Abstract
Mild traumatic brain injury (mTBI) is a major public health issue, representing 75–90% of all cases of TBI. In clinical settings, mTBI, which is defined as a Glascow Coma Scale (GCS) score of 13–15, can lead to various physical, cognitive, emotional, and psychological-related symptoms. To date, there are no pharmaceutical-based therapies to manage the development of the pathological deficits associated with mTBI. In this study, the neurotrophic and neuroprotective properties of glucose-dependent insulinotropic polypeptide (GIP), an incretin similar to glucagon-like peptide-1 (GLP-1), was investigated after its steady-state subcutaneous administration, focusing on behavior after mTBI in an in vivo animal model. The mTBI rat model was generated by a mild controlled cortical impact (mCCI) and used to evaluate the therapeutic potential of GIP. We used the Morris water maze and novel object recognition tests, which are tasks for spatial and recognition memory, respectively, to identify the putative therapeutic effects of GIP on cognitive function. Further, beam walking and the adhesive removal tests were used to evaluate locomotor activity and somatosensory functions in rats with and without GIP administration after mCCI lesion. Lastly, we used immunohistochemical (IHC) staining and Western blot analyses to evaluate the inflammatory markers, glial fibrillary acidic protein (GFAP), amyloid-β precursor protein (APP), and bone marrow tyrosine kinase gene in chromosome X (BMX) in animals with mTBI. GIP was well tolerated and ameliorated mTBI-induced memory impairments, poor balance, and sensorimotor deficits after initiation in the post-injury period. In addition, GIP mitigated mTBI-induced neuroinflammatory changes on GFAP, APP, and BMX protein levels. These findings suggest GIP has significant benefits in managing mTBI-related symptoms and represents a novel strategy for mTBI treatment.
Key words: : amyloid-β precursor protein, BMX, cognitive dysfunction, controlled cortical impact, GFAP, glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, mild traumatic brain injury, neuroinflammation
Introduction
Traumatic brain injury (TBI) impacts 10 million persons annually and is not only a global health problem but also a serious socioeconomic burden.1 Within the United States, TBI has become a major public and medical issue that affects an estimated 1.7 million persons with a cost of more than $76.5 billion to the US medical care system alone.2 TBI has a high morbidity, and no specific pharmaceutical-based treatments are available.3,4 Notably, mild TBI (mTBI), defined as a Glasgow Coma Scale (GCS) score of 13–15, constitutes 75–90% of all TBI survivors.5
Many patients with mTBI undergo a broad spectrum of neurological deficits. Frequently, there are profound functional deficits, such as headaches, dizziness, fatigue, irritability, memory problems, difficulty in concentration, depression, and anxiety, which may last from a few days up to few years.2,6–8 Further, mTBI-related cognitive impairments may significantly influence the quality of life, even after recovery from physical disabilities.9–13 These cognitive deficits may last over a prolonged period, leading to permanent neurocognitive dysfunction6 and may increase the risk for other neurodegenerative disorders, particularly dementia-related syndromes (e.g., Alzheimer disease [AD]).14
Previous studies have demonstrated that mTBI induces cognitive deficits in hippocampal-dependent learning and memory.9,15,16 Although the underlying mechanisms for these effects are unclear, the possible pathophysiological processes may involve cerebral ischemia/reperfusion, elevated levels of excitatory neurotransmitters, oxidative stress, and neuroinflammation. These processes could contribute to further neuronal degeneration, a disruption of synaptic connections and altered synaptic physiology.17–21
In our previous studies, we identified a TBI-associated biomarker, severity-dependent bone marrow tyrosine kinase gene in chromosome X (BMX), which is a nonreceptor protein-tyrosine kinase in the Tec family.22 BMX, also known as epithelial and endothelial tyrosine kinase (Etk), induces chronic inflammation via cytokine-mediated recruitment after ischemia.23–25
Among other potential TBI markers that are currently under investigation are glial fibrillary acidic protein (GFAP) and amyloid-β precursor protein (APP). GFAP, an intermediate filament protein, has a dual role that involves acute inflammation and chronic astrogliosis in glial scar formation after TBI.26 After TBI, GFAP is a well-characterized marker of activated astrocytes and reactive gliosis presence, with the potential to predict morbidity, mortality, and severity grading.27–30 This is of relevance, because previous studies have indicated that reactive astrocytes are implicated in the progression of AD,31,32 are sources of APP generation, and are both induced by and lead to amyloid-β (Aβ) plaque deposition.33,34
The accumulation of APP after TBI is a well described marker of axonal injury,35,36 which can lead to elevated Aβ production.37 In addition to neuronal loss, a hallmark of axonal injury, APP accumulation not only correlates with injury severity and post-TBI outcome,38,39 but also to impaired synaptic plasticity, and contributes to mTBI-related cognitive dysfunction when there is no overtly detectable cell loss.14,26,40
The selected measures of our studies therefore have relevance to two of the prime concerns of persons with mTBI: persistent cognitive dysfunction and the increasing risk of neurodegeneration.7,41,42 Currently, there are no available effective approved drugs to either control symptom progression after mTBI or offset pathways that ultimately lead to later neurodegenerative disorders.
Previous research has demonstrated that treatment with glucagon-like peptide-1 (GLP-1), an endogenous incretin, and long acting GLP-1 analogs provide neurotrophic and neuroprotective actions in many acute and chronic neurodegenerative models through GLP-1 receptor activation within the brain.43 Notably, GLP-1 receptor agonists have demonstrated favorable activity in cellular and animal models of mTBI.44–46
Glucose-dependent insulinotropic polypeptide (GIP) is a further incretin that, like GLP-1, stimulates glucose-dependent insulin secretion, increases insulin biosynthesis, and improves β-cell proliferation and survival via its cognate receptor.47 Hence, combined with GLP-1, GIP has been proposed as a treatment strategy for type 2 diabetes mellitus.48,49 Notably, GIP, similar to GLP-1 and agonists, appears to enter the brain, promotes synaptic plasticity via its reversal of hippocampal long-term potentiation (LTP) impairments,50 and has neuroprotective and neurotrophic properties,51 suggesting potential for halting or reversing neurodegenerative disorders.
In the current study, we used a number of novel experimental approaches to elucidate the impact of GIP on mTBI-induced behavioral deficits in an in vivo mTBI rat model. We then identified the possible regulatory mechanisms of GIP-related neuroprotection after mTBI.
Methods
Animal handling and preparation
Adult male Sprague-Dawley (SD) rats (250 to 300 g) were obtained from the National Laboratory Animal Center, Taipei, Taiwan, or from Taconic (Germantown, NY) for use in studies performed within Taipei Medical University and the Intramural Research Program of the National Institute on Aging, respectively. Rats were provided food and water ad libitum and were maintained on a 12 h light/dark cycle in a temperature- and humidity-controlled animal center at both medical institutions.
All experimental protocols were performed in accordance with either (1) the Guidelines for Animal Experiments of Taipei Medical University and the Guiding Principles for the Care and Use of Laboratory Animals approved by the Chinese Society of Laboratory Animal Sciences, Taiwan (LAC-100-0221), or (2) the Animal Care and Use Committee of the Intramural Research Program, National Institute on Aging (438-TGB-2016), and were in compliance with the guidelines for animal experimentation of the National Research Council (Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011) and the National Institutes of Health (DHEW publication 85-23, revised, 1995). A minimal number of rats were used for each study, and all efforts were made to minimize potential suffering.
Treatment groups
Modified surgical procedures were based on previously described methods.52 Anesthetized SD rats (tiletamine/zolazepam [10 mg/kg], and xylazine [2 mg/kg]) were placed in a stereotaxic frame. A craniotomy (5 mm in diameter) was performed in the right parietal cortex between bregma and lambda, 1 mm lateral from the midline. A mild grade TBI model was induced by a controlled cortical impact (CCI) device with a velocity of 2.5 m/sec at 1 mm depth. Body temperature was maintained at 37°C ± 1°C with a heating pad during surgery and afterward.
Human GIP or saline vehicle was delivered by a subcutaneous ALZET micro-osmotic pump that was implanted aseptically under anesthesia 2 days pre-injury. This delivered GIP over the course of 2 weeks at a rate of 21.58 or 38.85 μg/kg/day. There were three experimental groups, which included (1) mTBI animals saline-treated, (2) mTBI animals treated with the lower dose of GIP (21.58 μg/kg/day), and (3) mTBI animals treated with the higher dose of GIP (38.85 μg/kg/day).
Behavioral assessments
Several behavioral tests were chosen to identify the sensorimotor and cognitive impairments in our rat mTBI model. For cognition, long-term spatial memory was assessed by the Morris water maze (MWM), whereas recognition memory was evaluated by novel object recognition (NOR). The beam walking test and the adhesive removal test were used to evaluate sensorimotor functions in the rats with mTBI.
MWM
The MWM paradigm was used to evaluate long-term spatial memory.53 Animals were trained to find a hidden platform in a circular aluminum pool (150 cm in diameter and 75 cm deep) that was surrounded by visual cues placed at the same starting point over 5 consecutive days before mCCI. All animals were monitored by a video camera. Data were calculated by a computerized video tracking system. To test whether the function of visual acuity and muscle strength were affected after CCI, these tests were also conducted with visual water task.54
NOR
Recognition memory was evaluated using NOR, which is a task that uses a rat's innate preference to investigate a novel object versus an already known one.55 First, rats were habituated to an open field Plexiglas arena (59 × 59 × 40 cm) for 10 min. After habituation, rats were allowed to explore two identical objects for 5 min during the familiarization phase. After a 1 h intertrial interval, rats were returned to the arena and allowed to explore the familiar object (F), as well as a novel object (N). A preference index (i.e., the time spent exploring the novel object over the familiar one) was calculated as a percentage as follows: % = ([Time N - Time F]/[Time N + Time F]) × 100, where F and N represent the time spent near the familiar and novel objects, respectively.
Beam walking test
Motor coordination was evaluated by the beam walking test.56 Animals were trained before surgery to walk along a Plexiglas beam (120 cm long, 1.5 cm wide) toward a home cage located at one end of the beam. The latency to walk across the beam during five test trials after mTBI was calculated as a measure of motor coordination
Adhesive removal test
Sensorimotor function was evaluated by adhesive removal test.57 Rats were familiarized with the testing environment. Small dot-shaped stickers were placed on the contralateral forepaw. The time to remove the small adhesive stickers was recorded for the contralateral forelimb.
Biochemical analyses
Western blot
Animals were quickly anesthetized with sodium pentobarbitone intraperitoneally (60 mg/kg, Apoteksbolaget, Sweden) and then decapitated. The brain was removed, and cortical tissue was cut approximately 5 mm from the mCCI lesion epicenter and flash frozen in liquid nitrogen. Cortical samples were subsequently homogenized in a lysis buffer consisting of 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% Triton X-100, 0.1% SDS, and 1% mammalian protease inhibitor. The lysate was incubated on ice for 30 min and then centrifuged at 132,000 ×g for 5 min at 4°C. The supernatant was collected and the protein concentration was determined by BCA kit (Pierce, Rockford, IL).
For Western blot analysis, protein samples (30 μg) were resolved using a 10% polyacrylamide gel and then transferred to a nitrocellulose membrane. The membranes were incubated in a blocking buffer (5% nonfat dry milk/1% Tween 20 in TBS) for 1 h at room temperature. The membranes were then probed with specific primary antibodies (anti- β-actin, 1:2000, Chemicon; anti-GFAP, 1:1000, Chemicon; anti-BMX, 1:1000, BD Biosciences; anti-APP, 1:1000, Novus) overnight at 4°C. After a series of TBST washes, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (anti-rabbit or anti-mouse) for 1 h at room temperature. β-actin was used as a loading control.
Protein bands were then detected by enhanced chemiluminescence (ECL) Western blot detection reagents (Millipore). The immunoblots were quantified by UVP (Biospectrum Imaging System).
Immunofluorescence
Animals were perfused through the ascending aorta with 100 mL of cold physiologic saline, followed by 100 mL of ice-cold 4% w/v paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS). The brain was removed and post-fixed in the same fixative for 3 days followed by a series of incubations in 10%, 15%, and 30% sucrose. Sections were cut at a thickness of 30 μm, placed on slides, and stored at −20°C.
For immunostaining, the sections were washed several times with PBS and then incubated in a blocking buffer of 0.3% Triton X-100 and 4% normal goat serum for 1 h at room temperature. The sections were subsequently incubated with a mouse anti-GFAP antibody (reconstituted in PBS and 2% goat serum) at 4°C for 14–16 h, and then rinsed with PBS. Thereafter, they were incubated with an Alexa Fluor 555 fluorescent antibody for 1 h at room temperature. After several PBS washes, sections were mounted with Crystal Mount and analyzed by a Leica microscope. Controls consisted of omission of the primary antibody.
GIP plasma assay
Plasma concentrations of GIP were quantified in a parallel series of SD rats similarly implanted with a subcutaneous 2 week ALZET micro-osmotic pump containing either vehicle or the high dose GIP dose (38.85 μg/kg/day, n = 5/group). Animals were euthanized at 7 days, and blood was taken by cardiac puncture and immediately placed into iced heparinized tubes containing an excess of DPP4 inhibitor. These were directly centrifuged (10,000 × G at 4°C), the plasma removed and immediately frozen to −80°C. Samples were later thawed on wet ice and analyzed for human active GIP (1–42) by solid phase sandwich enzyme-linked immunosorbent assay (Immuno-Biological Laboratories (IBL)-America, Minneapolis, MN).
Experimental design summary and statistical analyses
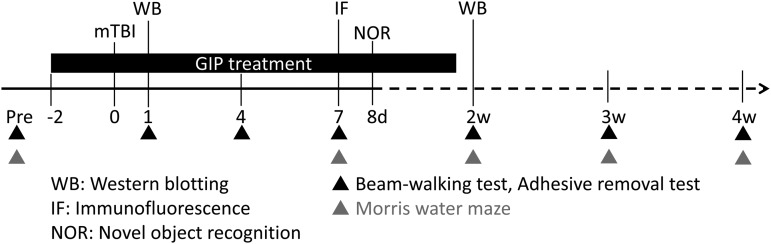
In our mTBI rat model, we investigated the effects of human GIP on behavioral impairments and mTBI-related cellular mechanisms (Fig. 1). Behavioral assessments and biochemical analyses were used to evaluate the therapeutic effects of GIP at multiple time points post-mTBI. Rats were initially pre-trained in each behavioral assay for at least 2 days to generate baseline data. Animals were then categorized into three groups: saline, and low or high dose of GIP.
FIG. 1.
Schematic diagram of behavioral assessments and biochemical analyses forrats with mild traumatic brain injury (mTBI) treated with vehicle or glucose-dependent insulinotropic polypeptide (GIP). GIP and sham treatments were delivered by implanted micro-osmotic pumps for 2 weeks implanted 2 days before injury. Behavioral tests included the beam walking test, the adhesive removal test, and the Morris water maze, which were performed on day 1, 4, 7, and every week after mTBI injury to evaluate a time course of the treatment effects. Biochemical evaluations were performed on days 1, 7, and 14 post-TBI lesion to identify any neuroprotective effects after GIP treatment.
Two days before mTBI injury, mTBI rats were treated with 21.58 or 38.85 μg/kg/day GIP over the course of 2 consecutive weeks delivered subcutaneously under steady-state conditions via an aseptically implanted mini pump. For functional outcomes, cognition and motor function were assessed on the fourth day post-injury, then at weekly intervals up to 4 weeks post-injury (i.e., 7, 14, 21, 28 days post-injury) using the same test conditions.
Further, to examine the possible regulatory mechanisms underlying GIP's neuroprotective effects after mTBI, we used Western blots and immunofluorescence to evaluate astrocytic activation, inflammatory responses, and axonal injury. Brains were collected at 1, 7, and 14 days post-injury for biochemical analyses.
Data were analyzed using SPSS (version 11.0) and are expressed as mean ± standard error of the mean values. The effects of GIP on mTBI were evaluated by a two-way repeated measures analysis of variance (ANOVARM), with “group” as a between-subjects factor and “time” as a within-subject factor. For the long-term effects on locomotion and behavioral scores, a repeated measures analysis of variance (ANOVA) assessed several time points (“pre- and post-injury intervention”) as the within-subjects factor and “treatment” (saline, low and high doses of GIP) as the between-subjects factor. Paired t tests were performed on the follow-up data. Post hoc Fisher least significant difference tests were used to compare between groups, as required. Effects were considered to be significant if p < 0.05.
Results
GIP improves cognitive dysfunction after mTBI
MWM
Rats were trained for 5 consecutive days before mTBI. Subsequently, long-term spatial memory was evaluated each week in the MWM until 1 month post-injury. The visual water tests showed that the latencies (p = 0.529) and swim speed (p = 0.172) were not different between groups, indicating that the visual acuity and muscle strength were not influenced by CCI and, importantly, are not confounding factors in the interpretation of other measures, such as that of cognitive function.
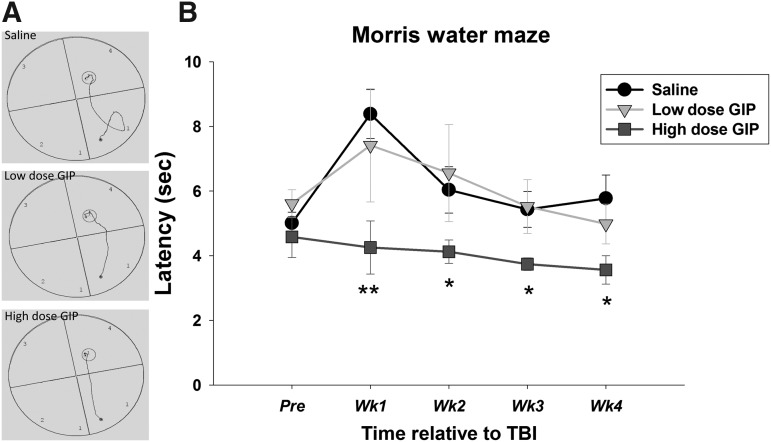
Figure 2 illustrates the subjects' path distance and average latency to find the hidden platform. These latencies were recorded during a 120 sec trial. In the event that an animal failed to reach the platform within the 120 sec trial, the latency was recorded as 120 sec. For latency to the hidden platform, an ANOVARM revealed a significant effect of group (F[2, 21] = 8.483, p = 0.002) and time (F[4, 84] = 5.741, p < 0.001), but not a group x time interaction (F[8, 84] = 1.500, p = 0.170).
FIG. 2.
The effect of GIP on long-term memory, evaluated by the Morris water maze (MWM) over 4 weeks. (A) Example tracking paths to find the hidden platform. (B) Average latency to find the hidden platform. All data are presented as mean ± SEM. Compared with vehicle treated-animals, those administered a low dose of GIP did not differ, whereas a high dose of GIP did. **p < 0.01 versus vehicle, *p < 0.05 versus vehicle. There were no significant differences between groups in the swimming time to a visible platform.
Specifically, mTBI rats administered saline exhibited a spatial memory deficit, because they had significantly longer latencies 1 week post-injury compared with their pre-injury latency (p = 0.003). mTBI rats that received the higher dose of GIP treatment had significantly decreased latencies 1 month post-injury when compared with the saline group (post 1-week: p = 0.003, post 2-weeks: p = 0.050, post 3-weeks: p = 0.023, and post 4-weeks: p = 0.030), suggesting that this high dose of GIP had beneficial effects on mTBI. There was little effect of the lower dose of GIP, showing dose dependency of GIP efficacy.
NOR
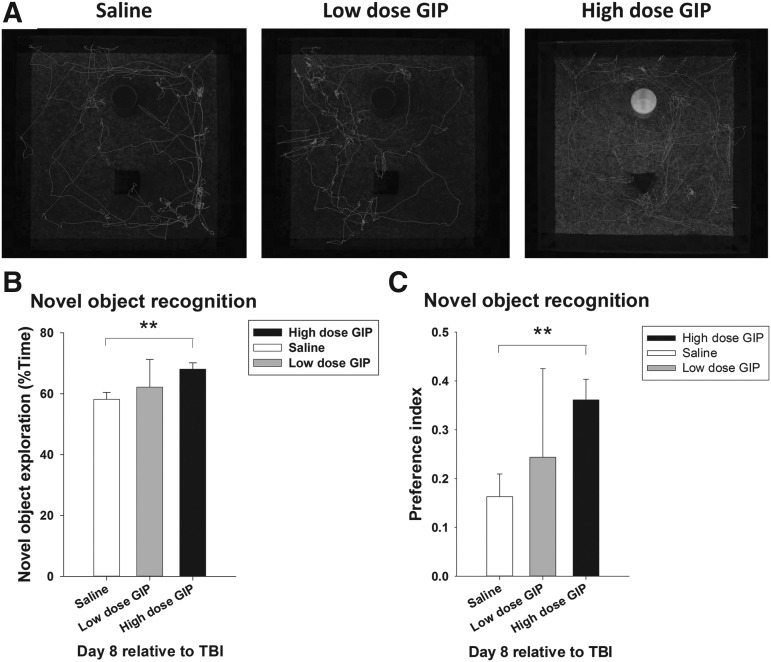
To evaluate recognition memory, the cumulative time spent with a familiar and a novel object was analyzed in mTBI rats in the NOR paradigm (Fig. 3A). An index of visual reference memory was calculated as the percent time spent exploring the novel object (Fig. 3B) and as a preference index (Fig. 3C) 1 week post-injury. mTBI rats treated with saline spent 58.15% ± 2.33 of their time exploring the novel object, with a preference index of 0.16 ± 0.05. In a group comparison (F[2, 26] = 3.978, p = 0.032), only rats receiving the higher dose of GIP had an elevated novelty preference, with a novel object exploration time of 68.06 sec ±2.11 and a novelty preference index of 0.36 ± 0.04, which was significantly greater than saline-treated TBI rats (p < 0.01).
FIG. 3.
The effect of glucose-dependent insulinotropic polypeptide (GIP) on short-term memory was evaluated by the novel object recognition task 8 days post-injury. (A) The tracking path for the exploration of the familiar objects. (B) The ratio of novel object exploration and (C) the preference index were compared between vehicle and GIP treatment groups. **p < 0.01 versus the vehicle group. TBI, traumatic brain injury.
The effect of GIP on sensorimotor function after mTBI
Adhesive removal test
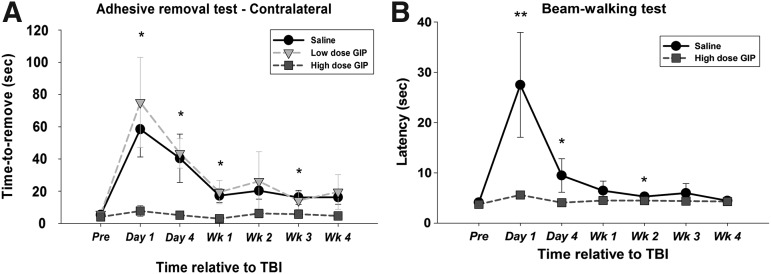
The sensory pathways in rats, as in man, are organized such that the contralateral rather than ipsilateral limbs are mostly dependent on cortical function. The removal for the adhesives did not differ before mTBI injury (p = 0.477). Therefore, mTBI injury induced sensorimotor deficits (Fig. 4A). The adhesive removal time in the saline group (F[6, 36] = 2.492, p = 0.040) was different between the early stage (p = 0.023), at 1 day post-injury, and the late stage (p = 0.041), showing some spontaneous recovery at 1 month post-injury after mTBI.
FIG. 4.
The effect of glucose-dependent insulinotropic polypeptide (GIP) on sensory function and gross fine motor coordination were evaluated by the adhesive removal test (A: contralateral limb) and the beam walking test (B) in a temporal analysis. All data are presented as mean ± standard error of the mean. Compared with vehicle, only the high dose of GIP had a significant difference. **p < 0.01 versus vehicle, *p < 0.05 versus vehicle. TBI, traumatic brain injury.
Removal times, however, were still significantly longer than pre-TBI values (Fig. 4A). The low dose of GIP treatment had no significant effect on removal time in the forelimbs compared with saline. In contrast, forelimbs in animals treated with the higher dose of GIP (F[1.472, 10.305] = 1.200) showed removal times not significantly different from pre-TBI values (Fig. 4A) (p = 0.325).
Beam walking test
The beam-walking test was used to assess the effects of GIP treatment on balance and fine motor coordination after mTBI (Fig. 4B). An ANOVARM revealed a significant effect of treatment (F[1, 12] = 8.269, p = 0.014) and time (F[1.291, 15.495] = 6.1559, p < 0.001) but not a treatment × time interaction (F[1.291, 15.495] = 3.099, p = 0.090).
In a further analysis of the main effect of time, the saline group showed a significant time effect (saline: F[6, 30] = 3.690, p = 0.007; high dose of GIP: F[1.077, 7.537] = 1.683, p = 0.149). A more detailed analysis using a two independent samples t test revealed that there were no differences before mTBI injury (saline: 4.138 sec ±0.284; high dose of GIP: 3.750 sec ±0.225); however, significant differences between the saline and high GIP groups were evident on day 1 (t = 0.010), day 4 (t = 0.014), and week 2 (t = 0.028) after mTBI challenge.
Plasma GIP levels
Subcutaneous steady-state GIP administration of 38.85 μg/kg/day (achieved by ALZET minipump) provided a plasma concentration of 58.6 ± 11.8 pmol/L GIP versus endogenous levels of 26.4 ± 2.1 pmol/L GIP in control animals administered vehicle. This represents a 2.2-fold elevation (p < 0.05) achieved by the subcutaneous administration of GIP.
Biochemical analysis
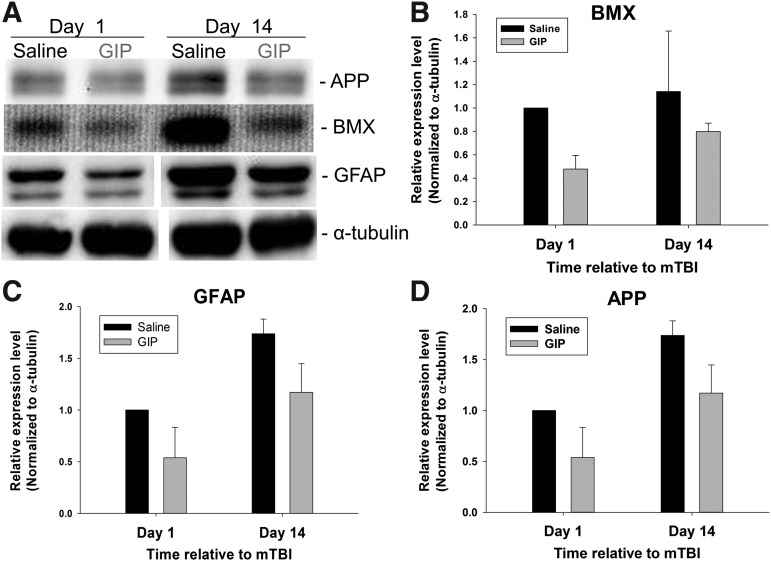
The mechanisms underlying GIP treatment in our mTBI animal model were evaluated by Western blot and immunofluorescence. A Western blot analysis of mTBI ipsilateral cortex lysate revealed an elevation in TBI biomarkers (BMX, GFAP, and APP) 1 day post-injury in mTBI challenged animals administered saline, compared with those given high dose GIP (Fig. 5 A–D). This elevation in biomarkers was maintained 14 days post-mTBI and, similarly, was mitigated by GIP.
FIG. 5.
The high dose of GIP reduced mTBI-induced increased expression of bone marrow kinase in chromosome X (BMX, also known as Etk), glial fibrillary acidic protein (GFAP), and amyloid-β precursor protein (APP). TBI, traumatic brain injury.
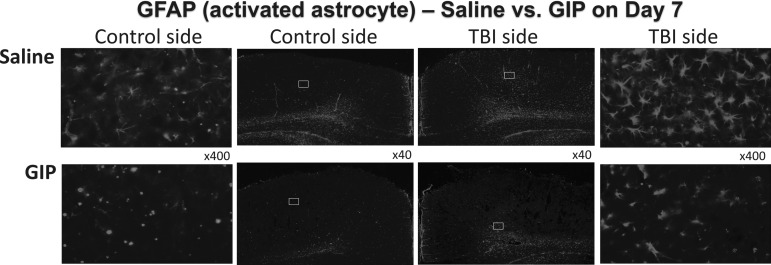
As evaluated in Figure 6, GFAP was largely localized to the mTBI impacted hemisphere, as evidenced by an increased GFAP fluorescence near the injury site (Fig. 6). In contrast, TBI-induced gliosis of GFAP immunoactivity was substantially mitigated in the high dose GIP treatment group.
FIG. 6.
Seven days after mTBI, the high dose of glucose-dependent insulinotropic polypeptide (GIP) reduced mild traumatic brain injury (mTBI)-induced increases in glial fibrillary acidic protein (GFAP) expression within the cortex, as evaluated by immunofluorescence in 30 μm thick sections with a specific antibody to GFAP (middle images, x40 magnification; left and right images are a higher magnification of the contralateral and ipsilateral hemispheres, x400).
Discussion
In the present study, we report on the efficacy of GIP, an incretin similar to GLP-1, to mitigate mTBI-induced behavioral deficits. Previous studies of a GLP-1 receptor agonist have demonstrated potential neuroprotective, neurotrophic, and antihyperglycemic effects to mitigate TBI-induced morbidity across TBI animal models at doses of translational relevance to humans.44 In this regard, exendin-4 (Ex-4), a GLP-1 receptor agonist resistant to hydrolysis by dipeptidyl peptidase-4 (DPP-4), has neuroprotective effects that occur independently of glycemic control, and works by increasing cyclic adenosine monophosphate (cAMP) levels and decreasing oxidative stress and inflammation.58–60 The therapeutic effect of Ex-4 was mediated by GLP-1 receptor (GLP-1R) activation, because it can be blocked by the selective GLP-1R antagonist Exendin (9–39) and was ineffective in GLP-1R knockout mice.60,61
GIP, like GLP-1, has a short half-life (approximately 7.3 min62) but has received less attention as a therapeutic strategy because the β-cell response to GIP may be reduced or markedly attenuated in persons with type 2 diabetes.63 The enhanced antihyperglycemic and insulinotropic efficacy of dual agonists that combine GIP and GLP-1 actions, relative to selective GLP-1 agonists, however, has reignited interest in the role of GIP and its targeting as a treatment strategy.64
In relation to TBI, recent studies have demonstrated that Ex-4 as well as liraglutide administration were well tolerated, ameliorated TBI-induced behavioral deficits and neuronal loss,46,65–67 and this effect appears associated with a low risk of hypoglycemia.68 Because these studies are in accord with earlier GLP-1R agonists mitigating mTBI impairments,45 the potential neuroprotective effect of GIP deserved evaluation for mTBI treatment.
GIP and its receptor have been implicated in neurogenesis,69,synaptic plasticity, behavioral improvements, such as cognition,70–73 sensory and motor physiological function,74 and axonal regeneration after sciatic nerve injury.75 GIP has also been purported to be involved in neurological diseases, including epilepsy, Parkinson disease, and Alzheimer disease.71,76,77 To validate the beneficial neurological actions of GIP on mTBI, we evaluated the use of GIP as a therapeutic agent in a mTBI rat model using mild CCI and showed significant effects of GIP on sensorimotor function and balance as well as hippocampus-dependent learning and memory.
Specifically, we found that mTBI-related spatial memory loss and recognition memory impairments were ameliorated by GIP treatment over time. In the MWM paradigm, the vehicle-treated group reached a plateau in their performance after 3–4 weeks. In contrast, high dose GIP-treated animals were much less affected by the mTBI lesion. In addition, in the vehicle-treated mTBI group, the latency to find the hidden platform within the MWM was maintained over time, whereas the mTBI animal group receiving the higher GIP dose time-dependently improved.
Our study supports the observations of increases in neuroplasticity after GIP intervention71 and that GIP treatment might be useful to reduce deficits after TBI. The lack of any differences in latency and swim speed between vehicle and GIP-treated mTBI groups to reach a visual platform within the MWM indicates that potential confounds in motor performance did not underlie the GIP induced improvement in cognitive measures. Further, mitigation in mTBI induced cognitive impairment was cross-validated within the NOR paradigm—an activity, unlike swimming, that is not so dependent on motor function.
In our appraisal of the longer term effects of GIP, we additionally evaluated its time-dependent actions on motor and sensory functions in rats with mTBI. Our results show that transient sensorimotor disturbances after mTBI were reduced after GIP treatment, particularly in the adhesive removal test (Fig. 4A) that requires dexterity, indicating that early GIP intervention can suppress these motor impairments.
In contrast, the time-dependent return of beam walking ability of mTBI vehicle mice at 1 week and after—subsequent to earlier impairment (Fig. 4B)—reiterates that potential motor deficits were not a confounding factor in the cognitive paradigms that were studied 1 week onward after mTBI. Together, these data add to a growing literature using basic and clinical research on the efficacy of incretins in early mTBI treatment to facilitate any spontaneous recovery and also provide further consideration about dosage required for potential neuroprotective treatment, particularly whether or not they are within the clinical realm.
In this regard, previous studies have shown that the GLP-1 agonist Ex-4 can increase and mediate cAMP in vitro as well as reduce TBI-related cognitive impairments in rodents at an in vivo dose of 21.1 μg/kg/day.61,65,78 This same dose proved effective in mitigating Alzheimer disease and ALS in animal models,79,80 and translates to approximately a third of the routine clinical dose of Ex-4 (Exenatide LAR) used in type 2 diabetes mellitus after normalization between species based on body surface area.
Because the successful mitigation of peripheral neuropathy in rats has been achieved by a subcutaneous steady-state administration of a GLP-1 dose of 20.7 μg/kg/day, and in light of the similar short half-lives of GLP-1 and GIP, we evaluated two doses of GIP (21.58 and 38.85 μg/kg/day) likewise administered under steady-state conditions (achieved by subcutaneous ALZET pump) to establish a GIP dose-response for its neuroprotective effects in our current study. Our results demonstrate that the mTBI-related deficits in cognition and sensorimotor function can be mitigated with the higher dose that achieved a plasma concentration of 58.6 ± 11.8 pmol/L, which elevated endogenous GIP levels in the rat by 2.2-fold. This concentration is readily achievable in humans.81
Alternative potential options to augment the effects of GIP are to lengthen its half-life. In this regard, the generation of DPP-4 resistant GIP analogues holds the potential to overcome the peptide's rapid N-terminal cleavage and inactivation, although GIP renal clearance is reported to additionally limit the peptide's activity.82 A number of modified analogs of GIP have been generated that demonstrate dramatic resistance to DPP-4.83–85 Several of these exhibit enhanced activity at the GIP receptor in cellular studies and display prolonged antihyperglycemic and insulin-releasing activities when administered to animal models of type 2 diabetes mellitus. Such agents are experimental, however, and hence are not currently available for translational studies in humans with TBI.
A further alternative is to augment endogenous GIP levels by use of a DPP-4 inhibitor, which has been described to elevate GIP levels in humans by twofold or greater.86 Such an elevation in humans would be in line with the 2.2-fold rise in plasma GIP levels evident in our rat mTBI study and could be further augmented by continuous subcutaneous administration of exogenous GIP, as has been achieved in human studies with GLP-1 over an extended period.87 Notably, administration of exogenous incretins does not appear to suppress their endogenous release,88 thereby providing our studies translational potential.
Neurodegenerative diseases are a well-established risk factor after TBI,89 and recent large epidemiological studies have identified mTBI as a risk factor for later development of dementia.42,90–93 The pathogenesis of chronic neurodegenerative diseases may be triggered and/or accelerated by systemic inflammation.94 Indeed, after TBI, neuroinflammation occurs at acute and chronic stages after primary injury and is considered a key secondary injury mechanism that contributes to progressive neurodegeneration and both short- and long-term neurological deficits.95,96,98,99
In line with this, earlier studies have demonstrated that long-term upregulation of inflammation occurs within the lesioned brain area in rats subjected to CCI TBI.100,101 In the light of the reported anti-inflammatory and neuroprotective roles of agents that impact insulin pathways99,102 as well as the anti-apoptotic, anti-inflammatory, and neuroprotective actions of incretins within the central nervous system (CNS),43,44,97 we evaluated the effects of GIP on neuroinflammatory markers as a mechanism underpinning its amelioration of CCI-induced cognitive and sensorimotor deficits in rats.
Our previous studies demonstrated that elevated BMX levels provide a useful indicator of TBI severity and is related to chronic inflammation.22 Administration of the higher dose of GIP mitigated the rise in BMX levels evident in our TBI rats and, notably, additionally ameliorated TBI induced elevations in GFAP and APP. The astrocyte marker, GFAP, is found exclusively within the CNS, particularly on reactive inflamed astrocytes (Fig. 6), and its elevation is considered a specific marker of CNS damage.27–30 In contrast, elevated APP is considered a sensitive and specific marker of damaged axons and impaired synapses after TBI challenge,14,26,40 and associates with injury severity and post-TBI outcome.38,39
An increasing number of studies have reported that early environmental induction of APP, as occurs with TBI46 or factors such as lead exposure, can trigger programmatic changes via alterations in gene expression or gene imprinting that may manifest as functional deficits, such as Alzheimer disease, later in life. Both neurons and activated astrocytes are sources of APP and Aβ, whose levels are both elevated by neuroinflammation and, additionally, drive neuroinflammation to potentially generate a self-propagating adverse cycle.103 In this regard, the action of GIP to mitigate neuroinflammatory markers and APP levels in brain after TBI can be considered beneficial.
The mechanism(s) underpinning the therapeutic effect of GIP in mTBI for cognitive impairments likely are multiple. In addition to anti-inflammatory actions, incretin activation of their G protein-coupled receptors leads to its interaction with the GTP-binding protein Gs, the activation of adenylyl cyclase and elevation of intracellular cAMP, and neurotrophic and protective actions that are chiefly mediated via protein kinase A (PKA) and PI3K signaling pathways.59,61,66
Kobori and associates104 suggested that TBI-induced working memory deficits may alter PKA activity in the medial prefrontal cortex. Titus and colleagues105 showed that synaptic plasticity dysfunction contributes to cognitive impairments by reducing cAMP levels after mTBI. Accordingly, down-regulation of the cAMP-PKA signaling pathway may play a key role in the impairment of short- and long-term memory after TBI. In the light of the central role that cAMP-PKA signaling plays in the anti-apoptotic actions of incretins,106 further studies are needed to validate the actions of GIP and its induced mechanisms to mitigate cognitive dysfunction as well as neurodegeneration after TBI.
In synopsis, we investigated the neuroprotective potency of GIP for protection against mTBI, focusing on neuroinflammation and neurodegeneration. Our results identified a mechanism for GIP as an mTBI therapy and suggest that GIP can reverse cognitive and motor impairments via the attenuation of mTBI-induced astrocytic activation, inflammation, and axonal injury. Previous findings suggest that GIP plays an important role in mediating synaptic plasticity and memory formation, while reducing cognitive deficits in various neurodegenerative diseases,107 and are in accord with our results.
Conclusion
This study demonstrates the efficacy of GIP in preventing sensorimotor and cognitive abnormalities in a rodent model of mTBI. GIP may ameliorate neuronal death and axonal injury after mTBI. Future pre-clinical studies are needed both to further identify GIP's mechanism of action and, importantly, to define its time-dependent therapeutic window of opportunity when administered after TBI. Such studies may lead to more effective drug therapies in humans.
Acknowledgments
This study was supported by the Ministry of Science and Technology, grant number MOST 103-2321-B-038-003, NSC 101-2321-B-038-004, NSC 102-2321-B-038-004 and NSC 101-2632-B-038-001-MY3, MOST 103-2314-B-038-038 and the Intramural Research Program of the National Institute on Aging.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 [PubMed] [Google Scholar]
- 2.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, NCfIPaC, ed: Atlanta, Ga [Google Scholar]
- 3.Garner J., and Brett S.J. (2007). Mechanisms of injury by explosive devices. Anesthesiol. Clin. 25, 147–160, x [DOI] [PubMed] [Google Scholar]
- 4.Howard P.K., and Shapiro S.E. (2011). Diagnosing and treating mild traumatic brain injury in children. Adv. Emerg. Nurs. J. 33, 274–278 [DOI] [PubMed] [Google Scholar]
- 5.Signoretti S., Vagnozzi R., Tavazzi B., and Lazzarino G. (2010). Biochemical and neurochemical sequelae following mild traumatic brain injury: summary of experimental data and clinical implications. Neurosurg. Focus 29, E1. [DOI] [PubMed] [Google Scholar]
- 6.Arciniegas D.B., Anderson C.A., Topkoff J., and McAllister T.W. (2005). Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr. Dis. Treat. 1, 311–327 [PMC free article] [PubMed] [Google Scholar]
- 7.Ponsford J., Cameron P., Fitzgerald M., Grant M., and Mikocka-Walus A. (2011). Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J. Neurotrauma 28, 937–946 [DOI] [PubMed] [Google Scholar]
- 8.Marshall S., Bayley M., McCullagh S., Velikonja D., and Berrigan L. (2012). Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can. Fam. Physician 58, 257–267, e128–140 [PMC free article] [PubMed] [Google Scholar]
- 9.Levin H.S. (1998). Cognitive function outcomes after traumatic brain injury. Curr. Opin. Neurol. 11, 643–646 [DOI] [PubMed] [Google Scholar]
- 10.Schultz B.A., Cifu D.X., McNamee S., Nichols M., and Carne W. (2011). Assessment and treatment of common persistent sequelae following blast induced mild traumatic brain injury. NeuroRehabilitation 28, 309–320 [DOI] [PubMed] [Google Scholar]
- 11.Oddy M., and Humphrey M. (1980). Social recovery during the year following severe head injury. J. Neurol. Neurosurg. Psychiatry 43, 798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddy M., Humphrey M. and Uttley D. (1978). Subjective impairment and social recovery after closed head injury. J. Neurol. Neurosurg. Psychiatry 41, 611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oddy M., Coughlan T., Tyerman A., and Jenkins D. (1985). Social adjustment after closed head injury: a further follow-up seven years after injury. J. Neurol. Neurosurg. Psychiatry 48, 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker K.R., and Tesco G. (2013). Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramlett H.M., Green E.J., and Dietrich W.D. (1997). Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 762, 195–202 [DOI] [PubMed] [Google Scholar]
- 16.Dixon C.E., Kochanek P.M., Yan H.Q., Schiding J.K., Griffith R.G., Baum E., Marion D.W., and DeKosky S.T. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 17.Lyeth B.G., Jenkins L.W., Hamm R.J., Dixon C.E., Phillips L.L., Clifton G.L., Young H.F., and Hayes R.L. (1990). Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 526, 249–258 [DOI] [PubMed] [Google Scholar]
- 18.McIntosh T.K., Juhler M., and Wieloch T. (1998). Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J. Neurotrauma 15, 731–769 [DOI] [PubMed] [Google Scholar]
- 19.Raghupathi R., Graham D.I., and McIntosh T.K. (2000). Apoptosis after traumatic brain injury. J. Neurotrauma 17, 927–938 [DOI] [PubMed] [Google Scholar]
- 20.Tweedie D., Milman A., Holloway H.W., Li Y., Harvey B.K., Shen H., Pistell P.J., Lahiri D.K., Hoffer B.J., Wang Y., Pick C.G., and Greig N.H. (2007). Apoptotic and behavioral sequelae of mild brain trauma in mice. J. Neurosci. Res. 85, 805–815 [DOI] [PubMed] [Google Scholar]
- 21.Frankola K.A., Greig N.H., Luo W., and Tweedie D. (2011). Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 10, 391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J.C., Chen K.Y., Yu Y.W., Huang S.W., Shih H.M., Chiu W.T., Chiang Y.H., and Shiau C.Y. (2012). Location and level of Etk expression in neurons are associated with varied severity of traumatic brain injury. PloS One 7, e39226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paavonen K., Ekman N., Wirzenius M., Rajantie I., Poutanen M. and Alitalo K. (2004). Bmx tyrosine kinase transgene induces skin hyperplasia, inflammatory angiogenesis, and accelerated wound healing. Mol. Biol. Cell 15, 4226–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmy A., Carpenter K.L., Skepper J.N., Kirkpatrick P.J., Pickard J.D., and Hutchinson P.J. (2009). Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J. Neurotrauma 26, 549–561 [DOI] [PubMed] [Google Scholar]
- 25.Palmer C.D., Mutch B.E., Workman S., McDaid J.P., Horwood N.J., and Foxwell B.M. (2008). Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkapp}B activity. Blood 111, 1781–1788 [DOI] [PubMed] [Google Scholar]
- 26.d'Avila J.C., Lam T.I., Bingham D., Shi J., Won S.J., Kauppinen T.M., Massa S., Liu J., and Swanson R.A. (2012). Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J. Neuroinflammation 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelinka L.E., Kroepfl A., Leixnering M., Buchinger W., Raabe A., and Redl H. (2004). GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma 21, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 28.Nylen K., Ost M., Csajbok L.Z., Nilsson I., Blennow K., Nellgard B., and Rosengren L. (2006). Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J. Neurol. Sci. 240, 85–91 [DOI] [PubMed] [Google Scholar]
- 29.Vos P.E., Jacobs B., Andriessen T.M., Lamers K.J., Borm G.F., Beems T., Edwards M., Rosmalen C.F., and Vissers J.L. (2010). GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 75, 1786–1793 [DOI] [PubMed] [Google Scholar]
- 30.Sofroniew M.V., and Vinters H.V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pike C.J., Cummings B.J., and Cotman C.W. (1995). Early association of reactive astrocytes with senile plaques in Alzheimer's disease. Exp. Neurol. 132, 172–179 [DOI] [PubMed] [Google Scholar]
- 32.Verkhratsky A., Olabarria M., Noristani H.N., Yeh C.Y., and Rodriguez J.J. (2010). Astrocytes in Alzheimer's disease. Neurotherapeutics 7, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funato H., Yoshimura M., Yamazaki T., Saido T.C., Ito Y., Yokofujita J., Okeda R., and Ihara Y. (1998). Astrocytes containing amyloid beta-protein (Abeta)-positive granules are associated with Abeta40-positive diffuse plaques in the aged human brain. Am. J. Pathol. 152, 983–992 [PMC free article] [PubMed] [Google Scholar]
- 34.Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., and Husemann J. (2003). Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 35.Gentleman S.M., Nash M.J., Sweeting C.J., Graham D.I., and Roberts G.W. (1993). Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci. Lett. 160, 139–144 [DOI] [PubMed] [Google Scholar]
- 36.Otsuka N., Tomonaga M., and Ikeda K. (1991). Rapid appearance of beta-amyloid precursor protein immunoreactivity in damaged axons and reactive glial cells in rat brain following needle stab injury. Brain Res. 568, 335–338 [DOI] [PubMed] [Google Scholar]
- 37.Janus C., Phinney A.L., Chishti M.A., and Westaway D. (2001). New developments in animal models of Alzheimer's disease. Curr. Neurol. Neurosci. Rep. 1, 451–457 [DOI] [PubMed] [Google Scholar]
- 38.Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., and Little D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 39.Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekmark-Lewen S., Flygt J., Kiwanuka O., Meyerson B.J., Lewen A., Hillered L., and Marklund N. (2013). Traumatic axonal injury in the mouse is accompanied by a dynamic inflammatory response, astroglial reactivity and complex behavioral changes. J. Neuroinflammation 10, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao X., and Chen J. (2011). Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp Neurol 70, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee Y.K., Hou S.W., Lee C.C., Hsu C.Y., Huang Y.S., and Su Y.C. (2013). Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PloS One 8, e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salcedo I., Tweedie D., Li Y., and Greig N.H. (2012). Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 166, 1586–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greig N.H., Tweedie D., Rachmany L., Li Y., Rubovitch V., Schreiber S., Chiang Y.H., Hoffer B.J., Miller J., Lahiri D.K., Sambamurti K., Becker R.E., and Pick C.G. (2014). Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 10, Suppl 1, S62–S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rachmany L., Tweedie D., Li Y., Rubovitch V., Holloway H.W., Miller J., Hoffer B.J., Greig N.H., and Pick C.G. (2013). Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age 35, 1621–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tweedie D., Rachmany L., Rubovitch V., Lehrmann E., Zhang Y., Becker K.G., Perez E., Miller J., Hoffer B.J., Greig N.H., and Pick C.G. (2013). Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp. Neurol. 239, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell J.E., and Drucker D.J. (2013). Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 [DOI] [PubMed] [Google Scholar]
- 48.Wu T., Rayner C.K., and Horowitz M. (2016). Incretins. Handb. Exp. Pharmacol. 233, 137–171 [DOI] [PubMed] [Google Scholar]
- 49.Tan T. and Bloom S. (2013). Gut hormones as therapeutic agents in treatment of diabetes and obesity. Curr. Opin. Pharmacol. 13, 996–1001 [DOI] [PubMed] [Google Scholar]
- 50.Gault V.A., Kerr B.D., Irwin N., and Flatt P.R. (2008). C-terminal mini-PEGylation of glucose-dependent insulinotropic polypeptide exhibits metabolic stability and improved glucose homeostasis in dietary-induced diabetes. Biochem. Pharmacol. 75, 2325–2333 [DOI] [PubMed] [Google Scholar]
- 51.Paratore S., Ciotti M.T., Basille M., Vaudry D., Gentile A., Parenti R., Calissano P., and Cavallaro S. (2011). Gastric inhibitory polypeptide and its receptor are expressed in the central nervous system and support neuronal survival. Cent. Nerv. System. Agents Med. Chem. 11, 210–222 [DOI] [PubMed] [Google Scholar]
- 52.Lin C.M., Lin J.W., Chen Y.C., Shen H.H., Wei L., Yeh Y.S., Chiang Y.H., Shih R., Chiu P.L., Hung K.S., Yang L.Y., and Chiu W.T. (2009). Hyaluronic acid inhibits the glial scar formation after brain damage with tissue loss in rats. Surg. Neurol. 72, Suppl 2, S50–S54 [DOI] [PubMed] [Google Scholar]
- 53.Wenk G.L. (2004). Assessment of spatial memory using the radial arm maze and Morris water maze. Curr. Protoc. Neurosci. Chapter 8, Unit 8.5A [DOI] [PubMed] [Google Scholar]
- 54.Prusky G.T., West P.W., and Douglas R.M. (2000). Reduced visual acuity impairs place but not cued learning in the Morris water task. Behav Brain Res 116, 135–140 [DOI] [PubMed] [Google Scholar]
- 55.Taglialatela G., Hogan D., Zhang W.R., and Dineley K.T. (2009). Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain Res. 200, 95–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 57.Barth T.M., Jones T.A., and Schallert T. (1990). Functional subdivisions of the rat somatic sensorimotor cortex. Behav. Brain Res. 39, 73–95 [DOI] [PubMed] [Google Scholar]
- 58.Darsalia V., Larsson M., Nathanson D., Klein T., Nystrom T., and Patrone C. (2015). Glucagon-like receptor 1 agonists and DPP-4 inhibitors: potential therapies for the treatment of stroke. J. Cereb. Blood Flow Metab. 35, 718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Tweedie D., Mattson M.P., Holloway H.W., and Greig N.H. (2010). Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J. Neurochem. 113, 1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry T., Lahiri D.K., Chen D., Zhou J., Shaw K.T., Egan J.M., and Greig N.H. (2002). A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharmacol. Exp. Ther. 300, 958–966 [DOI] [PubMed] [Google Scholar]
- 61.Li Y., Perry T., Kindy M.S., Harvey B.K., Tweedie D., Holloway H.W., Powers K., Shen H., Egan J.M., Sambamurti K., Brossi A., Lahiri D.K., Mattson M.P., Hoffer B.J., Wang Y., and Greig N.H. (2009). GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. U. S. A. 106, 1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deacon C.F., Nauck M.A., Meier J., Hucking K., and Holst J.J. (2000). Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 85, 3575–3581 [DOI] [PubMed] [Google Scholar]
- 63.Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., and Creutzfeldt W. (1993). Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finan B., Ma T., Ottaway N., Muller T.D., Habegger K.M., Heppner K.M., Kirchner H., Holland J., Hembree J., Raver C., Lockie S.H., Smiley D.L., Gelfanov V., Yang B., Hofmann S., Bruemmer D., Drucker D.J., Pfluger P.T., Perez-Tilve D., Gidda J., Vignati L., Zhang L., Hauptman J.B., Lau M., Brecheisen M., Uhles S., Riboulet W., Hainaut E., Sebokova E., Conde-Knape K., Konkar A., DiMarchi R.D., and Tschop M.H. (2013). Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 5, 209ra151. [DOI] [PubMed] [Google Scholar]
- 65.Eakin K., Li Y., Chiang Y.H., Hoffer B.J., Rosenheim H., Greig N.H., and Miller J.P. (2013). Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PloS One 8, e82016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y., Bader M., Tamargo I., Rubovitch V., Tweedie D., Pick C.G. and Greig N.H. (2015). Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice. J. Neurochem. 135, 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hakon J., Ruscher K., Romner B., and Tomasevic G. (2015). Preservation of the blood brain barrier and cortical neuronal tissue by liraglutide, a long acting glucagon-like-1 analogue, after experimental traumatic brain injury. PloS One 10, e0120074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monami M., Marchionni N., and Mannucci E. (2009). Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur. J. Endocrinol. 160, 909–917 [DOI] [PubMed] [Google Scholar]
- 69.Nyberg J., Anderson M.F., Meister B., Alborn A.M., Strom A.K., Brederlau A., Illerskog A.C., Nilsson O., Kieffer T.J., Hietala M.A., Ricksten A., and Eriksson P.S. (2005). Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. 25, 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faivre E., Gault V.A., Thorens B., and Holscher C. (2011). Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J. Neurophysiol. 105, 1574–1580 [DOI] [PubMed] [Google Scholar]
- 71.Figueiredo C.P., Pamplona F.A., Mazzuco T.L., Aguiar A.S., Jr., Walz R., and Prediger R.D. (2010). Role of the glucose-dependent insulinotropic polypeptide and its receptor in the central nervous system: therapeutic potential in neurological diseases. Behav. Pharmacol. 21, 394–408 [DOI] [PubMed] [Google Scholar]
- 72.Ding K.H., Zhong Q., Xie D., Chen H.X., Della-Fera M.A., Bollag R.J., Bollag W.B., Gujral R., Kang B., Sridhar S., Baile C., Curl W., and Isales C.M. (2006). Effects of glucose-dependent insulinotropic peptide on behavior. Peptides 27, 2750–2755 [DOI] [PubMed] [Google Scholar]
- 73.Tian J.Q., Wang Y., Lin N., Guo Y.J., Sun S.H., and Zou D.J. (2010). Active immunization with glucose-dependent insulinotropic polypeptide vaccine influences brain function and behaviour in rats. Scand. J. Immunol. 72, 1–7 [DOI] [PubMed] [Google Scholar]
- 74.Okawa T., Kamiya H., Himeno T., Seino Y., Tsunekawa S., Hayashi Y., Harada N., Yamada Y., Inagaki N., Seino Y., Oiso Y., and Nakamura J. (2014). Sensory and motor physiological functions are impaired in gastric inhibitory polypeptide receptor-deficient mice. J. Diabetes Investig. 5, 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buhren B.A., Gasis M., Thorens B., Muller H.W., and Bosse F. (2009). Glucose-dependent insulinotropic polypeptide (GIP) and its receptor (GIPR): cellular localization, lesion-affected expression, and impaired regenerative axonal growth. J. Neurosci. Res. 87, 1858–1870 [DOI] [PubMed] [Google Scholar]
- 76.Gault V.A., and Holscher C. (2008). Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. J. Neurophysiol. 99, 1590–1595 [DOI] [PubMed] [Google Scholar]
- 77.Holscher C. (2014). The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer's disease. Alzheimers Dement. 10, Suppl 1, S47–S54 [DOI] [PubMed] [Google Scholar]
- 78.Atkins C.M., Oliva A.A., Jr., Alonso O.F., Pearse D.D., Bramlett H.M., and Dietrich W.D. (2007). Modulation of the cAMP signaling pathway after traumatic brain injury. Exp. Neurol. 208, 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y., Duffy K.B., Ottinger M.A., Ray B., Bailey J.A., Holloway H.W., Tweedie D., Perry T., Mattson M.P., Kapogiannis D., Sambamurti K., Lahiri D.K., and Greig N.H. (2010). GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J. Alzheimers Dis. 19, 1205–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y., Chigurupati S., Holloway H.W., Mughal M., Tweedie D., Bruestle D.A., Mattson M.P., Wang Y., Harvey B.K., Ray B., Lahiri D.K., and Greig N.H. (2012). Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PloS One 7, e32008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamoi K., Shinozaki Y., Furukawa K., and Sasaki H. (2012). Potential correlation between plasma total GIP levels and body mass index in Japanese patients with types 1 or 2 diabetes mellitus. Endocr. J. 59, 353–363 [DOI] [PubMed] [Google Scholar]
- 82.Meier J.J., Nauck M.A., Kranz D., Holst J.J., Deacon C.F., Gaeckler D., Schmidt W.E., and Gallwitz B. (2004). Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes 53, 654–662 [DOI] [PubMed] [Google Scholar]
- 83.Irwin N., Green B.D., Gault V.A., Greer B., Harriott P., Bailey C.J., Flatt P.R., and O'Harte F.P. (2005). Degradation, insulin secretion, and antihyperglycemic actions of two palmitate-derivitized N-terminal pyroglutamyl analogues of glucose-dependent insulinotropic polypeptide. J. Med. Chem. 48, 1244–1250 [DOI] [PubMed] [Google Scholar]
- 84.Martin C.M., Irwin N., Flatt P.R., and Gault V.A. (2013). A novel acylated form of (d-Ala(2))GIP with improved antidiabetic potential, lacking effect on body fat stores. Biochim. Biophys. Acta 1830, 3407–3413 [DOI] [PubMed] [Google Scholar]
- 85.Tatarkiewicz K., Hargrove D.M., Jodka C.M., Gedulin B.R., Smith P.A., Hoyt J.A., Lwin A., Collins L., Mamedova L., Levy O.E., D'Souza L., Janssen S., Srivastava V., Ghosh S.S., and Parkes D.G. (2014). A novel long-acting glucose-dependent insulinotropic peptide analogue: enhanced efficacy in normal and diabetic rodents. Diabetes Obes. Metab. 16, 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herman G.A., Bergman A., Stevens C., Kotey P., Yi B., Zhao P., Dietrich B., Golor G., Schrodter A., Keymeulen B., Lasseter K.C., Kipnes M.S., Snyder K., Hilliard D., Tanen M., Cilissen C., De Smet M., de Lepeleire I., Van Dyck K., Wang A.Q., Zeng W., Davies M.J., Tanaka W., Holst J.J., Deacon C.F., Gottesdiener K.M., and Wagner J.A. (2006). Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J. Clin Endocrinol. Metab. 91, 4612–4619 [DOI] [PubMed] [Google Scholar]
- 87.Meneilly G.S., Greig N., Tildesley H., Habener J.F., Egan J.M., and Elahi D. (2003). Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care 26, 2835–2841 [DOI] [PubMed] [Google Scholar]
- 88.Elahi D., Ruff D.A., Carlson O.D., Meneilly G.S., Habener J.F., and Egan J.M. (2015). Does GLP-1 suppress its own basal secretion? Endocr. Res, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gardner R.C., and Yaffe K. (2015). Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell Neurosci. 66, 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gardner R.C., Burke J.F., Nettiksimmons J., Kaup A., Barnes D.E., and Yaffe K. (2014). Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 71, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graves A.B., White E., Koepsell T.D., Reifler B.V., van Belle G., Larson E.B., and Raskind M. (1990). The association between head trauma and Alzheimer's disease. Am. J. Epidemiol. 131, 491–501 [DOI] [PubMed] [Google Scholar]
- 92.Nordstrom P., Michaelsson K., Gustafson Y., and Nordstrom A. (2014). Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann. Neurol. 75, 374–381 [DOI] [PubMed] [Google Scholar]
- 93.Schofield P.W., Tang M., Marder K., Bell K., Dooneief G., Chun M., Sano M., Stern Y., and Mayeux R. (1997). Alzheimer's disease after remote head injury: an incidence study. J. Neurol. Neurosurg. Psychiatry 62, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perry V.H., Nicoll J.A., and Holmes C. (2010). Microglia in neurodegenerative disease. Nat. Rev. Neurol. 6, 193–201 [DOI] [PubMed] [Google Scholar]
- 95.Kumar A., and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 96.Cerecedo-Lopez C.D., Kim-Lee J.H., Hernandez D., Acosta S.A., and Borlongan C.V. (2014). Insulin-associated neuroinflammatory pathways as therapeutic targets for traumatic brain injury. Med. Hypotheses 82, 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lozano D., Gonzales-Portillo G.S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., and Borlongan C.V. (2015). Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 11, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lloyd E., Somera-Molina K., Van Eldik L.J., Watterson D.M., and Wainwright M.S. (2008). Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D.M., Sanberg P.R., Bickford P.C., Kaneko Y., and Borlongan C.V. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PloS One 8, e53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Acosta S.A., Diamond D.M., Wolfe S., Tajiri N., Shinozuka K., Ishikawa H., Hernandez D.G., Sanberg P.R., Kaneko Y., and Borlongan C.V. (2013). Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PloS One 8, e81585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spielman L.J., and Klegeris A. (2014). The role of insulin and incretins in neuroinflammation and neurodegeneration. Immunoendocrinology 1, e391 [Google Scholar]
- 102.Lindsay J.S., and Andis K. (2014). The role of insulin and incretins in neuroinflammation and neurodegeneration. Immunoendocrinol. 1, e391 [Google Scholar]
- 103.Tweedie D., Ferguson R.A., Fishman K., Frankola K.A., Van Praag H., Holloway H.W., Luo W., Li Y., Caracciolo L., Russo I., Barlati S., Ray B., Lahiri D.K., Bosetti F., Greig N.H., and Rosi S. (2012) Tumor necrosis factor-α synthesis inhibitor 3,6′-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. J. Neuroinflammation. 9, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kobori N., Moore A.N., and Dash P.K. (2015). Altered regulation of protein kinase a activity in the medial prefrontal cortex of normal and brain-injured animals actively engaged in a working memory task. J. Neurotrauma 32, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Titus D.J., Furones C., Kang Y., and Atkins C.M. (2013). Age-dependent alterations in cAMP signaling contribute to synaptic plasticity deficits following traumatic brain injury. Neuroscience 231, 182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim S.J., Nian C., Widenmaier S., and McIntosh C.H. (2008). Glucose-dependent insulinotropic polypeptide-mediated up-regulation of beta-cell antiapoptotic Bcl-2 gene expression is coordinated by cyclic AMP (cAMP) response element binding protein (CREB) and cAMP-responsive CREB coactivator 2. Mol. Cell. Biol. 28, 1644–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lennox R.R., Moffett C., Porter D.W., Irwin N., Gault V.A., and Flatt P.R. (2015). Effects of glucose-dependent insulinotropic polypeptide receptor knockout and a high-fat diet on cognitive function and hippocampal gene expression in mice. Mol. Med. Rep. 12, 1544–1548 [DOI] [PubMed] [Google Scholar]