Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most common comorbidities associated with overweight and metabolic syndrome (MetS). Importantly, NAFLD is one of its most dangerous complications because it can lead to severe liver pathologies, including fibrosis, cirrhosis and hepatic cellular carcinoma. Given the increasing worldwide prevalence of obesity, NAFLD has become the most common cause of chronic liver disease and therefore is a major global health problem. Currently, NAFLD is predominantly regarded as a hepatic manifestation of MetS. However, accumulating evidence indicates that the effects of NAFLD extend beyond the liver and are negatively associated with a range of chronic diseases, most notably cardiovascular disease (CVD), diabetes mellitus type 2 (T2DM) and chronic kidney disease (CKD). It is becoming increasingly clear that these diseases are the result of the same underlying pathophysiological processes associated with MetS, such as insulin resistance, chronic systemic inflammation and dyslipidemia. As a result, they have been shown to be independent reciprocal risk factors. In addition, recent data have shown that NAFLD actively contributes to aggravation of the pathophysiology of CVD, T2DM, and CKD, as well as several other pathologies. Thus, NAFLD is a direct cause of many chronic diseases associated with MetS, and better detection and treatment of fatty liver disease is therefore urgently needed. As non-invasive screening methods for liver disease become increasingly available, detection and treatment of NAFLD in patients with MetS should therefore be considered by both (sub-) specialists and primary care physicians.
Keywords: Nonalcoholic fatty liver disease, Metabolic syndrome, Diabetes mellitus type 2, Cardiovascular disease, Chronic kidney disease, Multisystem disease
Core tip: Given the increasing worldwide incidence of obesity and metabolic syndrome, non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease. Recent developments in the field have shown that NAFLD not only is a “liver disease” but also is the underlying cause of an increasing number of extrahepatic manifestations; thus, it should be treated as a multisystem disease. NAFLD is most prominently linked to chronic kidney disease, mellitus type 2 and cardiovascular disease, as well as a number of other severe chronic diseases. These findings demonstrate that NAFLD ranks amongst the most serious public health problems of our time.
INTRODUCTION
The overall incidence of nonalcoholic fatty liver disease (NAFLD) is increasing along with that of obesity and metabolic syndrome (MetS). Therefore, NAFLD is the most frequent form of chronic liver disease that is closely related to MetS and its individual components [diabetes mellitus type 2 (T2DM), dyslipidemia, obesity and arterial hypertension]. NAFLD is diagnosed in people who do not have a history of excessive urination, do not have any other secondary causes of chronic liver disease (autoimmune and metabolic liver diseases, viral hepatitis) and do not use medication that can lead to liver steatosis. The clinical presentation of NAFLD varies from simple steatosis to nonalcoholic steatohepatitis (NASH), liver cirrhosis and hepatocellular carcinoma (HCC). NASH-related cirrhosis and NASH-related HCC are expected to become the most common indications for liver transplantation in the near future. Despite NAFLD’s substantial effects on liver physiology, the major causes of death in patients with NAFLD are, in fact, malignant and cardiovascular diseases (CVD), whereas liver-related mortality is only the third cause of mortality in these patients[1-3]. However, recent studies have shown that NAFLD affects several extra-hepatic organs and regulatory pathways and may be an important underlying cause of mortality related to failure of other organs than the liver. Recent findings have indicated that NAFLD is a new risk factor for extrahepatic diseases, such as CVD, chronic kidney disease (CKD), colorectal carcinoma, T2DM and psoriasis. All major studies of the NAFLD-related chronic diseases during the past 10 years have involved CKD, CVD and T2DM. The clinical implication of this new evidence is that patients with NAFLD will benefit from more intensive surveillance and/or early treatment interventions to lower the incidence of diseases related to NAFLD[1-4]. In this review, we discuss the evidence linking NAFLD to extrahepatic diseases.
CARDIOVASCULAR MANIFESTATIONS OF NAFLD
Coronary artery disease (CAD) and stroke compose more than 75% of CVD, which is the leading cause of death worldwide[4,5]. Primary and secondary prevention of CVD are unsatisfactory throughout the world, and although the risk factors are well established, the incidence of CVD is still increasing. Abdominal obesity, arterial hypertension, T2DM and dyslipidemia are all features of MetS and have been repeatedly found in NAFLD. These findings prompt the question of whether NAFLD is a symptom of MetS or a consequence[6]. Numerous studies have examined this question, and the majority have suggested that NAFLD is the hepatic manifestation of the MetS[6]. Consequently, patients with these conditions are also at an increased risk for CVD because the common risk factors are shared[7-9]. Along with the epidemic of obesity, the prevalence of MetS and NAFLD is increasing globally and is emerging as a major public health problem.
Several retrospective hospital-based studies with reasonably long follow-up have reported that patients with NAFLD have a higher all-cause and CVD-related mortality than that in the general population[4,10-16]. Retrospective studies assessing the relationship between NAFLD and CVD risk are summarized in Table 1.
Table 1.
Principal retrospective studies of cardiovascular diseases mortality and morbidity risk in patients with nonalcoholic fatty liver disease (published in the past 10 years)
| Ref. | Study design | Study size | Diagnosis of NAFLD | Follow-up duration (yr) | Adjusted clinical variables | Major findings |
| Ekstedt et al[13] | Retrospective; Hospital-based | 129 | Histological | 13.7 | Matched for gender, age and country | NASH subjects (not those with simple steatosis) had higher rates of all-cause, CVD and liver-related mortality than the general population |
| Stepanova et al[15] | Retrospective; Population-based | 289 | Histological | 6.25 | No adjustments made | Higher risk of liver-related mortality in NASH than non-NASH. NAFLD and type II diabetes had the highest risk for overall and liver-related mortality |
| Ekstedt et al[14] | Retrospective; Community-based | 229 | Histological | 26.4 | NAFLD patients had increased risk of death, with a high risk of death from CVD and liver-related disease | |
| Fibrosis stage predicted all-cause, CVD and liver-related death | ||||||
| Rafiq et al[10] | Retrospective; Hospital-based | 173 | Histological | 13 | No adjustments made | Higher liver-related mortality but no difference in overall mortality (NASH vs simple steatosis) |
| Soderberg et al[11] | Retrospective; Hospital-based | 118 | Histological | 18 | Matched for gender, age and year | Increased total mortality in NAFLD was predominantly CV-related compared with matched reference population |
| Dunn et al[16] | Retrospective cohort | 2343 | Computed tomography | 5 | No significant association was found between NAFLD and risk of all-cause mortality and cause-specific | |
| (CVD, cancer and liver) mortality and morbidity. NAFLD patients | ||||||
| (steatosis > 30% on imaging) averaged 8 yr younger than those without NAFLD |
NAFLD: Nonalcoholic fatty liver disease; CVD: Cardiovascular diseases; NASH: Nonalcoholic steatohepatitis.
Table 2 summarizes the prospective studies investigating the relationship between NAFLD and CVD risk, which have indicated that most NAFLD patients experience CVD events in the long term[17-25].
Table 2.
Principal prospective studies of cardiovascular diseases mortality and morbidity risk in patients with nonalcoholic fatty liver disease (published in the past 10 years)
| Ref. | Study design | Study size | Diagnosis of NAFLD | Follow-up duration (yr) | Adjusted clinical variables | Major findings |
| Targher et al[17] | Prospective; Hospital-based | 2013 | Ultrasound | 6.5 | Gender, age, BMI, smoking status, diabetes duration, alcohol consumption, BP, HbA1c, TG, HDL, LDL cholesterol, GGT, use of medications (anti-hyperglycemic, antihypertensive, lipid-lowering, or anti-platelet drugs), and metabolic syndrome | NAFLD was independently associated with increased risk of nonfatal CVD events and CVD mortality |
| Hamaguch et al[18] | Prospective; Community-based | 1637 | Ultrasound | 5.0 | Gender, age, BMI, smoking, alcohol consumption, BP, TG, HDL, LDL cholesterol, MetS | NAFLD was independently associated with increased risk of nonfatal CVD events |
| Haring et al[19] | Prospective; Community-based | 4160 | Ultrasound | 7.3 | Gender, age, WC, diabetes, alcohol consumption, BP, physical activity, education level, civil status, equalized income, and Functional Co-morbidity Index | NAFLD was independently associated with increased risk of all-cause and CVD mortality in men |
| Wong et al[24] | Prospective; Hospital-based | 612 | Ultrasound | 1.8 | Gender, age, BMI, WC, smoking status, diabetes, alcohol consumption, BP, fasting glucose, ALT, TG, HDL, LDL cholesterol, creatinine | NAFLD was independently associated with an increased prevalence of CVD at baseline, but there was no significant association between NAFLD and risk of incident CVD events |
| Lazo et al[28] | Prospective; Population-based | 11371 | Ultrasound | 14.3 | Gender, age, ethnicity, BMI, education, smoking status, BP, alcohol consumption, physical activity, hypercholesterolemia diabetes | Independent increased risk of CVD but no significant association between NAFLD and all-cause and cause-specific (CVD, cancer and liver) mortality |
| Stepanova et al[22] | ||||||
| Zhou et al[20] | Prospective; Community-based | 3324 | Ultrasound | 4.0 | No adjustment made | Patients with NAFLD had about 3-fold higher rates of all-cause and CVD mortality than those without NAFLD |
| Treeprasertsuk et al[25] | Prospective; Community-based | 309 | Ultrasound/CT | 11.5 | Gender, age | Framingham risk score accurately predicted the higher 10-yr CAD risk in NAFLD patients and was the only variable significantly associated with the risk of developing new onset CVD events |
| Kim et al[23] | Prospective; Population-based | 11154 | Ultrasound and advanced fibrosis score systems | 14.5 | Gender, age, ethnicity, education, income, diabetes, BP, history of CVD, lipid-lowering medication, smoking status, WC, alcohol consumption, caffeine consumption, total and HDL cholesterol, transferrin saturation, and CRP | NAFLD was not associated with increased all-cause mortality. However, NAFLD with advanced hepatic fibrosis was independently associated with increased risk of all-cause mortality. Increase in mortality was almost entirely from CVD causes |
NAFLD: Nonalcoholic fatty liver disease; BMI: Body mass index; BP: Blood pressure; TG: Triglycerides; GGT: Gamma-glutamyl transferase; MetS: Metabolic syndrome; WC: Waist circumference; ALT: Alanine aminotransferase; CRP: C-reactive protein; CVD: Cardiovascular diseases; NASH: Nonalcoholic steatohepatitis.
EVIDENCE RELATING NAFLD TO CVD
NAFLD is linked to increased CVD risk; hence, it is critical to evaluate the markers of subclinical CVD and NAFLD. Subclinical atherosclerosis can be detected by several markers, such as increased carotid intima-media thickness, increased coronary artery calcification, impaired flow-mediated vasodilation and increased arterial stiffness, all of which have been investigated and strongly correlated with NAFLD, independently of classical CVD risk factors and MetS features[26].
Other factors associated with CVD found in NAFLD are endothelial dysfunction, impaired left ventricular (LV) function, epicardial fat, and clinical manifestations, such as obstructive sleep apnea syndrome and microangiopathic consequences[12].
CLINICAL AND SUBCLINICAL CVD AND NAFLD MARKERS
CVD risk in patients with NAFLD is related to increased carotid intima media thickness (IMT), which is a characteristic of subclinical atherosclerosis and an important predictor of myocardial infarction, and the presence of carotid atherosclerotic plaques[12,27-30]. The strong relationship between increased IMT and NAFLD has been confirmed in several studies indicating that NAFLD predicts an increased carotid IMT independently of traditional cardiometabolic risk factors[12,31-36].
Salvi et al[37] have evaluated the relationship between NAFLD and subclinical vascular damage and have found that NAFLD was associated with MetS in 48% of the cases. In the same paper, IMT values were strongly related to the number of metabolic syndrome factors.
In a study by Targher et al[38]; the severity of liver histology in NAFLD is strongly correlated with initial carotid atherosclerosis, independently of classical risk factors, thus indicating that the degree of liver damage may be important in atherosclerosis progression. Subclinical cardiovascular disease is shown to be closely associated with the severity of liver disease[38,39].
Arterial stiffness is an independent marker of symptomatic CVD and is measured by pulse wave velocity[40]. It indicates a loss of arterial elastic properties, which is linked to increased pulse pressure, LV hypertrophy, subendocardial ischemia, endothelial dysfunction and cardiac fibrosis[41]. Several studies have reported a strong association between NAFLD and impaired arterial elastic characteristics[36,37,40,42-44]. Furthermore, Lee et al[45] have found an independent association between arterial stiffness and the risk of NAFLD regardless of established CVD risk factors.
Circulatory endothelial dysfunction is one of the earliest-appearing markers of atherosclerosis. Disfunction is defined as brachial artery flow-mediated dilatation, and is significantly lower in patients with NAFLD compared with non-steatotic controls, thus indicating that NAFLD is associated with endothelial dysfunction, separately from MetS[44]. Villanova et al[46] have reported that NAFLD without MetS is strongly associated with increased carotid IMT.
The relationship between the degree of fatty liver severity and epicardial fat thickness has been described in obese NAFLD patients[12,47]. Epicardial fat correlates with MetS, abdominal visceral adiposity, atherosclerosis and CAD[12,48-50]. As a source of vasoactive products and cytokines, it may regulate coronary arterial tone and influence CAD, atrial fibrillation, systolic dysfunction and heart failure[12,51-55]. Cardiac steatosis has been found to be a strong predictor of LV diastolic dysfunction in a study where subjects with a higher intra-hepatic fat content had a significantly higher myocardial fat content[2,56].
Coronary artery calcification (CAC) ascertained by computed tomography is considered to be an independent marker of CVD risk[57]. A study conducted in 2007 in subjects with MetS identified an independent association of NAFLD with CAC[58]. Similar results have been reported by several other researchers[59-61].
NAFLD is also correlated with impaired LV diastolic dysfunction. Goland et al[62] were the first to report that NAFLD patients, in the absence of obesity, hypertension, and T2DM, have altered LV geometry and features of LV diastolic dysfunction. Fallo et al[63] have reported that NAFLD is significantly associated with insulin resistance and LV diastolic function, thus suggesting a parallel increase in metabolic and cardiac risk.
On the basis of the above findings, NAFLD may be an independent contributor to the development of atherosclerosis and other structural and functional cardiac alterations, which subsequently lead to clinical CVD and potentially an increased risk of congestive heart failure (HF).
Similar results have been shown in two large population-based cohort studies (more than 7000 subjects) in which serum liver enzyme-diagnosed NAFLD has been found to be independently associated with an increased incidence of HF[64-66].
Atrial fibrillation (AF) is one of the consequences of diastolic dysfunction. Few studies have examined the relationship between NAFLD and risk of incident AF in subjects with T2DM. NAFLD has been shown to be associated with an increased risk of AF, independently of gender, age, hypertension, LV hypertrophy and PR interval[12,67,68]. Additionally, a prospective study of nearly 1000 subjects has reported that NAFLD is an independent risk factor of AF[69].
NAFLD is also associated with prolonged QTc interval (strong predictor of ventricular arrhythmias and sudden cardiac death[70,71]) in T2DM even after adjusting for multiple known risk factors, which is a viable interpretation of the increased CVD mortality associated with NAFLD[2,72].
Echocardiographic findings of aortic valve sclerosis have been linked with NAFLD, independently of established CVD risk factors, in both diabetic and non-diabetic individuals[2,73,74]. Moreover, the ultrasonographic severity of NAFLD and LV diastolic dysfunction are significantly associated.
PATHOPHYSIOLOGICAL MECHANISMS LINKING NAFLD AND CVD
Although several well-established common risk factors explain the association between CVD and NAFLD, the precise pathophysiological mechanisms underlying this complex relationship are still unclear. Numerous pathophysiological mechanisms link these two diseases, including oxidative stress, atherogenic dyslipidemia, subclinical inflammation, insulin resistance, endothelial dysfunction and an abnormal adipokine profile, as shown in Figure 1[12].
Figure 1.
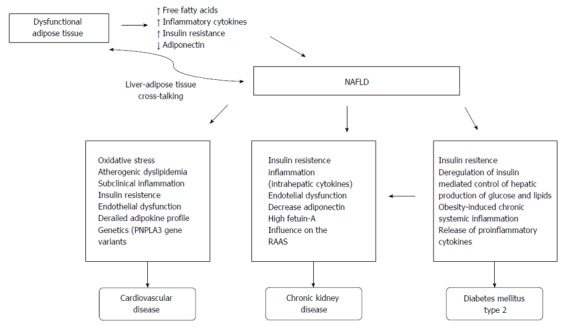
Pathophysiological mechanisms linking obesity, nonalcoholic fatty liver disease, cardiovascular disease, chronic kidney disease and diabetes mellitus type 2. NAFLD: Nonalcoholic fatty liver disease; RAAS: Renin-angiotensin-aldosterone system.
Many studies have shown that components of oxidative stress (possibly initiated by NAFLD) may be involved in the pathogenesis of CVD and increase CVD risk[75-78]. In patients with NAFLD, high levels of triglycerides and low levels of HDL-C have been observed, which indicates atherogenic dyslipidemia[12,79]. Atherogenic dyslipidemia in conjunction with insulin resistance and abdominal visceral adiposity constitutes the major factor involved in accelerated atherosclerosis[12,80-82].
Hepatic accumulation of lipids causes subacute liver inflammation[12]. Atherosclerosis has been correlated with liver damage, with increased levels in patients with simple steatosis and especially in NASH patients[12]. Additionally, patients with NAFLD have increased levels of circulating endothelial progenitor cells (EPC), such levels are associated with the severity of NAFLD. According to recent manuscript, these cells may play a role in the early natural history of NAFLD. EPC might be increased in an attempt to repair the endothelial damage resulting from metabolic alterations accompanying NAFLD[83].
Insulin resistance is a crucial contributor to NAFLD, MetS and atherosclerosis. Increased liver fat content appears to be the best independent predictor of IR in skeletal muscle, adipose tissue, and the liver[84,85]. Hence, with more advanced stages of NAFLD, liver fat is progressively increased, thus increasing the risk of CVD. NAFLD may act as a stimulus for further increased whole-body IR and dyslipidemia (with a typical overproduction of triglyceride and cholesterol-rich remnant particles), thereby leading to increased atherosclerosis[84,86].
Reduced adiponectin secretion, which has anti-atherogenic, anti-inflammatory and anti-fibrotic characteristics, in conjunction with increased leptin secretion, which has the opposite effects, promotes progression of fatty steatosis to NASH and CVD[12]. Additionally, TNF-a and IL-6 released from the abdominal visceral fat influence mitochondrial and endothelial dysfunction and increase free fatty acids levels, which contribute to atherosclerosis and CAD progression[12,87-89].
NAFLD AND CVD GENETICS
The underlying genetic bases of both NAFLD and CAD have been widely studied, and recenly reviewed by Li et al[90]. The authors have reviewed the literature on gene polymorphisms of the leptin receptor (LEPR), apolipoprotein C3 (APOC3), adiponectin-encoding gene (ADIPOQ), peroxisome proliferator-activated receptors (PPAR), sterol regulatory element-binding proteins (SREBP), microsomal triglyceride transfer protein (MTTP), transmembrane 6 superfamily member 2 (TM6SF2), tumor necrosis factors-alpha (TNF-α) and manganese superoxide dismutase (MnSOD), all of which have been linked to NAFLD and CAD.
Most consistently reported gene is patatin-like phospholipase-3 (PNPLA3, known as adiponutrin)[12]. Petta et al[91] have evaluated carotid atherosclerosis in NAFLD patients and have reported that the PNPLA3 GG genotype is associated with a higher severity of carotid atherosclerosis in younger subjects with NAFLD. PNPLA3 gene variants may increase lipid storage in the arterial walls and also induce release of ICAM-1, an endothelium-derived inflammatory marker that has been associated with myocardial infarction and stroke[12,92].
Key considerations
NAFLD is an independent contributor to CVD progression and is associated with an enhanced risk of cardiovascular disease, regardless of the established risk factors. The risk of CVD mortality may be greater in subgroups with a necro-inflammatory form of NAFLD (e.g., NASH) and advanced fibrosis, compared with subgroups with simple steatosis. Given the incidence of these diseases, it is of clinical and scientific interest to further elucidate the underlying mechanisms of liver progression to prevent CVD complications. NAFLD patients should be screened for CVD diseases, as has been proposed by many authors. Further studies should demonstrate how modification of the underlying liver disease progression may affect CVD outcome, to determine whether NAFLD treatment, and consequently the reversion of liver disease, can lower the incidence of CVD.
DIABETES MELLITUS AND NAFLD
Epidemiology of diabetes and NAFLD
T2DM is a multi-factorial disease, in which the body no longer properly responds to physiological insulin concentrations, generally as a result of chronic hypernutrition and obesity. Hepatic IR, in addition to chronic dyslipidemia, appears to be a key underlying mechanism of NAFLD[93]. Therefore, NAFLD and DM often occur within the same patient. Epidemiological studies have shown that 18%-33% of individuals with NAFLD also have T2DM and as many as 66%-83% of those with fatty liver disease have some form of insulin resistance[94-96]. In a study in which NAFLD patients were subjected to two-step hyperinsulinemic euglycemic clamp analysis all subjects with NAFLD were shown to be insulin resistant[97].
T2DM is a key risk factor for the development of severe liver diseases[95]. NAFLD patients with T2DM, compared with people without this disease, have a 2.6-fold higher risk of developing NASH[98]. This risk further increases with increasing body weight. The prevalence of NASH development in obese T2DM patients with NAFLD may be as high as 40%, whereas it is less than 5% in people without T2DM[99]. T2DM is linked to an accelerated rate of liver fibrosis progression[100] and strongly increases the risk of developing cirrhosis[101]. Miele and colleagues have shown that T2DM patients, compared with controls, have an almost threefold higher risk of developing hepatocellular carcinoma[102]. As a result, T2DM increases the risk of liver-related death in NAFLD patients by up to 22-fold[103].
Finally, a large prospective study has shown that NAFLD increased the chances of developing nonfatal coronary heart disease, ischemic stroke, or cardiovascular death by more than 50% in T2DM patients[104]. It is still unknown how NAFLD promotes cardiovascular disease in T2DM, and pharmacological therapy that increases the risk of developing CVD should therefore be avoided in this patient group[105,106].
Pathophysiology of diabetes and NAFLD
Although IR appears to be essential for the development of both fatty liver disease and T2DM, an inability of the liver to sense insulin is by itself does not induce NAFLD. This is illustrated by the observation that liver disease is common but not universal in T1DM patients[107]. Consistently with the “multiple hit” theory, additional risk factors, such as obesity, poor glycemic control and age, are associated with development of NAFLD in individuals with T1DM[108].
A key event in the development of NAFLD appears to be the deregulation of insulin-mediated control of hepatic production of glucose and lipids. In healthy individuals, insulin impairs gluconeogenesis while promoting lipogenesis. In NAFLD, especially in the context of T2DM, a paradoxical situation occurs in that IR results in a reduced ability to inhibit gluconeogenesis, whereas insulin-driven lipogenesis still occurs and is even enhanced. These circumstances have led to the hypothesis that hepatic IR is partial, limiting the Akt signaling arm while still allowing for SREBP1c activation[109]. Indeed, animal studies have shown that hepatic elimination of the insulin receptor does not result in higher levels of triglycerides (TG) in the liver or in circulation[110]. Defects in Akt signaling result in increased gluconeogenesis, whereas impaired activation of PKCλ is associated with hypolipidemia and decreased expression of SREBP1c[111].
To assess the model of selective hepatic post-receptor insulin resistance in humans, Semple RK and colleagues have studied a number of patients with rare mutations in either the insulin receptor or in the protein kinase Akt2[112]. Patients with primary defects at the level of the insulin receptor did not have metabolic dyslipidemia. In contrast, patients with Akt2 mutations showed marked metabolic dyslipidemia with elevated fasting TG, high VLDL/TG/cholesterol ratios, low HDL cholesterol levels and high small dense LDL levels. Additionally, liver fat and hepatic lipogenesis were strongly increased in these patients.
A second factor that contributes to the aggravation of NAFLD in T2DM is obesity-induced chronic systemic inflammation, which originates in visceral adipose tissue[113]. When adipocytes become hypertrophic as a result of hypernutrition, their access to proper oxygenation is reduced[114], and they become physically restricted by the stromal matrix[115]. Hypertrophic adipocytes therefore induce expression of stress ligands on their cell membrane, thus activating the local immune system and driving a sterile inflammatory response in adipose tissue[116]. As obesity progresses, adipose tissue inflammation is aggravated, ultimately leading to the release of pro-inflammatory cytokines[116-118]. The increased amount of free fatty acids (FFAs) in circulation, caused by IR, stimulates FFA uptake by the liver, thus contributing to lipid accumulation and inflammation in the liver and development of NAFLD[119].
In summary, the clinical complications of T2DM and NAFLD are both dependent on IR. However, differences in secondary aggravating factors indicate that treatment of one disease will not necessarily result in improvement in the symptoms of the other (Figure 1).
Key considerations
NAFLD is associated with a two to five times higher risk for development of T2DM. Many authors have suggested screening for T2DM in patients with NAFLD by periodically performing simple laboratory tests. Additionally, NAFLD patients with T2DM have a 2.6-fold higher risk of developing NASH compared with patients without this disease[98]. This risk further increases with body weight. The prevalence of NASH development in obese T2DM patients with NAFLD may be as high as 40%, whereas it is less than 5% in people without T2DM[99]. T2DM is associated with an accelerated rate of liver fibrosis progression[100] and strongly increases the risk of developing cirrhosis[101]. In addition, T2DM is associated with a higher prevalence of hepatic malignancies in NAFLD patients. As a result, T2DM increases the risk of liver-related death in NAFLD patients by up to 22-fold. Future studies should determine which screening regimens for liver disease should be applied to which T2DM patients. Additionally, it is not clear to what extent NAFLD contributes to T2DM and whether improvement of its clinical parameters might lower the risk for development of T2DM. Moreover, it is not known whether treatment of NAFLD could improve glycemic control in NAFLD patients who have developed T2DM.
NONALCOHOLIC FATTY LIVER DISEASE AND CHRONIC KIDNEY DISEASE
The prevalence of chronic kidney disease (CKD) is approximately 4.3%-13% in the general population, but it is expected to increase[4]. Because there is a high death risk primarily associated with adverse cardiovascular events, less than half of CKD patients develop end-stage renal disease (ESRD). Patients with CKD stage three may benefit the most from early referral strategies. There are many well-known risk factors that contribute to the progression of CKD, such as obesity, arterial hypertension, and T2DM. Recognizing groups of patients at risk for CKD is a clinical challenge. Owing to high morbidity, mortality and healthcare costs related to CKD, many scientists have sought to elucidate the underlying cause of CKD, and identification of modifiable risk factors for CKD has attracted increasing attention. Recent data have shown that IR and MetS are related to an increased incidence of albuminuria and CKD. The strongest evidence for this pathogenic link is that arterial hypertension and T2DM are the major etiological factors of CKD. As shown by many authors, NAFLD and CKD are related to the same group of cardiometabolic risk factors, including MetS and its individual components, and they are both associated with an increased risk of incident CVD events. CKD and NAFLD have several common traditional and non-traditional cardiometabolic risk factors, including higher hemostatic and plasma factors, lower circulating insulin-like growth factor-1 (IGF-1) levels, hyperuricemia, oxidative stress biomarkers and endothelial dysfunction. Recent studies have shown a correlation between CKD and NAFLD: there is a significantly higher prevalence of CKD in patients with NAFLD/NASH compared with patients without NAFLD/NASH[1,3,4,120-124]. Thus, it is not surprising that the connection between NAFLD, IR and MetS suggests that NAFLD may predict the occurrence of CKD. Moreover, data from the United Network Organ Sharing database from 2002-2011 have revealed an increase in NASH-related cirrhosis after simultaneous liver-kidney transplantation[1,123,125]. It is difficult to determine a causal relationship between CKD and NAFLD, owing to similarity in traditional risk factors for NAFLD and CKD.
EPIDEMIOLOGICAL EVIDENCE LINKING NAFLD AND CKD
On the basis of earlier observations that approximately 90% of patients with NAFLD have more than one component of MS, and 35%-75% of patients meet the diagnostic criteria, it is not surprising that patients with NAFLD may also have decreased renal function[126]. To date, many cross-sectional and retrospective studies involving patients with and without diabetes have shown that the prevalence of CKD in patients with NAFLD is increasing. In the majority of studies, the relationship between CKD and NAFLD is independent of co-morbidities, such as MetS and IR[127-131]. CKD is defined by decreased estimated glomerular filtration rate (eGFR) and/or the presence of significant proteinuria. By detecting elevated liver enzymes, such as gamma-glutamyl transferase (GGT) or alanine aminotransferase (ALT), or by imaging techniques, primarily abdominal ultrasound, researchers diagnosed NAFLD. Notably, approximately half of the patients’ aminotransferase levels, which are used as a marker of liver damage, were normal. Accordingly, in patients with normal aminotransferase values, NAFLD and liver fibrosis cannot be excluded; thus, the results of these studies should be carefully interpreted[121,126-131]. Although it is an invasive technique with possible sampling mistakes, liver biopsy remains the “gold standard” in diagnosing and staging NAFLD[126]. In the literature, few studies have used liver biopsy for detection and grading of NAFLD in the context of CKD. Interestingly, patients from a study with histologically defined NASH had lower eGFR and higher prevalence of CKD or abnormal albuminuria than matched controls. A study by Targher et al[132] has shown that individuals with biopsy-confirmed NASH had moderately decreased eGFR and more frequent abnormal albuminuria and CKD than the matched control group. Additionally, the severity of NASH histology was related to decreased kidney function, independently of traditional risk factors, MetS components and IR. Yilmaz et al[133] have come to similar conclusions.
Our group has examined the association between NAFLD and decreased kidney function. We have used transient elastography (TE) with controlled attenuation parameter (CAP) for NAFLD detection. In patients with CKD stages 3 and 4, we have found a high prevalence of NAFLD. In this study, the severity of liver steatosis defined by CAP had a negative correlation with kidney function[134,135]. Because one third of the population has NAFLD, it is not possible to perform liver biopsy in such large groups. Therefore, TE with CAP may be a reasonable screening method for NAFLD, but further studies are needed.
Another interesting study by Sesti et al[124] has evaluated whether the severity of liver fibrosis estimated by NAFLD fibrosis score is related to the higher prevalence of CKD in patients with NAFLD. eGFR and NAFLD fibrosis scores were assessed in 570 patients with ultrasonography-diagnosed NAFLD. Subjects with a high probability of liver fibrosis had a 5.1-fold increased risk of having CKD (OR = 5.13, 95%CI: 1.13-23.28, P = 0.03) compared with individuals at a low probability after adjustment for gender, age and BMI. After adjustment for statin therapy, glucose tolerance status and anti-hypertensive treatment in addition to gender, patients with a high probability of liver fibrosis had a 3.9-fold increased risk of CKD (OR = 3.94, 95%CI: 1.11-14.05, P = 0.03) compared with individuals at a low probability.
A remaining question regarding the connection between CKD and NAFLD is whether NAFLD is only a risk marker or a pathogenic factor of CKD development. Several prospective studies have evaluated the relationship between NAFLD and the development of CKD. These studies have shown that NAFLD is independently associated with an increased risk of CKD and/or microalbuminuria; the HR was 1.49 to 4.38[136-139]. Similar conclusions have been reached retrospective study by Jia et al[140]. Unfortunately, no studies have assessed whether changes in NAFLD (either development of the severe form of NAFLD or resolution of NAFLD) modify the risk of incidence of CKD during the follow-up period[3].
In 2014, a meta-analysis of 33 studies was performed with a total of over two thousand participants and has confirmed the relationship between NAFLD and CKD[141]. In this meta-analysis, NAFLD has been found to be correlated with an increasing risk of prevalent (OR = 2.12, 95%CI: 1.69-2.66) and incident (HR = 1.79, 95%CI: 1.65-1.95) CKD. Notably, NASH was associated with a higher prevalence (OR = 2.53, 95%CI: 1.58-4.05) and incidence (HR = 2.12, 95%CI: 1.42-3.17) of CKD than simple steatosis. Furthermore, advanced fibrosis was correlated with a higher prevalence (OR = 5.20, 95%CI: 3.14-8.61) and incidence (HR = 3.29, 95%CI: 2.30-4.71) of CKD than non-advanced fibrosis[141,142].
MECHANISM LINKING NAFLD TO CKD
Because NAFLD and CKD share many significant cardiometabolic risk factors, it should come as no surprise that these two diseases are closely associated. It is crucial to understand the mechanism linking these two conditions because new strategies for CKD prevention or treatment and its associated co-morbidities may arise from novel insights into the underlying mechanism. The key questions regarding the link between these two conditions are (1) whether shared cardiometabolic risk factors are the basis of the association; (2) or whether NAFLD itself may, independently of shared cardiometabolic risk factors, contribute to CKD development; and (3) or whether the CKD development risk is dependent on the degree of liver disease in patients with NAFLD. These questions will determine whether the risk is increased with the severity of liver disease, such as NASH and/or liver fibrosis presence or development[74].
Lipid and glucose metabolism is predominantly regulated by the liver, which is also a major source of inflammatory mediators linked with the pathogenesis of CKD and CVD. IR is the key pathophysiological factor for the development and progression of NAFLD. In addition, IR has been attributed to the development of CKD. Obesity is a known independent risk factor for CKD. Similarly, MetS and obesity have been described as strong predictors for the development of NAFLD[121]. The secretion of numerous classical biomarkers of inflammation and endothelial dysfunction is partly dependent on factors that are unregulated in the presence of IR and the MetS, and are principally produced by the liver. Therefore, a link between the liver and the inflamed adipose tissue, which releases FFA and secretes several proinflammatory adipokines, is likely. IR is a major pathogenic factor in the development of NAFLD and its associated comorbidities because it increases FFA uptake in the liver. As mentioned above, IR is a possible mechanism that links NAFLD and CKD, because it is a risk factor for CKD[3]. When there is an increased influx of FFA, and chronic inflammation is present, the target and contributing factor in systemic inflammation is probably the liver. Therefore, the increased expression of intrahepatic cytokines mediated by hepatocellular damage and fat-derived factors may have a role in the progression of CKD in NAFLD[74,121,126,143].
Other pathophysiological mechanisms may be contributing factors in CKD development in patients with NAFLD. NAFLD patients, for example, have decreased plasma adiponectin levels, which are inversely correlated with the degree of NAFLD. An inverse correlation has also been found between adiponectin levels and proteinuria[3,74,144]. Ix et al[144] have explained this association several years ago. According to the authors, the mechanism that leads to NAFLD and CKD, occurring due to fat, liver and kidney communication, is established through two serum proteins: fetuin-A and adiponectin, whose serum levels are associated with key components of MetS, but in opposite directions. High serum levels of fetuin-A have been found in patients with NAFLD and CKD. Fetuin-A inhibits adiponectin synthesis and is also an inductor of inflammatory signaling and IR, which are major factors driving T2DM, CVD and CKD development[3,74,144].
Another “puzzle” believed to be a link between NAFLD and CKD is the renin-angiotensin system (RAS). The expression levels of all RAS components in hepatic stellate cells (HSC) in healthy liver are very low. As such, HSCs are normally unable to secrete angiotensin II (Ang II). When activated by hepatic injury, HSCs and other hepatic myofibroblasts express numerous components of the RAS, including receptor of angiotensin-converting enzyme and the angiotensin 1 (AT1) receptor. As a result, contraction and proliferation of HSCs via the AT1 receptor are induced because HSCs can synthesize Ang II. In the liver, IR and de novo lipogenesis are promoted by Ang II, as well as the production of proinflammatory cytokines. A range of fibrogenic actions (which includes cell migration, cell proliferation and collagen synthesis) is also stimulated by Ang II. The hypothesis that HSCs are activated and differentiated into myofibroblasts through Ang II promotion has been supported by recent studies. In addition, Ang II promotes inflammatory cytokine release, as well as extracellular matrix protein deposition in excessive amounts (Figure 1)[121,145].
Key considerations
A popular area of scientific research is the emerging evidence linking NAFLD and CKD. Many authors agree that NAFLD (in particular, biopsy-proven NASH) is associated with a greater prevalence of CKD, in spite of the limitations of the studies that investigated the association between NAFLD and CKD. Furthermore, NAFLD is associated with an increased risk of prevalence and incidence of CKD. Simple steatosis is associated with a lower prevalence and incidence of CKD than the necroinflammatory form of NAFLD (e.g., NASH). Additionally, non-advanced fibrosis is associated with a lower prevalence and incidence of CKD than advanced fibrosis. These data suggest that CKD is associated with the stage of liver disease. According to the available literature, it is still not clear whether NAFLD is only an indicator of increased risk of CKD or is actively involved in the pathogenesis of CKD. However, most authors support the hypothesis that NAFLD is one of the pathophysiological mechanisms in the development and progression of CKD. Certainly, further prospective research is necessary to determine whether NAFLD is only an indicator of CKD or is actively involved in the pathogenesis of CKD[3,4,74,121,122,146].
The mechanisms linking NAFLD and CKD have not been completely elucidated, but these conditions share common cardiometabolic risk factors and the final clinical consequence of increased CVD morbidity and mortality. The prevalence of NAFLD is continuously increasing in concert with the epidemic of its risk factors: obesity and MetS and its individual components. The increase in simultaneous liver-kidney transplantation as well as the significant cost linked with CKD in the NAFLD population indicate that this relationship is a worthwhile target for screening and therapeutic intervention[3,4,74,121,122,146].
We expect that in the future, there will be an increase in CKD incidence, given the association of NAFLD and CKD. Treatment modalities that will lower the comorbidity burden linked with NAFLD may consequently decrease the risk of CKD. Future research should determine whether the treatment of liver disease will ultimately prevent or delay the development and progression of CKD. Moreover, future studies regarding NAFLD treatment should include the renal function as one end-point.
The major risk factor that defines the prognosis of all chronic liver disease, including NAFLD, is fibrosis. To identify patients who require stringent clinical surveillance to prevent development or progression of both liver and renal complications, researchers can use noninvasive methods to predict fibrosis in subjects with NAFLD. Further studies to identify noninvasive markers for practical detection of NAFLD in large populations are needed. TE with CAP may be useful. On the basis of current knowledge about the association between CKD and NAFLD, patients with NAFLD should benefit from regular (annual) screening for CKD, which may include an analysis of simple laboratory parameters, such as the determination of serum creatinine and albuminuria. Then, physicians who manage those patients should not only focus on liver disease but also recognize extra-hepatic manifestations of NAFLD. Additionally, physicians who manage patients with impaired renal function should also screen patients for NAFLD[3,4,74,121,122,124,126,146].
NONALCOHOLIC FATTY LIVER DISEASE AND COLORECTAL CANCER
Colorectal cancer (CRC) is one of the most common tumors worldwide. Inflammatory bowel disease and genetic factors are well-known risk factors for the development of CRC. Recent studies have shown that 75%-95% of all colorectal carcinomas are not caused by genetic factors but are related to lifestyle factors. Preventive colonoscopy examinations, detection of precursors (polyps) and polypectomy are necessary to reduce the development of invasive cancer[147-151].
The search for new risk factors for developing colon polyps as well as colon cancer is of interest to many researchers. Numerous studies support the hypothesis that patients with MetS have an increased risk of CRC and that IR is associated with an increased risk of developing CRC. MetS occurs as a result of the modern lifestyle. This hypothesis is supported by studies showing that some of the factors of IR (insulin and insulin-like growth factor 1 and 2) promote the progression of precursor CRC (colon polyps) to invasive adenocarcinoma. Therefore, some authors believe that patients with diabetes should undergo frequent endoscopic examinations of the colon[148-151].
Studies conducted in recent years have demonstrated the importance of MS, IR and chronic inflammation in the pathogenesis of CRC. Additionally, decreased levels of adiponectin promote malignant alteration of cells in the colon. On the other hand, the primary result of NAFLD is increased production of different proinflammatory factors and procoagulant factors from an “inflamed liver”. As stated previously, patients with NAFLD have reduced levels of protective adipokines and adiponectin. Therefore, common factors among NAFLD, colon polyps and CRC include IR, chronic inflammation, oxidative stress and decreased levels of adiponectin[2,4,147,148,149,150,152]. In recent years, an increasing number of publications have identified NAFLD as an independent risk factor for the development of adenomatous polyps and CRC[147-153]. For example, Lee et al[152] have analyzed 5517 patients, 833 of whom had NAFLD detected by abdominal ultrasound. The authors have found that patients with NAFLD had twice the risk of adenomatous polyps and a three times greater risk of developing CRC. The importance of the increased risk for developing CRC in patients with NAFLD is discussed in a study by Wong et al[153], who have used histopathological analysis to detect NAFLD and have found that the presence of NAFLD was associated with an increased incidence of adenomatous polyps and precancerous lesions of the colon. Notably, the researchers have found that patients with simple steatosis were not at increased risk. The authors have concluded that the presence of NAFLD is associated with a high incidence of colorectal adenomas and high-grade colorectal carcinomas. The largest number of adenomas was localized in the right colon. The authors of this study strongly support screening for CRC in these high-risk patients.
Key considerations
Research conducted to date has suggested a possible association between NAFLD and the risk of adenoma polyp development and CRC, although they are very heterogeneous. Future studies are necessary to confirm the risk of CRC and adenomas in patients with NAFLD. Further research should determine whether screening for CRC is necessary in these patients and whether it is necessary for young patients with MetS and NAFLD. Additionally, future studies should determine whether patients with NAFLD require more frequent and earlier preventive colonoscopy examinations to reduce morbidity and mortality from CRC.
NONALCOHOLIC FATTY LIVER DISEASE AND PSORIASIS
Psoriasis is a chronic inflammatory immune-mediated skin disease, which is associated with various comorbidities. Its prevalence in is 2%-3% worldwide[154-157]. Currently, psoriasis has been proposed to be not only disease of the skin but also a complex condition with multisystem involvement. Moreover, psoriasis is linked with MetS: cardiometabolic risk factors (e.g., hypertension, obesity, T2DM and dyslipidemia) are more common in patients with psoriasis than in the general population, thus conferring an increased CVD risk. It is believed that the underlying low and persistent inflammatory status with increased levels of pro-inflammatory cytokines (TNF-α, IL-6) is responsible for the metabolic dysregulation and for the higher CVD risk in this skin disease. Furthermore, preliminary data have shown that psoriasis is related to pro-thrombotic conditions. To date, multiple studies have confirmed the association between NAFLD and chronic plaque psoriasis[154-158]. Recent studies have shown that the prevalence of NAFLD, diagnosed either by imaging or by histology, is higher in patients with psoriasis (with prevalence up to 50% of these patients) compared with controls. Psoriasis is associated with NAFLD even after taking into account MetS components and other potential confounding factors. Several researchers believe that psoriatic patients are more likely to develop advanced forms of NAFLD than non-psoriatic controls and that NAFLD patients have more severe psoriasis than those without it[158-162]. A recent meta-analysis by Candia et al[163] of seven case-control studies has found that psoriatic patients have a twofold higher rate of NAFLD compared with people without psoriasis. Among patients with more severe psoriasis or psoriatic arthritis, this risk is even higher. However, it is important to note that the cross-sectional design of the abovementioned studies cannot ascertain the temporality and causality of the relationship between NAFLD and psoriasis[158].
Given that NAFLD and psoriasis, have a strong connection with MetS, these findings are not surprising. A chronic inflammatory state is the major characteristic of NAFLD patients. Thus, a low, subchronic inflammatory state may serve as the connection between NAFLD and psoriasis. Psoriasis and obesity are directly related; psoriasis predicts the development of obesity and MetS. Therefore, NAFLD and psoriasis may be linked by obesity itself, which may contribute to the development of further MetS components and comorbidities. Consistently with this hypothesis is the earlier observation that excess adipose tissue results in unbalanced pro- and anti-inflammatory mediators and that stronger inflammatory stimulation is responsible for the initiation of the subchronic inflammatory state. The pathogenesis of both psoriasis and NAFLD depends on several cytokines, both proinflammatory (TNF-α, IL-6, leptin, visfatin) and anti-inflammatory (adiponectin) cytokines. Additionally, adiponectin levels are lower in patients affected by both NAFLD and psoriasis than in patients affected by psoriasis without NAFLD[154,155-158,164-168]. Another hypothesis is that NAFLD itself can contribute to the severity of psoriasis by releasing proinflammatory cytokines from the liver[154].
Key consideration
The available literature indicates that the prevalence of NAFLD is higher in patients with psoriasis (up to 50% of these patients), regardless of coexisting MetS symptoms. Additionally, patients with psoriasis have a higher risk for developing the more severe forms of NAFLD. Therefore, patients with chronic plaque psoriasis should be carefully monitored and evaluated for NAFLD. The cross-sectional design of this study cannot determine the temporality and causality of the association between NAFLD and psoriasis. More studies are needed in the future to determine whether NAFLD is only an epiphenomenon of the coexisting MetS features or is an independent risk factor for the development and progression of psoriasis. Until then, patients with psoriasis would benefit from frequent NAFLD and MetS screening to decrease the incidence of CVD events and liver-related complications. Further studies are also necessary to elucidate the pathophysiological mechanisms connecting NAFLD with psoriasis. It must be considered that some medications can be hepatotoxic and have negative effects on metabolic comorbidities (including NAFLD). The data on the relationship between use of TNF-α inhibitor and NAFLD are contradictory. Consequently, more studies should be performed to examine the influence of biological agents on histological features of NAFLD in psoriatic patients. Until then, patients with psoriasis may benefit from lifestyle interventions, such as diet and exercise, as well as from treatment of associated MetS components[154].
NONALCOHOLIC FATTY LIVER DISEASE, POLYCYCSTIC OVARIAN SYNDROME AND OBSTRUCTIVE SLEEP APNEA SYNDROME
Polycystic ovary syndrome (PCOS) affects approximately 5%-11% women, and it is one of the most common endocrine disorders. The most important feature of PCOS is clinical and/or laboratorial hyperandrogenism. Hyperinsulinemia is very common amongst women with PCOS. Insulin and luteinizing hormone act synergistically, stimulating ovarian theca cells to increase androgen synthesis, inhibiting synthesis of the steroid hormone binding globulin by the liver and increasing the amount of free and biologically active testosterone in circulation. Because IR is the characteristic of MetS, several reports have shown that women with PCOS are more likely to have MetS c[169-171]. Other studies have shown that women with PCOS, regardless of their body weight, have a higher risk for the development of NAFLD. The results by Gutierrez-Grobe Y[172] suggest that NAFLD is more prevalent in postmenopausal and women with PCOS than those premenopausal ones. They have concluded that estrogens may have a protective effect of against NAFLD in women. Published studies of the coherence between PCOS and NAFLD are still very rare. Further studies are needed to confirm the relationships between these conditions[4,169].
In the past few years, several experiments have suggested the possibility of a connection between chronic intermittent hypoxia of obstructive sleep apnea syndrome (OSAS) and NAFLD[4]. Musso et al[173] have included eighteen cross-sectional studies (2183 participants) in their meta-analysis. They have found a correlation between OSAS and increased risk for NAFLD, NAS and fibrosis. In this meta-analysis, the pooled odds ratios (ORs) of OSAS for the presence of NAFLD, as defined by histology, radiology, and AST or ALT elevation, were 2.01 2.99, 2.36 and 2.60, respectively. The pooled ORs of OSAS for NASH, fibrosis-any stage, or advanced fibrosis in biopsy-proven NAFLD patients were 2.37, 2.16 and 2.30, respectively. The authors have emphasized the need for screening OSAS patients for the presence and severity of NAFLD[172]. However, further studies are needed.
CONCLUSION
During the past decade, it has been confirmed that NAFLD is more than just a “liver disease”. The vast amount of evidence linking NAFLD with other extrahepatic diseases indicates that it is a multisystem disease. On the basis of the literature, the strongest evidence exists between NAFLD and CKD, CVD and T2DM. The biggest question regarding the association between NAFLD, CVD, CKD and T2DM is whether NAFLD is only a risk factor or a pathogenic factor for these conditions. Currently, most of the studies and their authors support the hypothesis that NAFLD is one of the pathophysiological mechanisms in the development and progression of CVD, CKD and T2DM, but further prospective studies are needed[2,4].
NAFLD is a public health problem that increases in magnitude on a daily basis. Owing to its numerous potential extrahepatic manifestations, most (sub-)specialists, and even primary care physicians, should be aware of this condition, especially now that many diagnostic methods are available for screening in clinical practice[2,4].
Another interesting question is whether it is possible to prevent the appearance or progression of CKD, CVD and T2DM by treating liver conditions (e.g., NAFLD). Theoretically, treatment modalities that minimize the comorbidities associated with NAFLD may consequently decrease the risk of developing CKD, CVD and T2DM. Therefore, future studies should determine whether treatment of the liver would preclude or postpone the development and advancement of CKD, CVD and T2DM[2,4].
Preliminary research has convincingly revealed a connection between NAFLD with CRC and psoriasis. Further studies may determine whether screening for CRC is required in these patients and whether screening for CRC at young patients with MS (NAFLD) is necessary. These studies should answer the question of whether patients suffering from NAFLD require earlier preventive colonoscopy to decrease the mortality and morbidity due to CRC[2,4].
Despite the cumulative evidence that NAFLD (specifically NASH and fibrosis) is an important risk factor for extrahepatic disease, current guidelines have yet to include any recommendations for screening for these complications. As discussed above, adequately powered and prospective studies using comparable inclusion criteria (e.g., NAFLD diagnosis) will be required to confirm the incidence risk of specific extrahepatic disease among different NAFLD populations. In addition, future studies will be necessary to evaluate the cost effectiveness and risk: benefit balance of screening. However, until the results of these studies and new guidelines for obligatory screening are obtained, we strongly believe that NAFLD patients would benefit from CVD, CKD and T2DM screening by using simple and easily accessible clinical and laboratory methods. For screening for T2DM, we recommend frequently-used laboratory analyses, such as measuring fasting and postprandial serum glucose values and determination of Hb1Ac levels. Serum creatinine analyses as well as urine analysis are adequate and simple methods of screening for CKD. Finally, periodic measurements (at least once a year) of arterial blood pressure, lipid profile determination, body weight measurement and determination of central obesity would be good screening methods for CVD. Additionally, information on smoking history and physical activity could be included. Given the morbidity and mortality caused by CRC, patients with NAFLD would probably benefit from assessment of various risk factors, such as positive family history, smoking, alcohol consumption, and the presence of T2DM and obesity, as well as frequent colon symptom analysis[2,4].
In summary, NAFLD is a disease that extends beyond the liver, negatively affecting chronic diseases and substantially affecting people’s health. Future treatment of patients with NAFLD should therefore account for the both the benefits and risks of comorbidities associated with this important disease.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Peer-review started: June 30, 2016
First decision: July 29, 2016
Article in press: October 19, 2016
P- Reviewer: Mendez-Sanchez N, Penkova-Radicheva MP, Ratnasari N S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Bang KB, Cho YK. Comorbidities and Metabolic Derangement of NAFLD. J Lifestyle Med. 2015;5:7–13. doi: 10.15280/jlm.2015.5.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638–652. doi: 10.1053/j.ajkd.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174–1197. doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 7.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190–1200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 10.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 12.Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306–13324. doi: 10.3748/wjg.v20.i37.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 14.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 15.Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, McCullough A, Goodman Z, Younossi ZM. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2013;58:3017–3023. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 16.Dunn MA, Behari J, Rogal SS, O’Connell MR, Furlan A, Aghayev A, Gumus S, Saul MI, Bae KT. Hepatic steatosis in diabetic patients does not predict adverse liver-related or cardiovascular outcomes. Liver Int. 2013;33:1575–1582. doi: 10.1111/liv.12285. [DOI] [PubMed] [Google Scholar]
- 17.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50:1403–1411. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YJ, Li YY, Nie YQ, Huang CM, Cao CY. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis. 2012;13:153–160. doi: 10.1111/j.1751-2980.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 21.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, Chim AM, Yu CM, Yu J, Chan FK, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 25.Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945–950. doi: 10.1111/j.1478-3231.2011.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 27.Cobble M, Bale B. Carotid intima-media thickness: knowledge and application to everyday practice. Postgrad Med. 2010;122:10–18. doi: 10.3810/pgm.2010.01.2091. [DOI] [PubMed] [Google Scholar]
- 28.Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke. 2001;32:830–835. doi: 10.1161/01.str.32.4.830. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 30.Engström G, Melander O, Hedblad B. Carotid intima-media thickness, systemic inflammation, and incidence of heart failure hospitalizations. Arterioscler Thromb Vasc Biol. 2009;29:1691–1695. doi: 10.1161/ATVBAHA.109.193490. [DOI] [PubMed] [Google Scholar]
- 31.Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009;204:521–525. doi: 10.1016/j.atherosclerosis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Ramilli S, Pretolani S, Muscari A, Pacelli B, Arienti V. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol. 2009;15:4770–4774. doi: 10.3748/wjg.15.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammadi A, Sedani HH, Ghasemi-Rad M. Evaluation of carotid intima-media thickness and flow-mediated dilatation in middle-aged patients with nonalcoholic fatty liver disease. Vasc Health Risk Manag. 2011;7:661–665. doi: 10.2147/VHRM.S26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colak Y, Karabay CY, Tuncer I, Kocabay G, Kalayci A, Senates E, Ozturk O, Doganay HL, Enc FY, Ulasoglu C, et al. Relation of epicardial adipose tissue and carotid intima-media thickness in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24:613–618. doi: 10.1097/MEG.0b013e3283513f19. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Bi Y, Xu M, Ma Z, Xu Y, Wang T, Li M, Liu Y, Lu J, Chen Y, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol. 2012;32:2321–2326. doi: 10.1161/ATVBAHA.112.252957. [DOI] [PubMed] [Google Scholar]
- 37.Salvi P, Ruffini R, Agnoletti D, Magnani E, Pagliarani G, Comandini G, Praticò A, Borghi C, Benetos A, Pazzi P. Increased arterial stiffness in nonalcoholic fatty liver disease: the Cardio-GOOSE study. J Hypertens. 2010;28:1699–1707. doi: 10.1097/HJH.0b013e32833a7de6. [DOI] [PubMed] [Google Scholar]
- 38.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325–1330. doi: 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- 39.Kucukazman M, Ata N, Yavuz B, Dal K, Sen O, Deveci OS, Agladioglu K, Yeniova AO, Nazligul Y, Ertugrul DT. Evaluation of early atherosclerosis markers in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2013;25:147–151. doi: 10.1097/MEG.0b013e32835a58b1. [DOI] [PubMed] [Google Scholar]
- 40.Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, Aznaouridis K, Archimandritis A, Stefanadis C. Increased arterial stiffness and impaired endothelial function in nonalcoholic Fatty liver disease: a pilot study. Am J Hypertens. 2010;23:1183–1189. doi: 10.1038/ajh.2010.144. [DOI] [PubMed] [Google Scholar]
- 41.Quinn U, Tomlinson LA, Cockcroft JR. Arterial stiffness. JRSM Cardiovasc Dis. 2012;1:pii: cvd.2012.012024. doi: 10.1258/cvd.2012.012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Işilak Z, Aparci M, Kardeşoğlu E, Yiğiner O, Uz O, Sildiroglu O, Ozmen N, Yalçin M, Cingözbay BY, Cebeci BS. Abnormal aortic elasticity in patients with liver steatosis. Diabetes Res Clin Pract. 2010;87:44–50. doi: 10.1016/j.diabres.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Fotbolcu H, Yakar T, Duman D, Ozden K, Karaahmet T, Tigen K, Kurtoglu U, Dindar I. Aortic elastic properties in nonalcoholic fatty liver disease. Blood Press Monit. 2010;15:139–145. doi: 10.1097/MBP.0b013e328339e2c8. [DOI] [PubMed] [Google Scholar]
- 44.Catena C, Bernardi S, Sabato N, Grillo A, Ermani M, Sechi LA, Fabris B, Carretta R, Fallo F. Ambulatory arterial stiffness indices and non-alcoholic fatty liver disease in essential hypertension. Nutr Metab Cardiovasc Dis. 2013;23:389–393. doi: 10.1016/j.numecd.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, Lee HR. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:196–203. doi: 10.1007/s10620-011-1819-3. [DOI] [PubMed] [Google Scholar]
- 46.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 47.Iacobellis G, Barbarini G, Letizia C, Barbaro G. Epicardial fat thickness and nonalcoholic fatty liver disease in obese subjects. Obesity (Silver Spring) 2014;22:332–336. doi: 10.1002/oby.20624. [DOI] [PubMed] [Google Scholar]
- 48.Granér M, Siren R, Nyman K, Lundbom J, Hakkarainen A, Pentikäinen MO, Lauerma K, Lundbom N, Adiels M, Nieminen MS, et al. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98:1189–1197. doi: 10.1210/jc.2012-3190. [DOI] [PubMed] [Google Scholar]
- 49.Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, Cha S, Stepanek J, Hurst RT. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339–345. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Cikim AS, Topal E, Harputluoglu M, Keskin L, Zengin Z, Cikim K, Ozdemir R, Aladag M, Yologlu S. Epicardial adipose tissue, hepatic steatosis and obesity. J Endocrinol Invest. 2007;30:459–464. doi: 10.1007/BF03346328. [DOI] [PubMed] [Google Scholar]
- 51.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 52.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, Haluzikova D, Bosanska L, Vokurka M, Svacina S, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 53.Baker AR, Harte AL, Howell N, Pritlove DC, Ranasinghe AM, da Silva NF, Youssef EM, Khunti K, Davies MJ, Bonser RS, et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab. 2009;94:261–267. doi: 10.1210/jc.2007-2579. [DOI] [PubMed] [Google Scholar]
- 54.Nakazato R, Dey D, Cheng VY, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Min JK, Berman DS. Epicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery disease. Atherosclerosis. 2012;221:422–426. doi: 10.1016/j.atherosclerosis.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Khawaja T, Greer C, Chokshi A, Chavarria N, Thadani S, Jones M, Schaefle K, Bhatia K, Collado JE, Shimbo D, et al. Epicardial fat volume in patients with left ventricular systolic dysfunction. Am J Cardiol. 2011;108:397–401. doi: 10.1016/j.amjcard.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 56.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 57.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, Nasir K. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 58.Santos RD, Nasir K, Orakzai R, Meneghelo RS, Carvalho JA, Blumenthal RS. Relation of uric acid levels to presence of coronary artery calcium detected by electron beam tomography in men free of symptomatic myocardial ischemia with versus without the metabolic syndrome. Am J Cardiol. 2007;99:42–45. doi: 10.1016/j.amjcard.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 59.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 60.Chen CH, Nien CK, Yang CC, Yeh YH. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci. 2010;55:1752–1760. doi: 10.1007/s10620-009-0935-9. [DOI] [PubMed] [Google Scholar]
- 61.Mirbagheri SA, Rashidi A, Abdi S, Saedi D, Abouzari M. Liver: an alarm for the heart? Liver Int. 2007;27:891–894. doi: 10.1111/j.1478-3231.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 62.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–955. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 63.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, Ermani M, Catena C, Soardo G, Di Piazza L, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–653. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–1745. doi: 10.3748/wjg.v20.i7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhingra R, Gona P, Wang TJ, Fox CS, D’Agostino RB, Vasan RS. Serum gamma-glutamyl transferase and risk of heart failure in the community. Arterioscler Thromb Vasc Biol. 2010;30:1855–1860. doi: 10.1161/ATVBAHA.110.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wannamethee SG, Whincup PH, Shaper AG, Lennon L, Sattar N. Γ-glutamyltransferase, hepatic enzymes, and risk of incident heart failure in older men. Arterioscler Thromb Vasc Biol. 2012;32:830–835. doi: 10.1161/ATVBAHA.111.240457. [DOI] [PubMed] [Google Scholar]
- 67.Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, Zoppini G, Mantovani W, Barbieri E, Byrne CD. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8:e57183. doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Targher G, Mantovani A, Pichiri I, Rigolon R, Dauriz M, Zoppini G, Morani G, Vassanelli C, Bonora E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond) 2013;125:301–309. doi: 10.1042/CS20130036. [DOI] [PubMed] [Google Scholar]
- 69.Käräjämäki AJ, Pätsi OP, Savolainen M, Kesäniemi YA, Huikuri H, Ukkola O. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study) PLoS One. 2015;10:e0142937. doi: 10.1371/journal.pone.0142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 71.Robbins J, Nelson JC, Rautaharju PM, Gottdiener JS. The association between the length of the QT interval and mortality in the Cardiovascular Health Study. Am J Med. 2003;115:689–694. doi: 10.1016/j.amjmed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Pichiri I, Mantovani A, Zoppini G, Bonora E, Barbieri E, et al. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;24:663–669. doi: 10.1016/j.numecd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Bonapace S, Valbusa F, Bertolini L, Pichiri I, Mantovani A, Rossi A, Zenari L, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with aortic valve sclerosis in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e88371. doi: 10.1371/journal.pone.0088371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Targher G, Chonchol M, Zoppini G, Abaterusso C, Bonora E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J Hepatol. 2011;54:1020–1029. doi: 10.1016/j.jhep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Bhatia LS, Curzen NP, Byrne CD. Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol. 2012;27:420–428. doi: 10.1097/HCO.0b013e328354829c. [DOI] [PubMed] [Google Scholar]
- 76.Liu H, Lu HY. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol. 2014;20:8407–8415. doi: 10.3748/wjg.v20.i26.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krivis AF, Rabb JM. Cuprous complexes formed with isonicotinic hydrazide. Science. 1969;164:1064–1065. doi: 10.1126/science.164.3883.1064. [DOI] [PubMed] [Google Scholar]
- 78.Polimeni L, Del Ben M, Baratta F, Perri L, Albanese F, Pastori D, Violi F, Angelico F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J Hepatol. 2015;7:1325–1336. doi: 10.4254/wjh.v7.i10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227:429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nobili V, Alkhouri N, Bartuli A, Manco M, Lopez R, Alisi A, Feldstein AE. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr Res. 2010;67:665–670. doi: 10.1203/PDR.0b013e3181da4798. [DOI] [PubMed] [Google Scholar]
- 81.Alkhouri N, Carter-Kent C, Elias M, Feldstein AE. Atherogenic dyslipidemia and cardiovascular risk in children with nonalcoholic fatty liver disease. Clin Lipidol. 2011;6:305–314. doi: 10.2217/clp.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.da Luz PL, Favarato D, Faria-Neto JR, Lemos P, Chagas AC. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (Sao Paulo) 2008;63:427–432. doi: 10.1590/S1807-59322008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gutiérrez-Grobe Y, Gavilanes-Espinar JG, Masso-Rojas FA, Sánchez-Valle V, Páez-Arenas A, Ponciano-Rodríguez G, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Metabolic syndrome and nonalcoholic fatty liver disease. The role of endothelial progenitor cells. Ann Hepatol. 2013;12:908–914. [PubMed] [Google Scholar]
- 84.Brea A, Puzo J. Non-alcoholic fatty liver disease and cardiovascular risk. Int J Cardiol. 2013;167:1109–1117. doi: 10.1016/j.ijcard.2012.09.085. [DOI] [PubMed] [Google Scholar]
- 85.Akın L, Kurtoglu S, Yikilmaz A, Kendirci M, Elmalı F, Mazicioglu M. Fatty liver is a good indicator of subclinical atherosclerosis risk in obese children and adolescents regardless of liver enzyme elevation. Acta Paediatr. 2013;102:e107–e113. doi: 10.1111/apa.12099. [DOI] [PubMed] [Google Scholar]
- 86.Hashizume H, Sato K, Yamazaki Y, Horiguchi N, Kakizaki S, Mori M. A prospective study of long-term outcomes in female patients with nonalcoholic steatohepatitis using age- and body mass index-matched cohorts. Acta Med Okayama. 2013;67:45–53. doi: 10.18926/AMO/49256. [DOI] [PubMed] [Google Scholar]
- 87.Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 89.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 90.Li XL, Sui JQ, Lu LL, Zhang NN, Xu X, Dong QY, Xin YN, Xuan SY. Gene polymorphisms associated with non-alcoholic fatty liver disease and coronary artery disease: a concise review. Lipids Health Dis. 2016;15:53. doi: 10.1186/s12944-016-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petta S, Valenti L, Marchesini G, Di Marco V, Licata A, Cammà C, Barcellona MR, Cabibi D, Donati B, Fracanzani A, et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One. 2013;8:e74089. doi: 10.1371/journal.pone.0074089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paré G, Ridker PM, Rose L, Barbalic M, Dupuis J, Dehghan A, Bis JC, Benjamin EJ, Shiffman D, Parker AN, et al. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet. 2011;7:e1001374. doi: 10.1371/journal.pgen.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol. 2011;7:456–465. doi: 10.1038/nrendo.2011.72. [DOI] [PubMed] [Google Scholar]
- 94.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 95.López-Velázquez JA, Silva-Vidal KV, Ponciano-Rodríguez G, Chávez-Tapia NC, Arrese M, Uribe M, Méndez-Sánchez N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014;13:166–178. [PubMed] [Google Scholar]
- 96.Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 97.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 98.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 99.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 100.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, Angulo P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–1573. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miele L, Bosetti C, Turati F, Rapaccini G, Gasbarrini A, La Vecchia C, Boccia S, Grieco A. Diabetes and Insulin Therapy, but Not Metformin, Are Related to Hepatocellular Cancer Risk. Gastroenterol Res Pract. 2015;2015:570356. doi: 10.1155/2015/570356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 104.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22:1354–1358. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 105.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 106.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;(1):CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707–712. doi: 10.2337/dc06-1982. [DOI] [PubMed] [Google Scholar]
- 108.Leeds JS, Forman EM, Morley S, Scott AR, Tesfaye S, Sanders DS. Abnormal liver function tests in patients with Type 1 diabetes mellitus: prevalence, clinical correlations and underlying pathologies. Diabet Med. 2009;26:1235–1241. doi: 10.1111/j.1464-5491.2009.02839.x. [DOI] [PubMed] [Google Scholar]
- 109.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 110.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 112.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 114.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 115.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wensveen FM, Jelenčić V, Valentić S, Šestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Štimac D, Wunderlich FT, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 117.Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 118.Wensveen FM, Valentić S, Šestan M, Wensveen TT, Polić B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin Immunol. 2015;27:322–333. doi: 10.1016/j.smim.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 119.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Almeda-Valdes P, Aguilar-Olivos N, Uribe M, Méndez-Sánchez N. Common features of the metabolic syndrome and nonalcoholic fatty liver disease. Rev Recent Clin Trials. 2014;9:148–158. doi: 10.2174/1574887109666141216103908. [DOI] [PubMed] [Google Scholar]
- 121.Marcuccilli M, Chonchol M. NAFLD and Chronic Kidney Disease. Int J Mol Sci. 2016;17:562. doi: 10.3390/ijms17040562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Musso G, Tabibian JH, Charlton M. Chronic kidney disease (CKD) and NAFLD: time for awareness and screening. J Hepatol. 2015;62:983–984. doi: 10.1016/j.jhep.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 123.Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- 124.Sesti G, Fiorentino TV, Arturi F, Perticone M, Sciacqua A, Perticone F. Association between noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver disease. PLoS One. 2014;9:e88569. doi: 10.1371/journal.pone.0088569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014;98:216–221. doi: 10.1097/TP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 126.Orlić L, Mikolasevic I, Bagic Z, Racki S, Stimac D, Milic S. Chronic kidney disease and nonalcoholic Fatty liver disease-is there a link? Gastroenterol Res Pract. 2014;2014:847539. doi: 10.1155/2014/847539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Casoinic F, Sâmpelean D, Bădău C, Prună L. Nonalcoholic fatty liver disease--a risk factor for microalbuminuria in type 2 diabetic patients. Rom J Intern Med. 2009;47:55–59. [PubMed] [Google Scholar]
- 128.Hwang ST, Cho YK, Yun JW, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Rhee EJ, et al. Impact of non-alcoholic fatty liver disease on microalbuminuria in patients with prediabetes and diabetes. Intern Med J. 2010;40:437–442. doi: 10.1111/j.1445-5994.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- 129.Ahn AL, Choi JK, Kim MN, Kim SA, Oh EJ, Kweon HJ, Cho DY. Non-alcoholic Fatty Liver Disease and Chronic Kidney Disease in Koreans Aged 50 Years or Older. Korean J Fam Med. 2013;34:199–205. doi: 10.4082/kjfm.2013.34.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manco M, Ciampalini P, DeVito R, Vania A, Cappa M, Nobili V. Albuminuria and insulin resistance in children with biopsy proven non-alcoholic fatty liver disease. Pediatr Nephrol. 2009;24:1211–1217. doi: 10.1007/s00467-009-1134-9. [DOI] [PubMed] [Google Scholar]
- 131.Targher G, Kendrick J, Smits G, Chonchol M. Relationship between serum gamma-glutamyltransferase and chronic kidney disease in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001-2006. Nutr Metab Cardiovasc Dis. 2010;20:583–590. doi: 10.1016/j.numecd.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 132.Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5:2166–2171. doi: 10.2215/CJN.05050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, Ozdogan O, Duman D, Imeryuz N, Avsar E, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism. 2010;59:1327–1330. doi: 10.1016/j.metabol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 134.Mikolasevic I, Racki S, Bubic I, Jelic I, Stimac D, Orlic L. Chronic kidney disease and nonalcoholic Fatty liver disease proven by transient elastography. Kidney Blood Press Res. 2013;37:305–310. doi: 10.1159/000350158. [DOI] [PubMed] [Google Scholar]
- 135.Barshop NJ, Francis CS, Schwimmer JB, Lavine JE. Nonalcoholic fatty liver disease as a comorbidity of childhood obesity. Ped Health. 2009;3:271–281. doi: 10.2217/phe.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee DH, Jacobs DR, Gross M, Steffes M. Serum gamma-glutamyltransferase was differently associated with microalbuminuria by status of hypertension or diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2005;51:1185–1191. doi: 10.1373/clinchem.2004.045872. [DOI] [PubMed] [Google Scholar]
- 137.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–77. doi: 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 138.Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, Franchini M, Zoppini G, Muggeo M. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol. 2008;19:1564–1570. doi: 10.1681/ASN.2007101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang Y, Ryu S, Sung E, Woo HY, Oh E, Cha K, Jung E, Kim WS. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism. 2008;57:569–576. doi: 10.1016/j.metabol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 140.Jia G, Di F, Wang Q, Shao J, Gao L, Wang L, Li Q, Li N. Non-Alcoholic Fatty Liver Disease Is a Risk Factor for the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. PLoS One. 2015;10:e0142808. doi: 10.1371/journal.pone.0142808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghamar Chehreh ME, Vahedi M, Pourhoseingholi MA, Ashtari S, Khedmat H, Amin M, Zali MR, Alavian SM. Estimation of diagnosis and treatment costs of non-alcoholic Fatty liver disease: a two-year observation. Hepat Mon. 2013;13:e7382. doi: 10.5812/hepatmon.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kronenberg F. Emerging risk factors and markers of chronic kidney disease progression. Nat Rev Nephrol. 2009;5:677–689. doi: 10.1038/nrneph.2009.173. [DOI] [PubMed] [Google Scholar]
- 144.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Orlic L, Mikolasevic I, Lukenda V, Anic K, Jelic I, Racki S. Nonalcoholic fatty liver disease and the renin-angiotensin system blockers in the patients with chronic kidney disease. Wien Klin Wochenschr. 2015;127:355–362. doi: 10.1007/s00508-014-0661-y. [DOI] [PubMed] [Google Scholar]
- 146.Allen AM, Kim WR, Heimbach JK, Rule AD. Reply to: “Chronic kidney disease (CKD) and NAFLD: time for awareness and screening”. J Hepatol. 2015;62:984–985. doi: 10.1016/j.jhep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 147.Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis. J Gastrointest Oncol. 2014;5:440–446. doi: 10.3978/j.issn.2078-6891.2014.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin XF, Shi KQ, You J, Liu WY, Luo YW, Wu FL, Chen YP, Wong DK, Yuen MF, Zheng MH. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41:2989–2997. doi: 10.1007/s11033-014-3157-y. [DOI] [PubMed] [Google Scholar]
- 149.You J, Huang S, Huang GQ, Zhu GQ, Ma RM, Liu WY, Shi KQ, Guo GL, Chen YP, Braddock M, et al. Nonalcoholic fatty liver disease: a negative risk factor for colorectal cancer prognosis. Medicine (Baltimore) 2015;94:e479. doi: 10.1097/MD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stadlmayr A, Aigner E, Steger B, Scharinger L, Lederer D, Mayr A, Strasser M, Brunner E, Heuberger A, Hohla F, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med. 2011;270:41–49. doi: 10.1111/j.1365-2796.2011.02377.x. [DOI] [PubMed] [Google Scholar]
- 151.Hwang ST, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Won KH, Jin W. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol. 2010;25:562–567. doi: 10.1111/j.1440-1746.2009.06117.x. [DOI] [PubMed] [Google Scholar]
- 152.Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol. 2012;27:91–95. doi: 10.1111/j.1440-1746.2011.06816.x. [DOI] [PubMed] [Google Scholar]
- 153.Wong VW, Wong GL, Tsang SW, Fan T, Chu WC, Woo J, Chan AW, Choi PC, Chim AM, Lau JY, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60:829–836. doi: 10.1136/gut.2011.237974. [DOI] [PubMed] [Google Scholar]
- 154.Ganzetti G, Campanati A, Offidani A. Non-alcoholic fatty liver disease and psoriasis: So far, so near. World J Hepatol. 2015;7:315–326. doi: 10.4254/wjh.v7.i3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ganzetti G, Campanati A, Molinelli E, Offidani A. Psoriasis, non-alcoholic fatty liver disease, and cardiovascular disease: Three different diseases on a unique background. World J Cardiol. 2016;8:120–131. doi: 10.4330/wjc.v8.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Krueger G, Ellis CN. Psoriasis--recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol. 2005;53:S94–S100. doi: 10.1016/j.jaad.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 157.Rácz E, Prens EP. Molecular pathophysiology of psoriasis and molecular targets of antipsoriatic therapy. Expert Rev Mol Med. 2009;11:e38. doi: 10.1017/S146239940900129X. [DOI] [PubMed] [Google Scholar]
- 158.Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between Non-Alcoholic Fatty Liver Disease and Psoriasis: A Novel Hepato-Dermal Axis? Int J Mol Sci. 2016;17:217. doi: 10.3390/ijms17020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51:758–764. doi: 10.1016/j.jhep.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 160.Madanagobalane S, Anandan S. The increased prevalence of non-alcoholic fatty liver disease in psoriatic patients: a study from South India. Australas J Dermatol. 2012;53:190–197. doi: 10.1111/j.1440-0960.2012.00905.x. [DOI] [PubMed] [Google Scholar]
- 161.Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30:282–287. doi: 10.1111/jdv.13456. [DOI] [PubMed] [Google Scholar]
- 162.Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. 2015;40:722–727. doi: 10.1111/ced.12672. [DOI] [PubMed] [Google Scholar]
- 163.Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656–662. doi: 10.1111/jdv.12847. [DOI] [PubMed] [Google Scholar]
- 164.Gisondi P, Galvan A, Idolazzi L, Girolomoni G. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne) 2015;2:1. doi: 10.3389/fmed.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–E150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lestre S, Diamantino F, Veloso L, Fidalgo A, Ferreira A. Effects of etanercept treatment on lipid profile in patients with moderate-to-severe chronic plaque psoriasis: a retrospective cohort study. Eur J Dermatol. 2011;21:916–920. doi: 10.1684/ejd.2011.1548. [DOI] [PubMed] [Google Scholar]
- 167.Campanati A, Ganzetti G, Di Sario A, Damiani A, Sandroni L, Rosa L, Benedetti A, Offidani A. The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol. 2013;48:839–846. doi: 10.1007/s00535-012-0678-9. [DOI] [PubMed] [Google Scholar]
- 168.Feagins LA, Flores A, Arriens C, Park C, Crook T, Reimold A, Brown G. Nonalcoholic fatty liver disease: a potential consequence of tumor necrosis factor-inhibitor therapy. Eur J Gastroenterol Hepatol. 2015;27:1154–1160. doi: 10.1097/MEG.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Romanowski MD, Parolin MB, Freitas AC, Piazza MJ, Basso J, Urbanetz AA. Prevalence of non-alcoholic fatty liver disease in women with polycystic ovary syndrome and its correlation with metabolic syndrome. Arq Gastroenterol. 2015;52:117–123. doi: 10.1590/S0004-28032015000200008. [DOI] [PubMed] [Google Scholar]
- 170.Zueff LF, Martins WP, Vieira CS, Ferriani RA. Ultrasonographic and laboratory markers of metabolic and cardiovascular disease risk in obese women with polycystic ovary syndrome. Ultrasound Obstet Gynecol. 2012;39:341–347. doi: 10.1002/uog.10084. [DOI] [PubMed] [Google Scholar]
- 171.Brzozowska MM, Ostapowicz G, Weltman MD. An association between non-alcoholic fatty liver disease and polycystic ovarian syndrome. J Gastroenterol Hepatol. 2009;24:243–247. doi: 10.1111/j.1440-1746.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 172.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–409. [PubMed] [Google Scholar]
- 173.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]


