Abstract
Traumatic brain injury (TBI) at the moderate level of impact induces massive cell death and results in extensive dendrite degeneration in the brain, leading to persistent cognitive, sensory, and motor dysfunction. Our previous reports have shown that adult-born immature granular neurons in the dentate gyrus are the most vulnerable cell type in the hippocampus after receiving a moderate TBI with a controlled cortical impact (CCI) device. There is no effective approach to prevent immature neuron death or degeneration following TBI. Our recent study found that pretreatment of 7,8-dihydroxyflavone (DHF), a small molecule imitating brain-derived neurotrophic factor, protected immature neurons in the hippocampus from death following TBI. In the present study, we systemically treated moderate CCI-TBI mice or sham surgery mice with DHF once a day for 2 weeks via intraperitoneal injection, and then assessed the immature neurons in the hippocampus the 2nd day after the last DHF injection. We found that post-injury treatment of DHF for 2 weeks not only increased the number of adult-born immature neurons in the hippocampus, but also promoted their dendrite arborization in the injured brain following TBI. Thus, DHF may be a promising compound that can promote neurogenesis and enhance immature neuron development following TBI.
Key words: : brain-derived neurotrophic factor, cell survival, dendrite development, neurogenesis, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a serious public health problem in the United States. According to statistics from the Centers for Disease Control and Prevention, TBI is a contributing factor to one-third (30.5%) of all injury-related deaths in the U.S. Every year, at least 1.7 million TBIs occur either as an isolated injury or along with other injuries.1 In 2010, the total of direct and indirect medical costs was an estimated $80 billion.2 TBI is a significant problem because it induces not only direct damage, such as tissue lesions, but also indirect damage, including extensive cell death and dendritic degeneration.3,4 The secondary injury occurs throughout the brain, in areas such as the cortex and hippocampus in brains of both human5–7 and experimental animals.8–10 Secondary injuries contribute to further cognitive, sensory, and motor dysfunction.10,11 There is no U.S. Food and Drug Administration–approved therapeutic treatment for these disorders following TBI.
Recent research has identified neural stem cells (NSCs) in the adult mammalian hippocampus that can support neurogenesis throughout life, as demonstrated in rodents and primates, including humans.12–16 The pool of NSCs is a potential resource for repairing the damaged hippocampus following TBI. Our recent study found that TBI promotes NSC proliferation in the adult hippocampus.17 However, our follow up study did not find any increase in neurogenesis following TBI.18 Further study found that the adult-born immature neuron in the hippocampus is the cell type most vulnerable to TBI insult.19–21 Most immature neuron death occurs within 24 h of the initial insult.21 Disturbances in hippocampal neurogenesis may play a significant role in TBI-related pathologies, as the hippocampus is implicated in higher cognitive function22 and is frequently associated with post-traumatic seizure generation.23 Thus, in addition to other neuroprotection strategies, it is important to explore approaches preventing immature neuron death after TBI.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors. It is active broadly in the adult brain, but particularly active in the hippocampus.24 It has diverse and important functions on neurons, including promoting the survival of the mature25 and immature neurons.26–28 BDNF is the most abundant neurotrophin in the hippocampal formation of both the adult rodent and human cortex.29,30 When we conditionally knocked out BDNF in the hippocampus, we found that reduction of BDNF expression exacerbates both immature and mature neuron death after TBI.27 This result suggests that BDNF is involved in regulating the neuronal survival in the adult brain, and might potentially be used to protect immature neurons from death following TBI. Our unpublished data showed that direct injection of BDNF into the hippocampus decreased immature neuron death in a rodent TBI model, indicating that increasing the level of BDNF is a promising approach to prevent immature neuron death following brain injury.
However, direct injection of BDNF into the hippocampus is invasive. Further, since BDNF is a polypeptide growth factor, direct administration of BDNF into the brain also may cause an immune response and a further increase in inflammation following TBI. Therefore, an alternative molecule that can imitate BDNF function and be used non-invasively is essential. 7,8-Dihydroxyflavone (DHF), a small molecule imitating BDNF, has been shown to prevent neuron death.31 Treatment with DHF improves neurological functions in rodent models of TBI,32,33 stress,34 depression,35 aging,36 and Alzheimer's disease.37 We recently found that pre-injury treatment of DHF protected adult-born immature neurons from death induced by TBI.38 In this study, we tested the role of post-injury treatment with DHF in immature neuronal survival and their dendrite development following TBI.
Methods
Animal care
Mice (C57/BL6) were housed with a 12 h/12 h light/dark cycle and had free access to food and water ad libitum according to the principles outlined in Guidelines for Care and Use of Experimental Animals. They were used in experiments at an age of 8–10 weeks. All procedures were approved by the research institution's IACUC.
Controlled cortical impact traumatic brain injury
Male mice at 8–10 weeks old were subjected to moderate controlled cortical impact (CCI) injury or sham treatment, as we previously described,4,39 with the following exceptions: the amount of deformation was set at 1.0 mm and the piston velocity was controlled at 3.0 m/sec. These modifications resulted in a moderate level of injury using an electromagnetic model (Impact One TM Stereotaxic Impactor for CCI; Leica Microsystem). Briefly, the mice were anesthetized with avertin and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) prior to TBI. Using sterile procedures, the skin was retracted and a 4 mm craniotomy centered between the lambda and bregma sutures was performed. A point was identified midway between the lambda and bregma sutures and midway between the central suture and the temporalis muscle laterally. The skullcap was carefully removed without disruption of the underlying dura. Prior to the injury, the impacting piston was angled so that the impacting tip (3 mm in diameter) was perpendicular to the exposed cortical surface. Then, the mice received the impact. Sham (non-injured) animals received the craniotomy, but no CCI injury.
DHF treatment
Both TBI mice (total 15) and sham-injury mice (total 15) were randomized into three groups (n = 5 each group), and then were administered DHF (5mg/kg), dimethyl sulfoxide (DMSO) or phosphate-buffered saline (PBS) by intraperitoneal (i.p.) injection. The DHF was dissolved in 17% DMSO, so the DMSO group was used as vehicle control. The injection was given 1 h after CCI surgery, and then given daily for the next 2 weeks following surgery.
Pulse-labeling of the proliferating cells following TBI
The mice subjected to moderate TBI or sham surgery received DHF, DMSO, or PBS treatments as described above (n = 5, each group). Four hours before perfusion, mice received one injection of 5-bromo-2′-deoxyuridine (BrdU; 100 mg/kg in saline, i.p.; Sigma, St. Louis, MO) to pulse-label the proliferating cells. The animals were then deeply anesthetized and perfused transcardially with saline, followed by a cold fixative containing 4% paraformaldehyde (PFA) in PBS.
BrdU injection to label the birthday of immature neurons in the adult hippocampus following TBI
For labeling the newly-generated immature neurons, mice (n = 3 for sham mice and n = 3 for the TBI group) received one injection of BrdU (100 mg/kg in saline, i.p.) within 1 h after TBI surgery. These mice were sacrificed 2 weeks after TBI surgery. The BrdU labeled immature neurons with doublecortin (DCX) staining were selected for the dendrite quantification with 3-D imaging reconstruction.
Tissue processing
After perfusion, the brains were removed, post-fixed overnight in PFA, and cryoprotected for 48 h in 30% sucrose. Serial 30-μm thick coronal sections were cut using a cryostat (Leica CM 1950), and stored at −20°C. The sections were then processed for immunohistochemical analysis.
Immunochemistry
Free-floating sections were washed three times in PBS, and then the sections were incubated in blocking solution (0.1% Triton X-100, 1% bovine serum albumin, and 5% normal goat serum in PBS) for 2 h at 4°C, followed by 48 h incubation with primary antibody at 4°C. After 48 h, the sections were washed again with PBS (three times), and incubated at 4°C for 2 h with the secondary antibody. After treatment (10 min) with 4′, 6-diamidino-2-phenylindole, the sections were washed with PBS (three times), and attached to the slide. Finally, the dried slide was mounted using Fluorescent Mount G. For the BrdU staining, sections were pretreated with 2N HCl for 1 h at room temperature, and then soaked in 0.1 M borate buffer for 10 min (pH 8.4). After washing with PBS (three times), sections were ready for immunostaining. Primary antibodies and their final concentrations were as follows: anti-BrdU (1:400, rat, Accurate Chemical and Scientific), anti-DCX (1:1000, guinea pig; Millipore). Secondary antibodies and their final concentrations were as follows: goat anti rat, Alex 488, 1:800 (Invitrogen); goat anti guinea pig, Cy3, 1:800 (Jackson Laboratory).
Cell counting
Immunohistochemistry was performed simultaneously on sections to detect the target cells. For DCX-positive cell quantification, three sections at the epicenter were selected and images of dentate gyrus were taken. Every DCX-positive cell was counted with quantification results expressed as average cells/mm2 (n = 5).The experimenter was blinded to the treatment conditions.
For quantification of BrdU cells, series of every sixth section (30 μm thickness, 180 μm apart) through each hippocampus were processed. Every single BrdU positive cell (even the partial BrdU-positive nuclei at the borders of sections) in the dentate gyrus throughout the entire 30 μm sections was counted under a fluorescent microscope using the 40× objective through a whole series of sections (n = 5).The experimenter was blinded to the treatment conditions.
Dendrite quantification
DCX-stained sections were then used for dendrite morphology analysis; 40× images containing whole neurons and dendrite branches were taken. For neurons evaluated, all branches of the dendritic tree were reconstructed at 40× magnification using a motorized microscope (Zeiss Imager M2) with Neurolucida software (Microbrightfield, VT). A three-dimensional analysis of the reconstructed neurons was performed using NeuroExplorer software (Microbrightfield). More than 20 neurons were studied for each of the five animals in the six treatment groups. Several aspects of dendritic morphology were examined. To assess overall changes, total length of dendrite trees, average length of dendrite branches, and number of dendrite branches were compared across groups using two-way analysis of variance (ANOVA). The complexity of dendrite trees was assessed using Scholl analysis. The number of intersections of dendrites was calculated with concentric spheres positioned at radial intervals of 20 μm.
Statistical analysis
The collected data were expressed as mean ± standard error. For the immature neuron number and the dendrite morphology within six groups, two-way ANOVA followed by least significant difference (LSD) post hoc test was performed. For the cell proliferation within four groups, one-way ANOVA followed by LSD post hoc test was performed. For the dendrite morphology within two groups, Student's t-test was performed. For the Scholl analysis, repeated-measures test was performed. The significance level was set at p < 0.05. More details of statistical analysis are shown in Supplementary Table 1 (see online supplementary material at www.liebertpub.com).
Results
DHF treatment increased the number of adult-born immature neuron at 2 weeks after moderate TBI
Our previous study found that adult-born immature granular neurons in the dentate gyrus are the most vulnerable cell type in the hippocampus after receiving a moderate TBI.19,21 The number of immature neurons in the epicenter reduced about 50% at 24 h after moderate TBI.19 Our recent study found that pretreatment of DHF increased the number of immature neurons in the hippocampus following moderate TBI.38 To further investigate the neuroprotective role of DHF in a clinically relevant setting, here we assessed whether post-injury treatment of DHF protects immature neuron death and increases the number of newborn neurons in the hippocampus. Sham-operated mice or TBI-injured mice received injections of PBS, DMSO, or DHF in DMSO once a day consecutively for 2 weeks before sacrificed for analyzing immature neurons (n = 5 in each group). Our previous study showed that the highest number of dead immature neurons occurs at the epicenter, and the number of dead immature neurons reduces in both rostral and caudal directions within the hippocampus.19 Thus, in this study, three brain sections at the epicenter from each mouse were selected and immunohistochemistry was performed to observe changes in the immature neuron count 2 weeks after TBI. Antibodies against DCX, a specific immature neuron marker,40 were used.
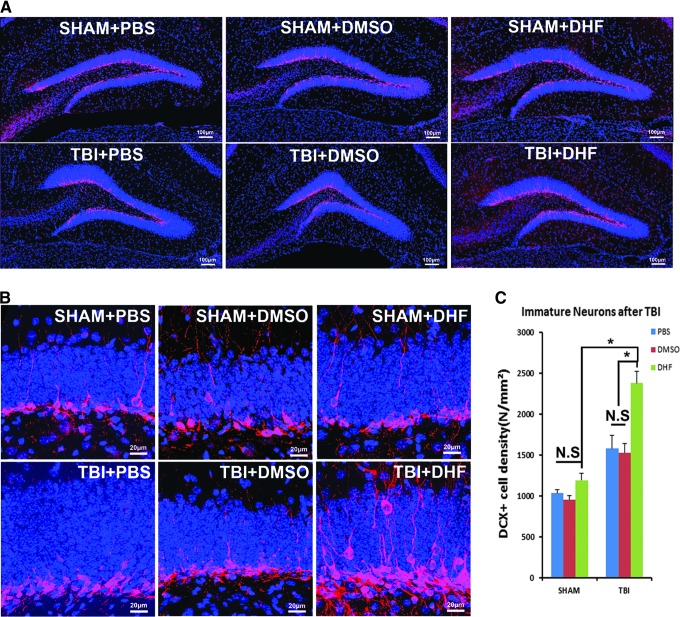
In low magnification, most of the DCX-positive cells were found to locate in the 1/3 inner layer of the granular cell layer (GCL) of the hippocampal dentate gyrus (HDG; Fig. 1A). High power images (40× magnification) at the single cell level clearly showed most of the DCX-positive immature neurons located in the inner region of the GCL, with dendrites projecting into the molecular layer (Fig. 1 B). TBI groups with DHF treatment had much more DCX-positive cells than the other TBI groups and sham groups (Fig. 1A, 1B).
FIG. 1.
7,8-Dihydroxyflavone (DHF) treatment increased newborn immature neuron number in the hippocampal dentate gyrus 2 weeks after traumatic brain injury (TBI). Doublecortin (DCX) was used to label immature neurons with red fluorescence. The sections were counterstained with 4′,6-diamidino-2-phenylindole (blue). (A) DCX staining (red) of the ipsilateral dentate gyrus in mice 2 weeks after TBI (20× image). (B) High power images of DCX staining in the dentate gyrus showed clear DCX-positive immature neurons in corresponding positions. (C) Quantification of DCX-positive cells (*p < 0.05). PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide. Color image is available online at www.liebertpub.com/neu
All DCX-positive cells of the dentate gyrus in three different epicenter sections were then counted for each group. The quantification showed that the density of DCX-positive cells in the three sham groups with different treatments were: sham+PBS, 1030 ± 39 cells/mm2; sham+DMSO, 951 ± 46 cells/mm2; and sham+DHF, 1190 ± 81 cells/mm2 (Fig. 1C). The density of DCX-positive cells in the three TBI-injured groups with different treatments were: TBI+PBS, 1576 ± 159 cells/mm2; TBI+DMSO, 1519 ± 119 cells/mm2; and TBI+DHF, 2377 ± 142 cells/mm2. Data were analyzed by two-way ANOVA followed by LSD post hoc test (Supplementary Table 1). The results showed that PBS, DMSO, and DHF did not change the density of DCX-positive cells in the hippocampus of sham-surgery mice (Fig. 1 C; p > 0.05; Supplementary Table 1). In contrast, DHF treatment dramatically increased the density of DCX-positive cells in the TBI-injured mice, compared with its corresponding sham surgery control (p < 0.05; Supplementary Table 1), while PBS or DMSO treatments did not significantly affect the density of DCX-positive cells in the TBI-injured mice. These results indicated DHF treatment dramatically increased the immature neuron count in the dentate gyrus of brains subjected to TBI at 2 weeks after being injured.
DHF did not increase cell proliferation at two weeks following TBI
DHF can mimic BDNF to activate its receptor, tyrosine-related kinase B (TrkB), which is expressed both in newborn neurons41 and NSCs.42 The increase in the immature neuron count with DHF can occur through aiding immature neurons in surviving TBI conditions, promoting NSC proliferation in the dentate gyrus, or a combination of the two previous mechanisms. To assess whether DHF promotes NSC proliferation, the sham-operated mice and TBI mice treated with PBS, DMSO, or DHF (n = 5) received one BrdU injection 4 h before sacrifice to pulse label the proliferating cells. Series of every sixth section (30 μm thickness, 180 μm apart) through each hippocampus (about 16 sections for each mouse) were selected to detect proliferating cells using immunostaining with antibody against BrdU. In low magnification images, BrdU-positive cells were detected in all groups, mainly located in the subgranular zone of the HDG, where the NSCs reside (Fig. 2A). High power images (40× magnification) clearly showed the single BrdU-positive cells in the HDG (Fig. 2B). Quantification showed that, compared with the sham group (850 ± 45 cells/DG), BrdU-positive cells did not significantly increase in the PBS-treated (905 ± 147 cells/DG) or DMSO-treated (968 ± 136 cells/DG) TBI groups (Fig. 2C; p > 0.05; Supplementary Table 1). The DHF-treated TBI group also did not show an increase in BrdU-positive cell count, compared with the PBS-treated and DMSO-treated TBI groups or the sham group (DHF, 935 ± 62 cells/DG; Fig. 2C; p > 0.05; Supplementary Table 1). These results indicated accumulated DHF treatment for 2 weeks did not promote NSC proliferation at sub-acute period following TBI. Thus, DHF treatment may likely induce an increase in immature neuron count by helping immature neurons survive following a TBI insult. However, it does not rule out the possibility that DHF treatment transiently promotes NSC proliferation at earlier acute period following TBI.
FIG. 2.
7,8-Dihydroxyflavone (DHF) did not increase cell proliferation 2 weeks after TBI. Immunostaining with antibody against 5-bromo-2′-deoxyuridine (BrdU) was used to detect the proliferating cells (green). The sections were counterstained with 4′,6-diamidino-2-phenylindole (blue). (A) Ipsilateral dentate gyrus 2 weeks after TBI with BrdU staining (20× images, green). (B) High magnification images of the dentate gyrus in corresponding positions showed individual BrdU-positive cells. (C) Quantification of BrdU-positive cells. PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide. Color image is available online at www.liebertpub.com/neu
TBI reduced dendritic arborization of immature neurons born after injury
Our previous studies found that TBI at the moderate level of impact not only induces cell death, it also results in extensive dendrite degeneration in those spared mature neurons in the HDG.4 We further found that TBI also impaired dendrite development in those adult-born immature neurons in the HDG.43 These immature neurons may be born either before or after injury, and they are at various developmental stages. Here, we further assessed the effect of TBI on dendrite development of immature neurons born after injury. We labeled the immature neurons born on the first day after TBI or sham surgery with BrdU, and then assessed their dendrite development at 2 weeks after TBI. DCX is highly expressed in the immature neurons and their developing dendrites, thus immunostaining with antibody against DCX was used to assess the developing dendrites of the immature neurons after sham operation (Fig. 3A) or CCI injury (Fig. 3B) at a moderate level of impact.
FIG. 3.
Traumatic brain injury (TBI) reduced dendritic arborization of immature neurons born after injury. Immunostaining with antibodies against both doublecortin (red) and 5-bromo-2′-deoxyuridine (green) was used to show the dendrite morphology of new generated immature neurons after TBI. The sections were counterstained with 4′,6-diamidino-2-phenylindole (blue). (A) New generated immature neurons of the sham-operated mice. (B) Immature neurons of the controlled cortical impact (CCI)–operated mice. (C) Reconstruction of the dendrites of immature newborn neurons 2 weeks after TBI. (D) Branch analysis of different groups. (E-F) Scholl analysis results of sham-operated mice and CCI-operated mice (*p < 0.05). Color image is available online at www.liebertpub.com/neu
High power images of DCX staining were captured and showed the clear newborn immature neurons with developing dendrites that projected towards the molecular layer in the HDG. The cells expressing both DCX and BrdU are immature neurons born at the first day after sham operation (Fig. 3A) or CCI-injury (Fig. 3B). In the sham control mice, an average of 4.0 ± 0.59 dendritic branches was observed in each immature neuron born on the first day after surgery and was allowed to survive for 2 weeks. The average number of dendritic branches reduced to 2.0 ± 0.15 after moderate TBI (p < 0.05; Supplementary Table 1). The total dendrite length in each immature neuron in sham-operated mice was 341.4 ± 40.9 μm. After TBI, this number reduced to 219.6 ± 11.4 μm. The reduction of the total dendrite length was significant (p < 0.05; Supplementary Table 1). While TBI did not significantly change the average length of each dendrite branch, the average length of each branch in the immature neuron of sham and TBI mice was 79.5 ± 2.0 μm and 109.8 ± 7.19 μm, respectively (Fig. 3D; p > 0.05; Supplementary Table 1).
Scholl analysis is a quantitative method for morphometric neuronal studies used for better understanding the dendritic branching characteristics of individual immature neurons in the HDG. Scholl analysis showed that dendrite complexity decreased in TBI mice, compared with sham mice (Fig. 3E, 3F; p < 0.05; Supplementary Table 1), especially at a distance from 100 μm to 160 μm (p < 0.05; Supplementary Table 1).These data further confirmed that TBI reduced dendrite arborization and complexity of immature neurons.
DHF treatment increases dendrite arborization of adult-born immature neurons after moderate TBI
BDNF has been reported to regulate primary dendrite formation44 and to play an important role in regulating the growth and branching of cortical dendrites in normal condition.45 To test whether activating the BDNF signaling pathway can increase dendrite branches of immature neurons following TBI, the sham-operated CCI-injured mice received DHF in DMSO (5mg/kg, i.p.) or DMSO as a control once a day for 14 consecutive days right after surgery. Immunostaining with antibody against DCX and digital reconstruction of the dendritic morphologies of individual immature neurons were then used to quantify the dendrite development following treatment after TBI. Fewer dendritic branches were observed in the hippocampus of the CCI-injured mice treated with PBS (Fig. 4D) or DMSO (Fig. 4E) than in hippocampus of the sham control mice treated with PBS (Fig. 4A) or DMSO (Fig. 4B). In contrast, treatment with DHF preserved the dendrite morphologies in the CCI-injured mice (Fig. 4F), compared with the sham-DHF group (Fig. 4C). These results showed that DHF might have a protective effect on dendrite degeneration or promote dendrite regrowth in immature neurons following TBI.
FIG. 4.
7,8-Dihydroxyflavone (DHF) treatment increases dendrites arborization of immature neurons following traumatic brain injury (TBI). Immunostaining with antibody against doublecortin was used to show the dendrite morphology of individual immature neurons (red) 2 weeks after TBI. The sections were counterstained with 4′,6-diamidino-2-phenylindole (blue). (A-C). Immature neurons of the sham-operated mice 2 weeks after receiving phosphate-bufered saline (PBS), dimethyl sulfoxide (DMSO), or DHF treatment. (D-F). Immature neurons of the CCI-injured mice 2 weeks after receiving PBS, DMSO, or DHF treatment. Color image is available online at www.liebertpub.com/neu
To further assess the effect of DHF on dendrite arborization of immature neurons, we reconstructed dendritic arbors of individual immature neurons (more than 20 neurons in each mice, five mice for each group) in the HDG from control mice and injured mice (Fig. 5A) and assessed their arborization (Fig. 5B) and complexity with Scholl analysis using Neurolucida software (Fig. 5C, 5D). After TBI, the average number of dendrite branches of TBI mice was slightly decreased, but not significantly different from the sham group (p > 0.05; Supplementary Table 1). The total dendrite length decreased from 267.19 ± 19.13 μm to 186.72 ± 11.74 μm (Fig. 5B; p < 0.05; Supplementary Table 1), and the average length of each branch was shorter (Fig. 5B; p < 0.05; Supplementary Table 1). Combined with those of Figure 3, these results showed that not only immature neuron born after TBI, but larger population of immature neuron in granule cell layer suffered from dendritic impairment.
FIG. 5.
7,8-Dihydroxyflavone (DHF) treatment increased dendrite complexity of newborn immature neurons 2 weeks after traumatic brain injury (TBI). (A) Reconstruction of the dendrites of immature newborn neurons 2 weeks after TBI. (B) Branch analysis of different groups following surgery. (C) Sholl analysis results of the TBI group with phosphate-buffered saline (PBS) treatment and the sham group with PBS treatment. (D) Sholl analysis results of the three TBI groups treated with dimethyl sulfoxide (DMSO) and DHF (**p < 0.01).
Scholl analysis (Fig. 5C) revealed that spared immature neurons showed reduced dendritic complexity, compared with the sham controls (Fig. 5C; p < 0.05; Supplementary Table 1). This decreased complexity was particularly evident at a distance between 80 and 160 μm from the soma (Fig. 5C; p < 0.05; Supplementary Table 1). These results indicate that TBI reduced the dendrite complexity of immature neurons, in addition to other injuries. This conclusion confirms that although a subpopulation of the immature neurons in the HDG was spared following moderate TBI, the dendrites of these neurons were extensively injured. Thus, the actual injury in the hippocampus was much more severe than previously assumed using cell death as an indicator.
Dendrite reconstruction showed an average of 4.00 ± 0.25, 4.00 ± 0.25, and 4.00 ± 0.39 dendrite branches in immature neurons of the sham-operated mice treated with PBS, DMSO and DHF, respectively (Fig. 5B). DHF treatment significantly increased the number of dendrite branches in immature neurons, compared with the PBS or DMSO control groups, in the CCI-injured mice (p < 0.05; Supplementary Table 1), while there was no significant difference between the three sham-operated groups (p > 0.05; Supplementary Table 1). The average number of dendrite branches increased from 3.00 ± 0.19 in the PBS-treated group or 3.00 ± 0.23 in the DMSO-treated group to 5.00 ± 0.32 in immature neurons in the DHF-treated group (Fig. 5 B; p < 0.05; Supplementary Table 1). DHF treatment also significantly increased the total dendrite length in immature neurons in the CCI-injured mice. The total dendrite length increased from 186.72 ± 11.74 μm in PBS-treated group or 188.80 ± 15.60 μm in DMSO-treated group to 270.89 ± 16.69 μm in DHF-treated group (Fig. 5B; p < 0.05; Supplementary Table 1). DHF treatment increased the average number of dendrite branches and the total length, but did not significantly increase the average length (Fig. 5B, p > 0.05; Supplementary Table 1). These data indicated a strong neuroprotective effect of DHF on dendrite degeneration following moderate TBI.
Scholl analysis showed that the mice treated with DHF underwent an increase of intersection crossing (p < 0.05; Supplementary Table 1), particularly evident at a distance from 40 μm to 120 μm (Fig. 5 D; p < 0.05; Supplementary Table 1). These results suggested that DHF treatment increased dendrite arbors of spared immature neurons in the dentate gyrus. DHF may preserve dendrite structure from injury or promote injury dendrites regrowth.
Discussion
TBI results in dramatic cell death and neurodegeneration.10,46 There is still a lack of effective pharmacological approaches that aim to protect them. Recent research has identified neural stem/progenitor cells (NSPCs) in the adult brain.14 New neurons are continuously generated from NSPCs throughout adulthood. These adult-born new neurons are a potential resource for repairing damages in the brain following TBI. The neurological function deficits are still substantial following TBI. This suggests that these innate processes are often unsuccessful. Excessive neuronal death may not be sufficiently compensated by enhanced NSC proliferation and subsequent neurogenesis. Thus, it is essential to identify approaches that can augment neurogenesis for post-traumatic brain repair.
BDNF is active broadly in the adult brain, and is particularly highly active in the hippocampus.24 It has diverse and important functions on neurons, such as helping the survival of the mature25 and immature neurons.26–28 DHF is a small molecule that can cross the blood–brain barrier and mimic BDNF functions.31,47,48 Reports, including research from our laboratory, showed that DHF can activate the BDNF receptor, TrkB, and increase its phosphorylation in the adult brain, particularly in the hippocampus following TBI.32,38,49 DHF further activates the PI3K pathway,32 promotes mitochondrial function in vitro,50 reduces dendrite degeneration in those spared mature neurons in the cortex,33 and improves post-trauma functions.32,33,49 Our previous report showed that pre-injury treatment of DHF protected immature neurons from death induced by moderate TBI.38 These prior reports suggest that DHF may be a promising chemical compound that promotes immature neuron survival after TBI.
New neurons are continuously generated from NSCs throughout adulthood. However, our recent studies showed that immature neurons are particularly vulnerable to TBI.19 About 50% of immature neurons in the hippocampus die within 24 h after TBI with a moderate CCI injury.19,21 Recent studies further show that TBI promotes NSPC proliferation in the adult,20,51–59 leading to increased generation of immature neurons. Thus, it is assumed that more immature neurons will be generated trying to compensate for TBI-induced immature neuron death. However, it is still not known whether the number of immature neurons can spontaneously recover to the sham control level, and how long it takes for the number of immature neurons in the hippocampus to restore to the sham control level.
Rola and colleagues showed that the number of immature neurons was still relatively lower than controls at 2 weeks after severe TBI.20 Here, we showed that the number of immature neurons in the hippocampus gradually recovers and is slightly higher than sham controls at 2 weeks following moderate CCI-TBI (Fig. 1B, 1C), but the difference is not statistically significant (Fig. 2C). It indicates that about 2 weeks are needed for the number of immature neurons in the hippocampus to return back to control level following moderate TBI. The neurological function deficits are still substantial at 2 weeks following moderate CCI-TBI. Thus, it is essential to identify approaches that can augment neurogenesis for post-traumatic brain repair.
TrkB is highly expressed in the adult-born immature neurons; however, its phosphorylation in the immature neurons is reduced by TBI.38 Previous studies have shown that DHF can restore TrkB phosphorylation in the hippocampus following TBI.32,38,58 Both pre-injury treatment38 and post-injury treatment (Fig. 1) of DHF increased the number of adult-born immature neurons in the hippocampus at 24 h and 2 weeks after moderate CCI-TBI respectively. It is likely that DHF protects immature neurons from death induced by TBI, since treatment of DHF for 2 weeks did not significantly increase NSC proliferation (Fig. 2). These data suggest that DHF may protect immature neurons from death following moderate TBI, and treatment with DHF may be a potential approach to augment neurogenesis following TBI.
Our previous studies found that TBI at the moderate level of impact not only induces cell death, it also results in extensive dendrite degeneration in those spared mature neurons in the cortex4 and immature neurons in the hippocampus.43 Here, we further confirmed that moderate CCI-TBI caused dramatic dendrite damage in immature neurons (Fig. 3). Dendrites provide a massive membrane surface for the post-synaptic spines and are the main input apparatus of a neuron. Their degeneration could likely cause disruption in synaptic transmission between neurons when they integrate into the neural network, and finally lead to neural circuitry disruption. There is no effective treatment available to re-grow dendrites.
It is known that embryonic neurons possess amazing dendritic elongation, branching, and regenerative capacities.60 Once fully matured, neurons switch from rapid dendrite growth to maintaining a remarkable dendritic morphology. During this transition, their dendrite regenerative capacity is significantly reduced. The neurons in the post-natal brain have limited capacity for dendrite regeneration. Thus, it is likely more effective to protect dendrites from injury or promote dendrite regeneration while they are still immature neurons.
BDNF has been reported to regulate primary dendrite formation44 and to play an important role in regulating the growth and branching of cortical dendrites.45 Overexpression of either BDNF or its high affinity receptor, TrkB,61 favors addition of dendrite branches close to the soma. BDNF also appears to regulate the dynamic stability of dendritic processes.62 These data strongly suggest that stimulation of BDNF signaling may protect dendrites from injury or restore dendrite development in the immature neurons. When we treated TBI-injured mice with DHF, we found that DHF has double effects on increasing the survival and preserving the dendritic morphologies of immature neurons following TBI (Fig. 4 and Fig. 5).
Conclusion
DHF is a small molecule with a molecular weight less than 500 daltons; thus, it can penetrate the blood–brain barrier and can be non-invasively administrated by intravenous or intraperitoneal injection. DHF may be a promising compound that can promote neurogenesis and enhance newborn neuron development following TBI. However, it is still not known whether those newborn neurons can differentiate into mature neurons, integrate into the neural circuitry, and contribute to post-traumatic functional recovery. DHF is eight times stronger in activating TrkB than BDNF itself.31 Therefore, it may have a stronger neuroprotective effect than BDNF, though it is not known whether strong activation of TrkB leads to an undesired effect. Although this small molecule has high translational potential, laboratories are still a long way from translating the results from basic research to clinical care in the future.
Supplementary Material
Acknowledgments
This work was supported by funding from Indiana Spinal Cord and Brain Injury Research Grants, the Ralph W. and Grace M. Showalter Research Award, Indiana University Biological Research Grant, and National Institutes of Health grants RR025761, 1R21NS072631, and R21NS075733 to J. Chen.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalization, and Deaths. Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 2.Finkelstein E.A., Corso P.S., and Miller T. (2006). The Incidence and Economic Burden of Injuries in the United States. Oxford University Press: New York [Google Scholar]
- 3.Gao X. and Chen J. (2011). Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 70, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X., Deng P., Xu Z.C., and Chen J. (2011). Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One 6, e24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isoniemi H., Kurki T., Tenovuo O., Kairisto V. and Portin R. (2006). Hippocampal volume, brain atrophy, and APOE genotype after traumatic brain injury. Neurology 67, 756–760 [DOI] [PubMed] [Google Scholar]
- 6.Ariza M., Serra-Grabulosa J.M., Junqué C., Ramírez B., Mataró M., Poca A., Bargalló N., and Sahuquillo J. (2006). Hippocampal head atrophy after traumatic brain injury. Neuropsychologia 44, 1956–1961 [DOI] [PubMed] [Google Scholar]
- 7.Wilde E.A., Bigler E.D., Hunter J.V., Fearing M.A., Scheibel R.S., Newsome M.R., Johnson J.L., Bachevalier J., Li X., and Levin H.S. (2007). Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate to severe traumatic brain injury. Dev. Med. Child Neurol. 49, 294–299 [DOI] [PubMed] [Google Scholar]
- 8.Saatman K.E., Feeko K.J., Pape R.L., and Raghupathi R. (2006). Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma 23, 1241–1253 [DOI] [PubMed] [Google Scholar]
- 9.Bonislawski D.P., Schwarzbach E.P., and Cohen A.S. (2007). Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol. Dis. 25, 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 11.Greve M.W. and Zink B.J. (2009). Pathophysiology of traumatic brain injury. Mount Sinai J. Med. 76, 97–104 [DOI] [PubMed] [Google Scholar]
- 12.Leuner B., Kozorovitskiy Y., Gross C.G., and Gould E. (2007). Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. U. S. A. 104, 17169–17173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn H.G., Dickinson-Anson H., and Gage F.H. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron H.A. and McKay R.D. (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417 [DOI] [PubMed] [Google Scholar]
- 15.Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., and Gage F.H. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 [DOI] [PubMed] [Google Scholar]
- 16.Kornack D.R. and Rakic P. (1999). Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. U. S. A. 96, 5768–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X., Enikolopov G., and Chen J. (2009). Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 219, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X. and Chen J. (2012). Moderate traumatic brain injury promotes neural precursor proliferation without increasing neurogenesis in the adult hippocampus. Exp. Neurol. 239, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X., Deng Bryant Y., Cho W., Carrico K.M., Hall E.D., and Chen J. (2008). Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 86, 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rola R., Mizumatsu S., Otsuka S., Morhardt D.R., Noble-Haeusslein L.J., Fishman K., Potts M.B., and Fike J.R. (2006). Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp. Neurol. 202, 189–199 [DOI] [PubMed] [Google Scholar]
- 21.Zhou H., Chen L., Gao X., Luo B., and Chen J. (2012). Moderate traumatic brain injury triggers rapid necrotic death of immature neurons in the hippocampus. J. Neuropathol. Exp. Neurol. 71, 348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pullela R., Raber J., Pfankuch T., Ferriero D.M., Claus C.P., Koh S.E., Yamauchi T., Rola R., Fike J.R., and Noble-Haeusslein L.J. (2006). Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev. Neurosci. 28, 396–409 [DOI] [PubMed] [Google Scholar]
- 23.Pitkanen A. and McIntosh T.K. (2006). Animal models of post-traumatic epilepsy. J. Neurotrauma 23, 241–261 [DOI] [PubMed] [Google Scholar]
- 24.Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., and Dean M. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A., Carnahan J., and Greenberg M.E. (1994). Requirement for BDNF in activity-dependent survival of cortical neurons. Science 263, 1618. [DOI] [PubMed] [Google Scholar]
- 26.Liu L., Cavanaugh J.E., Wang Y., Sakagami H., Mao Z., and Xia Z. (2003). ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc. Natl. Acad. Sci. U. S. A. 100, 8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X. and Chen J. (2009). Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J. Neurotrauma 26, 1325–1335 [DOI] [PubMed] [Google Scholar]
- 28.Kirschenbaum B. and Goldman S.A. (1995). Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc. Natl. Acad. Sci. U. S. A. 92, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisonpierre P.C., Belluscio L., Friedman B., Alderson R.F., Wiegand S.J., Furth M.E., Lindsay R.M., and Yancopoulos G.D. (1990). NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5, 501–509 [DOI] [PubMed] [Google Scholar]
- 30.Timmusk T., Belluardo N., Persson H., and Metsis M. (1994). Developmental regulation of brain-derived neurotrophic factor messenger RNAs transcribed from different promoters in the rat brain. Neuroscience 60, 287–291 [DOI] [PubMed] [Google Scholar]
- 31.Jang S.W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., and Ye K. (2010). A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. U. S. A. 107, 2687–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C.H., Hung T.H., Chen C.C., Ke C.H., Lee C.Y., Wang P.Y., and Chen S.F. (2014). Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS One 9, e113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao S., Gao X., Dong W., and Chen J. (2015). The role of 7,8-Dihydroxyflavone in preventing dendrite degeneration in cortex after moderate traumatic brain injury. Mol. Neurobiol. 2015. March 24; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andero R., Daviu N., Escorihuela R.M., Nadal R., and Armario A. (2012). 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus 22, 399–408 [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Chan C.B., Jang S.W., Pradoldej S., Huang J., He K., Phun L.H., France S., Xiao G., Jia Y., Luo H.R., and Ye K. (2010). A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J. Med. Chem. 53, 8274–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng Y., Liu Y., Wu M., Liu J., and Hu Q. (2012). Activation of TrkB by 7,8-dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J. Alzheimers. Dis. 31, 765–767 [DOI] [PubMed] [Google Scholar]
- 37.Devi L. and Ohno M. (2012). 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease. Neuropsychopharmacology 37, 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L., Gao X., Zhao S., Hu W., and Chen J. (2015). The small-molecule TrkB agonist 7, 8-dihydroxyflavone decreases hippocampal newborn neuron death after traumatic brain injury. J. Neuropathol. Exp. Neurol. 74, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romine J., Gao X., and Chen J. (2014). Controlled cortical impact model for traumatic brain injury. J. Vis. Exp. e51781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magavi S.S., Leavitt B.R., and Macklis J.D. (2000). Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955 [DOI] [PubMed] [Google Scholar]
- 41.Donovan M.H., Yamaguchi M., and Eisch A.J. (2008). Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus 18, 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babu H., Ramirez-Rodriguez G., Fabel K., Bischofberger J., and Kempermann G. (2009). Synaptic network activity induces neuronal differentiation of adult hippocampal precursor cells through BDNF signaling. Front. Neurosci. 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson S.W., Madathil S.K., Sama D.M., Gao X., Chen J., and Saatman K.E. (2014). Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J. Neuropathol. Exp. Neurol. 73, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dijkhuizen P.A. and Ghosh A. (2005). BDNF regulates primary dendrite formation in cortical neurons via the PI3-kinase and MAP kinase signaling pathways. J. Neurobiol. 62, 278–288 [DOI] [PubMed] [Google Scholar]
- 45.McAllister A.K., Lo D.C., and Katz L.C. (1995). Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803 [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto S.T., Longhi L., Saatman K.E., Conte V., Stocchetti N., and McIntosh T.K. (2004). Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 28, 365–378 [DOI] [PubMed] [Google Scholar]
- 47.Andero R., Heldt S.A., Ye K., Liu X., Armario A., and Ressler K.J. (2011). Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am. J. Psychiatry 168, 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson R.A., Lam M., Punzo A.M., Li H., Lin B.R., Ye K., Mitchell G.S., and Chang Q. (2012). 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J. Appl. Physiol. 112, 704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal R., Noble E., Tyagi E., Zhuang Y., Ying Z., and Gomez-Pinilla F. (2015). Flavonoid derivative 7,8-DHF attenuates TBI pathology via TrkB activation. Biochim. Biophys. Acta 1852, 862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal R., Tyagi E., Vergnes L., Reue K., and Gomez-Pinilla F. (2014). Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim Biophys Acta 1842, 535–546 [DOI] [PubMed] [Google Scholar]
- 51.Chirumamilla S., Sun D., Bullock M.R., and Colello R.J. (2002). Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma 19, 693–703 [DOI] [PubMed] [Google Scholar]
- 52.Rice A.C., Khaldi A., Harvey H.B., Salman N.J., White F., Fillmore H., and Bullock M.R. (2003). Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp. Neurol. 183, 406–417 [DOI] [PubMed] [Google Scholar]
- 53.Sun D., McGinn M.J., Zhou Z., Harvey H.B., Bullock M.R., and Colello R.J. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204, 264–272 [DOI] [PubMed] [Google Scholar]
- 54.Sun D., Colello R.J., Daugherty W.P., Kwon T.H., McGinn M.J., Harvey H.B., and Bullock M.R. (2005). Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma 22, 95–105 [DOI] [PubMed] [Google Scholar]
- 55.Dash P.K., Mach S.A., and Moore A.N. (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 63, 313–319 [DOI] [PubMed] [Google Scholar]
- 56.Ramaswamy S., Goings G.E., Soderstrom K.E., Szele F.G., and Kozlowski D.A. (2005). Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 1053, 38–53 [DOI] [PubMed] [Google Scholar]
- 57.Kernie S.G., Erwin T.M., and Parada L.F. (2001). Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J. Neurosci. Res. 66, 317–326 [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura S., Teramoto T., Whalen M.J., Irizarry M.C., Takagi Y., Qiu J., Harada J., Waeber C., Breakefield X.O., and Moskowitz M.A. (2003). FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J. Clin. Invest. 112, 1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braun H., Schafer K., and Hollt V. (2002). BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J. Neurotrauma 19, 975–983 [DOI] [PubMed] [Google Scholar]
- 60.Jan Y.N. and Jan L.Y. (2003). The control of dendrite development. Neuron 40, 229–242 [DOI] [PubMed] [Google Scholar]
- 61.Yacoubian T. and Lo D. (2000). Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat. Neurosci. 3. [DOI] [PubMed] [Google Scholar]
- 62.Horch H.W. (2004). Local effects of BDNF on dendritic growth. Rev. Neurosci. 15, 117–129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.