Abstract
This study examines the effects of manganese oxide octahedral molecular sieve chitosan microspheres (Fe3O4@OMS-2@CTS) on anaerobic and aerobic microbial communities during sewage biological treatment. The addition of Fe3O4@OMS-2@CTS (0.25 g/L) resulted in enhanced levels of operational performance for decolourization dye X-3B. However, degradation dye X-3B inhibition in the presence of Fe3O4@OMS-2@CTS was recorded as greater than or equal to 1.00 g/L. Illumina MiSeq high throughput sequencing of the 16 S rRNA gene showed that 108 genera were observed during the anaerobic process, while only 71 genera were observed during the aerobic process. The largest genera (Aequorivita) decreased from 21.14% to 12.65% and the Pseudomonas genera increased from 10.57% to 12.96% according to the abundance in the presence of 0.25 g/L Fe3O4@OMS-2@CTS during the anaerobic process. The largest Gemmatimonas genera decreased from 21.46% to 11.68% and the Isosphaerae genera increased from 5.8% to 11.98% according to the abundance in the presence of 0.25 g/L Fe3O4@OMS-2@CTS during the aerobic process. Moreover, the X-ray photoelectron spectroscopy results show that the valence states of Mn and Fe in Fe3O4@OMS-2@CTS changed during sewage biological treatment.
Metal oxide nanoparticles (NPs) have been widely applied in various industries (e.g., catalysis1, medicine2 and water/wastewater treatment3) and are among the most commonly used nanomaterials4. The excellent physiochemical properties of metallic/metal oxide NPs can be attributed to their high ratio of surface area to volume5. However, more metallic/metal oxide NPs are likely to accumulate in atmospheric, soil, or water environments. The toxic properties of metallic/metal oxide NPs can have adverse effects on human health and the environment6.
Metallic/metal oxide NPs can enter wastewater treatment plants (WWTPs) through discharge from various consumer products. Biological wastewater treatment methods have been employed for more than one hundred years7, and to date, they still maintain a prominent role in the environmental protection., Numerous bioreactor configurations had been operated for wastewater treatments, such as the widely used upflow anaerobic sludge blanket (UASB) and sequencing batch reactor (SBR)8. Both UASB and SBR had been successfully employed in wastewater treatment on many municipal and industrial fields for several decades9. Metallic/metal oxide NPs can have toxic effects on microorganisms within these biological wastewater treatment systems10. Photobacterium phosphoreum, a representative bacterium in aquatic environments, is inhibited by metallic/metal oxide NPs (e.g., Ag, TiO2, Fe3O4 and CeO2)11,12. Metallic/metal oxide NPs, namely, ZnO, Fe2O3, NiO, Cr2O3 and Co3O4, can affect microorganisms13,14. Antimicrobial mechanisms of the phototoxicity of nano-TiO2 involve nano-TiO2/bacteria surface interactions, the aggregation of nano-TiO2, and intrinsic photoactivity15.
Octahedral molecular sieve (OMS-2) NPs have extensive applications5 and present excellent organic dye decolourization capabilities in the presence of peroxymonosulfate (PMS)16. Unfortunately, OMS-2 catalysts cannot yet be applied, as it is difficult to recycle OMS-2 catalysts from solutions. Chitosan (CTS) is widely applied, as it is broadly available and inexpensive17,18. Currently, magnetic chitosan microspheres, which include combinations of magnetic nanoparticles with CTS, are of particular interest with respect to applications, as they are highly effective at separating and protecting magnetic nanoparticles from agglomeration19. However, the potential environmental impacts of manganese oxide octahedral molecular sieve chitosan microspheres (Fe3O4@OMS-2@CTS) are unknown. High-throughput sequencing methods (e.g., Illumina MiSeq high throughput sequencing) can be used to detect detailed information about microbial communities20. However, few studies have been conducted on the effects of OMS-2 NPs on efficiency and microbial communities of typical biological processes (UASB and SBR) during sewage treatment.
In this study, we examine the effects of Fe3O4@OMS-2@CTS on anaerobic and aerobic microbial communities during sewage biological treatment. First, the catalytic degradation of reactive brilliant red X-3B by Fe3O4@OMS-2@CTS was studied. Then, Fe3O4@OMS-2@CTS was added to anaerobic and aerobic sewage biological reactors to assess the degradation of reactive brilliant red X-3B. The anaerobic sewage biological reactor that was used was an upflow anaerobic sludge blanket (UASB), and the aerobic sewage biological reactor that was used had a sequencing batch reactor activated sludge process (SBR) mechanism. We applied the Illumina MiSeq sequencing technique to analyse the effects of NPs on anaerobic and aerobic microorganisms. Our observations serve as key data on the application of NPs in WWTPs.
Results and Discussion
Degradation of reactive brilliant red X-3B by Fe3O4@OMS-2@CTS /PMS during sewage biological treatment
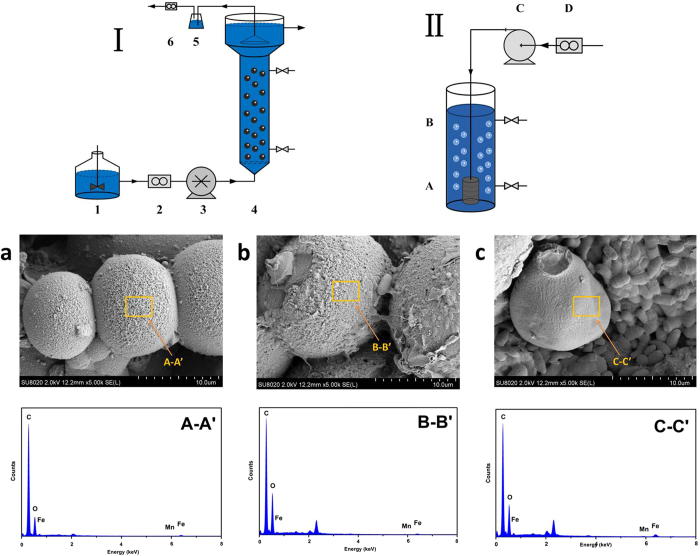
Liquid-phase reactions may be an efficient way to recover and recycle magnetic beads16. The magnetization curves of Fe3O4@OMS-2@CTS were measured, and the results are shown in Fig. S1 (Supplementary Information). The magnetization saturation value of pure Fe3O4 was measured at 107 emu/g, while that of Fe3O4@OMS-2@CTS was measured at approximately 18 emu/g. The SEM of Fe3O4@OMS-2@CTS is demonstrated in Fig. 1a (fresh material). The surface morphology of Fe3O4@OMS-2@CTS is clearly shown in Fig. S2 (a) of the Supplementary Information. A microporous and fibrous structure can be observed through section 1–1′ in Fig. S2 (1–1′). Figure 1b and c shows that the Fe3O4@OMS-2@CTS remained in the UASB reactor for 60 d and the SBR reactor for 60 d, respectively. In addition, EDS of Fe3O4@OMS-2@CTS through section A-A′ (Fig. 1 A-A′), B-B′ (Fig. 1 B-B′) and C-C′ (Fig. 1 C-C′) indicate that the main elements of Fe3O4@OMS-2@CTS are C and O due to the coating of chitosan, while Fe and Mn can also be detected (further confirmed by XPS analysis).
Figure 1. The first line:Schematic diagram of the upflow anaerobic sludge blanket (UASB) reactor device (I) and sequencing batch reactor activated sludge process (SBR) reactor device (II).
(I): 1 feed tank, 2 flow counter, 3 peristaltic pump, 4 reactor, 5 water-sealed bottle, and 6 wet gas flow metre (II): A aeration device, B reactor, C gas pump, D flow counter. The second line: (a) SEM image of fresh Fe3O4@OMS-2@CTS, (b) SEM image of used Fe3O4@OMS-2@CTS in UASB reactors, (c) SEM image of used Fe3O4@OMS-2@CTS in SBR reactors. The third line: A–A′. EDS spectra of fresh Fe3O4@OMS-2@CTS, B–B′. EDS spectra of used Fe3O4@OMS-2@CTS in UASB reactors, C–C′. EDS spectra of used Fe3O4@OMS-2@CTS in SBR reactors.
The XRD patterns of Fe3O4, CTS and Fe3O4@OMS-2@CTS were presented in Fig. S3 (a) of the Supplementary Information. The results of the FT-IR spectroscopy of OMS-2, Fe3O4, CTS and Fe3O4@OMS-2@CTS are shown in Fig. S3(b) of the Supplementary Information. Fe3O4@OMS-2@CTS exhibits as a core-shell structure, with chitosan acting as the shell and Fe3O4@OMS-2 as the core. Each component of Fe3O4@OMS-2@CTS played an important role in X-3B degradation: (1) Fe3O4 magnetizes the NPs for NP recovery, (2) OMS-2 was the primary catalytic site, which can activate PMS for X-3B degradation3, and (3) chitosan can first absorb X-3B onto the material for further degradation by OMS-2. Therefore, removal of X-3B was processed as two steps: X-3B was first absorbed by the microspheres of chitosan and then degraded by OMS-2/PMS. Without OMS-2 and PMS, chitosan can only absorb X-3B, but cannot degrade dyes, which has been confirmed by previous studies21,22,23.
The COD removal efficiency, decolourization ratio and COD balance levels of the UASB and SBR reactors are summarized in Table 1 for the steady operation stage. The data shown in Table 1 indicate that the UASB and SBR reactors were in good condition for dyeing wastewater processing. Although COD removal efficiencies were similar after addition of Fe3O4@OMS-2@CTS to both UASB and SBR, we found significantly different results on the decolourization ratio after adding Fe3O4@OMS-2@CTS to the UASB and SBR reactors. Figure 2a shows that the decolourization ratio in the UASB reactors could be enhanced at low concentrations of Fe3O4@OMS-2 @CTS (no more than 0.50 g/L), while the decolourization ratio can inhibit high concentrations of Fe3O4@OMS-2 @CTS (more than 0.50 g/L but no more than 2.00 g/L). The decolourization ratio in the UASB reactors was similar at concentrations of 0.25 g/L and 0.50 g/L, and the decolourization ratio in the UASB reactors was similar at concentrations of 1.00 g/L, 1.50 g/L and 0.50 g/L.
Table 1. COD removal efficiency, decolourization ratio and COD loading and effluent in different treatments.
| COD removal efficiency (%) | Decolourization ratio (%) | COD loading (g/L•d) | COD effluent (g/L•d) | |
|---|---|---|---|---|
| F1 (Control) | 87.61 ± 1.39 | 60.09 ± 0.81 | 4.0 | 0.49 |
| F2 (0.25 g/L) | 89.36 ± 1.43 | 64.63 ± 0.97 | 4.0 | 0.42 |
| F3 (0.50 g/L) | 91.79 ± 1.52 | 66.32 ± 0.93 | 4.0 | 0.33 |
| F4 (1.00 g/L) | 86.85 ± 1.36 | 58.02 ± 0.72 | 4.0 | 0.52 |
| F5 (1.50 g/L) | 86.41 ± 1.15 | 55.99 ± 0.81 | 4.0 | 0.54 |
| F6 (2.00 g/L) | 85.73 ± 1.26 | 56.51 ± 0.75 | 4.0 | 0.56 |
| G1 (Control) | 78.53 ± 1.27 | 79.10 ± 1.16 | 1.2 | 0.25 |
| G2 (0.25 g/L) | 85.82 ± 1.37 | 95.21 ± 1.28 | 1.2 | 0.16 |
| G3 (0.50 g/L) | 86.37 ± 1.32 | 95.30 ± 1.31 | 1.2 | 0.15 |
| G4 (1.00 g/L) | 84.54 ± 1.24 | 93.10 ± 1.42 | 1.2 | 0.18 |
| G5 (1.50 g/L) | 83.73 ± 1.21 | 84.81 ± 1.24 | 1.2 | 0.19 |
| G6 (2.00 g/L) | 82.31 ± 1.13 | 83.98 ± 1.19 | 1.2 | 0.20 |
Figure 2. The curve of decolourization ratio in different treatments.
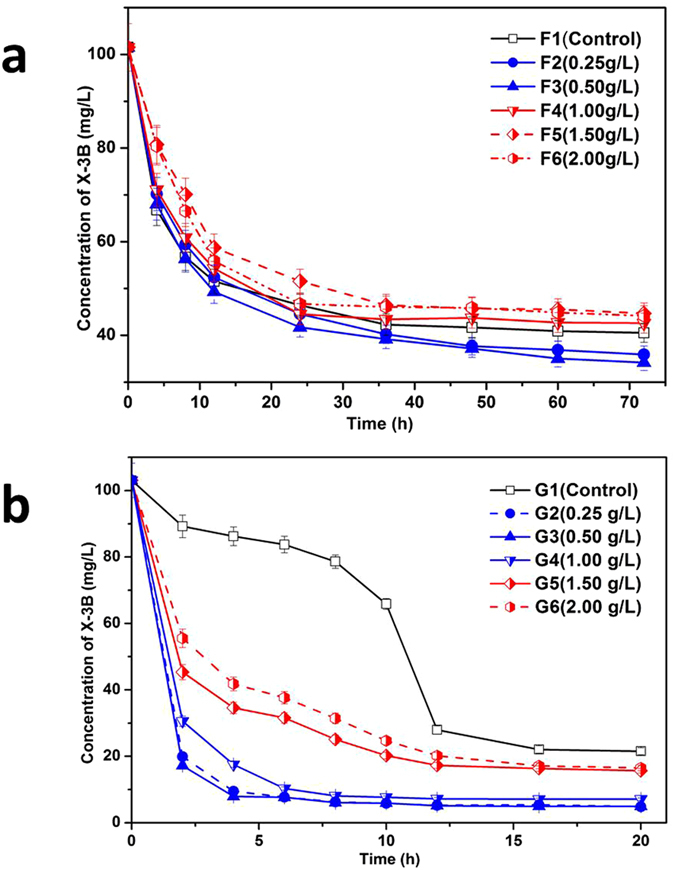
(a) Change of decolourization ratio in UASB reactors; (b) Change of decolourization ratio in SBR reactors.
However, the decolourization ratio in the SBR reactors would have been enhanced within the same Fe3O4@OMS-2 @CTS range (from 0.25 g/L to 2.00 g/L). Figure 2b shows that the maximum decolourization ratio occurred at a concentration of 0.50 g/L and that the minimum decolourization ratio occurred at a concentration of 2.00 g/L. All of the decolourization ratios with Fe3O4@OMS-2 @CTS (from 0.25 g/L to 2.00 g/L) were higher than the control value.
The different performances on decolourization ratio of these two bio-reactors are mainly due to their own characteristics. Both UASB and SBR reactors are important biological wastewater treatment technologies in WWTPs. UASB shows great resistances to treat wastewater containing biotoxicity compounds because of the dense structure of granular sludge24. The effect on the substrate in the UASB reactor should be small. However, SBR usually presents dynamic conditions, as the microbes in the reactor consistently vary along with the change of nutrients and essential elements25. After the addition of the material, significant enhancement on decolourization performance in SBR results from the following two reasons: (1) the activity of microbes in SBR was greatly enhanced due to the introduction of essential elements (Fe/Mn), and (2) the added material could also remove X-3B. This could explain the decolourization ratio result that indicates that the change of the decolourization ratio in UASB reactor was much less than that in SBR reactor with the same dosage of Fe3O4@OMS-2@CTS in both UASB and SBR reactors.
MiSeq-pyrosequencing results and microbial community structures
Figure 2 shows the results of the decolourization ratio, which indicated significant change in the different treatments. In UASB reactors, the decolourization ratios of F2 and F3 were similar, while the decolourization ratios of F4, F5 and F6 were similar. In SBR reactors, the decolourization ratios of G2, G3 and G4 were similar, while the decolourization ratios of G5 and G6 were similar. According to the decolourization ratio that was effected by the material dosage, samples collected from anaerobic (UASB-F1, UASB-F2 and UASB-F6) and aerobic reactors (SBR-G1, SBR-G2 and SBR-G6) were subjected to Illumina pyrosequencing.
Sequence information on the 6 samples is listed in Table 2. The Chao1/ace estimator and Simpson/Shannon diversity level are widely used microbial diversity indexes that reflect microbial phylotype richness levels and are calculated using a software mothur (http://www.mothur.org/) based on the sequence information. It is evident that Chao1 and Ace in the UASB were different from Chao1 and Ace in the SBR, demonstrating that Chao1 and Ace values of low concentration levels (0.25 g/L) of Fe3O4@OMS-2@CTS were higher than the control in the UASB and that the Chao1 and Ace of high concentrations (2.0 g/L) of Fe3O4@OMS-2@CTS were lower than the control. Both low (0.25 g/L) and high concentration (2.0 g/L) Fe3O4@OMS-2@CTS of the Chao1 and Ace were higher than the control in the SBR.
Table 2. Different microbial diversity indices in different treatments.
| Sample | Valid Sequence | Chao1 | Ace | Simpson | Shannon |
|---|---|---|---|---|---|
| F1 (Control) | 91102 | 1253 | 1282 | 0.066 | 4.005 |
| F2 (0.25 g/L) | 73983 | 1259 | 1289 | 0.058 | 4.067 |
| F6 (2.00 g/L) | 106594 | 1248 | 1254 | 0.048 | 4.061 |
| G1 (Control) | 97829 | 720 | 738 | 0.083 | 3.400 |
| G2 (0.25 g/L) | 83451 | 746 | 756 | 0.064 | 3.621 |
| G6 (2.00 g/L) | 81218 | 727 | 753 | 0.066 | 3.496 |
The behaviours and functions of bacteria may influence microbial activity and biomass, and rarefaction analyses can be used to compare and standardize the detected taxon richness levels between samples26. The rarefaction curves of samples in the UASB and SBR reactors are illustrated in Fig. S4 (Supplementary Information). We found similar patterns for the 3 samples in the UASB reactors and for the 3 samples in the SBR reactors. A Venn diagram that can be used to evaluate the distributions is presented in Fig. S5 (Supplementary Information). From the Venn diagram of samples in the UASB reactors, 1,149 OTU species occupied group UASB-F1 (Control), 1,048 OTU species occupied group UASB-F2 (0.25 g/L), and 1,163 OTU species occupied group UASB-F6 (2.0 g/L). The total richness level for all of the groups was measured at 1,321 OTUs. The Venn diagram shows that 820 OTUs containing 62.07% of the sequences were common among all of the samples in the UASB reactors.
The Venn diagram of the samples in the SBR reactors shows that 660 OUT species occupied group SBR-G1 (Control), 641 OTU species occupied group SBR-G2 (0.25 g/L), and 642 OTU species occupied group SBR-G6 (2.0 g/L). The total richness of all of the groups was 803 OTUs. The Venn diagram shows that 446 OTUs containing 54.79% of the sequences were common among all samples in the SBR reactors.
Taxonomic complexities of bacterial communities
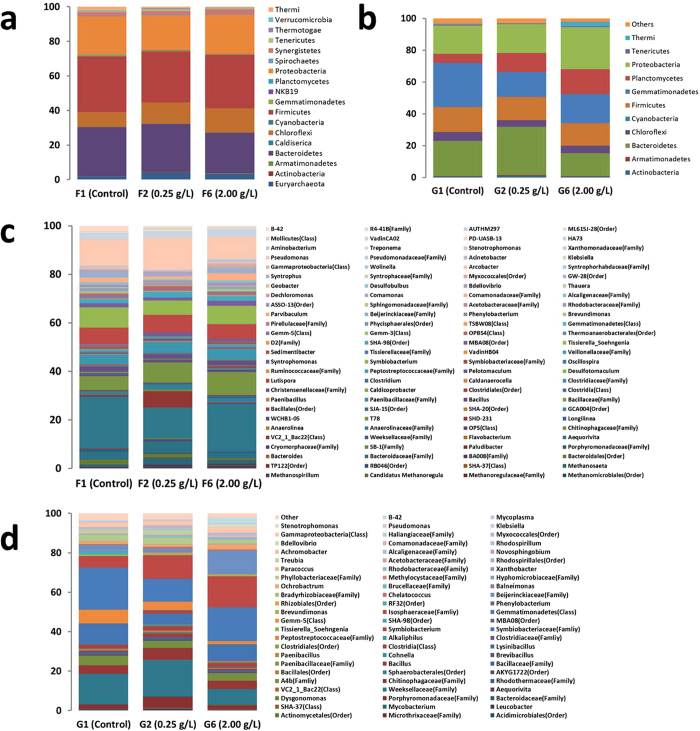
There were different abundance levels of bacteria associated with the 3 UASB reactors and 3 SBR reactors. The bacteria community abundance levels of the 3 UASB reactors and 3 SBR reactors at the phylum level are illustrated in Fig. 3a,b. We found 18 identified bacteria phyla from the 3 UASB reactors and 11 identified bacteria phyla from the 3 SBR reactors. Firmicutes, Bacteroidetes, Proteobacteria and Chloroflexi were the dominant phyla in bacteria from the 3 UASB reactors, and a change in the 4 dominant phyla was evident in the 3 UASB reactors. The Firmicutes percentages in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 32.27%, 29.53% and 30.76%, respectively. Percentages of Bacteroidetes in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were recorded at 28.37%, 27.73% and 23.55%, respectively. Percentages of Proteobacteria in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 28.37%, 27.73% and 23.55%, respectively. Percentages of Chloroflexi in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 8.51%, 12.41% and 14.23%, respectively. Thus, Firmicutes, Bacteroidetes, and Proteobacteria were weakened, while Chloroflexi was enriched during the Fe3O4@OMS-2@CTS treatments. Chloroflexi phylum is also a filamentous bacterium, thus potentially enhancing the anaerobic degradation of wastewater in a UASB27.
Figure 3. Relative read abundance of different bacterial community structure at phylum and genus levels in different treatments.
(a) Distribution of bacterial phyla (abundance ≥ 0.1%) in F1, F2 and F6 UASB reactors; (b) Distribution of bacterial phyla (abundance ≥ 0.1%) in G1, G2 and G6 SBR reactors; (c) Distribution of bacterial genera (abundance ≥ 0.1%) in F1, F2 and F6 UASB reactors; and (d) Distribution of bacterial genera (abundance ≥ 0.1%) in G1, G2 and G6 SBR reactors.
Gemmatimonadetes, Bacteroidetes, and Proteobacteria were the dominant bacteria phyla from the 3 SBR reactors, and changes in the 3 dominant phyla were also evident for the 3 SBR reactors. The percentages of Gemmatimonadetes in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 27.83%, 15.62% and 18.28%, respectively. The percentages of Bacteroidetes in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 22.22%, 30.33% and 14.48%, respectively. The percentages of Proteobacteria in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 17.72%, 18.12% and 26.37%, respectively; Gemmatimonadetes was weakened, while Proteobacteria was enriched during the Fe3O4@OMS-2@CTS treatments. Bacteroidetes was enriched in low concentrations and was weakened in high concentrations of Fe3O4@OMS-2 @CTS treatments. It has been reported that an increase in Bacteroidetes in microbiota is correlated with the alleviation of metabolic disorders, as it promotes microbial metabolism28. Proteobacteria was used as a sign of dysbiosis in bacterial communities29. Both Bacteroidetes and Proteobacteria could enhance biological removal of organic pollutants during SBR30.
The bacterial community abundance is analysed further at the genera level in Fig. 3c,d. We identified 108 genera in the bacteria from the 3 UASB reactors and 71 genera in the bacteria from the 3 SBR reactors. Pseudomonas, Aequorivita, Tissierella_Soehngenia, Sedimentibacter and T78 were the dominant phyla in the bacteria from the 3 UASB reactors, and a change in 5 dominant genera was found in the 3 UASB reactors.
The percentages of Pseudomonas in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 10.57%, 12.96% and 9.06%, respectively. The percentages of Aequorivita in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 21.14%, 12.65% and 19.55%, respectively. Pseudomonas and Aequorivita genera were also found in membrane bioreactors (MBRs)31. The percentages of Tissierella_Soehngenia in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 8.23%, 5.92% and 7.64%, respectively. The percentages of Sedimentibacter in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 7.01%, 7.35% and 6.11%. The percentages of T78 in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L) were measured at 5.79%, 8.37% and 9.47%, respectively. Thus, Pseudomonas, Sedimentibacter, Tissierella_Soehngenia and Aequorivita were weakened in the Fe3O4@ OMS-2@CTS treatments, while T78 was enriched in the Fe3O4@ OMS-2@CTS treatments.
Gemmatimonadetes (Class), Weeksellaceae (Family), Symbiobacteriaceae (Family), Isosphaeraceae (Family) and Gemm-5 (Class) were the dominant phyla in bacteria from the 3 SBR reactors, and changes in 5 dominant genera were found for the 3 SBR reactors. The percentages of Gemmatimonadetes (Class) in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 21.46%, 11.68% and 17.02%, respectively. The percentages of Weeksellaceae (Family) in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 15.36%, 18.38% and 7.90%, respectively. The percentages of Symbiobacteriaceae (Family) in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 10.48%, 5.58% and 8.51%, respectively. The percentages of Isosphaeraceae (Family) in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 5.80%, 11.98% and 15.80%, respectively. The percentages of Gemm-5 (Class) in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L) were measured at 6.82%, 4.16% and 1.42%, respectively. Thus, Gemmatimonadetes (Class), Symbiobacteriaceae (Family) and Gemm-5 (Class) were weakened in the Fe3O4@OMS-2@CTS treatments, while Isosphaeraceae (Family) was enriched in the Fe3O4@OMS-2@CTS treatments. Weeksellaceae (Family) was enriched in low concentrations and weakened in a high concentration of Fe3O4@OMS-2@CTS treatments.
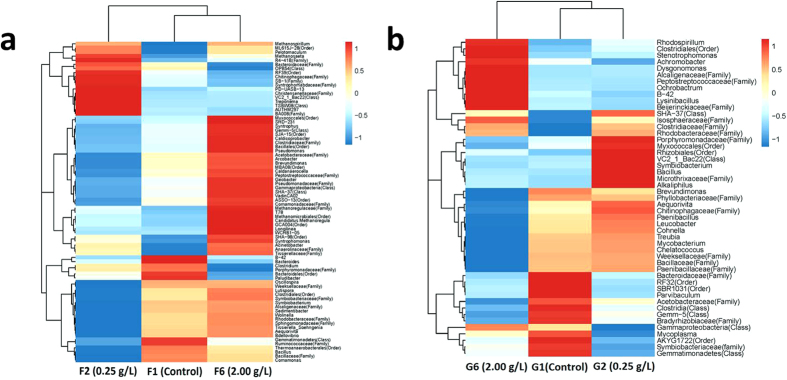
This microbial community heatmap can be applied to identify differences and similarities between bacterial communities in the UASB and SBR reactors (Fig. 4). As shown in Fig. 4a, 78 bacterial genera in UASB reactors were identified at the 0.2% abundance level. This shows that the bacterial community structures present clear differences in UASB-F1 (Control), UASB-F2 (0.25 g/L) and UASB-F6 (2.0 g/L). The genera were divided into three branches by species richness. From the top to the bottom of Fig. 4a, the first branch covers Methanospirillum to BA008 (Class) with 18 genera, the second branch covers Myxococcales (Order) to Tissierellaceae (Family) with 34 genera, and the third branch covers B-42 to Comamonas with 26 genera. According to Fig. 4a, the first branch was a high abundance branch in UASB-F2 (0.25 g/L), the second branch was a high abundance branch in UASB-F1 (Control), and the third branch was a high abundance branch in UASB-F6 (2.0 g/L).
Figure 4. Microbial community heatmap analysis in different treatment.
(a) Heat-map of the classified genera (abundance ≥ 0.2%) in F1, F2 and F6 UASB reactors; (b) Heat-map of the classified genera (abundance ≥ 0.2%) in G1, G2 and G6 SBR reactors.
From Fig. 4b, 49 bacterial genera in the SBR reactors were identified at the 0.2% abundance level. The results show that bacterial community structures presented clear differences in SBR-G1 (Control), SBR-G2 (0.25 g/L) and SBR-G6 (2.0 g/L). The genera were divided into three branches by species richness. From the top to the bottom of Fig. 4b, the first branch covers Rhodospirillum to Beijerinckiaceae (Family) with 11 genera; the second branch covers SHA-37 (Class) to Paenibacillaceae (Family) with 25 genera; and the third branch covers Bacteroidaceae (Family) to Gemmatimonadetes with 13 genera. According to Fig. 4b, the first branch was a high abundance branch in SBR-G6 (2.0 g/L), the second branch was a high abundance branch in SBR-G2 (0.25 g/L) and the third branch was a high abundance branch in SBR-G1 (Control).
Mechanism discussion
According to the previously described results, adding Fe3O4@OMS-2@CTS can affect UASB and SBR reactors, generally leading to enhanced X-3B decolourization ratio (Table 1 and Fig. 2). This is due to the microbial community in UASB and SBR changing after addition of Fe3O4@OMS-2 (Figs 3 and 4), i.e., a higher microbial diversity (Fig. 3) and change of predominant microorganisms in the reactors. Specifically, (1) the competing bacteria (e.g., Firmicutes, Bacteroidetes, and Proteobacteria in the UASB reactor; Gemmatimonadetes in the SBR reactor) were inhibited, contributing to the increase of microbial diversity; (2) the abundance of Chloroflexi bacteria in UASB reactor was enhanced, which promoted anaerobic degradation of wastewater27; (3) the increase of Proteobacteria bacteria in SBR reactor enhanced the biological removal of organic pollutants30 and Proteobacteria was used as a sign of dysbiosis in bacterial communities29; and (4) the abundance of Bacteroidetes bacteria was enriched in low concentrations and weakened in high concentrations of Fe3O4@OMS-2 @CTS treatments in the SBR reactor; the increase of Bacteroidetes bacteria could correlate with alleviation of metabolic disorders and promote microbial metabolism28. Compared to the control test without addition of material, this change on microbial community must be attributed to the NPs, which include: (1) NPs can incorporate into the mixed liquor suspended solids (MLSS) (containing the microbial community) and then change the microbial community; (2) bacteria could easily attach onto the microspheres of chitosan, and the effect of Fe3O4@OMS-2 would be enhanced; and (3) Fe and Mn originating from NPs could interact with the microbe, resulting in a reduction of Fe(III) and Mn(IV). Specifically, adding Fe3O4@OMS-2@CTS can enhance the decolourization ratio (not more than 0.50 g/L) and decrease it at high concentrations (more than 0.50 g/L and not more than 2.00 g/L) in UASB reactors. However, adding Fe3O4@OMS-2@CTS can enhance the decolourization ratio (not more than 2.00 g/L). This change in UASB and SBR reactors may result from the modulation of Fe3O4@OMS-2@CTS, which alters microbial communities in UASB and SBR reactors (Figs 3 and 4).
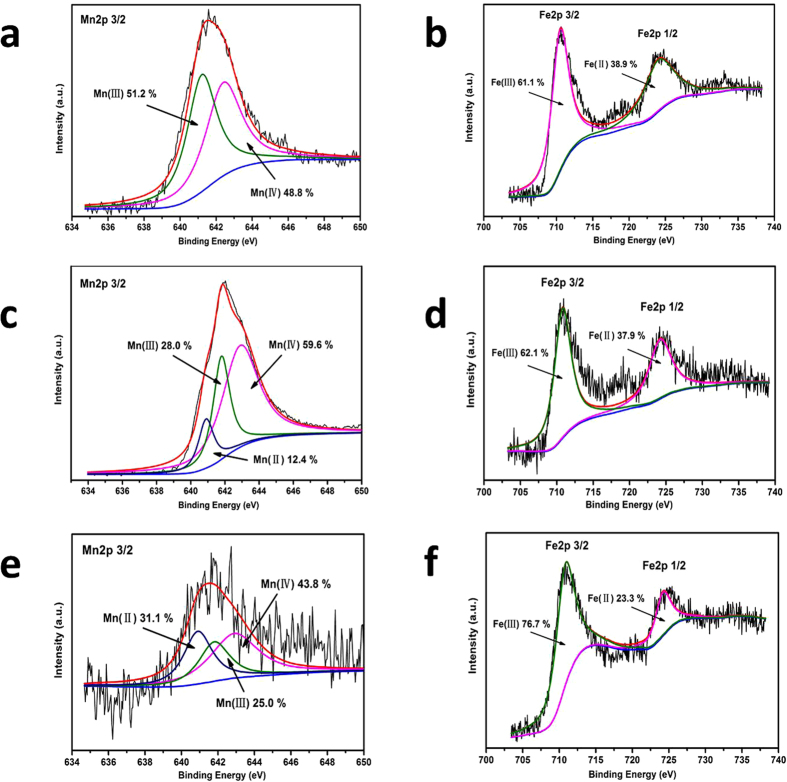
Fe3O4@OMS-2@CTS contains different oxidation states of manganese (e.g., Mn(IV), Mn(III) and Mn(II)) and oxidation states of iron (e.g., Fe(III) and Fe(II)). Microorganisms have the capacity for dissimilatory Fe(III) and Mn(IV) reduction, which involves transferring electrons to Fe(III) and Mn(IV)32. In addition, considering the extremely low concentrations of background Fe (0.5 μg/L) and Mn (1.88 μg/L) in the synthetic wastewater, Fe and Mn from NPs played a dominant role after material addition. Mn(III)/Mn(IV) oxide and Fe(III) oxide are relatively insoluble and stable, while Mn(II) oxide and Fe(II) oxide can be solubilized under acidic conditions. To investigate changes in Fe and Mn species in the Fe3O4@OMS-2@CTS, XPS spectra of Fe3O4@OMS-2@CTS were measured before and after the UASB and SBR experiments were conducted. For the Mn 2p3/2 spectra of Fe3O4@OMS-2@CTS, two peaks at 642.4 and 641.2 eV with a peak area ratio of 48.8:51.2 were found (Fig. 5a), and these may be designated Mn(III) and Mn(IV) oxidation states, respectively. The XPS spectra of Fe 2p are shown in Fig. 5b. We found two peaks centred at 710 and 724 eV indexed to Fe 2p3/2 and Fe 2p1/2 with a ratio of 61.1:38.9, which are consistent with the Fe(III) and Fe(II) oxidation states, respectively33.
Figure 5.
Changes of Fe3O4@OMS-2@CTS before and after in different treatments: (a) XPS spectra of Mn 2p of fresh Fe3O4@OMS-2@CTS; (b) XPS spectra of Fe 2p of fresh Fe3O4@OMS-2 @CTS; (c) XPS spectra of Mn 2p of used Fe3O4@OMS-2@CTS in UASB reactors; (d) XPS spectra of Fe 2p of used Fe3O4@OMS-2@CTS in UASB reactors; (e) XPS spectra of Mn 2p of used Fe3O4@OMS-2 @CTS in SBR reactors; and (f) XPS spectra of Fe 2p of used Fe3O4@OMS-2@CTS in SBR reactors.
After the UASB experiments were conducted, the third peak accredited to the Mn(II) oxidation states was identified in Fe3O4@OMS-2@CTS, and its atomic ratio was measured at 59.6:28.0:12.4 (Mn(IV): Mn(III): Mn(II)) (Fig. 5c). The atomic ratio of Fe(III) and Fe(II) in Fe3O4@OMS-2@CTS was measured at 62.1:37.9 (Fe(III): Fe(II)) (Fig. 5d) in UASB reactor, indicating almost no change of Fe states. However, after the SBR experiments were conducted, a third peak accredited to the Mn(II) oxidation states was identified in Fe3O4@OMS-2@CTS with an atomic ratio of 43.8:25.0:31.1 (Mn(IV): Mn(III): Mn(II)) (Fig. 5e). The atomic ratio of Fe(III) and Fe(II) in Fe3O4@OMS-2@CTS was measured at 76.7:23.3(Fe(III): Fe(II)) (Fig. 5f). The much higher Fe(III) ratio in SBR reactor was due to oxidation of Fe(II) by aeration, and interaction of Fe(II) with microorganism or metabolites.
The XPS results show that Mn(IV)/Mn(III) with redox were reduced to Mn(II) in both the UASB and SBR reactors, but Mn(IV)/Mn(III) with redox in the UASB reactors was less than that found in the SBR reactors. The XPS results also show a very small change in oxidation states of Fe(III)/Fe(II) in Fe3O4@OMS-2@CTS in the UASB reactors (only 2%) but a significant change in oxidation states of Fe(III)/Fe(II) in Fe3O4@OMS-2@CTS in the SBR reactors. Moreover, we should notice that XPS can only perform a surface analysis of the material; other characterizations such as X-ray absorption spectroscopy (XAS), extended X-ray absorption fine structure (EXAFS) and electron paramagnetic resonance (EPR) can be further conducted to deeply investigate the change of Mn/Fe during the reaction.
Mn(IV)/Mn(III) and Fe(III)/Fe(II) with redox offered reducing equivalents to the microorganisms and a broad variety of microorganisms can acquire energy from Mn(IV)/Mn(III) with redox. Emily et al. found that microorganisms, which were discovered from marine sediment in California, could utilize Mn(IV) and Fe(III) to oxidize methane and proved that Mn(IV) and Fe(III) could be used as electron acceptors in anaerobic oxidation of methane (AOM)34. Mukhopadhyay et al. reported that metal manganese blocked intracellular trafficking of Shiga toxin (STx) and affected its degradation in lysosome35.
Generally, the dissimilatory Mn(IV)-reducing microorganisms contain two major groups: (1) Mn(IV)-respiring microorganisms (FMR), which support growth by conserving energy from electron transfer to Mn(IV)5; and (2) non-Mn(IV)-respiring microorganisms (Non-FMR). FMR are widely dispersed throughout Bacteria and Archaea, which grow by oxidizing organic compounds, hydrogen or S0 associated with a reduction of Mn(IV)32. In this study, the addition of Fe3O4@OMS-2@CTS into the UASB and SBR reactors can promote FMR growth, such as Chloroflexi bacteria in UASB and Proteobacteria bacteria in SBR (Fig. 3). On the other hand, the terminal electron-accepting process (TEAP) of Mn(IV) reduction also occur in the non-FMR metabolic process, although they do not gain energy from the dissimilatory reduction of Mn(IV), e.g., Lactococcus spp36. Moreover, widespread reoxidation processes of Fe(II) are due to its biological utilization as an electron donor by aerobic bacteria37. Fe(II) in microorganisms plays a role in several key enzymatic activity, e.g., pyruvate-ferredoxin oxidoreductase, which plays a major role in fermentation38. Byrne et al. discovered that some microorganisms had the ability of utilization both Fe(III) and Fe(II) for growth and believed that Fe ions bound could be bioavailable as electron sources and electron sinks under different environment conditions. For example, Rhodopseudomonas palustris TIE-1 could oxidize magnetite nanoparticles by using light energy, while Geobacter sulfurreducens could reverse the process in co-cultures39. Microorganisms of both heterotrophic and autotrophic groups can turn nitrate into an electron acceptor40,41.
Conclusions
This study evaluated the effects of manganese oxide octahedral molecular sieve chitosan microspheres (Fe3O4@OMS-2@CTS) on anaerobic and aerobic microbial communities in typical sewage biological treatments, i.e., UASB and SBR. The decolourization ratio changes in the UASB reactors were found to be different from those observed in the SBR reactors. The microbial community changes in the UASB reactors were also different from those observed in the SBR reactors. Mn(IV)/Mn(III) and Fe(III)/Fe(II) with great redox were found in sewage biological treatments. This study provides basic information on the effect of nanoparticles on contaminant removal and microbial community change in the conventional biological wastewater treatment process. In the future, comparison on contaminants removal by pure nanoprocess, pure biological process, and the nanomaterial-enhanced biological process can be further investigated.
Methods
Preparation of Fe3O4@OMS-2@CTS catalyst
Fe3O4@OMS-2 was synthesized using a reflux method that is similar to a procedure reported elsewhere16. Mannich reaction methods were used to prepare the chitosan grafted ligand19. Further information on the characterization and synthesis of Fe3O4@OMS-2@CTS is provided in Text S1 (Supplementary Information).
Experimental setup
Six groups of anaerobic reactors (F1-F6) and six groups of aerobic reactors (G1-G6) were employed. The F1 of the anaerobic reactors and the G1 of the aerobic reactors formed the control group without the addition of Fe3O4@OMS-2@CTS. F2-F6 and G2-G6 formed the treatment groups with the addition of variable concentrations of Fe3O4@OMS-2@CTS. PMS (2KHSO5•KHSO4•K2SO4) was added to both the UASB and SBR operation and the dose of PMS was 0.125 g/L. Figure 1 presents a schematic diagram of the anaerobic and aerobic reactors. The anaerobic reactor was an upflow anaerobic sludge blanket (UASB), and the aerobic reactor employed a sequencing batch reactor activated sludge process (SBR). The parameters of UASB reactors and SBR reactors were listed in Text S2 (Supplementary Information).
Inoculated sludge and synthetic dyeing wastewater
Inoculated sludge was drawn from anaerobic and aerobic digesters of the sewage treatment plant based at Wuhan Textile University (Wuhan, China). The inoculated sludge was acclimated for 35 d to allow it to degrade X-3B dye. The inoculated sludge had a pH level of 6.5–7.2. The inoculated sludge included 4098 mg/L of mixed liquor suspended solids (MLSS) and 2665 mg/L of mixed liquor volatile suspended solids (MLVSS). The influent dyeing wastewater was synthesized with X-3B dye using the following stock solution: NH4HCO3 14.1 g/L, sucrose 90 g/L, K2HPO4•3H2O 1.62 g/L, KH2PO4 0.97 g/L and NaHCO3 33.3 g/L. The chemical oxygen demand (COD) level of the stock dyeing wastewater was measured at approximately 100 g/L. The desired COD concentration was then diluted with ingredients that had the following trace elements: CuSO4·5H2O 58, H3BO3 100, ZnSO4·7H2O 100, FeSO4·7H2O 750, NiSO4·6H2O 112, MnSO4·H2O 188, MgSO4·7H2O 5000, NaMo7O24·2H2O, 50, FeSO4·7H2O 50, CaCl2·2H2O 7500, and yeast 3000 μg/L. These were added before the stock dyeing wastewater was prepared for experimentation.
Analytical Methods
Degradation of reactive brilliant red X-3B by Fe3O4@OMS-2@CTS /PMS
All of the catalytic degradation experiments were performed in a 100-mL reactor at approximately 30 °C. Fe3O4@OMS-2@CTS was added to the reactor after adding desired amounts of X-3B and PMS (2KHSO5•KHSO4•K2SO4, Aladdin) in 50 mL of the aqueous solution. The reaction solution was repeatedly stirred with a magnetic stirrer that was coated with PTFE. Further information on degradation of X-3B by Fe3O4@OMS-2@CTS/PMS is listed in Text S3 and Fig. S6 (Supplementary Information).
Degradation of reactive brilliant red X-3B by Fe3O4@OMS-2@CTS /PMS during sewage biological treatment
The UASB reactors were first operated with COD of synthetic dyeing wastewater loading from 100 to 4,000 g/m•d for two months for the pre-acclimation of anaerobic microbiology. The COD values of both influents and effluents in all of the UASB reactors were monitored using standard methods. The UASB reactors were then operated over 4 phases, i.e., start-up (0–72 d), X-3B loading increase (73–121 d), recovery (122–140 d) and stable operation (141–219 d). On Day 149, Fe3O4@OMS-2@CTS of 0.25, 0.50, 1.00, 1.50 and 2.00 g/L were added to the F2–F6 reactors, and F1 served as the control group wherein no Fe3O4@OMS-2@CTS was spiked. The experimental schedule for COD and X-3B loadings of UASB is shown in Table S1 of the Supplementary Information section. The operation temperature of the UASB reactors was maintained at (30 ± 2 °C), the hydraulic retention time (HRT) was set to 18 ± 2 h, and the average cell residence time ranged from 20 to 40 d. On Day 209, samples were collected from the 6 groups of UASB reactors.
Freshly activated sludge was inoculated at a concentration of 4,000 ± 81 mg/L (MLSS) in the 6 SBR reactors. Fe3O4@OMS-2@CTS volumes of 0.25, 0.50, 1.00, 1.50 and 2.00 g/L combined with PMS (0.125 g/L) were also added to G2–G6 in the SBR reactors, with G1 serving as the control group wherein no Fe3O4@OMS-2@CTS was spiked. The detailed operating parameters for the 6 SBR reactors are listed in Table S2 of the Supplementary Information section.
Synthetic X-3B dyeing wastewater was continuously pumped through the SBR column. The flow rate of the SBR influent was set at 2.8 ± 0.1 mL/min. The 6 SBR reactors (G1–G6) were operated sequentially over 12 h cycles that consisted of 11.5 h of aeration time, 10 min of influent filling, 10 min of effluent withdrawal and 10 min of settling. The hydraulic residence time (HRT) was set to 20 h, and the exchange volume ratio discharged from the SBR reactors was set to 60%. There was a bubble aerator in the bottom of the SBR column, and the air flow rate was set to 1.5 L/min. The operating temperature of all of the SBR reactors was set to 30 ± 2 °C. On Day 60, the samples were collected from the SBR reactor.
The morphology characterization of Fe3O4@OMS-2@CTS in the UASB and SBR reactors was subjected to SU8020 Ultra-High-Resolution FE-SEM (Hitachi, Tokyo, Japan). The X-ray photoelectron spectroscopy of Fe3O4@OMS-2@CTS was conducted using a Thermo ESCALAB 250XI Multifunctional imaging electron spectrometer (Thermo Fisher Scientific, Waltham, USA). The anaerobic and aerobic microorganism genera were identified via Illumina MiSeq high-throughput sequencing.
Illumina high-throughput sequencing
The microbial DNA of liquor samples drawn from the anaerobic and aerobic reactors was isolated using an E.Z.N.A. Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA). The microbial DNA extracts were then stored at −20 °C for PCR amplification. The universal V4 regions of the 16 S rRNA genes primers were set to 520 F (5′-AYTGGGYDTAAAGNG-3′) and 802 R (5′-TACNVGGGTATCTAATCC-3′)5. The extracted DNA barcode and adapter with tags were added between the adapters and forward primers. Detailed information on PCR amplification is shown in Text S4 of Supplementary Information. The amplicons of PCR were then detected by 0.8% agarose gels with a loading level of 3 μL. The electrophoresis results of all of the amplicons were satisfactory with clear strap (Fig. S7, Supplementary Information). The multiplexed DNA libraries were homogenized to 10 nM and mixed at equal volumes. The mixed DNA libraries were then progressively and quantitatively diluted to 4–5 pM and then subjected to paired-end sequencing (2 × 250) on an Illumina MiSeq platform.
The obtained raw fastq were demultiplexed and quality-filtered via Qiime (version 1.7.0, http://qiime.org/) and Mothur (version 1.31.2, http: //www. mothur. org/) to obtain high-quality sequences for subsequent analysis. Operational Taxonomic Units (OTUs) were also clustered with 97% similarity using Qiime software.
Statistical analysis
A bicluster analysis of high-throughput sequencings was conducted using the pheatmap package available through the R software program. Statistical analysis was performed using Origin 8.1 and SPSS 19.0. Statistical significance was designated at a value of P < 0.05.
Additional Information
How to cite this article: Pan, F. et al. The effects of manganese oxide octahedral molecular sieve chitosan microspheres on sludge bacterial community structures during sewage biological treatment. Sci. Rep. 6, 37518; doi: 10.1038/srep37518 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors thank the Collaborative Innovation Plan of Hubei Province for Key Technology of Eco-Ramie Industry. This work was financially supported by the National Key Technology R&D Program on “research and demonstrations of key technologies for clean production in printing and dyeing” (2014BAC13B02) and through the Hubei Provincial Department of Education Young and Middle-Aged Talent Project (Q20151603).
Footnotes
Author Contributions F.P. and D.X. conceived the experiments, F.P., Y.Y., Q.W., Z.Z., M.W., Q.Z. and X. L. conducted the experiments, F.P., W.L., X.Y. and D.Z. analysed the data. All authors participated in writing the paper.
References
- Wang Q. et al. Synthesis, characterization, and catalytic evaluation of Co3O4/γ-Al2O3 as methane combustion catalysts: Significance of Co species and the redox cycle. Appl. Catal. B-Environ. 168–169, 42–50 (2015). [Google Scholar]
- Ling D., Lee N. & Hyeon T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Accounts Chem. Res. 48, 1276–1285 (2015). [DOI] [PubMed]
- Luo S. et al. Manganese oxide octahedral molecular sieve (OMS-2) as an effective catalyst for degradation of organic dyes in aqueous solutions in the presence of peroxymonosulfate. Appl. Catal. B-Environ. 164, 92–99 (2015). [Google Scholar]
- Wang D. et al. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 308, 328–334, (2016). [DOI] [PubMed] [Google Scholar]
- Pan F. et al. Effects of octahedral molecular sieve on treatment performance, microbial metabolism, and microbial community in expanded granular sludge bed reactor. Water Res. 87, 127–136 (2015). [DOI] [PubMed] [Google Scholar]
- Sajid M. et al. Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. R 22, 4122–4143 (2014). [DOI] [PubMed]
- Xiao Y., Araujo C. D., Sze C. C. & Stuckey D. C. Toxicity measurement in biological wastewater treatment processes: A review. J. Hazard. Mater. 286, 15–29 (2015). [DOI] [PubMed] [Google Scholar]
- Sun, F. et al. Integrating landfill bioreactors, partial nitritation and anammox process for methane recovery and nitrogen removal from leachate. Sci. Rep. UK 6, 27744 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Cascade degradation of organic matters in brewery wastewater using a continuous stirred microbial electrochemical reactor and analysis of microbial communities. Sci. Rep. UK 6, 27023 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor V., Elk M., Li X., Impellitteri C. A. & Santo Domingo J. W. Effects of Cr(III) and Cr(VI) on nitrification inhibition as determined by SOUR, function-specific gene expression and 16S rRNA sequence analysis of wastewater nitrifying enrichments. Chemosphere 147, 361–367 (2016). [DOI] [PubMed] [Google Scholar]
- Barrena R. et al. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75, 850–857 (2009). [DOI] [PubMed] [Google Scholar]
- Recillas S. et al. Use of CeO2, TiO2 and Fe3O4 nanoparticles for the removal of lead from water: Toxicity of nanoparticles and derived compounds. Desalination 277, 213–220 (2011). [Google Scholar]
- Zhang H. et al. Use of Metal Oxide Nanoparticle Band Gap To Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. ACS Nano 6, 4349–4368 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman T. Y., Radhika Rajasree S. R. & Kirubagaran R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotox. Environ. Safe. 113, 23–30 (2015). [DOI] [PubMed] [Google Scholar]
- Tong T. Z. et al. Effects of Material Morphology on the Phototoxicity of Nano-TiO2 to Bacteria. Environ. Sci. Technol. 47, 12486–12495 (2013). [DOI] [PubMed] [Google Scholar]
- Wei M. et al. The facile synthesis of a magnetic OMS-2 catalyst for decomposition of organic dyes in aqueous solution with peroxymonosulfate. New J. Chem. 39, 6395–6403 (2015). [Google Scholar]
- Denkbaş E. B., Kiliçay E., Birlikseven C. & Öztürk E. Magnetic chitosan microspheres: preparation and characterization. React. Funct. Polym. 50, 225–232 (2002). [Google Scholar]
- Qi J., Zhang G. & Li H. Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresource Technol. 193, 243–249 (2015). [DOI] [PubMed] [Google Scholar]
- Hossein Beyki M., Shemirani F. & Shirkhodaie M. Aqueous Co(II) adsorption using 8-hydroxyquinoline anchored γ-Fe2O3@chitosan with Co(II) as imprinted ions. Int. J. Biol. Macromol. 87, 375–384 (2016). [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang Y., Dai Y. & Xie S. Anaerobic biodegradation of nonylphenol in river sediment under nitrate- or sulfate-reducing conditions and associated bacterial community. J. Hazard. Mater. 286, 306–314 (2015). [DOI] [PubMed] [Google Scholar]
- Wan Ngah W. S., Teong L. C. & Hanafiah M. A. K. M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 83, 1446–1456 (2011). [Google Scholar]
- Azlan K., Wan Saime W. N. & Lai Ken L. Chitosan and chemically modified chitosan beads for acid dyes sorption. J. Environ. Sci. China 21, 296–302 (2009). [DOI] [PubMed] [Google Scholar]
- Yang S., Okada N. & Nagatsu M. The highly effective removal of Cs+ by low turbidity chitosan-grafted magnetic bentonite. J. Hazard. Mater. 301, 8–16 (2016). [DOI] [PubMed] [Google Scholar]
- Qiu G., Song Y., Zeng P., Duan L. & Xiao S. Combination of upflow anaerobic sludge blanket (UASB) and membrane bioreactor (MBR) for berberine reduction from wastewater and the effects of berberine on bacterial community dynamics. J. Hazard. Mater. 246–247, 34–43 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Evaluating the Role of Microbial Internal Storage Turnover on Nitrous Oxide Accumulation During Denitrification. Sci. Rep. UK 5, 15138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Chen L., Zhang R. & Lin K. High throughput sequencing analysis of the joint effects of BDE209-Pb on soil bacterial community structure. J. Hazard. Mater. 301, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- Nadais H. et al. Enhancing wastewater degradation and biogas production by intermittent operation of UASB reactors. Energy 36, 2164–2168 (2011). [Google Scholar]
- Barczynska R. et al. The effect of dietary fibre preparations from potato starch on the growth and activity of bacterial strains belonging to the phyla Firmicutes, Bacteroidetes, and Actinobacteria. J. Funct. Foods 19, Part A, 661–668 (2015). [Google Scholar]
- Shin N.-R., Whon T. W. & Bae J.-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503 (2015). [DOI] [PubMed] [Google Scholar]
- Kong Y., Xia Y., Nielsen J. L. & Nielsen P. H. Structure and function of the microbial community in a full-scale enhanced biological phosphorus removal plant. Microbiology 153, 4061–4073 (2007). [DOI] [PubMed] [Google Scholar]
- Guo X. et al. Correlation between microbial community structure and biofouling as determined by analysis of microbial community dynamics. Bioresource Technol. 197, 99–105 (2015). [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Holmes D. E. & Nevin K. P. In Adv. Microb. Physiol. Vol. Volume 49, 219–286 (Academic Press, 2004). [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang G., Bi X. & Chen X. Facile assembly of a hierarchical core@shell Fe3O4@CuMgAl-LDH (layered double hydroxide) magnetic nanocatalyst for the hydroxylation of phenol. J. Mater. Chem. A 1, 5934–5942 (2013). [Google Scholar]
- Beal E. J., House C. H. & Orphan V. J. Manganese- and Iron-Dependent Marine Methane Oxidation. Science 325, 184–187 (2009). [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S. & Linstedt A. D. Manganese Blocks Intracellular Trafficking of Shiga Toxin and Protects Against Shiga Toxicosis. Science 335, 332–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. B. Bioelectrochemical Mn (2) Leaching from Manganese Ore by Lactococcus Lactis SK071115. J. Microbiol. Biotechnol. 21, 154–161 (2011). [DOI] [PubMed] [Google Scholar]
- Straub K. L., Benz M., Schink B. & Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62, 1458–1460 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 192, 179–185 (2012). [Google Scholar]
- Byrne J. M. et al. Redox cycling of Fe(II) and Fe(III) in magnetite by Fe-metabolizing bacteria. Science 347, 1473–1476 (2015). [DOI] [PubMed] [Google Scholar]
- Hedrich S., Schlömann M. & Johnson D. B. The iron-oxidizing proteobacteria. Microbiology 157, 1551–1564 (2011). [DOI] [PubMed] [Google Scholar]
- D’Hondt S. et al. Distributions of Microbial Activities in Deep Subseafloor Sediments. Science 306, 2216–2221 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.