Abstract
Tau aggregation is a pathological feature of numerous neurodegenerative disorders and has also been shown to occur under certain conditions of traumatic brain injury (TBI). Currently, no effective treatments exist for the long-term effects of TBI. In some cases, TBI not only induces cognitive changes immediately post-injury, but also leads to increased incidence of neurodegeneration later in life. Growing evidence from our lab and others suggests that the oligomeric forms of tau initiate the onset and spread of neurodegenerative tauopathies. Previously, we have shown increased levels of brain-derived tau oligomers in autopsy samples from patients diagnosed with Alzheimer's disease. We have also shown similar increases in tau oligomers in animal models of neurodegenerative diseases and TBI. In the current study, we evaluated the presence of tau oligomers in blast-induced TBI. To test the direct impact of TBI-derived tau oligomer toxicity, we isolated tau oligomers from brains of rats that underwent either a blast- or a fluid percussion injury–induced TBI. Oligomers were characterized biochemically and morphologically and were then injected into hippocampi of mice overexpressing human tau (Htau). Mice were cognitively evaluated and brains were collected for immunological analysis after testing. We found that tau oligomers form as a result of brain injury in two different models of TBI. Additionally, these oligomers accelerated onset of cognitive deficits when injected into brains of Htau mice. Tau oligomer levels increased in the hippocampal injection sites and cerebellum, suggesting that tau oligomers may be responsible for seeding the spread of pathology post-TBI. Our results suggest that tau oligomers play an important role in the toxicity underlying TBI and may be a viable therapeutic target.
Key words: : neurodegeneration, tau oligomers, traumatic brain injury
Introduction
Each year, there are around 1.5 million new cases of traumatic brain injury (TBI) in the United States. 1 Of these patients, more than 200,000 require hospitalization, 80,000 are disabled, and 50,000 die.1 TBI causes nearly $50 billion in medical expenses and lost productivity annually,2 making it one of the major health care problems in the United States. There are more than 7.73 and 5.3 million4 people living with disabilities from TBI in the European Union and United States, respectively.5 Thus, whereas there are approximately 275,000 new hospitalizations for TBI in the United States annually,6 there are millions of TBI survivors in the United States and worldwide. In many patients, even mild TBI is a chronic disease that contributes to neurological, neuropsychological, and behavioral deficits for years post-injury.7 Additionally, TBI is a significant risk factor for subsequent development of dementia,8–10 even in patients with no post-traumatic cognitive impairments.11
TBI causes neuronal injury through processes that begin on impact and continue for days to months or years post-injury.12–14 There have been many hypotheses about the causes of this ongoing injury,14,15 including involvement of tau pathology.16–20 Recently, we proposed a possible mechanism, based on accumulation and spreading of a toxic form of tau protein, known as tau oligomers.21 A large body of research has suggested recently that toxicity of tau protein in neurodegenerative disorders, such as Alzheimer's disease (AD), does not depend on the fibrillar aggregates, neurofibrillary tangles, long known to be hallmarks of the disease. Rather, toxicity begins earlier when functional tau monomer misfolds to form soluble, oligomeric aggregates.22–27 In an earlier study, we reported rapid increases in levels of phosphorylated tau and tau oligomers that persisted in the brain for at least 2 weeks after experimental TBI in nontransgenic rats.21 However, though we showed that TBI correlates with increased tau oligomers, thus far there have been no studies to directly investigate the impact of tau oligomers derived from TBI models on toxicity associated with long-term deficits and neurodegeneration.
Therefore, in the present study, we characterized the structure and toxicity of tau oligomers in a second rodent model of TBI using an advanced blast simulator (ABS) device that produced a blast overpressure wave. Additionally, to test the hypothesis that tau oligomers derived from TBI can induce onset of cognitive impairment and spread of pathology, we isolated tau oligomers from brains of rats subjected to fluid percussion injury (FPI)-induced TBI, purified the proteins, and then injected the tau oligomers into brains of young, cognitively normal mice overexpressing nonmutated human tau protein (Htau mice).28 Here, we report that these TBI-harvested tau oligomers induced an accelerated onset of memory impairment. This finding was supported by our previous work in which tau oligomers harvested from brains of AD patients and injected into brains of wild-type (WT) and Htau mice produced significant memory deficits.26,29 Additionally, we observed increased levels of tau oligomers in multiple brain regions of mice that had been injected with the TBI-harvested tau oligomers. This increase in other brain regions may be attributed to seeding and a prion-like spreading effect of toxic tau oligomers.30
Methods
Animals
This study was conducted in a facility accredited by the American Association for the Accreditation of Laboratory Animal Care, and all experiments were performed in accord with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch (UTMB; Galveston, TX). Mice were bred at UTMB free of enrichment to prevent any effect on behavioral test performance. Mice and rats were housed at the UTMB animal care facility and maintained according to U.S. Department of Agriculture standards (12-h light/dark cycle, with food and water ad libitum).
Experimental design
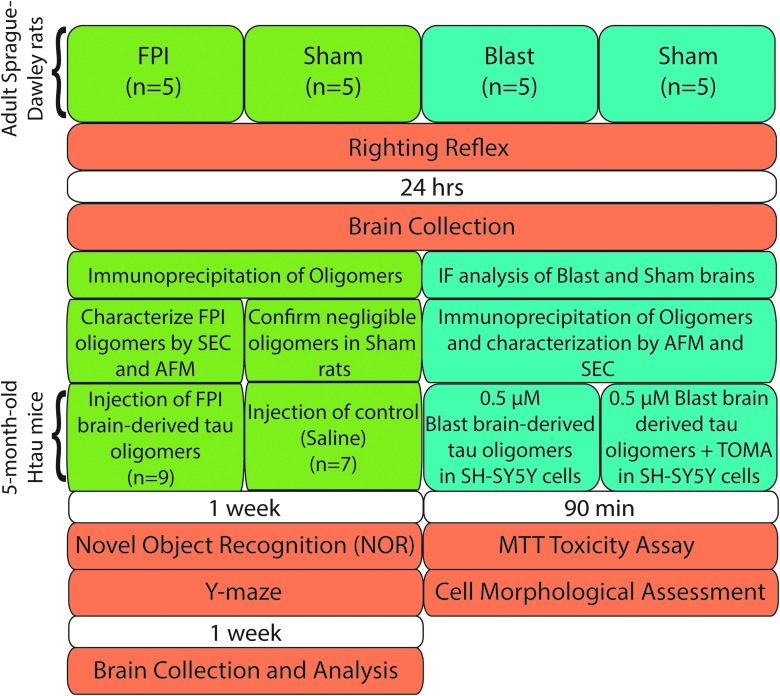
Experimental design is detailed in Figure 1.
FIG. 1.
Flow chart of experimental design characterizing tau oligomers and toxicity from FPI- and blast-injured rats. AFM, atomic force microscopy; FPI, fluid percussion injury; IF, immunofluorescence; MTT, methylthiazole tetrazolium; SEC, size-exclusion chromatography; TOMA, tau oligomer-specific monoclonal antibody.
Parasagittal fluid percussion injury
Male Sprague-Dawley (Charles River Laboratories, Wilmington, MA) rats (400–500 g) were anesthetized (4% isoflurane), intubated, mechanically ventilated with 1.5% isoflurane in O2/air (20:80) using a volume ventilator (NEMI Scientific, New England Medical Instruments, Medway, MA), and prepared for parasagittal FPI, as described previously.21,31 Rectal and temporalis muscle temperatures were maintained within a range of 37.5 ± 0.5°C. Rats were placed in a stereotaxic apparatus, and a craniotomy was trephined 1 mm lateral (right) of the sagittal suture midway between the lambda and bregma just before FPI or sham injury. Isoflurane was briefly discontinued until the rat displayed a withdrawal response to paw pinch. Immediately after the return of the withdrawal response, the rat was subjected to moderate (2.0 atmospheres [atm]) TBI using an FPI device (Custom Design & Fabrication, Richmond, VA). The transducer housing on our device is connected to the rat by an 18-mm nylon tube that fits into the cap (modified 20-gauge needle hub cemented into the craniotomy site with cyanoacrylic adhesive and hygienic dental acrylic), perpendicular to the craniotomy site. Sham-injured animals are prepared identically to TBI animals with the exception of the release of the pendulum. The height of the pendulum (set at 15.0 cm) determines the intensity of the injury (between 2.0 and 2.2 atm), and the fluid pressure pulse was recorded on an oscilloscope (TDS1002; Tektronix, Inc., Beaverton, OR). The peak pressure pulse was in the range of 250–360 mV, and the pressure wave duration was 25.0 ms. Post-TBI, the time it takes for the animal to regain three consecutive righting reflexes was recorded and then the animal was given isoflurane, and the skin incision was closed and the rodent was allowed to recover. Twenty-four hours post-TBI or sham injury, rats were humanely euthanized and brains were collected and immediately snap frozen and kept at −80°C.
Blast injury
Adult male Sprague-Dawley rats (Charles River Laboratories) weighing 350–400 g were anesthetized with 4% isoflurane in an anesthetic chamber, intubated, and mechanically ventilated with 1.5–2.0% isoflurane in O2/room air (20:80) using a volume ventilator. After intubation, the top of the scalp was shaved and foam plugs were placed into each ear. The ABS device is a shock tube designed by David Ritzel (Dyn-FX Consulting, Ltd., Amherstburg, Ontario, Canada) and produced by Steve Parks (ORA, Inc., Fredericksburg, VA). The ABS uses a compressed air driver to produce Friedlander-type shock over-/underpressure waves32 that closely replicate those recorded in open-field blasts.33 The ABS consists of a driver chamber, a blast wave expansion chamber, a specimen chamber, and a blast wave reflection suppressor. The driving chamber is separated from the expansion chamber by a Mylar membrane. Amplitudes of shock waves are determined by thickness of the membrane. The shock wave produced when the Mylar ruptures is propagated down the tube where it interacts with the experimental animal and then passes into a wave reflection suppressor. Just before the blast, each rat was disconnected from the ventilator, placed on a specimen tray with its head inside of the ABS device, and secured with Velcro strips. Immediately after the return of a withdrawal response to paw pinch, the rat was subjected to ABS blast injury (17–22 pounds per square inch) and removed from the specimen tray. The duration of the suppression of the righting reflex (RR) was measured as an indicator of injury severity, and the average RR range for mild blast in our lab is between 3 and 6 min. After the return of the RR, rats were placed in a cage in the laboratory and monitored. Twenty-four hours later, they were deeply anesthetized, decapitated, and brains were collected and immediately snap frozen and kept at −80°C.
Preparation of brain-derived tau oligomers
Tau oligomers were immunoprecipitated from brains of injured Sprague-Dawley rats with FPI (n = 5) and blast injury (n = 5), as previously described.21,26,31 Immunoprecipitation protocols were also completed for sham rats from both FPI and blast injury cohorts as a control (n = 5). Tosyl-activated magnetic Dynabeads (Dynal Biotech, Lafayette Hill, PA) were coated with 20 μg of antitau oligomer-specific polyclonal antibody T22 (1.0 mg/mL) diluted in 0.1 M of borate (pH 9.5) overnight at 37°C. Beads were washed (0.2 Mof Tris and 0.1% bovine serum albumin; pH 8.5) and then incubated with TBI rodent brain homogenate (phosphate-buffered saline [PBS] soluble fraction) with rotation at room temperature (RT) for 1 h. Beads were washed three times with PBS and eluted using 0.1 M of glycine (pH 2.8). pH was adjusted using 1 M of Tris (pH 8.0) and then fractions were centrifuged in a microcon centrifugal filter device with a molecular weight cutoff of 25 kDa (catalog no.: 42415; Millipore, Billerica, MA) at 14,000g for 25 min at 4°C. Oligomers were resuspended in sterile PBS. Protein concentration was measured using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Samples were then centrifuged once more in a microcon centrifugal filter device with a cutoff of 25 kDa at 14,000g for 25 min at 4°C. Oligomers were characterized by various methods, including size-exclusion chromatography (SEC) and atomic force microscopy (AFM), as previously described,21,31,34 and stored at −80°C. Oligomers were resuspended in PBS in order to obtain the desired concentration (0.18–1.2 mg/mL) and kept at 4°C for 15–30 min, then at RT for 10 min before use.
Stereotaxic injection of brain-derived tau oligomers
Five-month-old homozygous Htau mice (stock no.: 005491; The Jackson Laboratory, Bar Harbor, ME)28 were anesthetized with ketamine (80–100 mg/kg, intraperitoneally [i.p.]) and xylazine (10 mg/kg, i.p.) and placed in a stereotactic apparatus (Motorized Stereotaxic StereoDrive; Neurostar, Tübingen, Germanyt). The scalp of each mouse was then shaved and an incision was made through the midline to expose the skull. A craniotomy was drilled (–2.06 mm posterior, ±1.75 mm lateral, and 2.5 mm ventral to the bregma) into the skull of each mouse. A 5.0-μL Hamilton syringe was used to inject 2 μL of either 0.3 mg/mL of brain-derived tau oligomers or saline (n = 10 for each group) bilaterally into the dorsal hippocampus at an infusion rate of 0.2 μL/min, as described previously.26,33,27 The skin incision was closed using Vet-Bond, and mice were placed on a 37°C isothermal pad and continuously observed post-surgery until recovery. Attrition post-surgery left n = 7 for the control group and n = 9 for the oligomer-injected group.
Novel object recognition
The novel object recognition (NOR) task utilizes the natural tendency of rodents to preferentially explore novel objects and environments over those that are familiar. This task has been used previously to detect memory impairment in Htau mice.30,35 One week after brain-derived tau oligomer injection, animals were habituated to the NOR task (n = 7–9 mice per group). Mice were allowed to freely explore a white open-field arena (55 cm in diameter; 60 cm in height) for 15 min on the first day. The next day, mice were placed in the arena for the training phase with two identical objects, either spheres or cubes, and allowed to explore for 15 min. On the third day, mice were placed again in the arena for 15 min with one familiar object previously explored in the training phase and one novel object differing in color and shape, but sharing a common size and volume. After each trial, the apparatus was thoroughly cleaned using 70% ethanol and allowed to dry before placement of a new mouse. Trials were recorded and time spent exploring each object was measured using ANY-Maze software. Exploration was defined by head orientation within 2 cm of the object or physical contact with the object. The percentage of total time spent exploring the familiar object versus the novel object was measured. In order to control for any differences in exploratory behavior, the discrimination index was also calculated as the time spent exploring the familiar object subtracted from the time spent exploring the novel object, divided by the total time spent exploring both objects. Object exploration data were analyzed using GraphPad Prism sofware (version 5.04; GraphPad Software Inc., La Jolla, CA) by one-way t-test with a hypothesized mean of zero for the discrimination index.
Y-maze task
This task provides a measure of spatial working memory and is based on the innate preference of mice to alternate arms when exploring a new environment. At post-injection day 11, mice (n = 7–9 per group) were placed in a symmetrical Y-shaped maze. Arms were 40 cm long, 8 cm wide, and 12 cm high (San Diego Instruments, San Diego, CA), beige in color, nonreflective, and randomly designated A, B, or C. Each mouse was placed in an arm facing the center (arm A) and allowed to explore the maze for 8 min. The number of arms entered, as well as the sequence of entries, was recorded. A correct alternation occurred when the animal moved from the arm in which it began to the other two arms without retracing its steps (i.e., ABC or ACB). Spontaneous alternation, expressed as a percentage, was calculated by dividing the number of entries into all three arms on consecutive choices (correct choices) by number of arm entries subtracted by two, then multiplying the quotient by 100. A high spontaneous alternation rate is indicative of effective working memory given that animals must remember which arm was entered last in order to know not to re-enter it.
Tissue collection and immunofluorescence
One week after behavioral testing, mice injected with FPI brain-derived tau oligomers (n = 7–9) were anesthetized with CO2 and brains were collected. Brains from both FPI tau oligomer-treated mice and blast-injured rats were processed as follows for immunofluorescence (IF) analysis. Brains were embedded in optimal cutting temperature compound and sectioned using a cryostat. All sections were processed simultaneously under the same conditions. Sections (7 μm) were fixed in 4% paraformaldehyde. After blocking in bovine serum albumin for 1 h, sections were incubated overnight with antitau oligomer-specific polyclonal antibody, T22 (1:250). The following day, sections were washed in PBS three times for 10 min each and incubated with goat antirabbit immunoglobulin G (IgG) Alexa Fluor-488 (1:500; Invitrogen, Carlsbad, CA) for 1 h. Sections were then washed three times for 10 min each in PBS and either processed for cover-slipping or incubated overnight with total tau antibody, Tau-5 (1:100). Sections were washed in PBS three times for 10 min each before incubation with donkey antimouse IgG Alexa Fluor-568 (1:500; Invitrogen). Sections were washed and mounted using Fluoromount-G (Southern Biotech, Birmingham, AL) mounting medium with 4′,6-diamidino-2-phenylindole (Invitrogen). Sections were imaged using a Zeiss LSM 510 Meta confocal system (Carl Zeiss GmbH, Jena, Germany). Low-magnification images (20×) of the hippocampus and cerebellum were acquired for each sample. Three high-magnification images (100×) were taken at the hippocampus, frontal cortex, and cerebellum of each sample, and six cells were randomly selected from each image for quantification using ImageJ software (NIH, Bethesda, MD). The total level of T22 fluorescence was measured for each cell, as well as the level of background from three different regions around the cell without fluorescence. In order to correct the level of fluorescence for background and cell size, the background multiplied by the area of the cell was subtracted from the total fluorescence, as described previously.36 The corrected cell fluorescence was analyzed by one-way analysis of variance (ANOVA).
Cellular toxicity assay
Cell viability after treatment with ABS blast brain-derived tau oligomers was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) toxicity assay kit (Roche Diagnostics, Indianapolis, IN), as previously described.27 SH-SY5Y human neuroblastoma cells were maintained in Dulbecco's modified Eagle's medium and grown to confluence in a 96-well plate. Cells (∼10,000 per well) were treated with fresh media alone or containing either 0.5-μM tau oligomers or 0.5-μM tau oligomers pre-treated with an equal concentration of tau oligomer-specific monoclonal antibody (TOMA). After incubation for 90 min at 37°C, the cells were assayed using MTT colorimetric assay according to the manufacturer's directions. All measurements were made in triplicate. Results were analyzed by one-way ANOVA. Cells were also evaluated for morphological changes by microscopy.
Results
Brain tau oligomer levels are increased in blast-injured rats
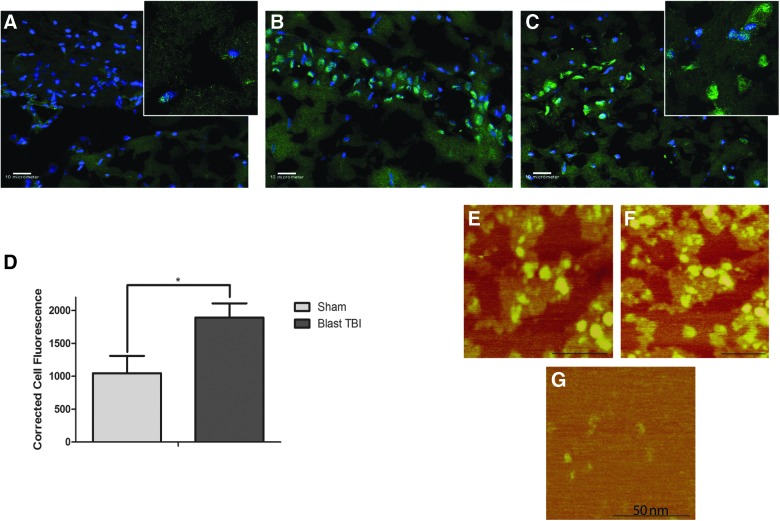
We investigated the presence of tau oligomers in the blast injury rat model 24 h post-injury. Using our novel tau oligomer-specific antibody, T22, we found that tau oligomers are present in high levels in the hippocampus of blast-injured rats compared to sham animals, which show low levels of tau oligomers (Fig. 2A–C). IF staining of individual cells with T22 was corrected for cell size and background fluorescence and quantified in blast injury and sham rat samples. Blast-injured rats exhibited significantly elevated levels of tau oligomers when compared to sham (p = 0.0217; Fig. 2D). In order to characterize tau oligomers formed after blast TBI, we isolated tau oligomers from rat brains 24 h post-injury by immunoprecipitation with the tau oligomer-specific antibody, T22, and analyzed them by AFM (Fig. 2E–G). The presence of a heterogeneous population of oligomers, but no fibrils, could be observed in samples immunoprecipitated from blast TBI brains, whereas negligible oligomers were found in samples isolated from sham rat brains.
FIG. 2.
Immunofluorescence with tau oligomer-specific antibody, T22 in hippocampal sections at 20× (100× magnification images inset) of sham (A) and blast-injured rats (B and C). Tau oligomers are significantly elevated in the hippocampus of blast-injured rats (B and C), when compared to sham animals (A), as measured by corrected total cell fluorescence analysis (p = 0.0217) (D). Tau oligomers immunoprecipitated with T22 from blast-injured rat brain homogenate can be seen by AFM (E and F), whereas sham animals do not exhibit characteristic oligomeric structures (G). AFM, atomic force microscopy; TBI, traumatic brain injury.
Single injection with traumatic brain injury–derived tau oligomers induces cognitive deficits in human tau mice
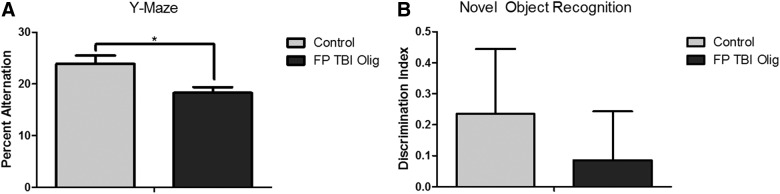
In order to test the cognitive impact of tau oligomers derived from TBI rodents, we isolated tau oligomers from FPI rats and characterized them by SEC and AFM.21 We then injected 0.6 μg of tau oligomers or 2 μL of PBS bilaterally in hippocampi of 5-month-old Htau mice. One week post-injection, mice were tested for cognitive deficits using the Y-maze and novel object recognition tasks. Mice injected with tau oligomers had a significantly lower percentage of spontaneous alternation than mice injected with PBS on the spatial memory task, the Y-maze (p = 0.0091; Fig. 3A), but differences in the novel object recognition task were not statistically significant (Fig. 3B).
FIG. 3.
Htau mice injected with TBI-derived tau oligomers exhibit significantly a lower percentage of spontaneous alternation of the Y-maze task compared to Htau mice injected with saline (p = 0.0091) (A). Mice injected with tau oligomers were not significantly impaired on the novel object recognition task (B). Htau, human tau; Olig, oligomer; TBI, traumatic brain injury.
Tau oligomer levels increase outside the site of injection in human tau mice
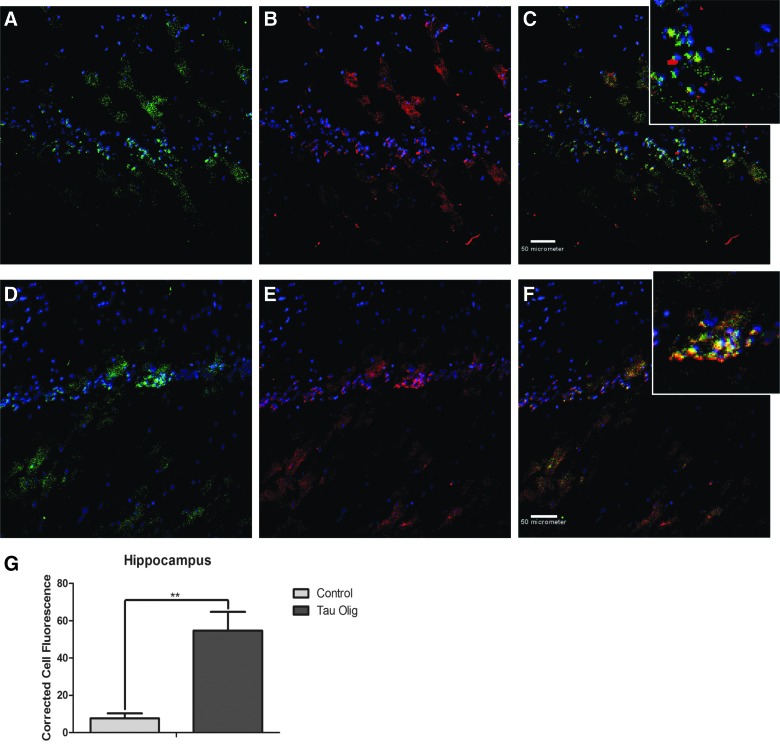
In order to determine whether tau oligomer levels differ between control and injected animals in specific brain regions, brain sections from Htau mice were analyzed by IF with T22 and Tau-5. Tau oligomers were present in the hippocampus of control Htau mice, but were significantly increased in hippocampi of mice injected with tau oligomers, as measured by the corrected total cell fluorescence of randomly selected cells in each sample (p = 0.0053; Fig. 4).
FIG. 4.
Tau oligomers detected with T22 (green) and total tau levels labeled with Tau-5 (red) in the hippocampus of control Htau (A–C) and TBI oligomer-injected Htau mice (D–F). Total fluorescence of individual cells labeled with T22, corrected for cell size and background fluorescence, showed that TBI tau oligomer-injected Htau brain samples had significantly higher levels of tau oligomers in the hippocampus (p = 0.0053) (G). Htau, human tau; Olig, oligomer; TBI, traumatic brain injury.
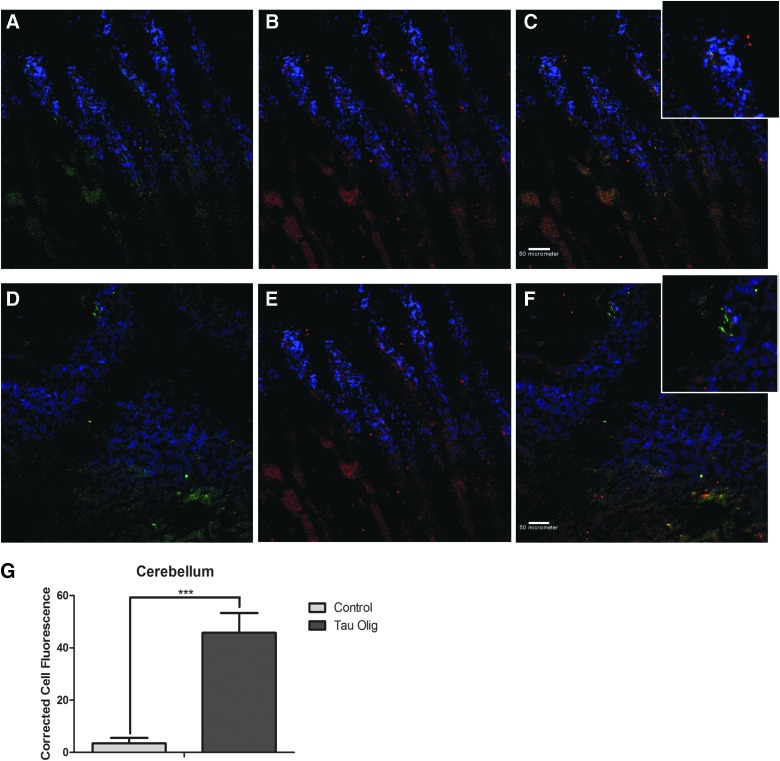
Negligible levels of tau oligomers were detected in the cerebellum of control Htau mice, but were significantly increased in the cerebellum of mice injected with tau oligomers, as measured by the corrected total cell fluorescence of randomly selected cells in each sample (p = 0.0005; Fig. 5). In order to test whether toxicity induced by injection with TBI brain-derived tau oligomers was dependent upon cell death, we analyzed brain sections from injected mice using Fluorojade, a dye that recognizes injured neurons. We found no effects of injection on the level on Fluorojade-positive cells (results not shown).
FIG. 5.
Tau oligomers detected with T22 (green) and total tau levels labeled with Tau-5 (red) in the cerebellum of control Htau (A–C) and TBI oligomer-injected Htau mice (D–F). Total fluorescence of individual cells labeled with T22, corrected for cell size and background fluorescence, showed that TBI tau oligomer-injected Htau brain samples had significantly higher levels of tau oligomers in the cerebellum (p = 0.0005) (G). Htau, human tau; Olig, oligomer; TBI, traumatic brain injury.
Blast traumatic brain injury–derived tau oligomers induce toxicity inhibited by tau oligomer-specific antibody
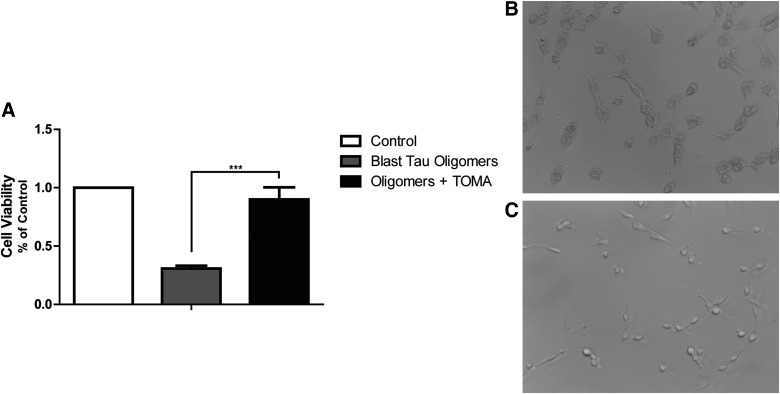
We treated SH-SY5Y neuroblastoma cells with tau oligomers isolated and characterized from blast TBI rats. We found that after 90 min, tau oligomer treatment led to a significant decrease in cell viability measured by the MTT cell toxicity assay when compared with cells given fresh media containing no tau oligomers (p = 0.0005). Moreover, we pre-incubated isolated tau oligomers with a TOMA and found that antibody treatment prevented the toxic effects of tau oligomers and cell viability did not differ from control cells given fresh media. Moreover, cells treated with blast TBI-derived tau oligomers exhibited an altered cellular morphology when compared to cells treated with tau oligomers pre-incubated with TOMA (Fig. 6).
FIG. 6.
Cell viability measured as a fraction of the absorbance detected by the MTT toxicity assay, in comparison to control cells, was analyzed for SH-SY5Y neuroblastoma cells treated with blast TBI-derived tau oligomers with and without TOMA (A). Cells given tau oligomers pre-incubated with TOMA had significantly higher cell viability when compared to TBI-derived tau oligomers alone (p = 0.0005). Cells administered TBI-derived tau oligomers alone (B) were compared to cells treated with tau oligomers pre-incubated with TOMA (C) and evaluated for morphological differences. MTT, methylthiazole tetrazolium; TBI, traumatic brain injury; TOMA, tau oligomer-specific monoclonal antibody.
Discussion
Blast exposure is the most common cause of combat-related TBIs among military personnel in the conflicts in Iraq and Afghanistan,37–39 and blast-induced neurotrauma may be associated with the subsequent development of chronic traumatic encephalopathy.40 Blast injury in humans and experimental animals is associated with increased levels of total and phosphorylated tau protein.18,40–42 We have previously shown that an increase in levels of tau oligomers results from fluid percussion TBI rats.21 However, the contribution of tau oligomers to blast TBI has not yet been investigated. Moreover, whether tau oligomers in TBI directly contribute to cognitive deficits and risk for neurodegenerative disease later in life is unknown. In this study, we characterized tau oligomer pathology in blast-injured rats. Tau oligomers detected with our novel tau oligomer-specific antibody, T22, are increased in the hippocampus of rats 24 h after blast injury. We were able to visualize tau oligomers immunoprecipitated from brains of blast-injured rats, but not in sham animals. Therefore, tau oligomers are an important component to blast injury pathology and may be a beneficial biomarker and therapeutic target. Moreover, an independent study of TBI found that tau oligomers were detected in brain samples at 12–24 h post-injury and by decreasing levels of phosphorylated and oligomeric, using an antibody against cis-p-tau, prevented the toxic effects of injury, further highlighting the potential role of tau oligomers in TBI.43
Tau has been highlighted as an important protein of interest in neurodegenerative diseases, such as AD and frontotemporal lobar dementia; however, it is clear that it may also be critical for induction of long-term effects of TBI and increased incidence of neurodegeneration later in life. Whereas the majority of tau mouse models express a mutated form of tau, tau pathology in AD and TBI occur in humans independent of any mutations. Therefore, more useful information relevant to TBI and increased incidence of AD could be gleaned using mice that do not express mutated tau leading to drastic neurodegeneration phenotypes early in age. Previously, we isolated oligomers from brains that had AD and other tauopathies27,36,44 and found that tau oligomers injected in WT mice exhibit toxic effects that can be protected with tau oligomer-specific antibodies, whereas treatment with tau monomer and fibrils does not lead to any phenotypic differences.27,30 However, WT mice maintain protection after antibody treatment for significantly longer periods than mice expressing human tau rather than mouse tau.30 This difference in benefit between WT and Htau mice suggests that brain-derived tau oligomers may have a lower ability to seed the misfolding of endogenous mouse tau when compared to human tau. Therefore, the Htau mouse model that expresses nonmutated human tau is a particularly good model for these conditions. Htau mice begin to shown memory deficits at around 12 months of age, but do not exhibit any detriments to motor skills, sensory abilities, or overall health.35 Whether tau oligomers from TBI lead to similar consequences as those derived from neurodegenerative tauopathies was unclear. Therefore, we examined the hypothesis that tau oligomers derived from TBI can induce onset of behavioral impairment and spread of pathology when injected into cognitively intact animals. We isolated tau oligomers from rats that underwent TBI and injected them into hippocampi of young, cognitively intact Htau mice. We found that injection of TBI-derived tau oligomers led to the rapid induction of deficits of spatial memory in mice, providing direct evidence supporting our hypothesis that tau oligomers induce many of the symptoms of TBI. We also detected tau oligomers in brain regions that were apart from the injection site, suggesting they may have spread to other areas of the brain. These results also provide a direct connection between occurrence of TBI and the increased prevalence of acquiring neurodegenerative disease later in life. Toxicity incurred in injected mice was found to be independent of cell death, indicating that toxicity from tau oligomers is likely attributed to effects occurring in the absence of cell death, such as synaptic and mitochondrial toxicity, as has been shown previously in tau models of neurodegeneration.26
Previously, we thoroughly examined the effects of recombinant tau oligomers as well as tau oligomers derived from neurodegenerative disease on neuroblastoma cells—a cell model used extensively to examine toxicity of amyloidogenic proteins—in comparison to tau monomer and tau fibrils. We have shown in repeated experiments that tau oligomers induce significantly higher levels of toxicity in these cells when compared to control as well as other forms of tau.25,29,34,45 In order to determine the toxicity of tau oligomers isolated from our ABS blast TBI model and whether toxicity can be prevented by pre-treatment with TOMA, we utilized the same procedure that we have established previously. We treated SH-SY5Y neuroblastoma cells with TBI brain-derived tau oligomers and saw a significant decrease in cell viability that was reversed when tau oligomers were pre-incubated with TOMA.
In addition to their role in toxicity in disease, tau oligomers are thought to contribute to the spread of pathology from affected regions of the brain to unaffected regions in neurodegenerative disease.30 We have shown that tau oligomers derived from AD are capable of propagating pathology from the injection site to connected brain regions,27 suggesting that oligomeric tau in TBI may similarly lead to proliferation of toxic forms of tau from the site of injury to other areas in the brain, leading to a worsening of symptoms in the time post-trauma. Here, we investigated tau oligomer levels in the hippocampal injection sites, as well as the frontal cortex and cerebellum. We found that tau oligomers begin to accumulate in the hippocampus and frontal cortex of control Htau mice, but are present in significantly higher levels throughout the hippocampus of injected mice (p = 0.0053). Importantly, tau oligomer levels were significantly increased in the cerebellum, but were not different in the frontal cortex (data not shown). Though more-extensive evaluation of brain regions will be needed in the future, these results suggest that tau oligomers in TBI may be capable of seeding the misfolding and aggregation of endogenous tau in the brain and spreading from one region to another. Previous studies have shown that tau aggregates are capable of propagating across synaptically connected, as well as neighboring, brain regions.46–48 Our data support the finding in neurodegenerative disease research that tau oligomers are associated with the endosomal/exosomal pathway and can be transferred from cell to cell in vitro,49 making them a likely source for initiation of the spread of pathology.
Our results highlight the importance of tau oligomers in TBI and suggest that TBI may share a common mechanism of toxicity with neurodegenerative tauopathies. Moreover, these data suggest that the increased prevalence of acquiring AD many years after occurrence of TBI may be attributed to the seeding and spread of tau oligomers released after neuronal injury. This has important implications for both treatment of TBI and for prevention of neurodegeneration late in life. We have previously shown that accelerated onset of cognitive dysfunction in Htau mice induced by the injection of AD-derived tau oligomers can be prevented by treating mice with a TOMA.30 Further study is warranted to determine whether targeting tau oligomers in TBI may be an effective therapeutic strategy.
Acknowledgments
This work was supported, in part, by the Mitchell Center for Neurodegenerative Disease, the Sealy Center for Vaccine Development, Darrell K. Royal Research Fund for Alzheimer's Disease, and the Institute for Rehabilitation and Research/Mission Connect. Additionally, these studies were completed as part of an interdisciplinary research team funded by The Moody Project for Translational Traumatic Brain Injury Research. The authors thank Prof. Giulio Taglialatela and Prof. Yogesh Wairkar for their valuable assistance with this project.
Author Disclosure Statement
R.K. has patent applications on the compositions and methods related to tau oligomers and antibodies. No competing financial interests exist for all other authors.
References
- 1.Thruman D., and Guerrero J. (1999). Trends in hospitalization associated with traumatic brain injury. JAMA 282, 954–957 [DOI] [PubMed] [Google Scholar]
- 2.Sosin D.M., Sniezek J.E., and Waxweiler R.J. (1995). Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA 273, 1778–1780 [PubMed] [Google Scholar]
- 3.Tagliaferri F., Compagnone C., Korsic M., Servadei F., and Kraus J. (2005). A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 148, 255–268 [DOI] [PubMed] [Google Scholar]
- 4.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 5.Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 6.Faul M., and Coronado V. (2015). Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 127, 3–13 [DOI] [PubMed] [Google Scholar]
- 7.Masel B.E., and DeWitt D.S. (2010). Traumatic brain injury: a disease process, not an event. J. Neurotrauma 27, 1529–1540 [DOI] [PubMed] [Google Scholar]
- 8.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsch-Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 9.Perry D.C., Sturm V.E., Peterson M.J., Pieper C.F., Bullock T., Boeve B.F., Miller B.L., Guskiewicz K.M., Berger M.S., Kramer J.H., and Welsh-Bohmer K.A. (2015). Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J. Neurosurg. 124, 511–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner R.C., Burke J.F., Nettiksimmons J., Goldman S., Tanner C.M., and Yaffe K. (2015). Traumatic brain injury in later life increases risk for Parkinson disease. Ann. Neurol. 77, 987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schofield P.W., Tang M., Marder K., Bell K., Dooneief G., Chun M., Sano M., Stern Y., and Mayeux R. (1997). Alzheimer's disease after remote head injury: an incidence study. J. Neurol. Neurosurg. Psychiatry 62, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith D.H., Chen X.H., Pierce J.E., Wolf J.A., Trojanowski J.Q., Graham D.I., and McIntosh T.K. (1997). Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma 14, 715–727 [DOI] [PubMed] [Google Scholar]
- 13.Bramlett H.M., and Dietrich W.D. (2007). Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 [DOI] [PubMed] [Google Scholar]
- 14.Osier N.D., Carlson S.W., DeSana A., and Dixon C.E. (2015). Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J. Neurotrauma 32, 1861–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramlett H.M., and Dietrich W.D. (2015). Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma 32, 1834–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivera A., Lejbman N., Jeromin A., French L.M., Kim H.S., Cashion A., Mysliwiec V., Diaz-Arrastia R., and Gill J. (2015). Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 72, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 17.Cheng J.S., Craft R., Yu G.Q., Ho K., Wang X., Mohan G., Mangnitsky S., Ponnusamy R., and Mucke L. (2014). Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS One 9, e115765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber B.R., Meabon J.S., Martin T.J., Mourad P.D., Bennett R., Kraemer B.C., Cernak I., Petrie E.C., Emery M.J., Swenson E.R., Mayer C., Mehic E., Peskind E.R., and Cook D.G. (2013). Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimers Dis. 37, 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran H.T., Sanchez L., Esparza T.J., and Brody D.L. (2011). Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One 6, e25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liliang P.C., Liang C.L., Lu K., Wang K.W., Weng H.C., Hsieh C.H., Tsai Y.D., and Chen H.J. (2010). Relationship between injury severity and serum tau protein levels in traumatic brain injured rats. Resuscitation 81, 1205–1208 [DOI] [PubMed] [Google Scholar]
- 21.Hawkins B.E., Krishnamurthy S., Castillo-Carranza D.L., Sengupta U., Prough D.S., Jackson G.R., DeWitt D.S., and Kayed R. (2013). Rapid accumulation of endogenous tau oligomers in a rat model of traumatic brain injury: possible link between traumatic brain injury and sporadic tauopathies. J. Biol. Chem. 288, 17042–17050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda S., Sahara N., Saito Y., Murayama S., Ikai A., and Takashima A. (2006). Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer's disease. Neurosci. Res. 54, 197–201 [DOI] [PubMed] [Google Scholar]
- 23.Maeda S., Sahara N., Saito Y., Murayama M., Yoshiike Y., Kim H., Miyasaka T., Murayama S., Ikai A., and Takashima A. (2007). Granular tau oligomers as intermediates of tau filaments. Biochemistry 46, 3856–3861 [DOI] [PubMed] [Google Scholar]
- 24.Patterson K. R., Remmers C., Fu Y., Brooker S., Kanaan N. M., Vana L., Ward S., Reyes J. F., Philibert K., Glucksman M. J., and Binder L. I. (2011). Characterization of prefibrillar tau oligomers in vitro and in Alzheimers disease. J. Biol. Chem. 286, 23063–23076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Sarmiento J., Troncoso J., Jackson G.R., and Kayed R. (2012). Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J. 26, 1946–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Clos A.L., Jackson G.R., and Kayed R. (2011). Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasagna-Reeves C.A., Castillo-Carranza D.L., Sengupta U., Guerrero-Munoz M.J., Kiritoshi T., Neugebauer V., Jackson G.R., and Kayed R. (2012). Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2, 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andorfer C., Kress Y., Espinoza M., de Silva R., Tucker K.L., Barde Y.A., Duff K., and Davies P. (2003). Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 86, 582–590 [DOI] [PubMed] [Google Scholar]
- 29.Castillo-Carranza D.L., Gerson J.E., Sengupta U., Guerrero-Muñoz M.J., Lasagna-Reeves C.A., and Kayed R. (2014). Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J. Alzheimers Dis. 40, Suppl. 1, S97–S111 [DOI] [PubMed] [Google Scholar]
- 30.Gerson J.E., and Kayed R. (2013). Formation and propagation of tau oligomeric seeds. Front. Neurol. 4, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 32.Friedlander F. G. (1946). The diffraction of sound pulses. I. Diffraction by a semi-infinite plate. Proc. R. Soc. Lond. A Math. Phys. Sci. 186, 322–344 [DOI] [PubMed] [Google Scholar]
- 33.Cernak I., and Noble-Haeusslein L.J. (2010). Traumatic brain injury: An overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 30, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasagna-Reeves C.A., Castillo-Carranza D.L., Guerrero-Muñoz M.J., Jackson G.R., and Kayed R. (2010). Preparation and characterization of neurotoxic tau oligomers. Biochemistry 49, 10039–10041 [DOI] [PubMed] [Google Scholar]
- 35.Polydoro M., Acker C.M., Duff K., Castillo P.E., and Davies P. (2009). Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J. Neurosci. 29, 10741–10749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerson J.E., Sengupta U., Lasagna-Reeves C.A., Guerrero-Muñoz M.J., Troncoso J., and Kayed R. (2014). Characterization of tau oligomeric seeds in progressive supranuclear palsy. Acta Neuropathol. Commun. 2, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galarneau M.R., Woodruff S.I., Dye J.L., Mohrle C.R., and Wade A.L. (2008). Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J. Neurosurg. 108, 950–957 [DOI] [PubMed] [Google Scholar]
- 38.Wojcik B.E., Stein C.R.Bagg K., Humphrey R.J., and Orosco J. (2010). Traumatic brain injury hospitalizations of US army soldiers deployed to Afghanistan and Iraq. Am. J. Prev. Med. 38, 1 Suppl., S108–S116 [DOI] [PubMed] [Google Scholar]
- 39.Bass C.R., Panzer M.B., Rafaels K.A., Wood G., Shridharani J., and Capehart B. (2012). Brain injuries from blast. Ann. Biomed. Eng. 40, 185–202 [DOI] [PubMed] [Google Scholar]
- 40.Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A., Upreti C., Kracht J.M., Ericsson M., Wojnarowicz M.W., Goletiani C.J., Maglakelidze G.M., Casey N., Moncaster J.A., Minaeva O., Moir R.D., Nowinski C.J., Stern R.A., Cantu R.C., Geiling J., Blusztajn J.K., Wolozin B.L., Ikezu T., Stein T.D., Budson A.E., Kowall N.W., Chargin D., Sharon A., Saman S., Hall G.F., Moss W.C., Cleveland R.O., Tanzi R.E., Stanton P.K., and McKee A.C. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Polo J.R., Rea H.C., Johnson K.M., Parsley M.A., Unabia G.C., Xu G.Y., Prough D., DeWitt D.S., Spratt H., and Hulsebosch C.E. (2015). A rodent model of mild traumatic brain blast injury. J. Neurosci. Res. 93, 549–561 [DOI] [PubMed] [Google Scholar]
- 42.McKee A.C., and Robinson M.E. (2014). Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 10, 3 Suppl., S242–S253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo A., Shahpasand K., Mannix R., Qiu J., Moncaster J., Chen C.H., Yao Y., Lin Y.M., Driver J.A., Sun Y., Wei S., Luo M.L., Albayram O., Huang P., Rotenberg A., Ryo A., Goldstein L.E., Pascual-Leone A., McKee A.C., Meehan W., Zhou X.Z., and Lu K.P. (2015). Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta U., Guerrero-Muñoz M.J., Castillo-Carranza D.L., Lasagna-Reeves C.A., Gerson J.E., Paulucci-Holthauzen A.A., Krishnamurthy S., Farhed M., Jackson G.R., and Kayed R. (2015). Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol. Psychiatry 78, 672–683 [DOI] [PubMed] [Google Scholar]
- 45.Lasagna-Reeves C., Senupta U., Castillo-Carranza D.L., Gerson J.E., Guerrero-Muñoz M.J., Troncoso J., Jackson G., and Kayed R. (2014). The formation of tau pore-like structures is prevalent and cell specific: possible implications for the disease phenotypes. Acta Neuropathol. Commun. 2, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., Jucker M., Goedert M., and Tolnay M. (2009). Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Calignon A., Polydoro M., Suarez-Calvet M., William C., Adamowicz D.H., Kopeikina K.J., Pitstick R., Sahara N., Ashe K.H., Carlson G.A., Spires-Jones T.L., and Hyman B.T. (2012). Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 73, 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L., Drouet V., Wu J.W., Witter M.P., Small S.A., Clelland C., and Duff (2012). Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J.W., Herman M., Liu L., Simoes S., Acker C.M., Figueroa H., Steinberg J.I., Margittai M., Kayed R., Zurzolo C., Di P.G., and Duff K.E. (2013). Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 288, 1856–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]