Abstract
Objectives
We sought to analyze the clinicopathologic features, recurrence patterns and survival outcomes of women with high-grade uterine cancer (UC) enrolled on The Gynecologic Oncology Group (GOG) LAP2 trial.
Methods
This is a post-hoc analysis of LAP-2 patients with grade 3 endometrioid adenocarcinoma (ENDO), uterine serous (USC), clear cell (CC) and carcinosarcoma (CS). Demographics, clinicopathologic features, and recurrence patterns, were compared by histology and surgical approach. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method.
Results
Of the 2600 patients enrolled in LAP-2, 753 patients had high-grade UC: 350 had ENDO, 289 had USC, 42 had CC and 72 had CS. Compared with the ENDO cohort, those with other high-grade subtypes were older (p < 0.001) and were more likely to have positive peritoneal cytology (p < 0.001), positive lymph nodes (p = 0.05) and higher disease stage on final pathology (p < 0.001). With a median follow-up time of 60 months, compared to patients with ENDO, those with USC, CCC and CS subtypes had higher recurrence rates (p < 0.001), extra-pelvic recurrences (p < 0.001) and poorer PFS (p < 0.001) and OS (p < 0.001). Those diagnosed with USC and CS experienced the worst survival outcomes (p = 0.003). Patterns of recurrence and survival were not different in those staged with LSC vs LAP. On multivariable analysis, age, stage, pelvic washings and Type II histology were independently and adversely associated with survival.
Conclusions
Women with apparent early-stage, USC and CS histologies have poorer outcomes than women with grade 3 endometrioid adenocarcinoma. Patterns of recurrence and survival were not impacted by surgical approach.
Keywords: LAP-2 trial, Uterine cancer, High-grade uterine cancer, Type II uterine cancer, Minimally invasive surgery
1. Introduction
Uterine cancer is not a single disease, but consists of several histologic subtypes, with different risk factors, precursor lesions, molecular and genetic profiles, and clinical outcomes. Epithelial uterine cancer has been historically classified into two subtypes, Type I and Type II, based on histologic and molecular characteristics [1,2]. Observations by Lauchlan, Hendrickson, and Bokhman led to the description of these two distinct types, with Type I, commonly referred to as the endometrioid type, comprising 80–90% of all uterine cancers, and Type II, most often non-endometrioid tumors, encompassing the remaining 10–20% of endometrial tumors [3–5]. The most common histologies of this latter subtype include uterine serous carcinoma (USC) and clear-cell carcinoma (CCC). Additionally, carcinosarcoma (CS), a biphasic, high-grade epithelial uterine tumor, behaves similarly to the Type II tumors and is often classified as such [6].
In general, Type II uterine neoplasms are associated with more aggressive tumor biology and clinical behavior than Type I tumors, accounting for more than 40% of all uterine cancer deaths. However, this has not been observed in all studies [3–5,7–8]. At least one study from the Gynecologic Oncology Group (GOG) suggested the association between tumor type and prognosis was weak for patients with advanced/recurrent uterine cancer [9]. Additionally, although it is often labeled as a Type II cancer, grade 3 endometrioid adenocarcinoma is an entity that may defy categorization, despite its endometrioid histotype. Some studies demonstrate that USC and CCC are associated with an unfavorable prognosis compared with grade 3 endometrioid cancer, while a recent clinicopathologic analysis revealed no difference in outcome between these histologies [7–8,10].
Survival outcomes for the GOG LAP-2 study, a multicenter phase III study of women with apparent early-stage, grade 1–3 uterine cancer randomized to laparoscopy versus laparotomy, have been previously reported [11]. While the authors suggest that histologic cell type should not necessarily be a factor in the decision to offer minimally invasive surgery, detailed data regarding the women with high-grade disease from the LAP-2 cohort remains unexplored. There are few large prospective studies describing the impact of high-risk uterine cancer histologies on patient prognosis. There are even fewer published reports evaluating the role of minimally invasive surgery in the management of high-grade uterine malignancies [12]. Therefore, the primary study aim was to examine the clinicopathologic features and prognostic differences among histologic cell types in women with high-grade uterine cancer enrolled in a cooperative group trial. A secondary aim was to compare the oncologic outcomes of this same population staged by minimally invasive approaches versus laparotomy.
2. Methods
This was an institutional review board exempt study. An ancillary analysis of the GOG LAP-2 trial, a multicenter phase III study of women with apparent early-stage uterine cancer randomized 2:1 to laparoscopy versus laparotomy was performed. Data was collected on patients with high-grade malignancies; specifically, grade 3 endometrioid, and those with Type II uterine cancers, including USC, CCC and carcinosarcoma. Representative slides from the hysterectomy specimens were submitted for central review and were performed by two members of the GOG Pathology Committee. Specimens containing >10% serous adenocarcinoma were designated as uterine serous carcinoma. Standardized pathology evaluation forms were to be completed prospectively by the local GOG pathologist, documenting the number of nodes removed and the number of positive nodes at each of four regions (right pelvic, left pelvic, right para-aortic, and left para-aortic). FIGO staging and prognostic criteria (depth of myometrial invasion, cervical involvement, lymphovascular invasion, metastatic sites, and peritoneal cytology results) were also collected prospectively, along with copies of pathology and cytology reports. We conducted an analysis comparing the grade 3 endometrioid tumors to the other three tumor histologies combined (collectively known as “other” Type II cancers) and an additional subset analysis stratified by individual histology. Demographics, clinicopathologic features, rates and patterns of recurrence, adjuvant treatment, progression-free survival (PFS) and overall survival (OS) were compared by histology and surgical staging approach (laparoscopy versus laparotomy).
Categorical variables were compared between the patient subgroups by the Pearson chi-square test [13], and continuous variables by the Wilcoxon–Mann–Whitney test [14] or the Kruskal–Wallis test [15]. Survival was estimated using the Kaplan–Meier [16] method. The Cox proportional hazards model [17] was used to evaluate independent prognostic factors identified by previous GOG studies, and to estimate their covariate-adjusted effects on survival. All statistical tests were two-tailed with the significance level set at a = 0.05, except where noted. Statistical analyses were performed using the R programming language and environment [18].
3. Results
Of 2600 patients enrolled in the LAP-2 trial, we identified 753 patients with high-grade disease: 350 were diagnosed with endometrioid; 289 with USC; 72 with carcinosarcoma; and 42 with CCC. Laparoscopy was performed in 507 patients and laparotomy in 246. Demographic and clinicopathologic data stratified by uterine tumor histology is shown in Table 1. Compared with the grade 3 endometrioid cohort, those with “other” Type II uterine cancers were more likely: older (median age 69.4 vs 61.8, respectively; p < 0.001); to have positive peritoneal cytology (20.2% vs 7.7%, respectively; p < 0.001); to have positive lymph nodes (22.6% vs 16.9%, respectively; p = 0.05); to have higher stage disease on final pathology (p < 0.001) and to undergo a conversion to laparotomy due to metastatic disease (34.3% vs. 23.3%; p = 0.008). With a median follow-up time of 60 months, patients with “other” Type II uterine cancers had almost double the recurrence rates (26.6% vs 13.7%; p < 0.001), were more likely to have a multisite or extra-pelvic recurrence (p < 0.001) and had poorer median PFS (59.3% vs 77.4%, p < 0.001) and median OS (65.5% vs 81.7%; p < 0.001) than the grade 3 endometrioid cohort (Fig. 1). This was despite the fact that the grade 3 endometrioid cohort received significantly less adjuvant therapy overall than those with other Type II malignancies (Table 2; p < 0.001). Specifically, patients with grade 3 endometrioid disease were: more likely to undergo observation after surgery (no therapy: 52.5% vs 41.7%, respectively; p < 0.001), less likely to receive chemotherapy (4.2% vs 18.8%, respectively; p < 0.001) or combination chemotherapy and radiation (8.4% vs 19.1%, respectively; p < 0.001), although more received radiation alone than the other Type II cohort (34.9% vs 20.4%, respectively; p < 0.001).
Table 1.
Patient clinicopathologic characteristics by histology.
| Endometrioid
|
Other
|
Test statistic
|
||
|---|---|---|---|---|
| N | N = 350 | N = 403 | ||
| Age years | 753 | 56.7 64.8 | 61.7 69.4 | p < 0.0011 |
| 73.3 | 75.9 | |||
| BMI kg/m2 | 752 | 23.4 26.7 | 23.8 27.4 | p = 0.1741 |
| 30.8 | 32.0 | |||
| Race/Ethnicity | 753 | p = 0.8052 | ||
| White | 87.7% (307) | 85.1% (343) | ||
| Hispanic | 3.4% (12) | 3.5% (14) | ||
| Black | 4.3% (15) | 6.2% (25) | ||
| Asian | 3.1% (11) | 3.5% (14) | ||
| Other | 1.4% (5) | 1.7% (7) | ||
| Performance status | 753 | p = 0.8052 | ||
| Normal, asymptomatic | 88.6% (310) | 88.8% (358) | ||
| Symptomatic, ambulatory | 10.6% (37) | 9.9% (40) | ||
| Symptomatic, in bed <50% | 0.9% (3) | 1.0% (4) | ||
| Symptomatic, in bed ≥50% | 0.0% (0) | 0.2% (1) | ||
| 2009 FIGO surgical stage | 747 | p < 0.0012 | ||
| IA | 48.7% (169) | 49.8% (199) | ||
| IB | 22.2% (77) | 10.2% (41) | ||
| II | 6.3% (22) | 4.8% (19) | ||
| IIIA | 4.0% (14) | 5.5% (22) | ||
| IIIC1 | 7.2% (25) | 8.2% (33) | ||
| IIIC2 | 8.6% (30) | 10.5% (42) | ||
| IVB | 2.9% (10) | 11.0% (44) | ||
| Positive peritoneal cytology | 723 | 7.7% (26) | 20.2% (78) | p < 0.0012 |
| Type of nodes removed | 744 | p = 0.6892 | ||
| No nodes | 0.3% (1) | 0.8% (3) | ||
| Para-aortic nodes only | 0.3% (1) | 0.3% (1) | ||
| Pelvic nodes only | 4.0% (14) | 5.3% (21) | ||
| Both pelvic and para-aortic nodes | 95.4% (332) | 93.7% (371) | ||
| Any + pelvic nodes | 753 | 16.0% (56) | 19.6% (79) | p = 0.1992 |
| Pelvic nodes retrieved | 753 | 13.0 17.0 | 11.5 17.0 | p = 0.2261 |
| 23.0 | 23.0 | |||
| L pelvic nodes retrieved | 743 | 5 8 12 | 5 8 11 | p = 0.6211 |
| R pelvic nodes retrieved | 741 | 6 9 12 | 6 9 12 | p = 0.3241 |
| Any + paraaortic nodes | 753 | 9.1% (32) | 11.7% (47) | p = 0.262 |
| Paraaortic nodes retrieved | 753 | 4 7 11 | 3 6 10 | p = 0.0521 |
| L paraaortic nodes retrieved | 729 | 2 3 6 | 1 3 5 | p = 0.1581 |
| R paraaortic nodes retrieved | 728 | 2 4 6 | 1 3 5 | p = 0.0311 |
| Any positive nodes | 753 | 16.9% (59) | 22.6% (91) | p = 0.052 |
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables.
N is the number of non–missing values. Numbers after percents are frequencies.
Tests used:
Wilcoxon test;
Pearson test.
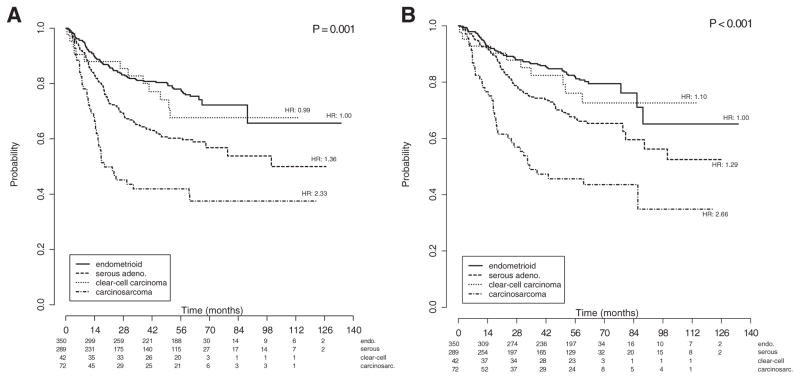
Fig. 1.
a: Kaplan–Meier curves of progression-free survival for all patients, stratified by histology group. Figures below months indicate the numbers of patients at risk. The p-value is from the Wald test to compare hazard ratios between the histology subgroups in the multivariate model. Legend: endo: grade 3 endometrioid; other: USC, CCC and carcinosarcoma. b: Kaplan–Meier curves of overall survival for all patients, stratified by histology group. Figures below months indicate the numbers of patients at risk. The p-value is from the Wald test to compare hazard ratios between the histology subgroups in the multivariate model. Legend: endo: grade 3 endometrioid; other: USC, CCC and carcinosarcoma.
Table 2.
Recurrence and survival by histology.
| Endometrioid
|
Other
|
Test statistic
|
||
|---|---|---|---|---|
| N | N = 350 | N = 403 | ||
| Adjuvant therapy | 728 | p < 0.001 | ||
| None | 52.5% (176) | 41.7% (164) | ||
| Chemotherapy | 4.2% (14) | 18.8% (74) | ||
| Radiation | 34.9% (117) | 20.4% (80) | ||
| Both | 8.4% (28) | 19.1% (75) | ||
| Recurrence | 753 | p < 0.001 | ||
| No | 86.3% (302) | 73.4% (296) | ||
| Yes | 13.7% (48) | 26.6% (107) | ||
| Site of recurrence | 753 | p < 0.001 | ||
| Trocar | 0.3% (1) | 0.2% (1) | ||
| Vagina | 2.0% (7) | 3.0% (12) | ||
| Pelvis | 1.4% (5) | 4.0% (16) | ||
| Abdomen | 1.4% (5) | 5.5% (22) | ||
| Liver | 0.3% (1) | 2.5% (10) | ||
| Lung | 2.6% (9) | 4.2% (17) | ||
| Bone | 0.0% (0) | 0.5% (2) | ||
| Nodal | 2.6% (9) | 2.0% (8) | ||
| Multiple | 2.0% (7) | 4.5% (18) | ||
| Unknown | 1.1% (4) | 0.2% (1) | ||
| No recurrence | 86.3% (302) | 73.4% (296) | ||
| Progression-free survival status | 753 | p < 0.001 | ||
| Censored | 77.4% (271) | 59.3% (239) | ||
| Progression or death | 22.6% (79) | 40.7% (164) | ||
| Overall survival status | 753 | p < 0.001 | ||
| Censored | 81.7% (286) | 65.5% (264) | ||
| Death | 18.3% (64) | 34.5% (139) | ||
| Cause of death | 203 | p = 0.213 | ||
| Disease | 60.9% (39) | 69.8% (97) | ||
| Other | 39.1% (25) | 30.2% (42) |
N is the number of non–missing values.Numbers after percents are frequencies. Test used: Pearson test
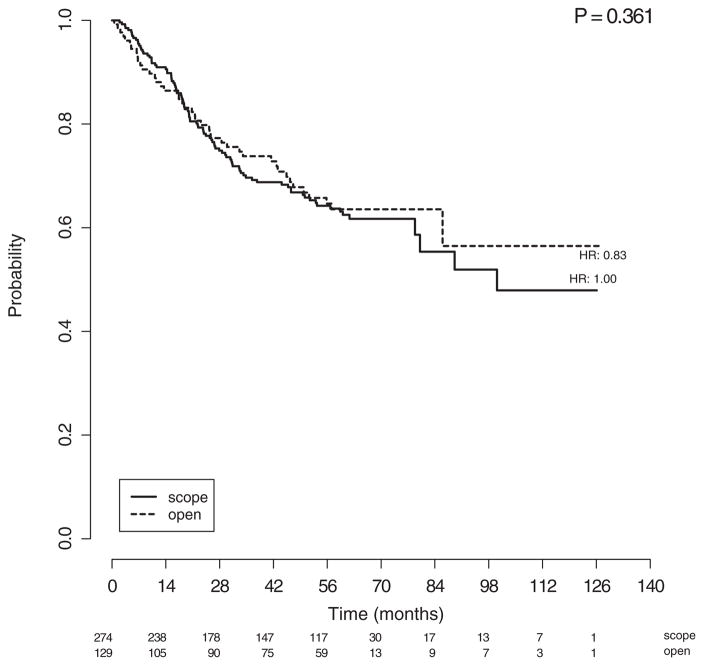
When examining outcomes of the patients with high-grade histologies by surgical approach (Table 3), there were no differences in age, race, body mass index, performance status, number or type of lymph nodes removed, number of positive lymph nodes, stage or adjuvant therapies between the laparoscopy and laparotomy cohorts. Recurrence rates (25.9% versus 27.9%, respectively), patterns of recurrence, PFS, and OS (Fig. 2) were also not significantly different in those staged with laparoscopy vs laparotomy (Table 3). Further, the incidence of trocarsite metastases was only 0.2% in the laparoscopy cohort.
Table 3.
Patterns and Rates of Recurrence and Survival by Laparoscopy vs. Laparotomy.
| Laparoscopy
|
Laparotomy
|
|||
|---|---|---|---|---|
| N | N = 274 | N = 129 | Test statistic | |
| Adjuvant therapy | 393 | p = 0.533 | ||
| None | 40.2% (107) | 44.9% (57) | ||
| Chemotherapy | 18.4% (49) | 19.7% (25) | ||
| Radiation | 20.3% (54) | 20.5% (26) | ||
| Both | 21.1% (56) | 15.0% (19) | ||
| Recurrence | 403 | p = 0.672 | ||
| No | 74.1% (203) | 72.1% (93) | ||
| Yes | 25.9% (71) | 27.9% (36) | ||
| Site of recurrence | 403 | p = 0.882 | ||
| Trocar | 0.4%(1) | 0.0% (0) | ||
| Vagina | 2.2% (6) | 4.7% (6) | ||
| Pelvis | 4.7% (13) | 2.3% (3) | ||
| Abdomen | 5.1% (14) | 6.2% (8) | ||
| Liver | 2.6% (7) | 2.3% (3) | ||
| Lung | 4.4% (12) | 3.9% (5) | ||
| Bone | 0.4% (1) | 0.8% (1) | ||
| Nodal | 1.8% (5) | 2.3% (3) | ||
| Multiple | 4.0% (11) | 5.4% (7) | ||
| Unknown | 0.4% (1) | 0.0% (0) | ||
| No recurrence | 74.1% (203) | 72.1% (93) | ||
| Progression-free survival status | 403 | p = 0.745 | ||
| Censored | 58.8% (161) | 60.5% (78) | ||
| Progression or death | 41.2% (113) | 39.5% (51) | ||
| Overall survival status | 403 | p = 0.737 | ||
| Censored | 65.0% (178) | 66.7% (86) | ||
| Death | 35.0% (96) | 33.3% (43) | ||
| Cause of death | 139 | p = 0.687 | ||
| Disease | 70.8% (68) | 67.4% (29) | ||
| Other | 29.2% (28) | 32.6% (14) |
N is the number of non–missing values. Numbers after percents are frequencies. Test used: Pearson test
Figure 2.
Kaplan–Meier curves of overall survival for patients with high-grade cancers, stratified by laparoscopic (scope) versus laparotomy (open) arms. Figures below months indicate the numbers of patients at risk. The p-value is from the Wald test to compare hazard ratios between the treatment subgroups in the multivariate model.
Survival stratified by histology is shown in Figs. 1a and 1b. Compared to those with grade 3 endometrioid, those with CCC experienced similar PFS and OS outcomes, while women with USC (PFS HR: 1.36; OS HR: 1.26) and carcinosarcoma (PFS HR: 2.12 and OS HR: 2.33) had poorer PFS (p < 0.004) and OS rates (p < 0.003), respectively. Those with carcinosarcoma experienced the worst survival outcomes. On multivariable analysis, age (PFS HR: 1.038, 95% CI 1.023–1.054, p < 0.001; OS HR: 1.052, 95% CI 1.035–1.070, p < 0.001), pelvic washings (PFS HR: 1.801, 95% CI: 1.248–2.598, p = 0.004; OS HR: 1.898, 95% CI: 1.274–2.287, p = 0.004); advanced stage (ref: stage IA; PFS stage IIIC1 HR: 2.854, 95% CI: 1.748–4.660, p < 0.001; OS stage IIIC1 HR: 2.692, 95% CI: 1.561–4.618, p < 0.001; PFS stage IIIC2 HR: 4.284, 95% CI: 2.640–6.592, p < 0.001; OS stage IIIC2 HR: 4.310, 95% CI 2.549–7.286, p < 0.001; PFS Stage IVB HR 11.976, 95% CI 6.890–20.818, p < 0.001; OS Stage IVB HR: 11.533, 95% CI 6.518–20.408, p < 0.001) and other Type II histologies (ref: grade 3 endometrioid; PFS HR: 1.483, 95% CI 1.086–2.026; p = 0.005; OS HR: 1.506, 95% CI 1.058–2.144; p = 0.014) were independently and adversely associated with both PFS and OS (Tables 4a and 4b, respectively). Choice of adjuvant therapy and surgical approach (Fig. 2) were not associated with survival outcomes.
Table 4a.
Multivariate progression-free survival analysis.
| HR | 2.5% | 97.5% | p | |
|---|---|---|---|---|
| Histology (endometrioid ref.) other | 1.483 | 1.086 | 2.026 | 0.013 |
| Age | 1.038 | 1.023 | 1.054 | <0.001 |
| Race (White ref.) Hispanic | 2.281 | 1.144 | 4.549 | 0.019 |
| Black | 1.202 | 0.647 | 2.236 | 0.560 |
| Other | 1.252 | 0.672 | 2.330 | 0.479 |
| Performance status (0 ref.) 1, 2, 3 | 1.467 | 0.977 | 2.205 | 0.065 |
| Positive washings (no ref.) yes | 1.801 | 1.248 | 2.598 | 0.002 |
| Stage (IA ref.) IB | 1.585 | 1.015 | 2.474 | 0.043 |
| II | 2.376 | 1.256 | 4.495 | 0.008 |
| IIIA | 1.522 | 0.711 | 3.257 | 0.279 |
| IIIC1 | 2.854 | 1.748 | 4.660 | <0.001 |
| IIIC2 | 4.284 | 2.640 | 6.952 | <0.001 |
| IVB | 11.976 | 6.890 | 20.818 | <0.001 |
| Adjuvant treatment (observation ref.) chemotherapy | 0.587 | 0.353 | 0.976 | 0.040 |
| Radiation | 0.841 | 0.577 | 1.224 | 0.366 |
| Both | 0.956 | 0.605 | 1.510 | 0.846 |
Table 4b.
Multivariate overall survival analysis.
| HR | 2.5% | 97.5% | p | |
|---|---|---|---|---|
| Histology (endometrioid ref.) other | 1.506 | 1.058 | 2.144 | 0.023 |
| Age | 1.052 | 1.035 | 1.070 | <0.001 |
| Race (White ref.) Hispanic | 2.702 | 1.297 | 5.629 | 0.008 |
| Black | 1.210 | 0.610 | 2.402 | 0.585 |
| Other | 1.342 | 0.672 | 2.679 | 0.405 |
| Performance status (0 ref.) 1, 2, 3 | 1.463 | 0.937 | 2.284 | 0.094 |
| Positive washings (no ref.) yes | 1.898 | 1.274 | 2.827 | 0.002 |
| Stage (IA ref.) IB | 1.262 | 0.752 | 2.116 | 0.379 |
| II | 1.672 | 0.784 | 3.566 | 0.183 |
| IIIA | 1.437 | 0.600 | 3.441 | 0.416 |
| IIIC1 | 2.692 | 1.569 | 4.618 | <0.001 |
| IIIC2 | 4.310 | 2.549 | 7.286 | <0.001 |
| IVB | 11.533 | 6.518 | 20.408 | <0.001 |
| Adjuvant treatment (observation ref.) chemotherapy | 0.481 | 0.272 | 0.850 | 0.012 |
| Radiation | 0.975 | 0.642 | 1.482 | 0.907 |
| Both | 1.091 | 0.662 | 1.799 | 0.733 |
4. Discussion
While most uterine corpus cancers are early-stage, low-grade Type I tumors with an excellent prognosis, grade 3 endometrioid adenocarcinoma, USC, CCC and carcinosarcoma have been identified as distinct, high-risk variants, each with a poorer prognosis than the Type I cancers [8,11]. Although these high-grade subtypes account for fewer than 25% of all uterine cancers, they collectively account for more than 50% of uterine cancer deaths [12,19]. Thus, it is paramount that cancer centers and cooperative trial groups focus greater attention on development of preventive oncology measures and innovative therapeutic strategies concentrated on these tumor types. Previous studies have shown that women with grade 3 endometrioid disease have better survival outcomes [20], other authors have not demonstrated a survival difference between this subtype and serous and clear cell histologies [19,21]. However, these reports are mostly small, retrospective series that lack power to detect significant differences among rare tumor histologies. The findings of one of the larger retrospective studies of the Surveillance, Epidemiology and End Results (SEER) database analysis [22] showed that five-year survival rates for USC and CCC were 72% and 80%, respectively, whereas the corresponding survival rate for grade 3 endometrioid cancers was 89% (p < 0.0001). Our ad hoc study of a Phase III GOG trial in 753 patients with surgically staged, high-grade uterine cancer adds to this literature. Compared to the grade 3 endometrioid adenocarcinoma cohort, those with apparent early-stage “other” Type II cancers were older and were more likely to have positive lymph nodes and higher disease stage on final pathology. It was not surprising that with more advanced disease evident at surgical staging, patients diagnosed with “other” Type II histologies had higher recurrence rates and poorer PFS and OS than the grade 3 endometrioid cohort. However, after controlling for age, stage, adjuvant treatment and other factors on multivariable analysis, “other” Type II histologies remained an independent factor adversely associated with survival (Tables 4a and 4b), with the poorer survival outcomes in this cohort largely attributed to the serous and carcinosarcoma histologies (Figs. 1a and 1b).
While the NRG/GOG has recently defined carcinosarcoma as a separate entity in terms of clinical trials development [23], with rare exception [24], women with uterine serous and clear cell histologies continue to be grouped together in most clinical trials with those who have grade 1–3 endometrioid cancers [25–29]. Questions remain, most importantly, does a significant difference in prognosis exist among these high-risk histologies, do the various high-risk tumors respond differently to adjuvant treatment, and ultimately, should these cell types be treated as separate disease entities? In a recent large GOG study exploring the etiologic heterogeneity of uterine cancer, those with Type II cancers were older, more often non-white and less often obese compared to women grade 1–2 endometrioid cancers [10]. Risk factors for grade 3 endometrioid carcinomas were generally similar to those identified for Type II cancers, although patients with grade 3 endometrioid tumors more often had histories of breast cancer without tamoxifen exposure while those with other Type II tumors, including carcinosarcoma and serous histologies, were more frequently treated with tamoxifen. These findings underscore the clinical and molecular distinctions between the low and high-risk subtypes as well as more nuanced etiologic differences among the high-risk histologies.
While some Phase III studies of advanced or recurrent uterine cancer have not demonstrated a difference in survival outcomes based upon grade or histology [9], no trial has been powered to evaluate the impact of Type II uterine histologies on outcome. Nevertheless, subgroup analysis of two GOG Phase III studies of women with advanced or recurrent uterine cancer, a clear difference in prognosis was observed based on histology and grade [26,27]. Randall et al. compared whole abdominal radiation to chemotherapy in women with advanced or recurrent endometrial cancer [26]. An exploratory multivariate analysis demonstrated that grade 3 tumors and serous histology were adversely associated with survival. A subsequent Phase III GOG study in the same population studying cisplatin, doxorubicin and whole pelvic radiation randomized with and without paclitaxel demonstrated poorer outcomes based on histology [27]. Unadjusted Kaplan-Meier estimates of recurrence-free survival by histology and grade demonstrated lower survival for patients with advanced or recurrent clear cell and serous histologies compared with endometrioid types [27]. Additionally, relative to those with grade 1 endometrioid adenocarcinoma, patients with grade 3 endometrioid disease had a recurrence-free survival hazard ratio of 3.12, which was similar to that of 3.45 for clear cell histology. However, serous histology had the most adverse survival impact, with a hazard ratio of 4.43. In the current ad hoc analysis, almost double the recurrence rate, a propensity for extra-pelvic recurrence, and a greater risk of cancer-specific mortality was observed in the “other” Type II cohort compared to the grade 3 endometrioid carcinoma cohort. These recent GOG/NRG studies in women with both early and advanced-stage uterine cancer demonstrate clear differences in treatment response as well as strong etiologic and prognostic distinctions in those with endometrioid carcinoma compared with women diagnosed with more high-risk histologies, providing compelling clinical evidence to support the development of distinct clinical trials for women with Type II histologies.
Recently, a comprehensive genomic analysis of nearly 400 endometrial tumors suggested that certain molecular characteristics, including mutation frequency, may complement current pathology methods and help distinguish between primary uterine tumor types, as well as provide insights into potential treatment approaches [28]. The study, led by investigators in The Cancer Genome Atlas Research Network, identified genomic similarities between endometrial and other cancers, including breast, ovarian, and colorectal cancers, and revealed four novel tumoral molecular uterine subtypes. These molecular classifications may to help better stratify patients for enrollment in clinical trials with targeted therapies. Given the substantial differences in genetic and molecular profiles between the “Type II” uterine tumors, should the dichotomous Type I and II classification be abandoned? This is not yet clear and cannot be answered by our study. However, the promise of tumor histology being complemented–or replaced altogether–by genomic tumoral classifications is likely to be realized in the near future.
Finally, the GOG LAP-2 protocol randomized over 2600 women with endometrial cancer to laparotomy versus laparoscopy and reported fewer postoperative moderate or severe adverse events, shorter hospital stays, less pain, earlier resumption of normal activities and improved short-term quality of life in the laparoscopy cohort [11]. The estimated 5-year OS was nearly identical between the cohorts at 89.8%. However, this and other randomized studies of minimally invasive surgery in uterine cancer were focused on patients with relatively low-risk, grade 1–2 endometrial tumors. Our current ad hoc LAP-2 analysis demonstrates that survival is not compromised for patients with high-grade uterine cancers staged via laparoscopy, which corresponds with a retrospective, multi-institutional study performed at high-volume U.S. cancer centers demonstrating similar results [12]. Further, trocar site recurrences were not a significant concern, occurring in <1% of patients treated with laparoscopy. Recognizing that women diagnosed with Type II, high-grade uterine cancer are usually older, possess comorbidities and are more likely to require adjuvant therapies than those with Type I malignancies, there is great interest in minimizing surgical morbidity in this population [12]. While more patients with Type II disease are found to have extra-uterine disease at primary surgery and should be counseled that conversion rates to laparotomy might be higher in this population, minimally invasive surgery should be the preferred surgical modality in this high-risk patient cohort in the absence of obvious extra-uterine disease or other contraindications to laparoscopy.
An additional surgical observation in our study was that positive peritoneal cytology (i.e., washings) correlated strongly and adversely with PFS and OS on multivariate analysis. While positive peritoneal cytology in early-stage uterine cancer does not appear to influence prognosis in those with low-grade disease (and for this reason, was removed from the 2009 FIGO staging criteria) [29], it is more likely that in those with high-risk histologies, positive washings are not a random event and are more prognostic of outcome. Consideration should be given to performance of peritoneal cytology in all women with a pre-operative diagnosis of high-grade uterine cancer, as the information gained from this procedure may influence decision-making regarding adjuvant therapies.
Study weaknesses include the ad hoc analysis with its intrinsic limitations and that study participants received a heterogeneous array of adjuvant therapies. Study strengths include the prospectively collected data from a phase III GOG study, the large sample size of study subjects with high-grade uterine cancer and that tumor specimens had undergone central pathology review by GOG pathologists.
In conclusion, women with apparent early-stage, Type II uterine cancer—especially those with serous and carcinosarcoma histologies—are older, more likely to have advanced-stage disease on final pathology, higher recurrence rates and poorer survival outcomes than women with grade 3 endometrioid adenocarcinoma. Despite the biological aggressiveness of these high-risk histologies, women with high-grade uterine cancer staged via laparoscopy had similar patterns of recurrence and survival as those staged by laparotomy. Given the significant molecular and prognostic differences among these histologic cell types, future cooperative group initiatives should focus on development of distinct treatment trials for patients with Type II malignancies that include histologic and genomic tumoral classifications so that outcomes for women with rare, but deadly, uterine malignancies can be improved.
HIGHLIGHTS.
Women with early-stage uterine serous and carcinosarcoma have poor survival.
Recurrence patterns were similar in those staged with laparoscopy vs laparotomy.
Survival is similar for high-grade uterine cancer patients staged via laparoscopy.
Footnotes
These findings were presented at the Society of Gynecologic Oncology's 45th Annual Meeting on Women's Cancer in Tampa, Florida, March 22–25, 2014.This study was supported by National Cancer Institute grants to the University of Oklahoma (1CA65221), the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1U10 CA180822) and NRG Operations (U10CA180868). The following Gynecologic Oncology Group (GOG) institutions participated in the GOG 2222 (LAP2) study: Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, University of Pennsylvania Cancer Center, University of California at San Diego, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, University of California Medical Center at Irvine, Tufts–New England Medical Center, Rush–Presbyterian–St Luke's Medical Center, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Women's Cancer Center, University of Oklahoma, Tacoma General Hospital, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Fletcher Allen Health Care, University of Wisconsin Hospital, Women and Infants Hospital, and Community Clinical Oncology Program.
Conflicts of interest
Dr. Nick Spirtos receives per capita reimbursement to the Women's Cancer Center, travel supplement as Gyn Onc Committee Chair, co-operative research funding on a per capita basis from GOG Foundation, Inc., and royalties from Wiley Health Services for Bonney's Gynaecological Surgery.
Dr. Michael Pearl receives grant money from the GOG/NRG. He also serves as a consultancy for Ethicon, provided expert testimony for legal expert review, and has grants/grants pending from NIH, small foundations, as well as royalties from VitaTex.
Dr. David O'Malley serves as a consultant for Yondelis on the Sarcoma Advisory Board, AstraZeneca on the Ovarian Cancer Advisory Board, Clovis on the Ovarian Cancer Advisory Board, Genentech/Roche on the Ovarian Cancer Advisory Board and Eisai on the Advisory Board.
Dr. Robert Mannel receives grant monies from GOG-LAP2 grant. He also serves on the Advisory Board as a consultant for Medimmune, Oxigene, Endocyte, Bayer and Clovis.
All other coauthors have no conflicts of interest to declare.
Contributor Information
Amanda N. Fader, Email: afader1@jhmi.edu.
James Java, Email: james.j.java@gmail.com.
Meaghan Tenney, Email: mtenney@babies.bsd.uchicago.edu.
Stephanie Ricci, Email: RICCIS@ccf.org.
Camille C. Gunderson, Email: camille-gunderson@ouhsc.edu.
Sarah M. Temkin, Email: stemkin1@jhmi.edu.
Nick Spirtos, Email: nspirtos@wccenter.com.
Christina L. Kushnir, Email: christinalkush@gmail.com.
Michael L. Pearl, Email: michael.pearl@sbumed.org.
Oliver Zivanovic, Email: zivanovo@mskcc.org.
Krishnansu S. Tewari, Email: ktewari@uci.edu.
David O'Malley, Email: omalley.46@osu.edu.
Ellen M. Hartenbach, Email: emharten@wisc.edu.
Chad A. Hamilton, Email: chad.a.hamilton.mil@health.mil.
Natalie S. Gould, Email: ngould@wccenter.com.
Robert S. Mannel, Email: Robert-Mannel@ouhsc.edu.
William Rodgers, Email: whr9001@nyp.org.
Joan L. Walker, Email: joan-walker@ouhsc.edu.
References
- 1.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010;21:1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogen-esis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 5.Moore KN, Fader AN. Uterine papillary serous carcinoma. Clin Obstet Gynecol. 2011;54:278–291. doi: 10.1097/GRF.0b013e318218c755. [DOI] [PubMed] [Google Scholar]
- 6.Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, Leitao MM, PMA, Poveda A, et al. Gynecologic Cancer Inter-Group (GCIC): consensus review for uterine and ovarian carcinosarcoma. Int J Gynecol Cancer. 2014 Nov;24(9 Suppl 3):S55–S60. doi: 10.1097/IGC.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 7.Prat J, Gallardo A, Cuatrecasas M, Catasús L. Endometrial carcinoma: pathology and genetics. Pathology. 2007;39:72–87. doi: 10.1080/00313020601136153. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Kim TJ, Lee YY, Choi CH, Lee JW, Bae DS, Kim BG. A comparison of uterine papillary serous, clear cell carcinomas, and grade 3 endometrioid corpus cancers using 2009 FIGO staging system. J Gynecol Oncol. 2013;24(2):120–127. doi: 10.3802/jgo.2013.24.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMeekin DS, Filiaci V, Thigpen JT, Gallion HH, Fleming GF, Rodgers WH, et al. The relationship between histology and outcome in advanced and recurrent endo-metrial cancer patients participating in first line chemotherapy trials: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007 Jul;106:16–22. doi: 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013 May;129(2):277–284. doi: 10.1016/j.ygyno.2013.02.023. http://dx.doi.org/10.1016/j.ygyno.2013.02.023 (Epub 2013 Feb 26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Lap2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fader AN, Seamon LG, Escobar PF, Frasure HE, Havrilesky LA, Zanotti KM, et al. Minimally Invasive Surgery versus laparotomy in women with high-grade endome-trial cancer: a multi-institutional study at high volume centers. Gynecol Oncol. 2012;126:180–185. doi: 10.1016/j.ygyno.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philos Mag. 1900;50(Series 5):157–175. [Google Scholar]
- 14.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 15.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 18.R Core Team. R, A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. ( http://www.R-project.org/) [Google Scholar]
- 19.Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004;11:117–142. doi: 10.1097/00125480-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous carcinoma, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593–596. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Alektiar KM, McKee A, Lin O, Venkatraman E, Zelefsky MJ, McKee B. Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO grade 3 cancer? Int J Radiat Oncol Biol Phys. 2002;1(54):79–85. doi: 10.1016/s0360-3016(02)02913-9. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton C, Cheung MK, Osann K, Chen L, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://clinicaltrials.gov/ct2/show/NCT00954174.
- 24.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 25.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus fllgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 26.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 27.Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:543–552. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, ADC, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcionoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tebeau PM, Popowski Y, Verkooijen HM, Bouchardy C, Ludicke F, Usel M, et al. Positive peritoneal cytology in early-stage endometrial cancer does not influence prognosis. Br J Cancer. 2004;91:720–724. doi: 10.1038/sj.bjc.6602035. [DOI] [PMC free article] [PubMed] [Google Scholar]