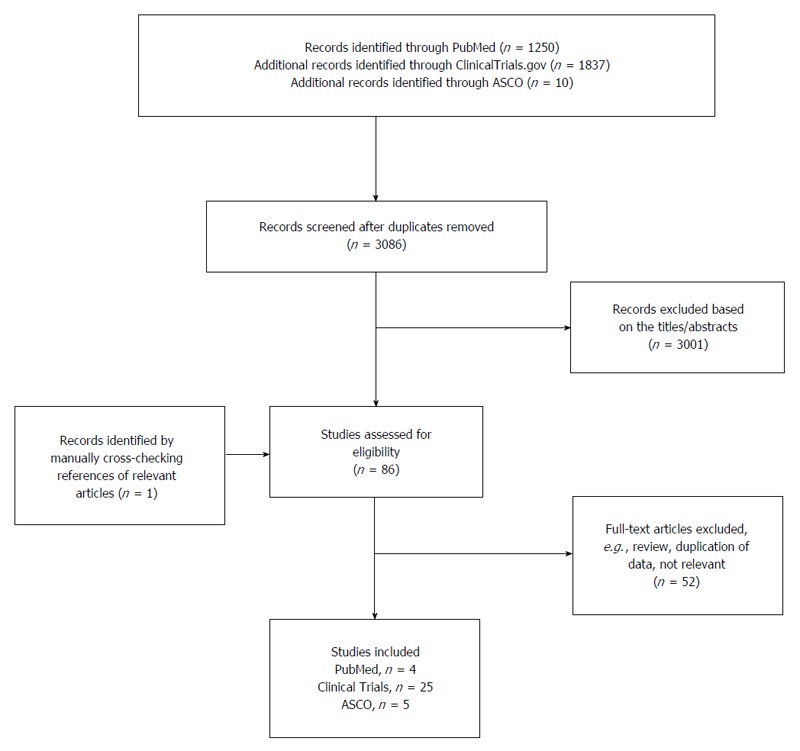
Flow diagram of article selection. ASCO: American Society of Clinical Oncology’s.
RESULTS
Out of 1250 identified search records in the PubMed database, three articles were selected and one was further retrieved by manually cross-checking bibliographies of relevant articles. In total, four articles were included. An additional 1837 records were identified through United States National Institutes of Health Clinical Trials, resulting in another 25 references. Furthermore, five out of 10 abstracts from “Multidisciplinary Treatment, Translational Research of Cancer of the Pancreas, Small Bowel and Hepatobiliary Tract” from the 2009-2016 Gastrointestinal Cancer Symposiums met the criteria for this study and were therefore selected. Results from clinical trials are presented in Table 1, Table 2, Table 3. Results from the meeting abstracts and articles are presented in Tables 4 and 5, respectively. An overview of the checkpoint molecules, their potential expression and interaction and their targeting drugs are presented in Figure 2.
Table 1.
Clinical trials on cytotoxic T lymphocyte antigen 4
| Target molecule | Drug name | Study phase | Study design | Status | Condition | Intervention | Cohort | Estimated enrollment | Dose | End point | Study Arm | Adverse event | Response | Survival | Ref. |
| CTLA-4 | Ipilimumab (BMS-734016/MDX-010) | 1 | NA | Active | PC | Palliative | Locally advanced | 28 | DE | Safety (MTD, DLT) | Ipilimumab, Gemcitabine | NA | NA | NA | NCT01473940 |
| Not recruiting | Metastatic | Efficacy (RR, PFS, OS) | |||||||||||||
| 2 | Randomized | Recruiting | PC | Palliative | Metastatic | 92 | 3 mg/kg | Safety (AE) | Ipilimumab, GVAX (Arm A) | NA | NA | NA | NCT01896869 | ||
| Efficacy (OS, PFS, ORR, DoR) | FOLFIRINOX (Arm B) | ||||||||||||||
| Tremelimumab (CP-675/CP-675,206) | 2 | NA | Recruiting | PC, BlC, BC | Palliative | Metastatic | 77 | NA | Safety | Tremelimumab (Arm A) | NA | NA | NA | NCT02527434 | |
| Efficacy (ORR, DoR, DCR, PFS, OS, BoR) | Durvalumab (Arm B) | ||||||||||||||
| Tremelimumab, Durvalumab (Arm C) | |||||||||||||||
| 2 | Randomized | Recruiting | PC | Palliative | Metastatic | 130 | NA | Safety | Durvalumab (Arm A) | NA | NA | NA | NCT02558894 | ||
| Efficacy (ORR, DoR, DCR, PFS) | Durvalimab, Tremelimumab (Arm B) | ||||||||||||||
| PK | |||||||||||||||
| Antidrug Antibody Presence | |||||||||||||||
| 1 | Randomized | Recruiting | PC, NSCLC, HNSCC | Palliative | Locally advanced | 108 | NA | Safety (AE, DLT) | Durvalumab, Mogamulizumab (Arm A) | NA | NA | NA | NCT02301130 | ||
| Metastatic | Tremelimumab, Mogamulizumab (Arm B) | ||||||||||||||
| 1 | Non-randomized | Recruiting | PC, NSCLC, BC, MM | Palliative | Metastatic | 30 | 1 mg/kg | Safety (AE) | Durvalumab, Tremelimumab, Radiotherapy | NA | NA | NA | NCT02639026 | ||
| Efficacy |
ORR: Overall response rate; DLT: Dose limiting toxicity; AE: Adverse event; MTD: Maximum tolerance dose; NA: Not available.
Table 2.
Clinical trials on programmed cell death 1
| Target molecule | Drug name | Study phase | Study design | Status | Condition | Intervention | Cohort | Estimated enrollment | Dose | End point | Study Arm | Adverse event | Response | Survival | Ref. |
| PD-1 | Nivolumab (BMS-936558/MDX-1106/ONO-4538) | 1|2 | Randomized | Recruiting | PC | Neoadjuvant | Resectable | 50 | 3 mg/kg | Safety | Cy/GVAX ( Arm A) | NA | NA | NA | NCT02451982 |
| Adjuvant | Efficacy (IRAEs, OS, DFS) | Cy/GVAX, Nivolumab (Arm B) | |||||||||||||
| Median-[IL17A] in Vaccine-induced Lymphoid Aggregates | |||||||||||||||
| 1|2 | Non-randomized | Recruiting | PC, NSCLC, RCC, CrC, EC, UC | Palliative | Metastatic | 49 | NA | Safety | Nivolumab, Temsirolimus (Arm A) | NA | NA | NA | NCT02423954 | ||
| Efficacy | Nivolumab, Irinotecan (Arm B) | ||||||||||||||
| RD | Nivolumab, Irinotecan, capecitabine (Arm C) | ||||||||||||||
| 1 | Non-randomized | Recruiting | PC, NSCLC, CrC, MM, HNSCC, GBM | Palliative | Locally advanced | 270 | 3 mg/kg | Safety (AE) | Phase 1a: | NA | NA | NA | NCT02526017 | ||
| Efficacy (OS; mOS, oyOS, DOR, PFS, ORR; CR, PR) | FPA008 (Arm A) | ||||||||||||||
| Tolerability | FPA008, Nivolumab (Arm B) | ||||||||||||||
| RD | Phase 1b: | ||||||||||||||
| PK | MTD/RD FPA008, Nivolumab | ||||||||||||||
| Immunogenicity | |||||||||||||||
| PDA biomarkers | |||||||||||||||
| 1 | Non-randomized | Recruiting | PC, OC, BC, CrC, RCC, MM, PrC, NSCLC | Palliative | Locally advanced | 300 | 3 mg/kg | Safety | DE AM0010 (Arm A) | NA | NA | NA | NCT02009449 | ||
| Metastatic | Tolerability | DE AM0010, Paclitaxel/Docetaxel, Carboplatin/Cisplatin (Arm B) | |||||||||||||
| PK | DE AM0010, FOLFOX, (Arm C) | ||||||||||||||
| DE AM0010, gemcitabine/nab-paclitaxel (Arm D) | |||||||||||||||
| DE AM0010, Capecitabine (Arm E) | |||||||||||||||
| DE AM0010, Paclitaxel (Arm F) | |||||||||||||||
| DE AM0010, Pazopanib (Arm G) | |||||||||||||||
| DE AM0010, Pembrolizumab (Arm H) | |||||||||||||||
| DE AM0010, Nivolumab (Arm I) | |||||||||||||||
| DE AM0010, Gemcitabine/carboplatin (Arm J) | |||||||||||||||
| Pembrolizumab (MK-3475/SCH 900475) | 2 | NA | Not recruiting | PC | Palliative | Locally advanced | 54 | 200 mg | Safety (IRAEs) | Cy/GVAX, Pembrolizumab, SBRT | NA | NA | NA | NCT02648282 | |
| Efficacy (DMFS, OS, LPFS) | |||||||||||||||
| 2 | Randomized | Active | PC | Palliative | Locally advanced | 76 | NA | Safety (TEAE) | ACP-196 (Arm A) | NA | NA | NA | NCT02362048 | ||
| Not recruiting | Metastatic | Efficacy | ACP-196, Pembrolizumab (Arm B) | ||||||||||||
| 1|2 | Randomized | Recruiting | PC | Neoadjuvant | Resectable | 56 | 200 mg | Safety (DLT) | Pembrolizumab, Capecitabine, Radiotherapy | NA | NA | NA | NCT02305186 | ||
| Borderline resectable | Efficacy (DFS, OS, RR) | ||||||||||||||
| [TILs] | |||||||||||||||
| 1|2 | Non-randomized | Recruiting | PC, SCLC, OC, BC, Sarcoma | Palliative | Metastatic | 90 | 2 mg/kg | Safety (AE) | Pembrolizumab, Gemcitabine (Arm A) | NA | NA | NA | NCT02331251 | ||
| Efficacy (ORR, OS, PFS) | Pembrolizumab, Gemcitabine, Docetaxel (Arm B) | ||||||||||||||
| RD | Pembrolizumab, Gemcitabine, Nab-paclitaxel (Arm C) | ||||||||||||||
| Pembrolizumab, Gemcitabine, Vinorelbine (Arm D) | |||||||||||||||
| Pembrolizumab, Irinotecan (Arm E) | |||||||||||||||
| Pembrolizumab, Liposomal, Doxorubicin (Arm F) | |||||||||||||||
| 1 | Non-randomized | Recruiting | PC | Palliative | Locally advanced | 50 | 200 mg | Safety | Pembrolizumab, Defactinib, Gemcitabine | NA | NA | NA | NCT02546531 | ||
| Metastatic | Efficacy | ||||||||||||||
| 1 | NA | Active | PC, RC, NSCLC, BlC, ASN, RCC, CC, HCC, BC, MM, HNSCC, Sarcoma | Palliative | Metastatic | 12 | NA | Safety | Pembrolizumab, p53MVA | NA | NA | NA | NCT02432963 | ||
| Not recruiting | Efficacy (Clinical Response) | ||||||||||||||
| Tolerability | |||||||||||||||
| 2 | Non-randomized | Suspended | PC, ChC, GeC, CrC, HCC | Palliative | Metastatic | 290 | 2 mg/kg | Safety | Pebrolizumab, Young TIL, Aldesleukin, Cyclophosphamide, Fludarabine | NA | NA | NA | NCT01174121 | ||
| Efficacy | |||||||||||||||
| 1 | NA | Recruiting | PC | Palliative | Locally advanced | 9 | 2 mg/kg | Safety (DLT) | Pembrolizumab, Reolysin, Gemcitabine/Irinotecan/Leucovorin with 5-FU | NA | NA | NA | NCT02620423 | ||
| Metastatic | Efficacy (ORR, PFS, OS) | ||||||||||||||
| 1|2 | NA | Recruiting | PC, ChC, GeC, CrC | Palliative | Locally advanced | 128 | DE | Safety | Pembrolizumab, mFOLFOX6, Celecoxib | NA | NA | NA | NCT02268825 | ||
| Metastatic | Efficacy (RR, PFS, OS) | ||||||||||||||
| 1 | Non-randomized | Recruiting | PC, OC, BC, CrC, RCC, MM, PrC, NSCLC | Palliative | Locally advanced | 300 | 3 mg/kg | Safety | DE AM0010 (Arm A) | NA | NA | NA | NCT02009449 | ||
| Metastatic | Tolerability | DE AM0010, Paclitaxel/Docetaxel, Carboplatin/Cisplatin (Arm B) | |||||||||||||
| PK | DE AM0010, FOLFOX, (Arm C) | ||||||||||||||
| DE AM0010, gemcitabine/nab-paclitaxel (Arm D) | |||||||||||||||
| DE AM0010, Capecitabine (Arm E) | |||||||||||||||
| DE AM0010, Paclitaxel (Arm F) | |||||||||||||||
| DE AM0010, Pazopanib (Arm G) | |||||||||||||||
| DE AM0010, Pembrolizumab (Arm H) | |||||||||||||||
| DE AM0010, Nivolumab (Arm I) | |||||||||||||||
| DE AM0010, Gemcitabine/carboplatin (Arm J) | |||||||||||||||
| Pidilizumab (CT-011) | 2 | NA | Suspended | PC | Adjuvant | Resectable | 29 | 3 mg/kg | Safety | Pidilizumab, Gemcitabine | NA | NA | NA | NCT01313416 | |
| Efficacy (Median DFS) | |||||||||||||||
| 1 | Non-randomized | Withdrawn | PC, OC, BC, CC, Sarcoma | Palliative | NA | 0 | DE | Safety | Pidilizumab, p53 Vaccine | NA | NA | NA | NCT01386502 | ||
| Efficacy |
AE: Adverse event; NA: Not available.
Table 3.
Clinical trials on programmed cell death ligand 1
| Target molecule | Drug name | Study phase | Study design | Status | Condition | Intervention | Cohort | Estimated enrollment | Dose | End point | Study Arm | Adverse event | Response | Survival | Ref. |
| PD-L1 | Durvalumab (MEDI4736) | 2 | Non-randomized | Not recruiting | PC, GeC, OC, NSCLC, BC, RCC, CrC | Palliative | Metastatic | 136 | 750 mg > 30 kg, | Safety Efficacy (ORR, PFS, OS) | Durvalumab | NA | NA | NA | NCT02669914 |
| 10 mg/kg < 30 kg | |||||||||||||||
| 2 | NA | Recruiting | PC, BlC, BC | Palliative | Metastatic | 77 | NA | Safety | Tremelimumab (Arm A) | NA | NA | NA | NCT02527434 | ||
| Efficacy (ORR, DoR, DCR, PFS, OS, BoR) | Durvalumab (Arm B) | ||||||||||||||
| Tremelimumab, Durvalumab (Arm C) | |||||||||||||||
| 1 | Non-randomized | Recruiting | PC, HNSCC, MM, CrC, BC, CEC | Palliative | Locally advanced | 40 | NA | Safety (AE) | Durvalumab, Selumetinib | NA | NA | NA | NCT02586987 | ||
| Metastatic | Efficacy (ORR, BoR, DoR) | ||||||||||||||
| Tolerability | |||||||||||||||
| PK | |||||||||||||||
| 2 | Randomized | Recruiting | PC | Palliative | Metastatic | 130 | NA | Safety | Durvalumab (Arm A) | NA | NA | NA | NCT02558894 | ||
| Efficacy (ORR, DoR, DCR, PFS) | Durvalimab, Tremelimumab (Arm B) | ||||||||||||||
| PK | |||||||||||||||
| Antidrug Antibody Presence | |||||||||||||||
| 1|2 | Non-randomized | Recruiting | PC | Palliative | Metastatic | 26 | NA | Safety (DLT) | Durvalumab , nab-Paclitaxel, Gemcitabine (Arm A) | NA | NA | NA | NCT02583477 | ||
| Efficacy (ORR, DoR, DCR, PFS) | Durvalumab, AZD5069 (Arm B) | ||||||||||||||
| PK | |||||||||||||||
| 1 | Randomized | Recruiting | PC, NSCLC, HNSCC | Palliative | Locally advanced | 108 | NA | Safety (AE, DLT) | Durvalumab, Mogamulizumab (Arm A) | NA | NA | NA | NCT02301130 | ||
| Metastatic | Tremelimumab, Mogamulizumab (Arm B) | ||||||||||||||
| 1 | Non-randomized | Recruiting | PC, NSCLC, BC, MM | Palliative | Metastatic | 30 | 1 mg/kg | Safety (AE) | Durvalumab, Tremelimumab, Radiotherapy | NA | NA | NA | NCT02639026 | ||
| Efficacy |
NA: Not available.
Table 4.
Immune checkpoint inhibitors based on American Society of Clinical Oncology's meeting abstracts
| Target molecule | Drug name | Study phase | Study design | Status | Condition | Intervention | Cohort | Estimated enrollment | Dose | End point | Study Arm | Adverse event | Response | Survival | Ref. |
| CTLA-4 | Tremelimumab | 1 | Non-randomized | Recruiting | PC | Palliative | Metastatic | 60 | NA | Safety | Durvalumab, SBRT (Arm A) | NA | NA | NA | Duffy et al[28] |
| (CP-675/CP-675,206) | Efficacy (ORR, PFS) | Tremelimumab, SBRT (Arm B) | |||||||||||||
| Tolerability | Durvalumab, Tremelimumab, SBRT (Arm C) | ||||||||||||||
| PK | |||||||||||||||
| PD-1 | Nivolumab | 2 | Randomized | Recruiting | PC | Palliative | Metastatic | 94 | 3 mg/kg | Safety (TRT) | Cy/GVAX/Nivolumab, CRS-207/Nivolumab (Arm A) | NA | NA | NA | Le et al[25] |
| (BMS-936558/MDX-1106/ONO-4538) | Efficacy (OS, PFS, RR) | Cy/GVAX, CRS-207 (Arm B) | |||||||||||||
| 1 | Randomized | Recruiting | PC, NSCLC, BC | Palliative | Locally advanced | 138 | 3 mg/kg | Safety (DLT, TEAEs) | Nivolumab, nab-Paclitaxel (Arm A) | NA | NA | NA | Firdaus et al[45] | ||
| Metastatic | Efficacy (PFS, OS, DCR, ORR, DR) | Nivolumab, nab-Paclitaxel, Gemcitabine (Arm B) | |||||||||||||
| Pembrolizumab | 1|2 | Non-randomized | Recruiting | PC, GeC, OC, NSCLC, BC, BlC, MM, HNSCC, | Palliative | Locally advanced | 400 | 200 mg | Safety | Part A: Pembrolizumab, DE Pexidartinib (PLX3397) | NA | NA | NA | Wainberg et al[50] | |
| (MK-3475/SCH 900475) | Metastatic | Efficacy (ORR; CR, PR) | Part B: RD Pexidartinib (PLX3397), Pembrolizumab | ||||||||||||
| PD-L1 | Durvalumab (MEDI4736) | 1 | Non-randomized | Recruiting | PC | Palliative | Metastatic | 60 | NA | Safety | Durvalumab, SBRT (Arm A) | NA | NA | NA | Duffy et al[31] |
| Efficacy (ORR, PFS) | Tremelimumab, SBRT (Arm B) | ||||||||||||||
| Tolerability | Durvalumab, Tremelimumab, SBRT (Arm C) | ||||||||||||||
| PK | |||||||||||||||
| 1|2 | NA | Recruiting | PC, NSCLS, mBC | Palliative | Metastatic | 160 | NA | Safety (AE, DLT) | Part 1: Durvalumab, DLT Ibrutinib | NA | NA | NA | Borazanci et al[74] | ||
| Efficacy | Part 2: Durvalumab, RD Ibrutinib | ||||||||||||||
| Tolerability | |||||||||||||||
| RD | |||||||||||||||
| PK |
ORR: Overall response rate; DLT: Dose limiting toxicity; NA: Not available.
Table 5.
Immune checkpoint inhibitors based on articles
| Target molecule | Drug | Study phase | Study design | Condition | Intervention | Cohort | Enrollment | Dose | End point | Study Arm | Adverse event | Response | Survival | Ref. |
| CTLA-4 | Ipilimumab | 2 | Non-randomized | PC | Palliative | Locally advanced | 27 | 3 mg/kg | Efficacy (ORR; CR, PR) | Ipilimumab | 11% Grade 3-4 irAEs (Colitis: 1, Encephalitis: 1, Hypophysitis: 1) | No RECIST-response | NA | Royal et al[23] |
| (BMS-734016/MDX-010) | Metastatic | SD: delayed progression, RECIST-progressive disease (n:1) | ||||||||||||
| 1 | Randomized | PC | Palliative | Locally advanced | 30 | 10 mg/kg | Safety (AE) | Ipilimumab (Arm A) | 73%, 80% irAEs (Arm A, B) | SD: growth < 20% growth cut-off, 7w (n:1) , 22w (n:1) (Arm A) regression 17w (n:1), stabilization 59w (n:1), 71w (n:1) (Arm B) | mOS (95%CI:) IPI vs IPI, GVAX: 3.6 (2.5-9.2), 5.7 (4.3-14.7); | Le et al[24] | ||
| Metastatic | Efficacy (OS, ORR) | Ipilimumab, GVAX (Arm B) | 20% Grade 3-4 irAEs (Colitis: 1, GBS: 1, Nephritis: 1) (Arm A), (Rash: 1, Colitis: 1, Pneumonitis: 1) (Arm B) | HR = 0.51, 95%CI: 0.23-1.08, P = 0.072. | ||||||||||
| irAEs (p: 0.037) | yOS (95%CI:) IPI vs IPI, GVAX: 7% (1%-45%), 27% (11%-62%) | |||||||||||||
| Tremelimumab (CP-675/CP-675,206) | 1 | Non-randomized | PC | Palliative | Metastatic | 34 | 15 mg/kg | Safety (AE, DLT, MTD) | Tremelimumab DE (C6, C10, C15), Gemcitabine | Grade 3-4 irAEs (Asthenia: 1, Nausea: 1, Diarrhea: 1, Anemi: 1, Pruritus: 1, Hypertransaminasemia: 1) (C 10), (Asthenia: 3, Nausea: 2, Diarrhea:1, Anemi: 1, Neutropenia: 2, Hypertransaminasemia: 1, Thrombocytopenia:2) (C 15) SAE:11 (Dehydration-diarrhea: 1, ACS: 1, PE: 1, Hyperbilirubinemia: 1, Hematemesis: 1) (C10) (AKI: 1, GIB: 1, Hyperbilirubinemia: 2) (C15) | PR: 8w (n:2) (10.5%: 2/19) | mOS (95%CI:) C6 (6 mg/kg Tremelimumab), C10 (10 mg/kg Tremelimumab), C15 (15 mg/kg Tremelimumab): 5.3 (1.2-14.6), 8.0 (2.3-16.9), 7.5 (6.0-9.5) | Aglietta et al[27] | |
| Efficacy (OS, OR, PFS) | SD: > 10w [n:7 (n:2 completed study)] | |||||||||||||
| PD-L1 | BMS-936559 | 1 | Non-randomized | PC, NSCLC, MM, CrC, OC, GeC, RRC, BC | Palliative | Locally advanced | 207 (14 PDA) | DE | Safety (AE, MTD, DLT) | BMS-936559 | NA | No objective PDA-response | NA | Brahmer et al[79] |
| Metastatic | Efficacy (ORR) | |||||||||||||
| PK |
PDA: Pancreatic ductal adenocarcinoma; MTD: Maximum tolerance dose; ORR: Overall response rate; DLT: Dose limiting toxicity; NA: Not available; CTLA-4: Cytotoxic T lymphocyte antigen 4; PD-L1: Programmed cell death ligand 1.
Figure 2.
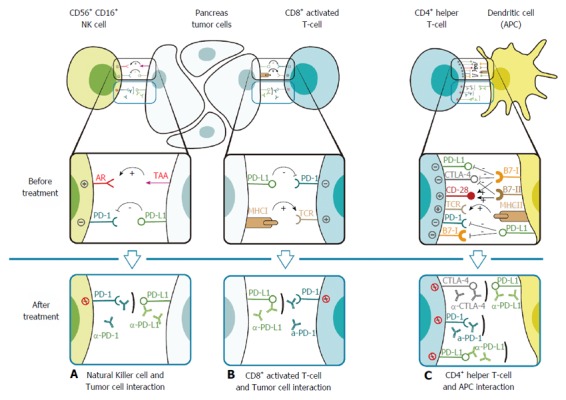
Check-point inhibition. A: Natural killer cell (NK cell) interacts with a PDA cell and gets activated through the binding of its activating receptor (AR) with PDA’s tumor associated antigen (TTA). Tolerance occurs when the programmed-death-receptor (PD-1) molecule on the NK cell interacts with its ligand, the programmed-death-ligand (PD-L1), on the PDA cell. Treatment with monoclonal antibody to bind these inhibitory proteins such as either α-PD-1 (Nivolumab, Pembrolizumab, Pidilizumab) or α-PD-L1 (Druvalumab, BMS-936559) prevents this interaction; B: CD8+ T cell interacts with a PDA cell and gets activated through the binding of its T cell receptor (TCR) with PDA’s major histocompatibility complex (MHC). Tolerance occurs when the PD-1 molecule on the NK cell interacts with PD-L1 on the PDA cell. Treatment with monoclonal antibody to bind these inhibitory proteins such as either α-PD-1 (Nivolumab, Pembrolizumab, Pidilizumab) or α-PD-L1 (Druvalumab, BMS-936559) prevents this interaction, resulting in suppression of T cell tumor attack; C: CD4+ helper T cell interacts with an antigen presenting cell (APC) through the binding of its TCR with APC’sMHC that present TAA. The co-stimulatory APC-signal-binding is induced by the CD28-B7-I/II interaction. The inhibitory checkpoint molecules: PD-L1, PD-1 and CTLA-4 are either presented before CD4+ activation or upregulated after activation and might result in inhibition and anergy of the helper T cell via PD-L1–PD-1, PD-L1–B7-1 and/or CTLA-4–B7-I/II interactions. Treatment with monoclonal antibody to bind these inhibitory proteins such as either α-PD-1 (Nivolumab, Pembrolizumab, Pidilizumab), α-PD-L1 (Druvalumab, BMS-936559) or α-CTLA-4 (Ipilimumab, Tremelimumab) can prevent this interaction, thus maintain antitumor activity.
CTLA-4
CTLA-4 is a molecule expressed and upregulated on activated CD4+, CD8+ T cells and T-regulatory FOXP3+, CD4+, CD25+ cells (Tregs)[9,10]. It is a member of the costimulatory B7 family of receptor signals (homolog to the CD28 receptor) critical in regulation and development of T-cells in the adaptive immune response[11]. Similar to CD-28, CTLA-4 binds to APCs ligands B7.1 (CD80) and B7.2 (CD86) after MHC-TCR binding in APC-T-cell-interaction[12,13]. CTLA-4 binds to these ligands competitively with a higher affinity than CD28[13].
Triggering of CTLA-4 result in downregulation of immune response and maintaining of the peripheral resistance by inhibiting co-stimulation and dephosphorization of the MHC-TCR-interaction. CTLA-4 does this through activation of protein phosphatases, PP2A and SHP-2 and removal of CD80 and CD86 ligands on APC surface[14-16]. This is in contrast to its homologous Ig-member, CD-28, which potentiates immune response by T cell proliferation, activation, differentiation, cell migration and preventing T cell apoptosis[16-18].
The main function of the molecule is to suppress immune responses by Tregs and to downregulate effector T cells[19]. This immunosuppressive function of CTLA-4 is especially prominent in the tumor microenvironment of PDA[20].
Blockade of CTLA-4 has two contradictory effects. It can result in immune response progression with effector T cell enhancement, Treg depletion and consequent tumor reduction. However, there is also a risk of autoimmunity development[19-21].
Currently, two human anti-CTLA-4-antibodies, Ipilimumab and Tremelimumab have been approved for use in cancer treatment.
Ipilimumab
Ipilimumab (BMS-734016, MDX-010) is a humanized monoclonal IgG1 immunoglobulin antibody, developed against CTLA-4-molecules on T cells. It binds to CTLA-4 and prevents T cell suppression by the inhibitory immune checkpoints, resulting in a cytotoxic T-lymphocyte antitumor-mediated immune response[22].
In PDA, a phase II trial, of Ipilimumab was conducted by Royal et al[23] for 27 patients with locally advanced or metastatic disease. A 3 mg/kg single dosage was administered each third week with 4 doses per course. It did not demonstrate any objective response according to the standard response evaluation criteria in solid tumors (RECIST). However, delayed progression in one patient was noted with radiographic response in both the primary tumor and the metastatic tumors. Three patients reported episodes of colitis, encephalitis, hypophysitis, grade 3-4 immune-related adverse events (irAEs), with one resulting in treatment-related death.
Preclinical data suggest synergetic effects of Ipilimumab when combined with “GVAX”; an immune response stimulating, granulocyte macrophage colony stimulating factor (GM-CSF) gene transfected tumor-cell vaccine. In a two-armed, randomized, phase I study of 30 patients with advanced PDA, Le et al[24] reported greater overall survival in patients treated with Ipilimumab and GVAX compared to patients treated with Ipilimumab alone. A 10 mg/kg single dosage of Ipilimumab was administered in Arm A and a 10 mg/kg dosage of Ipilimumab with GVAX was administered in Arm B. The results met the criteria for stable disease (SD) according to RECIST in four patients (Arm A, B) and one further according to the immune-related response criteria (irRC) (Arm B). In treatment Arm A, two patients with constant disease progression reported SD for seven and 22 wk. In treatment Arm B, three patients reported SD, with 17 wk of regression in one, 59 wk of stabilization in one and till week 71 in another. Median overall survival was reported as 3.6 months (95%CI: 2.5-9.2) and 5.7 mo (95%CI: 4.3-14.7), (HR = 0.51, 95%CI: 0.23-1.08, P = 0.072), and one-year overall survival to 7% (1%-45%), and 27% (11%-62%), in Arm A and in Arm B respectively. Furthermore, 73% vs 80% of the patients reported irAEs in Arm A and Arm B. In total 20% grade 3-4 irAEs were reported, including three episodes of colitis, Guillain-Barre syndrome and nephritis (Arm A) and three episodes of colitis, rash and pneumonitis (Arm B). According to Le et al[24] these rates were similar to previous studies testing Ipilimumab 10 mg/kg. The conclusion was that even though insufficient data was conducted to conclude whether Ipilimumab alone, or in combination therapy result in better clinical outcome, combination therapy of Ipilimumab and GVAX had the potential for efficacy improvement in patients with longer life expectancy and that immune checkpoint therapy should start early in the PDA treatment course.
A phase II multicenter study of Ipilimumab and GVAX treatment in metastatic PDA patients is ongoing in patients who have been earlier treated with FOLFIRINOX-chemotherapy (Arm A) or patients continuously treated with FOLFIRINOX (Arm B) (NCT01896869). Survival comparison between continuous FOLFIRINOX-treatment vs FOLFIRINOX followed by Ipilimumab and GVAX is the primary end point[25].
NCT01473940 is another clinical trial studying safety and efficacy of Ipilimumab, when administered together with the cytostatic Gemcitabine hydrochloride, in locally advanced, or metastatic, unresectable PDA patients. Researchers in this trial are hypothesizing the synergetic effect of this combination[26].
Tremelimumab
Tremelimumab (CP-675, CP-675,206) is a humanized monoclonal IgG2 immunoglobulin antibody also developed against CTLA-4-molecules on T-cells. It binds to CTLA-4 and inhibits immune checkpoint mediated T-cell suppression, resulting in cytotoxic T-lymphocyte antitumor-mediated immune response[22].
In a non-randomized, dose increasing, phase I study of Tremelimumab and Gemcitabine hydrochloride for 34 patients with advanced PDA, a partial response in two patients was demonstrated. The patients received a 15 mg/kg Tremelimumab treatment-cycle, consisting of one dose of Tremelimumab each 84th-day along with up to twelve doses of Gemcitabine hydrochloride 1000 mg/m2 for three weeks followed with one week of rest. Stable disease over ten weeks was reported in seven patients, where two patients completed the study. Median overall survival was 7.4 mo (95%CI: 5.8-9.4), with 7.5 mo (95%CI: 6.0-9.5) in the Tremelimumab 15 mg/kg study-arm. However, Aglietta et al[27] did not demonstrate any objective response according to RECIST. Also the confidence interval was wide and overlapped due to limited participants in the respective study-arms. Moreover, the most common grade 3-4 adverse events were asthenia (11.8%), and nausea (8.8%).
In metastatic PDA, a three-armed, phase I, non-randomized, clinical trial testing Tremelimumab alone or in combination with Durvalumab (another immune checkpoint inhibitor discussed later) together with stereotactic body radiation therapy (SBRT) is conducted. The primary objectives are to determine safety, tolerability and efficacy of immune checkpoint therapy together with SBRT. SBRT is expected to enhance Durvalumab’s and/or Tremelimumab’s immune checkpoint inhibitory effects resulting in systemic anti-tumor effects[28].
Tremelimumab is currently being tested for safety and efficacy in two randomized phase I and II trials for locally advanced and metastatic PDA, together with Morgalizumab [a monoclonal antibody against C-C chemokine receptor 4 (CCR4) (NCT02301130)] and together with Durvalumab (NCT02558894)[29,30].
NCT02639026 is a phase I, non-randomized trials, that studies the safety and efficacy of Tremelimumab and Durvalumab in combination with hypofractionated radiation therapy in patients with metastatic PDA[31,32].
NCT02527434 is a phase II clinical trial studying safety and efficacy of Tremelimumab alone, or combined with Durvalumab, in patients with metastatic PDA[33].
PD-1
PD-1 is an immune checkpoint molecule expressed on activated T cells, B cells, NK cells, monocytes and DCs[34]. Like CTLA-4, PD-1 is a member of the CD28 family of receptor providing costimulatory signals, critical in regulation and development of T cells in the adaptive immune response[12]. PD-1 binds to APCs programmed death ligands PD-L1 and PD-L2, and to solid tumor cell ligands PD-L1[34]. The strength of this binding depends on the strength of the TCR-signal, where low TCR-MHC interaction results in great PD-1 binding[35]. When bound to its ligands, PD-1 induces effector T cell inhibition through downstream, kinase inhibition, TCR decreased signaling and a decrease in INF-γ, IL-2 secretion by phosphatase SHP1, SHP2 phosphorylation[35,36].
PD-1 is, similar to CTLA-4, expressed on CD4+ Tregs, where it increases immunosuppression[37]. Its function in the immune response is described as limiting T-cell activity in peripheral tissues, in both the inflammatory infectious response and in potential autoimmunity immune responses[34,35]. Within the PDA tumor microenvironment, as well as in the peripheral blood of PDA patients, PD-1 molecules are largely expressed on tumor infiltrating lymphocytes (TILs), such as CD4+ T-cells, resulting in the tumor exerting a potentiated immune resistance by inducing T-cell apoptosis, and promoting tumor growth[38,39]. PD-1 is further described to create T-cell anergy among cognate antigen-specific T cells due to chronic antigen exposure in cancer tumors[40].
Besides this expression, PD-1 is expressed on other activated immune-cells, such as NK cells and B cells, in which it limits its lytic activity[34,41]. This results in immune suppression. Also, this is thought to result in enhancement of tumor immune surveillance[42].
Conversely, blockade of PD-1 results in immune response progression, with antitumor immunity[39,43].
Since its discovery in 1992 PD-1 has been another promising immune-checkpoint target molecule in cancer tumor immunotherapy to enhance intratumoral immune response[34].
In PDA, the human PD-1-antibody-drugs Pidilizumab, Nivolumab and Pembrolizumab are now tested in clinical trials.
Nivolumab
Nivolumab (BMS-936558, MDX-1106, ONO-4538) is a humanized monoclonal IgG4 immunoglobulin antibody developed against PD-1-checkpoint-molecule-receptors on T cells, NK cells and B cells. It binds to and inhibits either PD-1-PD-L1 or PD-1-PD-L2 cell interaction, resulting in immune function reinstating by activation of NK cells and cytotoxic T lymphocyte (CTL) antitumor immune response[22].
In previously chemotherapy-treated patients, with PDA, a 1:1 randomized, two-armed, phase II study, of Cyclophosphamide-GVAX-vaccine, (CY/GVAX) and CRS-207 (a live-attenuated Listeria monocytogenes-expressing mesothelin drug) is being conducted both with and without Nivolumab. Patients receiving two doses of CY/GVAX and Nivolumab, together with four doses of CRS-207 and Nivolumab (Arm A) will be compared with patients receiving two doses of CY/GVAX and four doses of CRS-207 (Arm B). Overall survival is the primary objective of the study. CY/GVAX-vaccine together with CRS-207, have shown promising results in PDA-patients by priming tumor antigen-specific T cells, through Cyclophosphamide Treg-inhibitor-function; GVAX induced immune response-function against PDA tumor antigens via its GM-CSF-expressing- modified allogeneic pancreatic cancer cells; and CRS-207 enhanced antitumor PDA immune response-function by NK cells and T cells through its tumor-associated mesothelin antigens. Le et al[44] expect this combination therapy, together with Nivolumab to result in priming tumor antigen-specific T cells and simultaneously blocking immune-checkpoints.
In another ASCO-symposium Firdaus et al[45] presented their two-armed, two-part, phase I study of Nivolumab and Nab-paclitaxel-cytostatic, with and without Gemcitabine in patients with advanced PDA. Patients administered 125 mg/m2 Nab-paclitaxel and 3 mg/kg Nivolumab (Arm A), will be compared with patients administered 125 mg/m2 Nab-paclitaxel, 1000 mg/m2 Gemcitabine and 3 mg/kg Nivolumab (Arm B). Evaluation of dose limiting toxicity (DLT) (Part I), together with evaluation of the safety of Nab-paclitaxel/Nivolumab combination (Part I and II) are the primary objectives in this still recruiting clinical trial.
Furthermore, Nivolumab is currently being tested for safety and efficacy in two, phase I and II trials for resectable, (NCT02451982) and metastatic (NCT02423954) PDA. In NCT02451982 patients with resectable PDA are randomized to receive 200 mg/m2 CY/GVAX (Arm A) or 200 mg/m2 CY/GVAX together with 3 mg/kg Nivolumab (Arm B)[46]. NCT02423954 is a non-randomized three-armed study. In Arm A, patients are given 25 mg Temsirolimus (a protein kinase inhibitor) and Nivolumab every 14th day. In Arm B, 150 mg/m2 Irinotecan-cytostatic and Nivolumab every 14th day. In Arm C, 175 mg/ m2 Irinotecan is administered on day one then every 14 d. A dosage of 1000 mg Capecitabine-cytostatic is given on days 1-5, on days 6-7, off each 7 d period in combination with Nivolumab[47].
Two phase I, non-randomized trials in locally advanced or metastatic PDA patients are being conducted (NCT02009449, NCT02526017). NCT02009449, a study of AM0010 (recombinant human IL-10) is being conducted with one cohort treated with 20 μg/kg AM0010 daily subcutaneous injections, together with 3 mg/kg Nivolumab on day one of each 14-d cycle to study dose escalation, where safety and tolerability of AM0010 in patients with advanced solid tumors, dosed daily as a monotherapy or in combination with chemotherapy or immunotherapy, is evaluated[48]. NCT02526017 is a study of FPA008 (a monoclonal humanized antibody) with monotherapy every other week. This is compared to combination therapy consisting of FPA008 and 3 mg/kg Nivolumab every second week. Safety of FPA008 with Nivolumab is being evaluated[49].
Pembrolizumab
Pembrolizumab (MK-3475, SCH 900475) is also a humanized monoclonal IgG4 immunoglobulin antibody, developed against the PD-1-checkpoint-molecule-receptor on T cells. It binds to and inhibits either PD-1-PD-L1 or PD-1-PD-L2 cell interaction, resulting in activation of a CTL antitumor immune response[22].
In a two-part immunotherapy clinical trial, testing Pexidartinib [a colony-stimulating factor 1 receptor (CSF1R) inhibitor] and Pembrolizumab in patients with PDA and other solid tumors, safety, efficacy, pharmacokinetics and pharmacodynamics is to be evaluated. In part I, a safety and tolerability combination-dose will be established; 200 mg Pembrolizumab each third week, with daily 200 mg oral doses of Pexidartinib. Thereafter, overall response rate (ORR) and progression free survival (PFS) will, in solid tumor-patients with the given established combination-dose, be evaluated. Pexidartinib is expected to target tumor associated macrophages (TAMs) and Myeloid-derived suppressor cells (MDSCs). TAMs enhance tumor growth and contribute to tumor resistance to radiation and chemotherapy. MDSCs suppress tumor surveillance and cause resistance to PD-1-checkpoint therapy[50].
Three other phase I and II clinical trials are currently running.
NCT02305186 is a randomized, safety and efficacy study. 200 mg of Pembrolizumab is given every third week during concurrent neoadjuvant Capecitabine-cytostatic treatment and radiation therapy in resectable PDA patients[51]. NCT02331251 is a non-randomized, safety and efficacy study of 2 mg/kg Pembrolizumab given every third week, together with a different cytostatic in different study arms in metastatic PDA[52]. NCT02268825 is a safety and efficacy dose-escalating study were phase I evaluates the maximum tolerance dose (MTD) of Pembrolizumab in combination with mFOLFOX6-cytostatic. Phase II combines an additional dose of Celecoxib (NSAID) to determine the clinical benefit rate, the objective response rate, PFS and overall survival in locally advanced or metastatic PDA[53].
Two phase II clinical trials of Pembrolizumab in patients with locally advanced or metastatic PDA are being conducted. NCT02648282 is a safety and efficacy study of combination therapy using 200 mg Pembrolizumab together with 200 mg/m2 Cyclophosphamide-cytostatic and GVAX-vaccine (CY/GVAX) and SBRT[54]. NCT02362048 is an active, non-recruiting, two-armed, randomized safety and efficacy study where ACP-196 alone (Arm A) and ACP-196 in combination with Pembrolizumab (Arm B) are studied[55].
Four phase-I clinical trials of Pembrolizumab in PDA are being conducted.
NCT02546531 and NCT02009449 are two non-randomized, safety and efficacy trials in locally advanced and metastatic PDA. In NCT02546531, Pembrolizumab is tested together with Gemcitabine and Defactinib in advanced PDA. Defactinib, a selective cancer stem cell inhibitor, directed against focal adhesion kinase is hoped to reduce stromal fibrosis in tumors during immune-checkpoint-therapy[56]. NCT02009449 is a dose escalation study of AM0010 (recombinant human IL-10) administered in incremental dosages through daily subcutaneous injections, together with 2 mg/kg Pembrolizumab on day one of each 14-d cycle. Safety and tolerability of AM0010 in patients with advanced solid tumors, either as a monotherapy or in combination with chemotherapy or immunotherapy, is evaluated[48].
NCT02432963 and NCT02620423 are two phase-I Pembrolizumab studies. NCT02432963 is an active, non-recruiting safety trial of Pembrolizumab in combination with p53MVA (a gene-modified virus vaccine) administered to enhance antitumor immune response and reduce tumor growth in patients with metastatic PDA[57]. NCT02620423 is a safety and efficacy study of Pembrolizumab 2 mg/kg in combination with Reolysin (a human oncolytic reovirus that lysates cancer cells) and with chemotherapy in locally advanced or metastatic PDA[58].
NCT01174121 is a non-randomized, phase-II trial of Pembrolizumab, 2 mg/kg Pembrolizumab, 60 mg/kg per day Cyclophosphamide, along with 25 mg/m2 per day Fludarabine-cytostatic, Aldesleukin (a humanised IL-2-molecule) and tumor infiltrating cells (TILs) was conducted to study if tumor reduction could be reached by immunotherapy and TILs in PDA. The study was suspended[59].
Pidilizumab
Pidilizumab (CT-011, MDV9300) is a humanized monoclonal IgG1 immunoglobulin antibody developed against PD-1-molecule-receptors on T cells, NK cells, and B cells. It binds to and inhibits either PD-1-PD-L1 or PD-1-PD-L2 cell interaction, resulting in activation of CTL and an NK-cell antitumor immune response[22]. In PDA, two clinical trials, NCT01313416, and NCT01386502 of Pidilizumab were partially conducted, then suspended and withdrawn[60,61].
PD-L1
PD-L1 is a molecule expressed on solid tumors (such as PDA) and on tumor-infiltrating dendritic cells and macrophages[62,63]. PD-L belongs to the B7 family consisting of PD-L1 (CD274/B7-H1) and PD-L2 (CD273/B7-DC)[63,64]. PD-L1 is a co-inhibitory ligand that binds to PD-1 and to CD80 (the co-stimulatory molecule to CD28 and co-inhibitory molecule to CTLA4 of T-cells) on T-cells and APCs[65]. When bound to its receptors, PD-L1 induces effector T-cell inhibition, apoptosis, anergy and exhaustion via PD-1-PD-L1 interaction[39,40,66]. Immunosuppression is enhanced through inhibitory receptor-signaling via B7-1-molecules in the B7-1-PD-L1 binding. However, this is a finding that not have been observed in tumors[34]. Moreover PD-L1 expression was observed to increase Treg infiltration in the PDA tumor microenvironment, also resulting in immune suppression[67].
PD-L2 is primarily expressed on dendritic cells and macrophages[68].
PD-L1 is the major PD-L-molecule expressed on solid tumors[39]. The expression of PD-L1 on PDA tumor cells is induced via CD8+ T-cell INFγ-secretion[69,70]. The expression of PD-L1 molecules in PDA is associated with assessed tumor proliferation, accelerated tumor cell carcinogenesis and drug resistance, defining the tumor as highly malignant[71].
Blockade of PD-L1 is described to result in significant immune response progression, with enhanced T-cell activation[72]. In PDA enhanced upregulation and secretion of INF-γ, cytokines and proteases by infiltrated and activated CD8+ T-cell in the tumor microenvironment was reported, after PD-L1 blockade[73]. Furthermore, as already stated, blockade of the PD-1 to PD-L1-pathway, results in tumor and antitumor immunity reduction by Treg depletion. Likewise, blockade of the CD80 to PD-L1-pathway results in immune response enhancement. The CD80-PD-L1-pathway is reported to be a bidirectional interaction of CD80 and PD-L1, where CD80, as stated above, interacts co-stimulatory with CD28-receptor and co-inhibits CTLA4-receptor on T-cells. PD-L1 also interact with PD-1, indicating a specific and significant T-cell response-inhibition, regulation and tolerance[34].
Thus, PD-L1 is another promising immune checkpoint target molecule to enhance intratumoral immune response in cancer tumor immunotherapy. In PDA, Durvalumab, and BMS-936559, human anti-PD-1-antibody-drugs, are now tested in clinical trials.
Durvalumab
Durvalumab (MEDI4736) is a humanized FC optimized monoclonal IgG1 immunoglobulin antibody, developed against PD-L1-checkpoint-ligand on solid tumors and TILs, such as dendritic cells and macrophages. When bound, Durvalumab inhibits PD-1 cell interaction, potentially resulting in T cell upregulation and antitumor immune potentiation against PD-L1 expressing tumors by CTL response exertion. To avoid antibody-dependent cytotoxicity or complement-dependent cytotoxicity Durvalumabs FC-region is modified[22,74].
As stated above, Duffy et al[28] have, in metastatic PDA, conducted a three-armed, phase I, non-randomized clinical trial testing Durvalumab alone or in combination with Tremelimumab, together with SBRT. The primary objectives were to determine safety, tolerability and efficacy of immune checkpoint therapy together with SBRT. SBRT is expected to enhance Durvalumab’s and/or Tremelimumab’s immune checkpoint inhibitory effects, resulting in systemic anti-tumor effects.
In a phase Ib/II clinical trial, Borazanci et al[74] expect to evaluate the safety, tolerability and efficacy of Durvalumab in combination with Ibrutinib (a Burton’s tyrosine kinase inhibitor). Preclinical data suggest that Ibrutinib inhibit tumor cell proliferation via angiogenesis and collagen deposition in TME by PDA mast cell degranulation[75]. DLT in phase I will determine recommended phase II dosage to treat up to 160 patients.
In two non-randomized, phase I clinical trials, Durvalumab is tested in locally advanced and metastatic PDA (NCT02586987) or metastatic PDA (NCT02639026). NCT02586987 is an open label, multicenter study, assessing safety, tolerability and anti-tumor activity[76]. NCT02639026 studies safety and efficacy of Tremelimumab and Durvalumab in combination with hypofractionated radiation therapy[32].
NCT02301130 is a randomized, two-armed, phase I trial studying safety and efficacy. In Arm A, Durvalumab is combined with Mogamulizumab (monoclonal antibody against CCR4). In Arm B, Tremelimumab is combined with Mogamulizumab[29]. Two phase II randomized clinical trials of Durvalumab in metastatic PDA studying safety and efficacy together with Tremelimumab are conducted[30,33]. Two non-randomized, safety and efficacy clinical trials of Durvalumab in metastatic PDA were conducted. NCT02669914 is a phase II non-recruiting study of Durvalumab against refractory or recurrent brain metastases from solid tumors such as in PDA[77]. NCT02583477 is a two-armed, phase Ib/II recruiting study of Durvalumab evaluated in combination with gemcitabine and nab-paclitaxel-cytostatics or in combination with the C-X-C motif chemokine receptor 2 (CXCR2) reversible antagonist with antineoplastic activity potentiality, AZD5069, in patients with metastatic PDA[78].
BMS-936559
BMS-936559 is a Bristol-Myers Squibb humanized monoclonal IgG4 antibody directed against PD-L1-checkpoint-ligand on solid tumors and TILs such as dendritic cells and macrophages. BMS-936559 inhibits PD-1 and CD80 cell interaction. In a phase I, non-randomized safety and efficacy study, Brahmer et al[79] reported objective response rates of 6% to 17% and prolonged disease stabilization rates of 12% to 41% at 24 wk in advanced cancers patients, when treated with BMS-936559. The study consisted of 207 patients with locally advanced, or metastatic cancer; 75 with NSCLC, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 7 with gastric cancer, four with breast cancer, and 14 with PDA. BMS-936559 was tested with escalating doses ranging from 0.3 mg/kg to 10 mg/kg. In patients with melanomas, objective response was observed in 29% at a dose of 3 mg/kg. In PDA, no objective response was observed.
FURTHER IMMUNE CHECKPOINT MOLECULES
Besides the above defined inhibitory lymphocyte receptors (CTLA-4, PD-1) and the ligand (PD-L1), other immune checkpoint molecules have been observed to be expressed on tumors and TILs in the tumor microenvironment. Lymphocyte activation gene 3 (LAG-3), B and T lymphocyte attenuator (BTLA/CD272), T cell membrane protein 3 (TIM-3/HAVCR2), adenosine A2a receptor (A2aR) and the B7-family inhibitory receptors (B7-H3/CD276, B7-H4/B7S1) are immune checkpoint molecules described to exert immune suppression[80-84]. B7-H3 is a molecule expressed on tumors, while B7-H4 is expressed on monocytes and macrophages[84]. TIM-3 is expressed on T helper cells and co-expressed with PD-1 on CD8+ cells, preventing immune response[82]. A2aR interacts with adenosine, resulting in T-cell suppression[83]. LAG-3 is an inhibitory receptor molecule expressed on NK cells, APCs, Tregs, T-cells, B cells[80]. In TIL anergic or exhausted T cells, LAG-3 is co-expressed with PD-1[80]. It binds to MHC-II molecules on CD4+ and CD8+, resulting in immune suppression[80]. Blockade of LAG-3 is reported to slow tumor growth and act in synergy with anti-PD-1 antibodies[80,85].
In PDA one non-randomized, phase-I clinical trial testing IMP321 (an LAG-3 immune checkpoint blocker) was conducted, but was terminated, due to company manufacturing production inability[86].
DISCUSSION
In regulating the human immune system, immune checkpoint molecules are considered essential. CTLA-4, PD-1 and PD-L1 are three well-described inhibitory checkpoint molecules expressed on immune cells in the pancreatic ductal adenocarcinoma tumor microenvironment. Together with other molecules derived by the tumor cells and different tumor-specific antigens, these checkpoint inhibitors create a heterogeneous protein expressing tumor, able to thwart immune surveillance and suppress and evade immune response. Checkpoint inhibition may enhance the T-cell response against the tumor. Hence immune checkpoint therapy is expected to have a place in the treatment arsenal for solid tumors such as pancreatic ductal adenocarcinoma. In patients with melanoma and lung cancer, treatments with immune checkpoint therapy have shown promising results in terms of tumor regression, objective response rate and overall survival progression, whereby FDA approval has been received.
Among patients with pancreatic ductal adenocarcinoma, immune checkpoint therapies also appear to be effective. Royal et al[23], noted delayed progression in one patient when treated with 3 mg/kg Ipilimumab. Le et al[24] observed stable disease in five patients (two Arm A, three Arm B), four according to RECIST (two Arm A, two Arm B) and one according to irRC (Arm B), when treated with 10 mg/kg Ipilimumab alone (Arm A) or in combination with GVAX-vaccine (Arm B). Furthermore greater overall survival of 5.7 mo in patients treated with Ipilimumab and GVAX-vaccine, compared to 3.6 mo of Ipilimumab monotherapy was in this study noted. Aglietta et al[27] demonstrated partial response in two patients with advanced pancreatic ductal adenocarcinoma, receiving 15 mg/kg Tremelimumab. Moreover a median overall survival of 7.4 mo with Tremelimumab was observed. Brahmer et al[79] reported objective response rates of 6% to 17%, and prolonged disease stabilization rates of 12% to 41% at 24 wk in advanced cancers patients of melanoma and NSCLC, but not in PDA, when treated with BMS-936559.
However, these studies are limited, both in numbers and enrolled patients. These results of treatment with immune checkpoint inhibitors in PDA instead indicate that further research is needed. The number of currently running clinical trials described in this analysis serves as evidence that immune checkpoint therapy is a new and novel treatment perspective in the fight against PDA. A couple of trials are combining two of the checkpoints inhibitors, Durvalumab and Tremelimumab[29-33]. Other trials are testing checkpoint inhibitors, in combination with cytostatics[54,60]; in combination with vaccine[25,57,87]; or as monotherapy[30,49]. Currently, no results from these trials are presented.
Further knowledge is needed regarding the tumor of pancreatic ductal adenocarcinoma, its tumor microenvironment, its immune response and carcinogenesis to determine the activated immunosuppressors within an individual patient tumor in order to develop a personalized combination treatment. Improved evaluation of key-molecules within the tumor microenvironment of PDA, may as Bailey et al[8] reported, enable distinctions of PDA, where distinct combination therapies can be given in order to increase efficacy and response rate. The repertoire of immune checkpoints is expanding with further research. LAG-3 is a checkpoint molecule found to have been under investigation in a clinical trial by blocking it with IMP321, an immune checkpoint blocker, which was subsequently terminated due to company manufacturing production inability[86]. Identifying and blocking this molecule, as well as other novel reported checkpoint molecules, for example TIM-3 and BTLA might result in improved efficacy in treating the cancer, since these molecules are reported being co-expressed with PD-1[82]. As stated above, Le et al[24] reported that combination therapy of Ipilimumab and GVAX-vaccine improved overall survival compared to Ipilimumab monotherapy. Vaccine therapies are described to potentiate associated antigen specific T-cells in the tumor microenvironment. Likewise, cytostatic and radiation therapies are described to result in tumor cell death with antigen releases, resulting in T-cell activation in the tumor microenvironment. In order to create and above all maintain the tumor microenvironment as an immunogenic environment, checkpoint inhibitors might be essential.
Outside the scope of this paper, but still worth mentioning is the finding of McGranahan et al[2]. It describes the potential of combining immune checkpoint therapy in advanced cancers which both show sensitivity to immune checkpoint therapy and also express multiple tumor-antigens such as clonal neoantigens, and CD8+ specific TILs (that recognize these clonal neoantigens) and identifying and potentiating these cells. Such an approach could make possible a general potentiation of a CD8+ T-cell immune response against the whole tumor. This could lead to a new generation of personalized cancer treatment that could treat and perhaps even cure a cancer like pancreatic ductal adenocarcinoma.
This particular research is in its infancy. While the results are rather bleak the promise of future success lies in one important fact: that the treatment is leveraging the body’s own immune response to a tumor. Great strides have been made in medicine, but we do not yet fully understand the wonders of the immune system and the force that drives the healing process within the human body.
CONCLUSION
Pancreatic cancer is one of the most treatment resistant human malignancies. Due to its heterogenic tumor microenvironment it has long been considered as a non-immunogenic cancer. However, in recent years more emphasis has been put on the importance of the immune system and the immune escape mechanisms of pancreatic cancer. Novel immune-based treatment strategies have been developed. One of the more promising types of immunotherapies includes immune checkpoint inhibition, which is currently being tested in clinical trials in combination with vaccines, radiation or cytotoxic agents. If proven clinically beneficial, immune checkpoint therapy may become a valuable tool in our armamentarium.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Peer-review started: July 26, 2016
First decision: September 12, 2016
Article in press: October 19, 2016
P- Reviewer: Kleeff J, Somani P S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennock GK, Chow LQ. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist. 2015;20:812–822. doi: 10.1634/theoncologist.2014-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet LR, Hagemann T, Andrew G, Mudan S, Marabelle A. Have lessons from past failures brought us closer to the success of immunotherapy in metastatic pancreatic cancer? Oncoimmunology. 2016;5:e1112942. doi: 10.1080/2162402X.2015.1112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazhin AV, Shevchenko I, Umansky V, Werner J, Karakhanova S. Two immune faces of pancreatic adenocarcinoma: possible implication for immunotherapy. Cancer Immunol Immunother. 2014;63:59–65. doi: 10.1007/s00262-013-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 7.Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, Bruno M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40:513–522. doi: 10.1016/j.ctrv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 9.Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 10.Fokas E, O’Neill E, Gordon-Weeks A, Mukherjee S, McKenna WG, Muschel RJ. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochim Biophys Acta. 2015;1855:61–82. doi: 10.1016/j.bbcan.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 13.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachsmann MB, Pop LM, Vitetta ES. Pancreatic ductal adenocarcinoma: a review of immunologic aspects. J Investig Med. 2012;60:643–663. doi: 10.231/JIM.0b013e31824a4d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 18.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 19.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 21.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National cancer institute. NCI Drug Dictionary. Available from: http://www.cancer.gov/publications/dictionaries/cancer-drug.
- 23.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le D. A Phase 2, Multicenter Study of FOLFIRINOX Followed by Ipilimumab With Allogenic GM-CSF Transfected Pancreatic Tumor Vaccine in the Treatment of Metastatic Pancreatic Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01896869.
- 26.Mulcahy M. Ipilimumab and Gemcitabine Hydrochloride in Treating Patients With Stage III-IV or Recurrent Pancreatic Cancer That Cannot Be Removed by Surgery. Available from: https://clinicaltrials.gov/ct2/show/NCT01473940.
- 27.Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750–1755. doi: 10.1093/annonc/mdu205. [DOI] [PubMed] [Google Scholar]
- 28.Duffy AG, Makarova-Rusher OV, Drew Pratt DEK, Alewine C, Fioravanti S, Walker M, Carey S, Figg WD, Steinberg SM, Anderson V, et al. A pilot study of immune checkpoint inhibition (tremelimumab and/or MEDI4736) in combination with radiation therapy in patients with unresectable pancreatic cancer. Available from: http://meetinglibrary.asco.org/content/160088-173.
- 29.Study of Mogamulizumab MEDI4736 and Mogamulizumab Tremelimumab in Subjects w/ Advanced Solid Tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT02301130.
- 30.O’Reilly EM. Phase II Study of MEDI4736 Monotherapy or in Combinations With Tremelimumab in Metastatic Pancreatic Ductal Carcinoma. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02558894.
- 31.Duffy AG. Immune Checkpoint Inhibition (Tremelimumab and/or MEDI4736) in Combination With Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02311361.
- 32.O’Hara MH. Trial Of Hypofractionated Radiotherapy In Combination With MEDI4736 And Tremelimumab For Patients With Metastatic Melanoma And Lung, Breast And Pancreatic Cancers. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02639026.
- 33.Padmanee S. Study of Tremelimumab in Patients With Advanced Solid Tumors. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02527434.
- 34.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 37.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komura T, Sakai Y, Harada K, Kawaguchi K, Takabatake H, Kitagawa H, Wada T, Honda M, Ohta T, Nakanuma Y, et al. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact. Cancer Sci. 2015;106:672–686. doi: 10.1111/cas.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 40.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 41.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 42.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le DT, Crocenzi TS, Uram JN, Lutz ER, Laheru DA, Sugar EA, Vonderheide RH, Fisher GA, Ko AH, Murphy AL, et al. Randomized phase II study of the safety, efficacy, and immune response of GVAX pancreas vaccine (with cyclophosphamide) and CRS-207 with or without nivolumab in patients with previously treated metastatic pancreatic adenocarcinoma (STELLAR) Available from: http://meetinglibrary.asco.org/content/146694-156.
- 45.Firdaus I, Waterhouse DM, Gutierrez M, Wainberg ZA, George B, Kelly K, Bekaii-Saab TS, Carrizosa DR, Soliman HH, Fraser CD, et al. nab-paclitaxel (nab-P) + nivolumab (Nivo) ± gemcitabine (Gem) in patients (pts) with advanced pancreatic cancer (PC) Available from: http://meetinglibrary.asco.org/content/159332-173.
- 46.Zheng L. Neoadjuvant/Adjuvant GVAX Pancreas Vaccine (With CY) With or Without Nivolumab Trial for Surgically Resectable Pancreatic Cancer. Available from: https: //clinicaltrials.gov/ct2/show/study/NCT02451982.
- 47.Khemka V. Study of Nivolumab Plus Chemotherapy in Patients With Advanced Cancer (NivoPlus) (NivoPlus) Available from: https://clinicaltrials.gov/ct2/show/study/NCT02423954.
- 48.Oft M. A Phase 1 Study of AM0010 in Patients With Advanced Solid Tumors. Available from: https: //clinicaltrials.gov/ct2/show/NCT02009449.
- 49.Hambleton J. Study of FPA008 in Combination With Nivolumab in Patients With Selected Advance Cancers (FPA008-003) Available from: https://clinicaltrials.gov/ct2/show/study/NCT02526017.
- 50.Wainberg ZA, Eisenberg PD, Sachdev JC, Weise AM, Kaufman DR, Hutchinson M, Tong S, Aromin I, Hu-Lieskovan S, Patnaik A. Phase 1/2a study of double immune suppression blockade by combining a CSF1R inhibitor (pexidartinib/PLX3397) with an anti PD-1 antibody (pembrolizumab) to treat advanced melanoma and other solid tumors. Available from: http://meetinglibrary.asco.org/content/159443-173.
- 51.Rahma O. Safety and Immunological Effect of Pembrolizumab in Resectable or Borderline Resectable Pancreatic Cancer (UVA-PC-PD101) Available from: https://clinicaltrials.gov/ct2/show/NCT02305186.
- 52.Khemka V, Weiss G. Study of Pembrolizumab Plus Chemotherapy in Patients With Advanced Cancer (PembroPlus) Available from: https://clinicaltrials.gov/ct2/show/study/NCT02331251. [DOI] [PMC free article] [PubMed]
- 53.Phase I/IIA Study MK-3475 With Chemotherapy in Patients With Advanced GI Cancers (MK-3475 GI) Available from: https://clinicaltrials.gov/ct2/show/NCT02268825.
- 54.Zheng L. Study With CY, Pembrolizumab, GVAX, and SBRT in Patients With Locally Advanced Pancreatic Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02648282.
- 55.Indamdar S. ACP-196 Alone and in Combination With Pembrolizumab in Subjects With Advanced or Metastatic Pancreatic Cancer (KEYNOTE144) Available from: https: //clinicaltrials.gov/ct2/show/NCT02362048.
- 56.Wang-Gillam A. Defactinib Combined With Pembrolizumab and Gemcitabine in Patients With Advanced Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02546531.
- 57.Chung V. Vaccine Therapy and Pembrolizumab in Treating Patients With Solid Tumors That Have Failed Prior Therapy. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02432963.
- 58.Mahalingam D. Study of Pembrolizumab With REOLYSIN® and Chemotherapy in Patients With Advanced Pancreatic Adenocarcinoma. Available from: https://clinicaltrials.gov/ct2/show/NCT02620423.
- 59.Rosenberg SA. Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients With Metastatic Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01174121.
- 60.Khleif SN. Gemcitabine and CT-011 for Resected Pancreatic Cancer. Available from: https://ClinicalTrials.gov/show/NCT01313416.
- 61.CT-011 and p53 Genetic Vaccine for Advanced Solid Tumors. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01386502.
- 62.Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 63.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 64.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 65.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, W Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 68.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 69.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-γ. Cancer Immunol Immunother. 2011;60:1529–1541. doi: 10.1007/s00262-011-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song X, Liu J, Lu Y, Jin H, Huang D. Overexpression of B7-H1 correlates with malignant cell proliferation in pancreatic cancer. Oncol Rep. 2014;31:1191–1198. doi: 10.3892/or.2013.2955. [DOI] [PubMed] [Google Scholar]
- 72.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 73.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 74.Borazanci EH, Hong DS, Gutierrez M, Rasco DW, Reid TR, Veeder MH, Tawashi A, Lin J, Dimery IW. Ibrutinib durvalumab (MEDI4736) in patients (pts) with relapsed or refractory (R/R) pancreatic adenocarcinoma (PAC): A phase Ib/II multicenter study. Available from: http://meetinglibrary.asco.org/content/160089-173.
- 75.Massó-Vallés D, Jauset T, Serrano E, Sodir NM, Pedersen K, Affara NI, Whitfield JR, Beaulieu ME, Evan GI, Elias L, et al. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675–1681. doi: 10.1158/0008-5472.CAN-14-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jänne PA. A Study to Assess the Safety, Tolerability, Pharmacokinetics and Anti-tumour Activity of Ascending Doses of Selumetinib in Combination With MEDI4736 in Patients With Advanced Solid Tumours. Available from: https://clinicaltrials.gov/ct2/show/NCT02586987.
- 77.Govindan R. MEDI4736 (Durvalumab) in Patients With Brain Metastasis From Epithelial-derived Tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT02669914.
- 78.Sun W. Phase Ib/II Study of MEDI4736 Evaluated in Different Combinations in Metastatic Pancreatic Ductal Carcinoma. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02583477.
- 79.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother. 2012;61:917–926. doi: 10.1007/s00262-011-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawkins WG. Lag-3 and Gemcitabine for Treatment of Advanced Pancreas Cancer. Available from: https://clinicaltrials.gov/ct2/show/study/NCT00732082.
- 87.Le DT. GVAX Pancreas Vaccine (With CY) and CRS-207 With or Without Nivolumab. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02243371.


