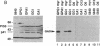Abstract
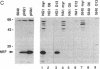
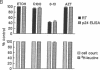
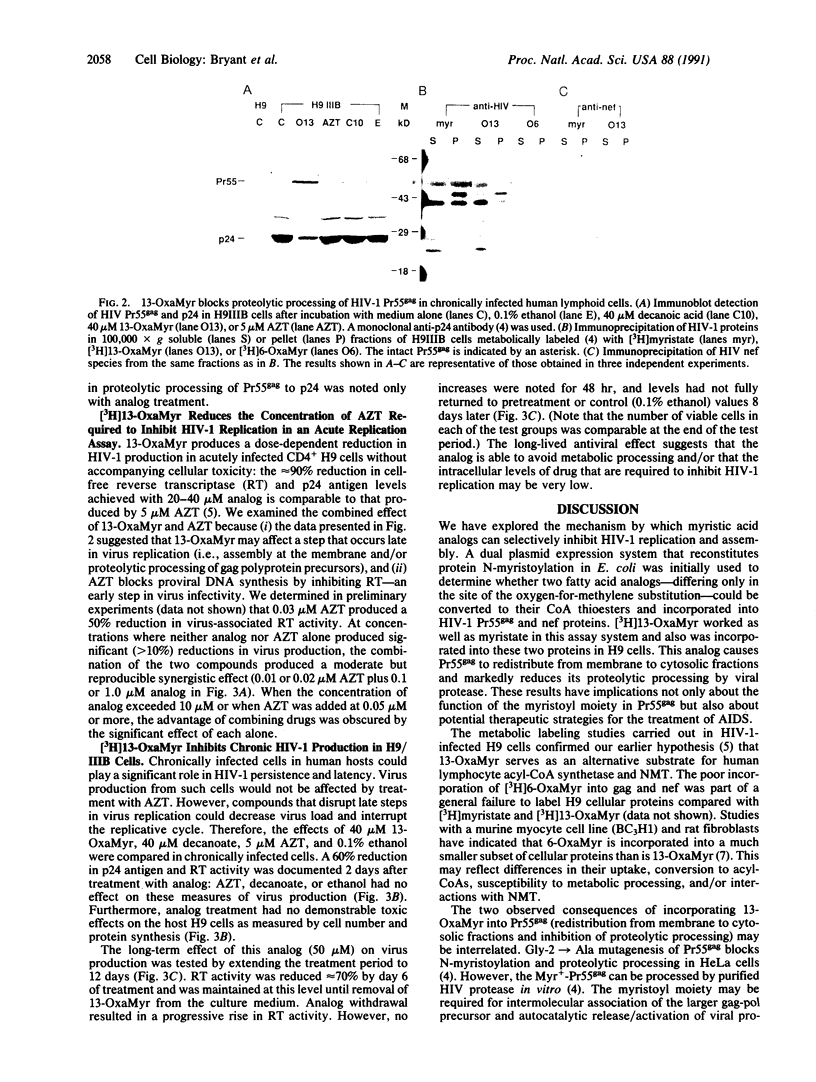
Covalent linkage of myristate (tetradecanoate; 14:0) to the NH2-terminal glycine residue of the human immunodeficiency virus 1 (HIV-1) 55-kDa gag polyprotein precursor (Pr55gag) is necessary for its proteolytic processing and viral assembly. We have shown recently that several analogs of myristate in which a methylene group is replaced by a single oxygen or sulfur atom are substrates for Saccharomyces cerevisiae and mammalian myristoyl-CoA:protein N-myristoyltransferase (EC 2.3.1.97; NMT) despite their reduced hydrophobicity. Some inhibit HIV-1 replication in acutely infected CD4+H9 cells without accompanying cellular toxicity. To examine the mechanism of their antiviral effects, we performed labeling studies with two analogs, 12-methoxydodecanoate (13-oxamyristate; 13-OxaMyr) and 5-octyloxypentanoate (6-oxamyristate; 6-OxaMyr), the former being much more effective than the latter in blocking virus production. [3H]Myristate and [3H]13-OxaMyr were incorporated into Pr55gag with comparable efficiency when it was coexpressed with S. cerevisiae NMT in Escherichia coli. [3H]6-OxaMyr was not incorporated, even though its substrate properties in vitro were similar to those of 13-OxaMyr and myristate. [3H]13-OxaMyr, but not [3H]6-OxaMyr, was also efficiently incorporated into HIV-1 Pr55gag and nef (negative factor) in chronically infected H9 cells. Analog incorporation produced a redistribution of Pr55gag from membrane to cytosolic fractions and markedly decreased its proteolytic processing by viral protease. 13-OxaMyr and 3'-azido-3'-deoxythymidine (AZT) act synergistically to reduce virus production in acutely infected H9 cells. Unlike AZT, the analog is able to inhibit virus production (up to 70%) in chronically infected H9 cells. Moreover, the inhibitory effect lasts 6-8 days. These results suggest that (i) its mechanism of action is distinct from that of AZT and involves a late step in virus assembly; (ii) the analog may allow reduction in the dose of AZT required to affect viral replication; and (iii) combinations of analog and HIV-1 protease inhibitors may have synergistic effects on the processing of Pr55gag.
Full text
PDF


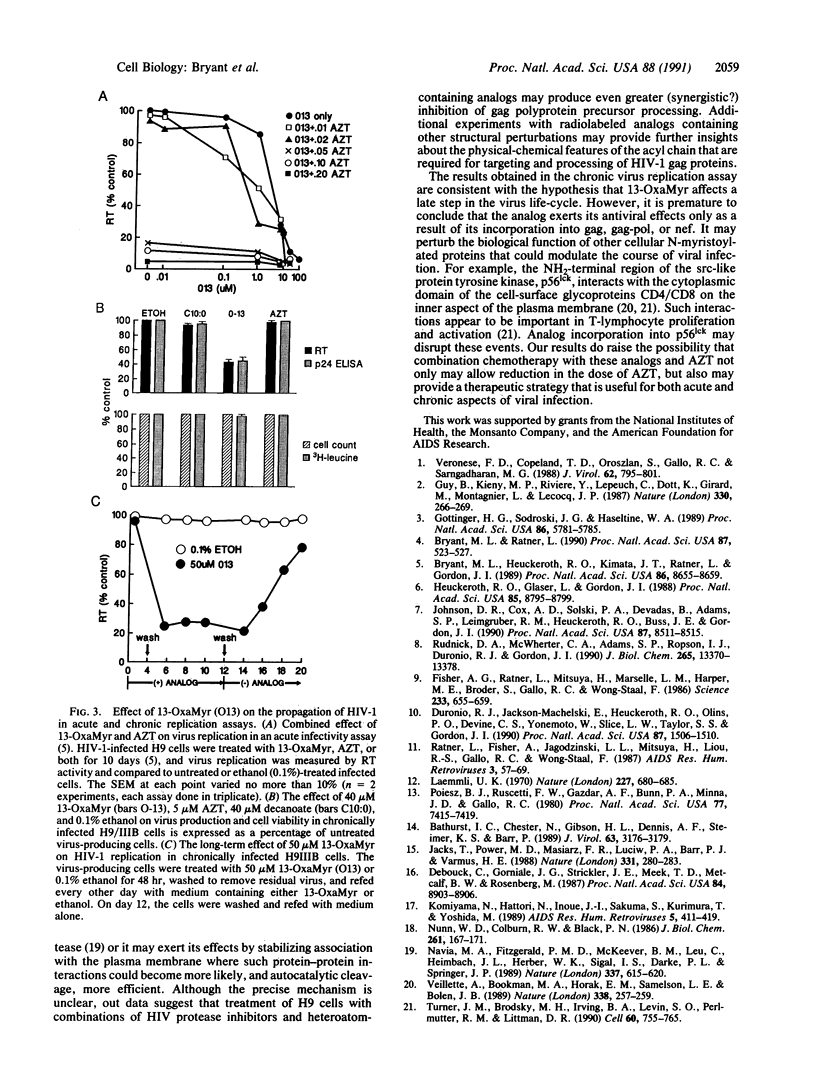
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bathurst I. C., Chester N., Gibson H. L., Dennis A. F., Steimer K. S., Barr P. J. N myristylation of the human immunodeficiency virus type 1 gag polyprotein precursor in Saccharomyces cerevisiae. J Virol. 1989 Jul;63(7):3176–3179. doi: 10.1128/jvi.63.7.3176-3179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. L., Heuckeroth R. O., Kimata J. T., Ratner L., Gordon J. I. Replication of human immunodeficiency virus 1 and Moloney murine leukemia virus is inhibited by different heteroatom-containing analogs of myristic acid. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8655–8659. doi: 10.1073/pnas.86.22.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio R. J., Jackson-Machelski E., Heuckeroth R. O., Olins P. O., Devine C. S., Yonemoto W., Slice L. W., Taylor S. S., Gordon J. I. Protein N-myristoylation in Escherichia coli: reconstitution of a eukaryotic protein modification in bacteria. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Ratner L., Mitsuya H., Marselle L. M., Harper M. E., Broder S., Gallo R. C., Wong-Staal F. Infectious mutants of HTLV-III with changes in the 3' region and markedly reduced cytopathic effects. Science. 1986 Aug 8;233(4764):655–659. doi: 10.1126/science.3014663. [DOI] [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Glaser L., Gordon J. I. Heteroatom-substituted fatty acid analogs as substrates for N-myristoyltransferase: an approach for studying both the enzymology and function of protein acylation. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8795–8799. doi: 10.1073/pnas.85.23.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Cox A. D., Solski P. A., Devadas B., Adams S. P., Leimgruber R. M., Heuckeroth R. O., Buss J. E., Gordon J. I. Functional analysis of protein N-myristoylation: metabolic labeling studies using three oxygen-substituted analogs of myristic acid and cultured mammalian cells provide evidence for protein-sequence-specific incorporation and analog-specific redistribution. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8511–8515. doi: 10.1073/pnas.87.21.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama N., Hattori N., Inoue J., Sakuma S., Kurimura T., Yoshida M. Nucleotide sequences of gag and env genes of a Japanese isolate of HIV-1 and their expression in bacteria. AIDS Res Hum Retroviruses. 1989 Aug;5(4):411–419. doi: 10.1089/aid.1989.5.411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Colburn R. W., Black P. N. Transport of long-chain fatty acids in Escherichia coli. Evidence for role of fadL gene product as long-chain fatty acid receptor. J Biol Chem. 1986 Jan 5;261(1):167–171. [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Fisher A., Jagodzinski L. L., Mitsuya H., Liou R. S., Gallo R. C., Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987 Spring;3(1):57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- Rudnick D. A., McWherter C. A., Adams S. P., Ropson I. J., Duronio R. J., Gordon J. I. Structural and functional studies of Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase produced in Escherichia coli. Evidence for an acyl-enzyme intermediate. J Biol Chem. 1990 Aug 5;265(22):13370–13378. [PubMed] [Google Scholar]
- Turner J. M., Brodsky M. H., Irving B. A., Levin S. D., Perlmutter R. M., Littman D. R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988 Mar;62(3):795–801. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]