Abstract
Techniques to monitor the oxygen partial pressure (pO2) within implanted tissue-engineered grafts (TEGs) are critically necessary for TEG development, but current methods are invasive and inaccurate. In this study, we developed an accurate and noninvasive technique to monitor TEG pO2 utilizing proton (1H) or fluorine (19F) magnetic resonance spectroscopy (MRS) relaxometry. The value of the spin-lattice relaxation rate constant (R1) of some biocompatible compounds is sensitive to dissolved oxygen (and temperature), while insensitive to other external factors. Through this physical mechanism, MRS can measure the pO2 of implanted TEGs. We evaluated six potential MRS pO2 probes and measured their oxygen and temperature sensitivities and their intrinsic R1 values at 16.4 T. Acellular TEGs were constructed by emulsifying porcine plasma with perfluoro-15-crown-5-ether, injecting the emulsion into a macroencapsulation device, and cross-linking the plasma with a thrombin solution. A multiparametric calibration equation containing R1, pO2, and temperature was empirically generated from MRS data and validated with fiber optic (FO) probes in vitro. TEGs were then implanted in a dorsal subcutaneous pocket in a murine model and evaluated with MRS up to 29 days postimplantation. R1 measurements from the TEGs were converted to pO2 values using the established calibration equation and these in vivo pO2 measurements were simultaneously validated with FO probes. Additionally, MRS was used to detect increased pO2 within implanted TEGs that received supplemental oxygen delivery. Finally, based on a comparison of our MRS data with previously reported data, ultra-high-field (16.4 T) is shown to have an advantage for measuring hypoxia with 19F MRS. Results from this study show MRS relaxometry to be a precise, accurate, and noninvasive technique to monitor TEG pO2 in vitro and in vivo.
Keywords: : 19F-MRS, oximetry, tissue-engineered graft, relaxometry, islet transplantation, oxygen measurement
Introduction
Tissue-engineered grafts (TEGs) have the potential to treat numerous devastating diseases including type 1 diabetes.1–6 Pancreatic islet transplantation is a potential treatment for type 1 diabetes, but favorable long-term outcomes are inconsistent because intrahepatic delivery of islets is not optimal.6–10 Additionally, current demand for donor pancreata far exceeds supply and allotransplantation requires a lifetime of immunosuppression.7,9,10 Macroencapsulated TEGs may ameliorate or eliminate these limitations by allowing the use of alternative cell sources (such as porcine or stem cell-derived islets) and providing an immunoisolating membrane.1–6
Macroencapsulation of islets within a TEG offers many advantages; however, oxygenation is a critical limitation for the development of large-scale and high-cell density implanted TEGs.11 Noninvasive monitoring of the oxygen partial pressure (pO2) within TEGs is critically important because of the deleterious effects of long- and short-term exposure of islet tissue to hypoxia or anoxia.2,7,8,11–13 Lack of oxygen is the most critical issue preventing widespread utilization of macroencapsulated islet transplantation, and delivery of exogenous oxygen has been shown to enhance TEG survival and function in vivo.2,14,15 Noninvasive monitoring of pO2 can improve TEG development and allow the evaluation of TEG viability16 and local changes in pO2 with the delivery of supplemental oxygen (DSO). Oxygen measurements are especially important in TEGs that have not revascularized due to either lack of time or the presence of an immunoisolating membrane. Additionally, pO2 measurements may detect oxygenation-altering events such as cell proliferation, inflammation (including fibrous capsule formation), and vascularization.17
Eppendorf needle probes utilizing polarographic techniques remain the gold standard for in vivo oxygen measurements.18,19 These needle probes, however, are invasive, magnetic resonance (MR)-unsafe, and only measure the oxygen at a single point necessitating multiple measurements to map the average oxygenation of a given volume. Additionally, these probes may consume oxygen during operation and may introduce oxygen to the system during the mechanically invasive measurement procedure. Luminescence-based fiber-optic (FO) sensors are an MR-compatible oxygen-measurement technique providing equivalent results when compared with Eppendorf probes while remaining invasive and limited to single-point measurements.20 Optical and photoacoustic lifetime-imaging techniques remain applicable only to superficial oxygen measurements.19,21,22 Nuclear medicine techniques require the injection of radioactive tracers, often require an on-site accelerator, and the results remain difficult to quantify.18,19,23
Magnetic resonance spectroscopy (MRS) allows for noninvasive, real-time, aggregate oxygen measurements in vivo. The spin-lattice relaxation rate constant (R1) is sensitive to oxygen concentration as a result of dipole–dipole interactions between the nucleus and the unpaired electrons in the dissolved oxygen gas.18,24,25 Because R1 is sensitive to oxygen concentration rather than pO2, R1 measurements are independent of gas solubility, but can easily be converted to pO2 values using Henry's law.26 Perfluorinated compounds (PFCs) are commonly used as MRS oxygen probes because of their high oxygen solubility and hydrophobic nature.25,26 The hydrophobicity prevents the R1 of such compounds from being influenced by confounding factors in vivo such as variable ion concentrations or pH. The most prevalent and promising PFC probes found in literature include perfluoro-15-crown-5-ether (PFCE), hexafluorobenzene (HFB), perfluorodecalin (PFD), perfluorooctyl bromide (PFOB), and perfluorotributylamine (PFTBA).24,25,27–40 Hexamethyldisiloxane (HMDSO), a 1H probe, has shown promise as well.37,41
MRS methods utilizing these probes have been used to both measure and image oxygen in vivo, but have had limited clinical applications due to large measurement errors and difficulty with molecule delivery to target tissues. Both the R1 and oxygen sensitivity (change in R1 for a given change in pO2 at a specified temperature) of the MRS probes are affected by temperature and static magnetic field strength making a proper calibration critical to minimizing pO2 measurement error. This study used temperature-compensating multiparametric calibrations to reduce measurement error.27 Additionally, most previous 19F-MRS oximetry studies evaluated PFCs directly injected into the tissue of interest37,40 or intravenously into tumors that often have chaotic and ineffective vasculature,27,28,30,31,34,35 which resulted in inhomogeneous distributions of the probes. An additional benefit of measuring pO2 within TEGs, therefore, is the removal of limitations due to ineffectual probe delivery.
Previous studies have successfully measured the pO2 of encapsulated cell systems in vivo. PFC-loaded alginate beads implanted into the rodent kidney capsule, peritoneal cavity, and quadriceps femoris muscle reveal a hypoxic environment unconducive to islet survival.38,42–44 Macroencapsulated cell systems, however, are often implanted into the subcutaneous space2 and these studies, limited to microencapsulation, did not examine the oxygen availability of this site.
In this study, we first measured the oxygen and temperature sensitivities of several MRS oxygen probes to choose the most appropriate probe for our application. We then developed MRS techniques to measure encapsulated TEG pO2 in vitro and implanted into a subcutaneous pocket of a rat in vivo. Additionally, both techniques were validated with FO pO2 probes. Finally, we demonstrated that changes in TEG pO2 resulting from DSO can be monitored with our MRS relaxometry technique.
Materials and Methods
19F and 1H R1 measurement
19F-MRS spectra were acquired with a 16.4 T MR system (Agilent Technologies, Santa Clara, CA) using a custom-built single-loop surface coil tuned to 656.8 MHz. For HMDSO, the coil was tuned to 1H (698.2 MHz). A standard inversion-recovery pulse sequence was used to measure R1. Adiabatic half-passage (90°) and inversion (180°) pulses were used to ensure uniform flip angles. For each scan, the magnetization recovery was interrogated after various delay times. Single spectra were acquired for in vitro experiments, but in vivo data were averaged (N = 2) to increase the signal-to-noise ratio (SNR) and reduce errors. The unapodized spectra were processed by automatically correcting the phase, baseline, and drift using VnmrJ (Agilent Technologies). The peaks were then integrated and the peak areas were fit to Equation (1) using a three-parameter nonlinear regression with GraphPad Prism (v5; GraphPad Software, Inc., La Jolla, CA). Equation (1) is the solution to the Bloch equation for the longitudinal magnetization (Mz) during an inversion-recovery pulse sequence when repetition time (TR) >>T1 and echo time <<T2, where α and β are fitting constants and t is the recovery time. The additional fitting constant was utilized because it can compensate for imperfect inversion and has been previously and successfully applied to 19F relaxometry.27,34,35,42,44
 |
PFC and HMDSO MRS calibration
Various probes were tested for oxygen and temperature sensitivity using neat compounds. To calibrate each PFC (PFCE [Exfluor Research Corporation, Round Rock, TX], HFB [Sigma-Aldrich, St. Louis, MO], PFD [Fluoromed, L.P, Round Rock, TX], PFOB [Sigma-Aldrich], PFTBA [Sigma-Aldrich]), and HMDSO (Sigma-Aldrich), 1.8 mL of the selected molecule was placed into a 2 mL vial. The vial was placed in the center of the surface coil; the coil was tuned, and inserted into the magnet. PFCE, HFB, and HMDSO benefit from processing only a single resonance. For PFD, PFOB, and PFTBA (which have multiple resonances), the I-,45 θ-,29 and δ-29 resonances, respectively, were interrogated. The sample was then bubbled with a gas of known oxygen concentration for 30 min while the temperature was held constant. The magnet was shimmed and R1 was then measured as described above and delay times were optimized to each sample's R1.46,47 Measurements were made for five different pO2s (0, 38, 76, 114, and 160 mmHg) at three different temperatures (21, 37, and 45°C). An FO temperature probe (SA Instruments, Inc., Stony Brook, NY) verified the temperature of the sample. Each condition was measured in replicate (N = 2–6) with a TR of at least 5/R1. The R1 was assumed to be a function of the pO2, temperature (T), and four fitting constants [A, B, C, and D; Eq. (2)] as previously described.27 This model accounts not only for the dipolar interactions of the dissolved oxygen gas, but for the effects of temperature on the correlation time and dissolved oxygen concentration as well. Fitting constant A is a result of dipole–dipole interactions between the magnetic moments of the nuclei of the probes (the most significant energy-transfer mechanism leading to relaxation in the anoxic probes). Fitting constant B is attributable to additional dipole–dipole interactions between the nuclei of the probes and the unpaired electrons of the dissolved oxygen gas, which quickens relaxation. Temperature alters the correlation time (the time scale on which these magnetic field fluctuations occur) and necessitates fitting constant C. Fitting constant D is due to cross-interactions of oxygen and temperature, mainly the temperature dependence of the Henry constant. Many of the physical processes associated with the fitting constants are complex, but yield a surprisingly linear relationship for the temperatures and pO2s typically encountered during in vivo experiments.27 R1 measurements were fit to the multiparametric calibration equation using a Levenberg–Marquardt algorithm (OriginLab, Northampton, MA).
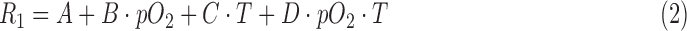 |
TEG construction
TEGs were prepared by loading a macroencapsulation device with an oxygen-sensitive matrix. PFCE was chosen for the probe because of its high oxygen sensitivity, high nuclear molarity (equivalent nuclei per volume), and excellent reported biocompatibility.32,40 Fluorine-loaded fibrin gels were prepared with 75 μL of porcine plasma (Sigma-Aldrich) emulsified with 75 μL of PFCE. The mixture was emulsified by manual agitation, injected into a 40 μL immunoisolation device (TheraCyte, Inc., Laguna Hills, CA) using a 250-μL precision syringe (Hamilton Company, Reno, NV), and then cross-linked with 5% v/v bovine thrombin solution. The thrombin solution was made by diluting concentrated topical thrombin solution (GenTrac, Inc., Middleton, WI) in phosphate-buffered saline with calcium and magnesium (PBS++) (Gibco, Thermo Fisher Scientific, Waltham, MA). The cell access port was trimmed short, and sealed with adhesive (Dermabond; Ethicon, Inc., Somerville, NJ).
19F-MRS TEG calibration
TEGs were prepared as described above and placed into a 50 mL conical tube filled with PBS++. An FO oxygen probe (Ocean Optics, Dunedin, FL) was inserted into the TEG for simultaneous comparison with 19F-MRS-measured pO2. The vial was placed in the center of the coil; the coil was tuned, and inserted into the magnet. The sample was then bubbled with a gas of known oxygen concentration for 30 min while the temperature was held constant. The magnet was shimmed and R1 was then measured as described above with 10 different delay times (0.05, 0.1, 0.15, 0.25, 0.5, 0.75, 1, 2, 4, and 5 s). Measurements were made for five different pO2s (0, 38, 76, 114, and 160 mmHg) at three different temperatures (21, 37, and 45°C) as verified with the FO temperature probe as described above. Each condition was measured in replicate (N = 6) with a TR of 6 s. The R1 was again fit to the multiparametric calibration equation using a Levenberg–Marquardt algorithm.
TEG implantation
All animal research was approved and overseen by the University of Minnesota Institute for Animal Care and Use Committee (IACUC). A total of nine male Lewis rats were implanted with a PFCE-loaded TEG. On the day of implantation, rats were anesthetized with inhalation isoflurane using a respirator and vaporizer. The surgical sites were shaved with an electric clipper, and the skin was surgically prepped with chlorhexidine or equivalent antiseptic. A 1.5 cm incision was made in the skin on the dorsal side, just inferior to the scapulae, and perpendicular to, but symmetric across the medial line. Using gentle blunt dissection, a small pocket was created adjacent to the vertebral column just large enough to accommodate the device snugly. The pocket was rinsed and lubricated with saline, and the device was tucked into the pocket. The device was completely fabricated using plastic (PTFE) materials, designed for flexibility and biocompatibility. The incision was closed with absorbable suture in a normal fashion using at least two layers and taking care to make sure the device components were not directly below the incision. Surgical glue was applied over the closed incision as a barrier. NSAIDs (meloxicam, 1 mg/kg) were administered by subcutaneous injection to minimize pain and inflammation for three or more days following surgery. Animals were monitored daily during recovery for up to 14 days until the incision was fully healed.
In vivo oximetry in rats
The subcutaneous TEG pO2 of six rats was evaluated 1, 4, 8, 15, 22, and 29 days postimplantation. On the day of measurement, rats were anesthetized with inhalation isoflurane, immobilized onto a holder with the device centered over the custom-built surface coil, stabilized at a core body temperature of 37°C ± 0.2°C as measured with a rectal thermocouple, and inserted into the 16.4 T horizontal-bore magnet. Animals were continuously monitored during scanning with anesthesia depth and forced-air heater temperature adjusted as needed. After the coil was tuned and the magnet shimmed, R1 measurements (N = 6) were made with the inversion-recovery sequence with adiabatic pulses used for TEG calibration. The R1 of each measurement was then converted to a pO2 using the multiparametric calibration previously determined in vitro. Following completion of the study, animals were promptly euthanized using CO2 for device explant and gross evaluation.
Validation of 19F-MRS in vivo pO2 measurements
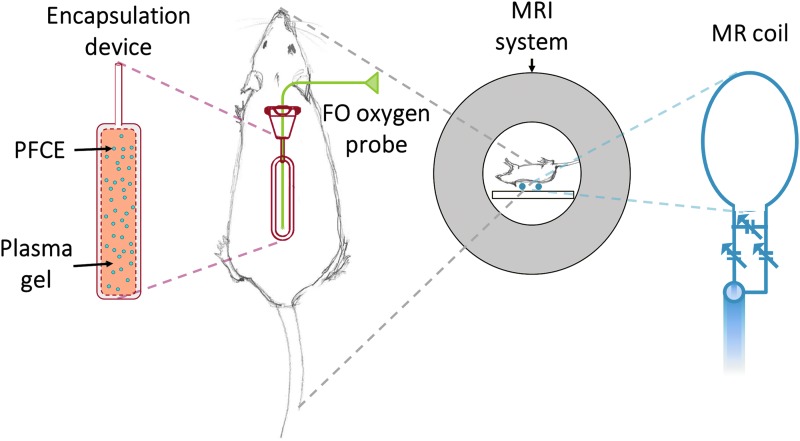
The 19F-MRS pO2 measurements of a subset of TEGs (N = 3) were validated with an FO probe in vivo. These devices were equipped with an additional access port (In-Line access port; Access Technologies, Skokie, IL). On the day of scanning, rats were anesthetized and a transcutaneous catheter was inserted into the access port. A previously calibrated ultra-thin 250 μm FO probe (Ocean Optics) was then threaded through the catheter into the TEG and the rat was inserted into the MR system (Fig. 1). FO pO2 was measured simultaneously with the MRS pO2 measurement technique for direct comparison.
FIG. 1.
Illustration of a macroencapsulation device with access port (red) in a dorsal subcutaneous pocket of a Lewis rat. For FO measurements, the 250-μm thin-fiber oxygen probe (green) was inserted transcutaneously through the skin and access port into the cell compartment of the device. The 19F-MRS coil (blue) was placed on the dorsal side of the anesthetized rat to detect the 19F signal of the PFCE within the device. FO, fiber optic; MRS, magnetic resonance spectroscopy.
TEGs with DSO
A small group of rats (N = 3) were acclimated with a harness and tether apparatus (Instech Laboratories, Plymouth Meeting, PA) for 2 weeks before implantation with a TEG modified for DSO. The harness and tether apparatus contains a swivel, and counter-balance system to allow for animal movement around the cage, and normal feeding, drinking, and grooming behaviors. The TEGs were modified to include an internal compartment and cannulae for transcutaneous oxygen delivery in vivo. Briefly, two additional internal membranes were added to the macroencapsulation device previously described to create a three-chamber device with an internal oxygen compartment, and two distinct cell compartments superior and inferior to this central oxygen compartment. Further, three additional access ports were added to the device to allow access to each compartment. Two access ports provided access to the central compartment to allow for oxygen gas infusion and effusion while two additional ports provided access to the superior and inferior cell compartments. TEGs for DSO were prepared by loading the cell compartments of the modified devices with the acellular PFCE-plasma gel emulsion described above.
The TEGs were then implanted in the subcutaneous space as described above leaving the oxygen compartment access cannulae protruding through the incision for attachment to the harness and tether apparatus. The tether system was connected through the cage lid to an electrochemical oxygen generator (Giner, Inc., Newton, MA) through a specialized manifold to regulate and monitor the delivery of oxygen gas to the implanted devices. Pure humidified oxygen gas (760 mmHg) was continuously delivered through the harness and tether apparatus to the modified TEG for the duration of the study. Extended sections of tubing were used to continue DSO during pO2 assessment, which was measured with 19F-MRS 1 day postimplantation. DSO was then discontinued for 1 day and the pO2 of the TEG was reassessed with 19F-MRS. R1 measurement and conversion to pO2 was as described above except for the addition of a 760 mmHg pO2 to the calibration procedure.
Comparative data collection and analysis
A thorough review of literature was conducted to allow a comparison of our findings and determine whether the static magnetic field strength (B0) affects R1 oxygen sensitivity, R1 temperature sensitivity, or anoxic R1 of PFCE. Data were collected from relevant publications identified using electronic databases (PubMed and Google Scholar) and references cited in relevant publications. Data collected included B0, change in PFCE R1 with oxygen concentration, change in PFCE R1 with temperature, and PFCE anoxic R1. Results were excluded if any information other than change in PFCE R1 with temperature was not available. It was assumed that creating an emulsion with PFCE does not change its relaxation. After compilation, data were fitted assuming a linear relationship between B0 and the parameters of interest. These fittings were then used to determine the effect of B0 on the contrast-to-noise ratio (CNR) between anoxia and three partial pressures of oxygen (15, 40, and 100 mmHg). In calculating the CNR, it was assumed that proton densities were equal, T2 effects could be ignored, SNR increases linearly with B0, and TR = 5 ms. The excitation angle was adjusted for optimal T1 contrast as previously described.48
Results
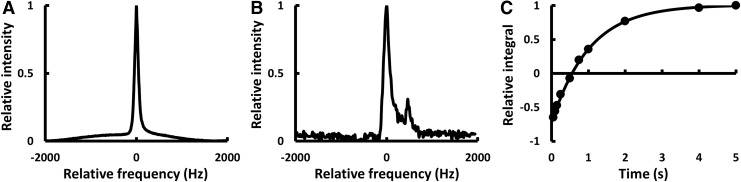
MRS spectra were successfully acquired both in vitro (Fig. 2A) and in vivo (Fig. 2B). Multiple peaks were occasionally observed in vivo, possibly due to animal motion or uneven redistribution of the PFCE within the immunoisolation devices over time. R1 was then determined using Equation (1) (Fig. 2C). The three-parameter equation that compensates for incomplete inversion was found to provide better fitting with smaller errors than a two-parameter equation. R1 determination was found to be highly repeatable and fitting errors were typically <0.5% in vitro and <5% in vivo. The in vivo errors were found to be greater due to respiratory motion and poorer SNR.
FIG. 2.
Typical 19F-MRS spectra from PFCE within an immunoisolation device (A) in vitro (SNR = 529; FWHM = 91 Hz) and (B) in vivo (SNR = 40.5; FWHM = 157.8 Hz). (C) The fitted inversion-recovery curve from the experiment that produced the in vivo spectrum (α = 0.861; β = 0.146; R1 = 0.985 s−1). FWHM, full width at half maximum; SNR, signal-to-noise ratio.
To evaluate the potential MRS probes, R1 data for each probe were fit to the multiparametric calibration equation previously described [Eq. (2)]. Probe characteristics and calibration results are summarized in Table 1. All probes had distinct advantages and weaknesses, making the choice of probe highly dependent on application. PFCE (chosen for our TEG studies) was confirmed to have a high number of equivalent nuclei (the number of nuclei in a molecule contributing to the MRS peak), high nuclear molarity (the number of nuclei [moles] contributing to an MRS peak per liter of compound), high oxygen sensitivity (change in R1 with pO2), high temperature sensitivity (change in R1 with temperature), and high anoxic R1 (fast relaxation without oxygen).
Table 1.
Results of the Calibration of the PFCs and HMDSO Revealed Differences in pO2 Sensitivity at 37°C, Anoxic Temperature Sensitivity, and Anoxic R1 at 37°C Between the Different Probes
| Probe | Equivalent nuclei | Nuclear molarity (M) | pO2 sensitivitya(10−5 mmHg−1·s−1) | Temperature sensitivityb(10−4 °C−1·s−1) | R1,a,b(s−1) |
|---|---|---|---|---|---|
| PFCE | 20 | 61 | 223.4 ± 15 | −197.4 ± 2.5 | 0.943 ± 0.014 |
| HFB | 6 | 52 | 133.2 ± 18 | −41.90 ± 2.7 | 0.388 ± 0.015 |
| PFD-I | 2 | 8 | 188.0 ± 38 | −76.90 ± 6.4 | 0.271 ± 0.031 |
| PFOB-θ | 3 | 12 | 199.6 ± 6.8 | −106.1 ± 1.4 | 0.657 ± 0.007 |
| PFTBA-δ | 9 | 25 | 226.0 ± 78 | −289.9 ± 16 | 1.551 ± 0.080 |
| HMDSO | 18 | 85 | 101.1 ± 11 | −3.013 ± 2.1 | 0.116 ± 0.011 |
37°C.
Anoxia.
PFCs, perfluorinated compounds; pO2, oxygen partial pressure.
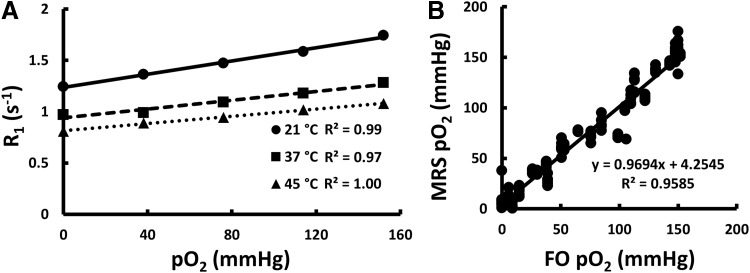
PFCE was then incorporated into TEGs and the R1 was again found to be directly proportional to pO2 with the slope and intercept dependent on temperature (Fig. 3A). A multiparametric calibration equation containing R1, pO2, and temperature [Eq. (2)] was successfully extrapolated (Table 2). 19F-MRS oxygen measurements correlated well (p < 0.0001) with FO oxygen measurements in vitro (Fig. 3B). These data validate 19F-MRS as an accurate and reliable measurement technique in vitro.
FIG. 3.
In vitro oxygen calibration and validation using 19F-MRS relaxometry and FO oxygen techniques. (A) Calibration of PFCE-loaded TEGs demonstrated that R1 changes linearly with pO2. The slope and intercept of the calibration line changed with temperature. These values were used to generate the multiparametric calibration coefficients. The error bars are contained within the markers. (B) Plotting 19F-MRS against FO in vitro oxygen measurements showed a highly correlated relationship. These results validate the success of an accurate and reliable calibration (Pearson's R = 0.98, slope = 0.97, intercept = 4.3 mmHg). pO2, oxygen partial pressure; TEG, tissue-engineered graft.
Table 2.
Fitting Parameters Resulting from the Neat PFCE Calibration and the TEG Calibrations Used for the No DSO and DSO R1 Measurements
| A (s−1) | B (10−3 mmHg−1·s−1) | C (10−2 °C−1·s−1) | D (10−5 mmHg−1·°C−1·s−1) | |
|---|---|---|---|---|
| Neat | 1.67 ± 0.01 | 3.64 ± 0.11 | −1.97 ± 0.03 | −3.80 ± 0.27 |
| TEG, No DSO | 1.65 ± 0.02 | 4.46 ± 0.19 | −1.93 ± 0.05 | −5.67 ± 0.53 |
| TEG, DSO | 1.66 ± 0.03 | 3.85 ± 0.06 | −1.98 ± 0.08 | −4.62 ± 0.16 |
DSO, delivery of supplemental oxygen; TEG, tissue-engineered graft.
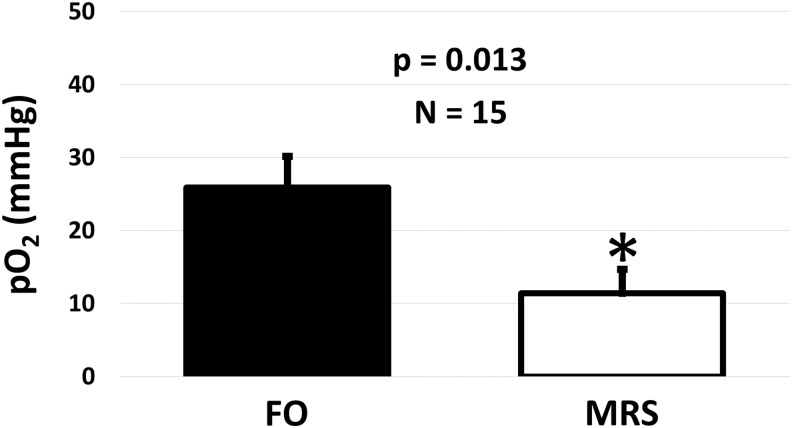
In vivo validation using simultaneous FO and 19F-MRS techniques for measuring pO2 within implanted TEGs showed that FO probes consistently measured significantly higher oxygen levels than 19F-MRS when compared using a two-tailed, paired Student's t-test (Fig. 4). The pO2s measured with the FO oxygen probe were higher than 19F-MRS measurements, but all measurements indicated a hypoxic or anoxic condition in vivo. The variability of FO oxygen measurements was found to be greater than 19F-MRS measurements.
FIG. 4.
Average in vivo pO2 measured in implanted TEGs over 29 days using FO and 19F-MRS techniques. Oxygen levels measured with both methods were less than venous blood pO2 (40 mmHg), but there was a significant difference between methods with the FO probes measuring an average of 14 ± 6 mmHg higher than 19F-MRS (*indicates significant difference p < 0.05).
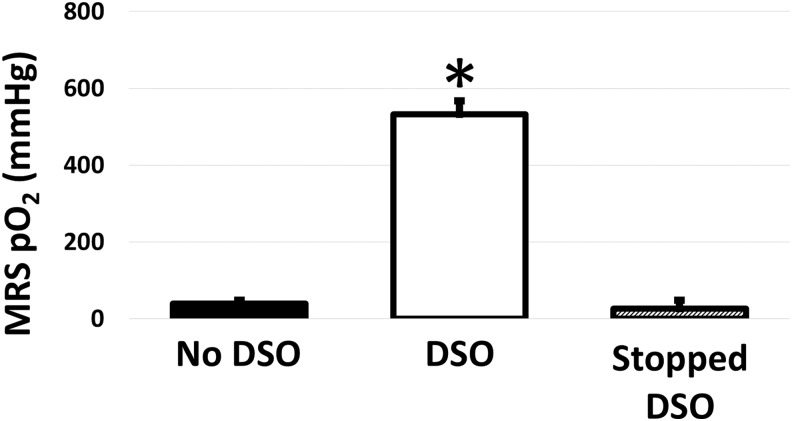
Oxygen gas was directly supplied to a subset of in vivo TEGs (N = 3) to ensure that 19F-MRS could accurately detect a local increase in oxygenation. It was observed that TEGs with DSO had significantly higher pO2s (p < 0.0001), as expected, with the supplemental oxygen increasing the TEG pO2 from 39 ± 10 to 532 ± 35 mmHg as measured with 19F-MRS. With the cessation of DSO, TEG pO2 returned to the hypoxic levels previously seen without oxygen delivery (26 ± 22 mmHg; Fig. 5).
FIG. 5.
In vivo pO2 in implanted TEGs without DSO, with DSO, and after DSO was stopped as measured noninvasively with 19F-MRS. DSO increased pO2 in implanted TEGs. After DSO was discontinued, the reassessed pO2 showed a return to low pO2 previously seen without oxygen delivery. (*indicates a significant difference, p < 0.0001, when compared to the No DSO and Stopped DSO conditions). DSO, delivery of supplemental oxygen.
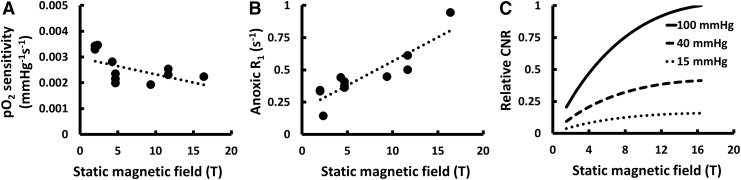
To compare our PFCE calibration results to other published values and evaluate the effect of B0 on 19F-MRS relaxometry for the measurement of pO2, a comprehensive literature review was conducted. Eleven publications reporting information on PFCE R1 oxygen sensitivity were identified.28,30–36,38–40 One was excluded for inconsistent reporting of data38 and another was excluded for results that differed from other reported data by several standard deviations,40 leaving eleven reported calibrations28,30–36,39 that were combined with our data for analysis. Oxygen sensitivity decreased (−6.39 × 10−5 mmHg−1·s−1/T; Pearson's R = −0.53; Fig. 6A), anoxic R1 increased (3.74 × 10−2 s−1/T; Pearson's R = 0.89; Fig. 6B), and calculated oxygen CNR increased (Fig. 6C) with increasing B0. Only three additional publications reported the R1 temperature sensitivity of PFCE,28,30,35 which prevented a meaningful analysis.
FIG. 6.
Predicted changes in oxygen sensitivity, anoxic R1, and theoretical oxygen CNR in an optimized gradient-echo image as a function of static magnetic field strength in Tesla (T) resulting from an analysis of our measurements combined with previously reported measurements. It was found that (A) oxygen sensitivity decreases, (B) anoxic R1 increases, and (C) theoretical oxygen CNR in an optimized gradient-echo image increases with increasing static magnetic field strength. When transitioning from 1.5 to 16.4 T, the results predict a 33% decrease in oxygen sensitivity at 37°C, a 223% increase in anoxic R1, and a 387% increase in CNR between 0 and 100 mmHg. CNR, contrast-to-noise ratio.
Discussion
Accurate oxygen measurements are critical to the development of TEGs for the treatment of diabetes and other diseases. Traditional sensors for oxygen measurement in vivo use polarographic needle or FO fluorescence-quenching probes. These traditional probes are invasive, often inaccurate, and only provide a single-point measurement at the tip of the probe itself, and, therefore, multiple mechanically disruptive measurements are required to obtain an average pO2 measurement with small errors. This work demonstrates a method for noninvasive oxygen measurements within TEGs in vivo using MRS that overcomes many of these limitations, and offers an accurate, noninvasive, and temperature-compensating technique for TEG pO2 measurements in vivo.
Our methods were optimized and adapted for measurements within macroencapsulated TEGs at ultra-high-field 16.4 T MRS to maximize signal intensities. We examined a number of potential oxygen probes and found that PFCE offers the best combination of high oxygen sensitivity, high nuclear molarity, and reported biocompatibility. Our data also demonstrate the sensitivity of R1 to both oxygen and temperature. Additionally, the oxygen sensitivity of the probes were found to be dependent on temperature through Henry's law, necessitating that accurate MRS oxygen measurement techniques address this multiparametric physical nature. Controlling and measuring the temperature of an implanted oxygen probe is technically challenging, but due to the high sensitivity of MRS relaxation rates to both oxygen and temperature, temperature must be considered to measure pO2 accurately and precisely.
To account for the effects of both oxygen and temperature on R1, we used a multiparametric oxygen and temperature calibration using acellular TEGs loaded with a PFCE-impregnated plasma-gel matrix. High accuracy and precision in calibration were achieved by measuring R1 using a surface coil, a standard inversion recovery pulse sequence with adiabatic pulses, and a three-parameter fit accounting for incomplete inversion. These measurements correlated well with measurements from an FO oxygen probe, suggesting that our MRS technique could reliably measure the pO2 of in vitro samples with a high level of accuracy. Despite these complex calibrations, the in vivo oxygen measurements were still susceptible to errors in temperature measurement, and MRS methods for in vivo oximetry would greatly benefit from improved temperature measurement techniques, especially if the temperature measurements could be localized to the area of interest. An MRS method to locally measure temperature in vivo would be an ideal complement to MRS oximetry, and development in this area would greatly improve the sensitivity and accuracy of the described methods.
Our technique was validated in vivo with a series of rats implanted with TEGs that were modified with the addition of an access port to allow for insertion of an FO oxygen probe. This modification allowed for simultaneous pO2 measurements using FO and MRS at various time points. A paired comparison between FO and MRS oxygen measurements indicated a significant difference in the measured in vivo pO2 within implanted TEGs. While this difference is significant, both measurement techniques confirm a hypoxic oxygen level within the TEG in vivo. The disparity is due to critical differences between the FO and MRS methods. The FO oxygen probe provides a single-point measurement of pO2 within the TEG, while MRS provides a volume-averaged measurement of the internal pO2. This difference makes the FO probe method susceptible to local variabilities in oxygen levels, and probe placement is critical for measurements with FO probes. It is also important to note that the FO probe requires invasive intrusion into the TEG itself to obtain an oxygen measurement. The probe was inserted into the central compartment of the TEG, and this disturbs the matrix within the TEG. The FO probe is also likely introducing oxygen to the TEG during the insertion process, as it will be acclimated to atmospheric pO2 and opens access to the external environment during the insertion procedure, which will likely contribute to measurement error. These likely contributed to the measurements with the FO oxygen probe having higher standard deviations when compared to the MRS technique. Despite the differences observed with these in vivo studies, MRS was found to provide reliable and reproducible measurements of internal TEG pO2, and offers significant advantages over traditional invasive pO2 measurement techniques.
For further confirmation of MRS pO2 measurement techniques in vivo, the macroencapsulation device was modified to allow for continuous DSO to the TEG in vivo. MRS pO2 measurements suggest a dramatic increase in local pO2 in the TEGs with DSO. The results of these preliminary studies prove that MRS oxygen measurements can be used to measure the pO2 of therapeutically relevant macroencapsulated TEGs in vivo. These measurements can be used to optimize these types of interventions, and to determine the optimal parameters for DSO.
The literature review clearly demonstrates that MRS relaxometry can be successfully applied to measure pO2 at a variety of B0, but that ultra-high-field is often advantageous for MRS pO2 measurements. While our review suggests the oxygen sensitivities of MRS probes decrease with increasing B0, there is a larger increase in SNR (and CNR) due to the increasing field and increase in R1. For example, when increasing from 1.5 to 16.4 T, oxygen sensitivity decreases by a factor of 1.5 at 37°C, but the anoxic R1 increases by a factor of 3.2 and the CNR between anoxia and 100 mmHg increases by a factor of 4.9.
In conclusion, these studies confirm that MRS can be an accurate and noninvasive technique to monitor TEG pO2 for long durations postimplantation. A multiparametric calibration was successfully developed to compensate for the temperature variability that is encountered during measurements, and this method improved the measurements of pO2. Rigorous validations of our MRS technique with FO probes both in vitro and in vivo confirmed reliable measurements of internal TEG pO2, and that this method can detect both natural physiological changes in the oxygen environment and interventional changes such as DSO. This technique will be a valuable tool to study the natural progression of oxygenation within implanted TEGs and to evaluate the success of DSO for increasing the internal pO2 of implanted TEGs.
Acknowledgments
This work was supported in part by the Minnesota Lions Diabetes Foundation, the Schott Family Foundation, the Schulze Family Foundation, JDRF 5-2013-141, and NIH grants P41 EB015894 and S10 RR025031. The authors would like to thank all members of the Center for Magnetic Resonance Research for their thoughtful discussions, especially Drs. Bruce Hammer and Louis Kidder. Additional thanks to Jody Janecek, Dr. Leah Steyn, and Jessica Einstein.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ludwig B., Reichel A., Steffen A., Zimerman B., Schally A.V.V., Block N.L.L., et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci 110, 19054, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colton C.K. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev 67, 93, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Yang H.K., and Yoon K.-H. Current status of encapsulated islet transplantation. J Diabetes Complications 29, 1, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Iacovacci V., Ricotti L., Menciassi A., and Dario P. The bioartificial pancreas (BAP): biological, chemical and engineering challenges. Biochem Pharmacol 100, 12, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Weir G.C. Islet encapsulation: advances and obstacles. Diabetologia 56, 1458, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Scharp D.W., and Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 67, 35, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Emamaullee J.A., and Shapiro A.M.J. Factors influencing the loss of β-cell mass in islet transplantation. Cell Transplant 16, 1, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Suszynski T.M., Avgoustiniatos E.S., and Papas K.K. Intraportal islet oxygenation. J Diabetes Sci Technol 8, 575, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahearn A.J., Parekh J.R., and Posselt A.M. Islet transplantation for Type 1 diabetes: where are we now? Expert Rev Clin Immunol 11, 59, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Plesner A., and Verchere C.B. Advances and challenges in islet transplantation: islet procurement rates and lessons learned from suboptimal islet transplantation. J Transplant 2011, 1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papas K.K., Avgoustiniatos E.S., and Suszynski T.M. Effect of oxygen supply on the size of implantable islet-containing encapsulation devices. Panminerva Med 58, 72, 2016 [PubMed] [Google Scholar]
- 12.Dionne K.E., Colton C.K., and Yarmush M.L. Effect of oxygen on isolated pancreatic tissue. ASAIO Trans 35, 739, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Papas K.K., Avgoustiniatos E.S., Tempelman L.A., Weir G.C., Colton C.K., Pisania A., et al. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc 37, 3412, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Barkai U., Weir G.C., Colton C.K., Ludwig B., Bornstein S.R., Brendel M.D., et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant 22, 1463, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Evron Y., Zimermann B., Ludwig B., Barkai U., Colton C., Weir G., et al. Oxygen supply by photosynthesis to an implantable islet cell device. Horm Metab Res 47, 24, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Suszynski T.M., Avgoustiniatos E.S., Stein S.A., Falde E.J., Hammer B.E., and Papas K.K. Assessment of tissue-engineered islet graft viability by fluorine magnetic resonance spectroscopy. Transplant Proc 43, 3221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suszynski T.M. Tissue-Engineered Islet Graft Design and Assessment. Minneapolis, MN: University of Minnesota, 2012. [Google Scholar]
- 18.Zhao D., Jiang. L.P., and Mason R. Measuring changes in tumor oxygenation. Methods Enzymol 386, 378, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Vikram D.S., Zweier J.L., and Kuppusamy P. Methods for noninvasive imaging of tissue hypoxia. Antioxid Redox Signal 9, 1745, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Shaw A.D., Li Z., Thomas Z., and Stevens C.W. Assessment of tissue oxygen tension: comparison of dynamic fluorescence quenching and polarographic electrode technique. Crit Care 6, 76, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amao Y. Probes and polymers for optical sensing of oxygen. Microchim Acta 143, 1, 2003 [Google Scholar]
- 22.Shao Q., and Ashkenazi S. Photoacoustic lifetime imaging for direct in vivo tissue oxygen monitoring. J Biomed Opt 20, 36004, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordji-Nejad A., Möllenhoff K., Oros-Peusquens A.M., Pillai D.R., and Shah N.J. Characterizing cerebral oxygen metabolism employing oxygen-17 MRI/MRS at high fields. MAGMA 27, 81, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Parhami P., and Fung B. Fluorine-19 relaxation study of perfluoro chemicals as oxygen carriers. J Phys Chem 87, 1928, 1983 [Google Scholar]
- 25.Ruiz-Cabello J., Barnett B.P., Bottomley P.A., and Bulte J.W.M. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 24, 114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riess J.G. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol 33, 47, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Mason R.P., Shukla H., and Antich P.P. In vivo oxygen tension and temperature: simultaneous determination using 19F NMR spectroscopy of perfluorocarbon. Magn Reson Med 29, 296, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Dardzinski B.J., and Sotak C.H. Rapid tissue oxygen tension mapping using 19F inversion-recovery echo-planar imaging of perfluoro-15-crown-5-ether. Magn Reson Med 32, 88, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Shukla H.P., Mason R.P., Woessner D.E., and Antich P.P. A comparison of three commercial perfluorocarbon emulsions as high-field 19F NMR probes of oxygen tension and temperature. J Magn Reson Ser B 131, 1995 [Google Scholar]
- 30.Helmer K.G., Han S., and Sotak C.H. On the correlation between the water diffusion coefficient and oxygen tension in RIF-1 tumors. NMR Biomed 11, 120, 1998 [DOI] [PubMed] [Google Scholar]
- 31.van der Sanden B.P.J., Heerschap A., Simonetti A.W., Rijken P.F.J.W., Peters H.P.W., Stuben G., et al. Characterization and validation of noninvasive oxygen tension measurements in human glioma xenografts by 19F-MR relaxometry. Int J Radiat Oncol 44, 649, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Duong T.Q., and Kim S.G. In vivo MR measurements of regional arterial and venous blood volume fractions in intact rat brain. Magn Reson Med 43, 393, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Duong T.Q., Iadecola C., and Kim S.G. Effect of hyperoxia, hypercapnia, and hypoxia on cerebral interstitial oxygen tension and cerebral blood flow. Magn Reson Med 45, 61, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Fan X., River J.N., Zamora M., Al-Hallaq H.A., and Karczmar G.S. Effect of carbogen on tumor oxygenation: combined fluorine-19 and proton MRI measurements. Int J Radiat Oncol Biol Phys 54, 1202, 2002 [DOI] [PubMed] [Google Scholar]
- 35.McNab J.A., Yung A.C., and Kozlowski P. Tissue oxygen tension measurements in the Shionogi model of prostate cancer using 19F MRS and MRI. MAGMA 17, 288, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Gross J.D.D., Long R.C.R.C., Constantinidis I., and Sambanis A. Monitoring of dissolved oxygen and cellular bioenergetics within a pancreatic substitute. Biotechnol Bioeng 98, 261, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kodibagkar V.D., Wang X., and Mason R.P. Physical principles of quantitative nuclear magnetic resonance oximetry. Front Biosci 13, 1371, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Goh F., and Sambanis A. In vivo noninvasive monitoring of dissolved oxygen concentration within an implanted tissue-engineered pancreatic construct. Tissue Eng Part C Methods 17, 887, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goh F. The use of perfluorocarbons in encapsulated cell systems: their effect on cell viability and function and their use in noninvasively monitoring the cellular microenvironment. Atlanta, GA: Georgia Institute of Technology, 2011. [Google Scholar]
- 40.Mignion L., Magat J., Schakman O., Marbaix E., Gallez B., and Jordan B.F. Hexafluorobenzene in comparison with perfluoro-15-crown-5-ether for repeated monitoring of oxygenation using 19F MRI in a mouse model. Magn Reson Med 69, 248, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kodibagkar V.D., Cui W., Merritt M.E., and Mason R.P. Novel 1H NMR approach to quantitative tissue oximetry using hexamethyldisiloxane. Magn Reson Med 55, 743, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Nöth U., Gröhn P., Jork A., Zimmermann U., Haase A., and Lutz J. 19F-MRI in vivo determination of the partial oxygen pressure in perfluorocarbon-loaded alginate capsules implanted into the peritoneal cavity and different tissues. Magn Reson Med 42, 1039, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann U., Nöth U., Gröhn P., Jork A., Ulrichs K., Lutz J., et al. Non-invasive evaluation of the location, the functional integrity and the oxygen supply of implants: 19F nuclear magnetic resonance imaging of perfluorocarbon-loaded BA2+-alginate beads. Artif Cells Blood Substit Immobil Biotechnol 28, 129, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Goh F., Long R., Simpson N., and Sambanis A. Dual perfluorocarbon method to noninvasively monitor dissolved oxygen concentration in tissue engineered constructs in vitro and in vivo. Biotechnol Prog 27, 1115, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Fung B.M. Selective detection of multiplets via double quantum coherence: A19F NMR study of perfluorodecalin. Org Magn Reson 21, 397, 1983 [Google Scholar]
- 46.Bernassau J-M., and Hyafil F. Choice of delay time sequence in spin-lattice relaxation time measurements by inversion-recovery. J Magn Reson 40, 245, 1980 [Google Scholar]
- 47.Weiss G.H., and Ferretti J.A. Optimal design of relaxation time experiments. Prog Nucl Magn Reson Spectrosc 20, 317, 1988 [Google Scholar]
- 48.Buxton R., Edelman R., Rosen B., Wismer G., and Brady T. Contrast in rapid MR imaging: T1- and T2-weighted imaging. J Comput Assist Tomogr 11, 7, 1987 [DOI] [PubMed] [Google Scholar]