Abstract
To obtain new information about Streptococcus pyogenes intrahost genetic variation during invasive infection, we sequenced the genomes of 2,954 serotype M1 strains recovered from a nonhuman primate experimental model of necrotizing fasciitis. A total of 644 strains (21.8%) acquired polymorphisms relative to the input parental strain. The fabT gene, encoding a transcriptional regulator of fatty acid biosynthesis genes, contained 54.5% of these changes. The great majority of polymorphisms were predicted to deleteriously alter FabT function. Transcriptome-sequencing (RNA-seq) analysis of a wild-type strain and an isogenic fabT deletion mutant strain found that between 3.7 and 28.5% of the S. pyogenes transcripts were differentially expressed, depending on the growth temperature (35°C or 40°C) and growth phase (mid-exponential or stationary phase). Genes implicated in fatty acid synthesis and lipid metabolism were significantly upregulated in the fabT deletion mutant strain. FabT also directly or indirectly regulated central carbon metabolism genes, including pyruvate hub enzymes and fermentation pathways and virulence genes. Deletion of fabT decreased virulence in a nonhuman primate model of necrotizing fasciitis. In addition, the fabT deletion strain had significantly decreased survival in human whole blood and during phagocytic interaction with polymorphonuclear leukocytes ex vivo. We conclude that FabT mutant progeny arise during infection, constitute a metabolically distinct subpopulation, and are less virulent in the experimental models used here.
INTRODUCTION
Streptococcus pyogenes is a Gram-positive bacterium that causes a range of diseases in humans, including pharyngitis, superficial and deep skin infections, acute rheumatic fever, poststreptococcal glomerulonephritis, bacteremia, and necrotizing fasciitis (“flesh-eating disease”) (1, 2). Globally, there are over 700 million group A streptococcus (GAS) infections annually (3). It has been estimated that 9,000 to 11,500 cases of invasive GAS disease occur each year in the United States, resulting in 1,000 to 1,800 deaths annually (4; http://www.cdc.gov). Rheumatic fever, necrotizing fasciitis, and toxic shock syndrome are responsible for the high morbidity and mortality rates due to GAS infections (5).
Advances in DNA sequencing and related technologies have facilitated genome-wide dissection of genetic events involved in colonization (6), immune evasion (7), virulence (8–10), and the evolution and spread of highly virulent S. pyogenes clones (11–13). To advance our understanding of S. pyogenes molecular-pathogenesis events occurring during invasive infection, we performed whole-genome sequencing of 2,954 isolates recovered from a nonhuman primate model of necrotizing fasciitis. The resulting genome sequence data stimulated us to construct an isogenic fabT deletion mutant strain and to compare its global transcriptome and virulence attributes with those of the wild-type parental strain.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli strains were grown in Luria-Bertani (LB) medium at 37°C unless otherwise indicated. The LB medium was supplemented with ampicillin (Fisher Scientific; 150 μg/ml) or chloramphenicol (Acros Organics; 20 μg/ml) as needed. GAS was grown in Todd-Hewitt broth (Bacto Todd-Hewitt broth; Becton Dickinson and Co.) supplemented with 0.2% yeast extract (THY medium). The THY medium was supplemented with chloramphenicol (20 μg/ml) as needed. Trypticase soy agar supplemented with 5% sheep blood (Becton Dickinson and Co.) was used as required. Polymyxin B was purchased from Sigma. Growth experiments were performed as described previously (8).
Routine DNA manipulation and analysis.
Standard protocols or the manufacturer's instructions were used to isolate plasmid DNA for restriction endonuclease, DNA ligase, PCR, and other enzymatic treatments of DNA. The enzymes were purchased from New England BioLabs, Inc. (NEB) (Beverly, MA). Plasmid DNA was purified using the Qiaprep Spin and Qiaquick gel extraction kits (Qiagen). Q5 high-fidelity DNA polymerase (NEB) was used as the high-fidelity PCR enzyme. For chromosomal-DNA extraction, S. pyogenes was grown to an optical density (OD) of ∼1.0, 800 μl of the culture was centrifuged for 1 min at 13,200 rpm (high speed), and the pellets were suspended in 800 μl water. Bacteria were lysed with a Fastprep 96 MP instrument (Biomedicals, Santa Ana, CA) according to the manufacturer's instructions. The lysates were centrifuged for 2 min at high speed, and 600 μl of the supernatant was added to 1.5-ml Eppendorf tubes containing an equal volume of ice-cold isopropanol. After incubation at −20°C for 2 h, the tubes were centrifuged at high speed for 30 min, the supernatant was discarded, and the pellets were washed with 70% ice-cold ethanol. After a final high-speed centrifugation for 30 min, the supernatant was decanted, and the pellets were dried by incubating the inverted tubes for ∼30 min. The pellets were suspended in 50 to 100 μl of water, and 1 μl of this chromosomal DNA was used for subsequent PCRs.
Routine DNA sequencing was performed with a 3730 xl DNA analyzer (Applied Biosystems) and a BigDye Terminator v3.1 Cycle Sequencing kit (Life Technologies). Oligonucleotides were purchased from Sigma-Aldrich. The sequences of relevant primers used in PCR-based cloning are shown in Table S1 in the supplemental material. The genomes of all final clones were sequenced for verification and to rule out spurious mutations.
Experimental animal infection.
A nonhuman primate model of necrotizing fasciitis was used (14). Four adult cynomolgus macaques (Macaca fascicularis; Charles River BRF) were inoculated intramuscularly in the anterior thigh to a uniform depth with 1 × 108 CFU/kg of body weight of the S. pyogenes emm1 strain MGAS2221. Strain MGAS2221 was used because it has a wild-type allele (i.e., the most common allele) for all major transcriptional regulators, it is genetically representative of contemporary epidemic serotype M1 strains, and it has been used extensively in animal experiments (12). The animals were observed continuously, sacrificed at 24 h postinoculation, and necropsied. At necropsy, the quadriceps muscle was removed en bloc, serially sectioned in 0.5-cm slices, and visually inspected. Biopsy specimens of grossly purulent tissue (∼0.5 g each) were collected from the inoculation site, 1 cm from the caudal margin, and 1 cm from the cephalic margin. Each muscle sample was homogenized (Omni International), serially diluted in sterile phosphate-buffered saline (PBS), and plated in quadruplicate on Trypticase soy agar supplemented with 5% sheep blood (Becton Dickinson and Company). Following incubation at 37°C with 5% CO2 for 18 h, colonies were counted. Colonies (n = 768) from each animal were saved for whole-genome sequencing. To avoid introducing bias due to colony phenotype, every colony was taken from plates prepared from the dilution that produced approximately 100 to 200 colonies/plate. The study protocol (AUP-0521-0027) was approved by the Institutional Animal Care and Use Committee, Houston Methodist Research Institute.
For analysis of the virulence of the fabT deletion mutant strain, two adult cynomolgus macaques (M. fascicularis; Charles River BRF) were used, as previously described (12, 14). Each animal was anesthetized and inoculated intramuscularly in the anterior thighs. The right quadriceps muscle was inoculated at a marked site with wild-type strain MGAS2221, and the left quadriceps muscle was inoculated at a marked site with the fabT-deficient strain MGAS2221ΔfabT. The animals were observed continuously, sacrificed at 24 h postinoculation, and necropsied. At necropsy, the quadriceps muscle was removed en bloc, serially sectioned, and visually inspected. Tissues from the inoculation site and cephalic margin (1 cm from the distal margin) were collected for enumeration of CFU and microscopic examination using standard methods. The study protocol was approved by the Houston Methodist Research Institute (protocol number AUP-0115-0004).
Whole-genome sequencing and polymorphism analysis.
Sequencing libraries were prepared from 768 colonies collected from the infected muscle of each animal (3,072 colonies in total) as described previously (12, 15). Genome sequence data were obtained with a HiSeq 2000 instrument (Illumina, Inc.) according to the manufacturer's instructions. The sequence reads were corrected using Musket (16) and mapped to the reference serotype M1 strain MGAS5005 (GenBank accession number CP000017) using SMALT (available at http://www.sanger.ac.uk/resources/software/smalt/). Single nucleotide polymorphisms (SNPs) and insertions and deletions (indels) were identified using FreeBayes (available at http://www.github.com/ekg/Freebayes/) as described previously (17). The nature of the SNPs (coding/noncoding, synonymous/nonsynonymous, etc.) was determined using SNPfx.pl (a PERL script developed in house).
Construction of an isogenic fabT deletion mutant strain.
To construct a fabT deletion mutant from wild-type emm1 strain MGAS2221 (MGAS2221ΔfabT), we replaced the entire coding region of the fabT gene with the coding region of the cat gene (encoding chloramphenicol acetyltransferase) from plasmid pDC123. Thus, the fabT promoter drives the expression of the cat gene in the final construction. This strategy allowed the use of positive selection for the gene replacement through a double-crossover recombination event by plating target cells on chloramphenicol. In addition, we avoided complications, such as interruption of a putative operon or creation of polar effects on downstream genes that could occur by introduction of a plasmid backbone into this DNA region via a single-crossover event. The gene fabH, which is located immediately downstream from fabT, is typically essential (18). All relevant primer DNA sequences, along with a depiction of the steps in mutant strain construction, are shown in Fig. S1 in the supplemental material.
Primers JE66 and JE67 were used to amplify a 1,964-bp DNA fragment containing the 435-bp fabT gene and 760 bp upstream (5′ to fabT) and 769 bp downstream (3′ to fabT) from MGAS2221 chromosomal DNA. The JE66 DNA sequence is located internal to the phaB gene upstream from fabT (see Fig. S1 in the supplemental material). Similarly, the JE67 DNA sequence is located internal to the fabH gene downstream from fabT. We designed the primers internal to these genes to avoid amplifying the full-length version of either phaB or fabH, thus minimizing the likelihood of cloning complications due to toxicity.
The 1,964-bp PCR product was used as a template for construction of the fabT deletion mutant using combinatorial PCR (19). Briefly, the region 5′ to fabT was amplified with primers JE66 and JE69; to amplify the region 3′ to fabT, we used primers JE67 and JE63. The 5′ ends of primers JE69 and JE63 contain 15 bp that are complementary to the 5′ and 3′ ends of the cat gene, respectively. The 651-bp cat gene was amplified from pDC123 with primers JE68 and JE62 as forward (FWD) and reverse (REV) primers, respectively. These primers contain 15 bp at their 5′ ends complementary to the immediate DNA sequences located upstream and downstream of fabT. Thus, we made three contiguous PCR fragments: (i) upstream of fabT, (ii) the cat gene, and (iii) downstream of fabT (see Fig. S1 in the supplemental material). Combinatorial PCR was performed in two sequential steps. First, the upstream fabT PCR product and the cat gene DNA were combined using primers JE66 and JE62. Second, the downstream fabT PCR fragment was used as a template, together with this PCR fragment, to generate a new 2,180-bp PCR fragment containing the cat gene (instead of fabT plus the sequences upstream and downstream of fabT), using the external JE66 and JE67 primers. Finally, the fragment was reamplified using FWD primer JE70, which has the same DNA sequence as JE66 and a BamHI restriction site, and REV primer JE67. This constituted the PCR fragment used for allelic exchange.
Upon digestion with BamHI, the target PCR fragment was cloned into pBluescript (pBSII KS) digested with BamHI and SmaI and subsequently excised as a BamHI-HindIII fragment and cloned into the GAS suicide vector pBBL740, which had been digested with BamHI and HindIII. E. coli JM109 was used for all cloning experiments. The final recombinant plasmid was extracted from 100 ml of JM109 cells, digested with BamHI, extracted from a gel, column purified, and electroporated into strain MGAS2221. Transconjugants and controls were inoculated onto THY plates containing chloramphenicol. PCR analysis of transconjugants confirmed that the fabT gene had been successfully replaced.
Two sets of primers were used to confirm that the fabT gene had been successfully replaced with cat. Primers JE1 and JE5 generate a PCR product that extends 143 bp upstream and 100 bp downstream of fabT. When we used wild-type MGAS2221 chromosomal DNA as a template, we generated the expected 678-bp PCR fragment. In contrast, the size of the PCR fragment amplified from both candidate mutant strains was 894 bp, as expected. Primers JE12 and JE9 were used to verify that the DNA region flanking the deleted fabT gene was intact. DNA sequencing confirmed that both clones contained the correct chromosomal replacement. Further, whole-genome sequencing of both mutant strains (MGAS2221ΔfabT-1 and MGAS2221ΔfabT-2) further confirmed that (i) the replacement was in the appropriate chromosomal location within the Fab gene cluster and the DNA sequence was correct and (ii) there were no DNA sequences corresponding to the plasmid backbone in the chromosome. We arbitrarily chose strain MGAS2221ΔfabT-1 for further analysis, which for simplicity we designated strain MGAS2221ΔfabT.
Genome-wide transcriptome-sequencing (RNA-seq) analysis.
The wild-type M1 strain MGAS2221 and the isogenic fabT deletion mutant MGAS2221ΔfabT were grown overnight in 10 ml of THY medium with 5% CO2 at 35°C or 40°C. Aliquots (0.6 ml) from overnight cultures were used to inoculate 30 ml of prewarmed THY medium in 50-ml conical tubes (1:50 dilution) at 35°C and 40°C. Each strain was grown in triplicate, and samples were collected when the absorbance at 600 nm reached an OD of 1.0 and exactly 2 h after the OD reached 1.4, corresponding to the mid-exponential and stationary phases of growth, respectively. Bacteria from the stationary phase or mid-exponential phase, 1 and 2 ml, respectively, were added to RNAprotect Bacteria Reagent (Qiagen) and centrifuged at 4,000 rpm for 10 min. The supernatant was discarded, and the bacterial pellet was frozen in liquid nitrogen and stored at −80°C.
The frozen cell pellets were thawed on ice and suspended in 100 μl of RNase-free Tris-EDTA (TE) buffer, pH 7.0 (Ambion), for RNA extraction. The bacteria were added to 2-ml tubes containing lysis matrix B (MP Biomedicals) and lysed using a FastPrep-96 instrument (MP Biomedicals). The RNA was extracted with an RNeasy minikit (Qiagen) following the manufacturer's instructions. The extracted RNA was subjected to three consecutive DNase treatments with Turbo DNA-free (Ambion), and the quality of the total RNA was evaluated with an Agilent 2100 Bioanalyzer and 2100 Expert software and with RNA Nano chips (Agilent Technologies). For the subsequent steps, we used the ScriptSeq Complete kit for bacteria (Epicentre), and rRNA was depleted with Ribo-Zero magnetic beads. The quality of the rRNA-depleted RNA was evaluated with RNA Pico chips (Agilent Technologies) and an Agilent 2100 Bioanalyzer. The cDNA libraries were prepared with indexed reverse primers from the ScriptSeq Index PCR primer kit (Epicentre) and purified with AMPureXP beads (Agencourt, Beckman Coulter, Inc.). The quality of the cDNA libraries was evaluated with DNA high-sensitivity chips (Agilent Technologies) and an Agilent 2100 Bioanalyzer. For each sample, the cDNA library concentration was measured fluorometrically with Qubit dsDNA HS assay kits (Invitrogen). The cDNA libraries were diluted, pooled, and analyzed with an Illumina HiSeq 2500 instrument.
We obtained an average of 107 reads/sample for the 24 samples (wild-type and fabT mutant strains grown at 35°C and 40°C, with two time points recorded at the mid-exponential and stationary phases and each sample grown in triplicate). Adapter contamination was removed from the FASTQ sequence files using Trimmomatic (20), and the RNA-seq data were analyzed with CLC Bio CLC Genomics Workbench version 7.0 (Qiagen). Reads were mapped to the genome of the emm1 reference strain MGAS5005, excluding rRNA and tRNA genes. Gene expression values from each strain and set of conditions were compared. The Baggerly test and Bonferroni correction were used to test for differential transcript expression. We considered a gene to be differentially expressed when the change in the transcript level differed by at least 1.5-fold between strains and the P value was less than 0.05.
Spot dilution assays.
The wild type, the fabT deletion mutant, MGAS1253, and MGAS25770 were grown overnight to stationary phase, diluted 1:50 in the morning, and grown to an OD of 0.6 to 0.8 in THY medium. Serial dilutions were made in fresh THY broth, and 2.5 μl of each diluted solution was inoculated onto the surfaces of THY plates containing the appropriate polymyxin B concentration. The plates were incubated for approximately 24 h and scored. The experiments were repeated in triplicate.
Survival of S. pyogenes strains in whole human blood.
Bactericidal assays with whole human blood were performed using a standard phagocytosis method with minor modifications (21). The wild-type M1 strain MGAS2221 and the isogenic fabT deletion mutant were grown to early mid-exponential phase in THY medium with 5% CO2 at 37°C (OD600, ∼0.150 to 0.220). The bacteria were diluted to 10−4 in sterile saline. A total volume containing 500 μl blood and 50 μl diluted bacteria (∼103 bacteria/ml blood) was dispensed into a 1.5-ml Eppendorf tube. Heparin was used at 5 U/ml. All studies with human blood and polymorphonuclear leukocytes (PMNs) were performed in accordance with a protocol (01-I-N055) approved by the Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases, National Institutes of Health. All subjects gave written informed consent prior to participation in the study.
GAS survival following exposure to purified PMNs.
Human PMNs were purified from heparinized blood of healthy donors using a standard method (22). The wild-type strain MGAS2221 and the isogenic fabT deletion mutant were grown to early mid-exponential phase in THY medium with 5% CO2 at 37°C (OD600, ∼0.150 to 0.220). A total volume of 600 μl containing 106 PMNs, 106 bacteria, and 10% autologous serum in HEPES-buffered RPMI 1640 medium was dispensed into a 1.5-ml Eppendorf tube. The assay mixtures were rotated gently at 37°C for 1 h or 3 h, at which time saponin (0.1% final concentration) was added to each assay mixture, and the tubes were placed on ice for 15 min. An aliquot of each tube was diluted appropriately and plated on THY agar plates, and colonies were counted the next day. The percent GAS survival was determined by comparing CFU from assays with PMNs to CFU from assays without PMNs. Data at each time point were analyzed with a paired t test (Prism 6 for Windows; GraphPad Prism; GraphPad Software Inc.).
Accession number(s).
The sequence data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra) under accession number SRP087644 and in the NCBI Gene Expression Omnibus (GEO) under accession number GSE86854.
RESULTS
Identification of acquired polymorphisms in serotype M1 strain MGAS2221 during host infection.
With the goal of analyzing intrahost genomic variation during experimental necrotizing fasciitis infection, four animals were inoculated intramuscularly with the serotype M1 strain MGAS2221. This infection model has been used extensively and recapitulates human disease (14, 23, 24). Strain MGAS2221 was used because its genome has been sequenced to high quality, it has been used in many previous studies of necrotizing fasciitis, and it lacks mutations in major regulatory genes (25–28).
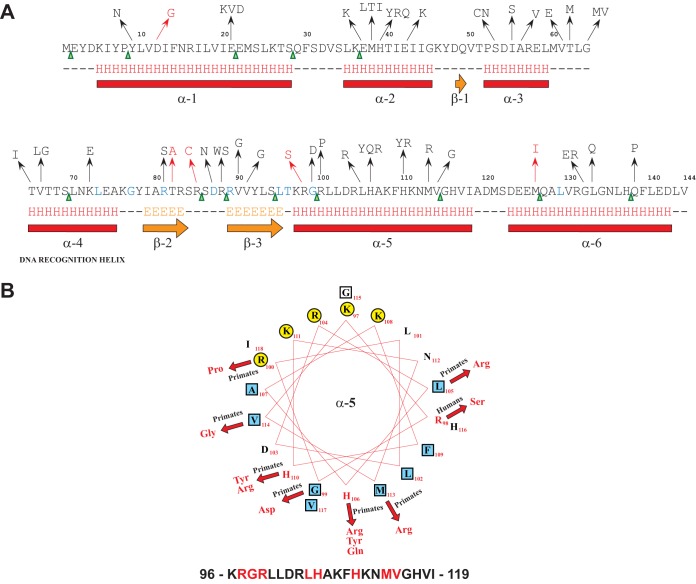
Twenty-four hours after infection, we recovered 768 colonies per animal and performed whole-genome sequencing. High-quality sequencing data were obtained for 698, 760, 743, and 753 strains from each animal, resulting in whole-genome sequence data for 2,954 strains. Analysis of the sequence data found that approximately 20% of these strains had one single nucleotide change, and only a small percentage (47 of 2,954 strains [1.6%]) had two or more changes. There was a strongly nonrandom genomic distribution of polymorphisms in that ∼50% were located in fabT (Spy_1495), a 435-nucleotide gene encoding a transcriptional repressor of fatty acid (FA) synthesis. All the identified nonsynonymous FabT amino acid substitutions are shown in Fig. 1A. Sixty of 66 SNPs (91%) were nonsynonymous, and 6 were synonymous (9%). In addition, among the 36 indels, 4 were in frame (11%) and 32 (89%) would result in a frame-shifted mutant FabT protein. Thirty of the 144 amino acids encompassing FabT (∼21%) had amino acid replacements, of which 20 were single, 6 were double (where either of 2 amino acids replaced the wild-type amino acid residue), and 4 were triple replacements (Fig. 1A and B). Thus, we identified a total of 44 amino acid replacements at 30 positions in the FabT protein. Several of these mutations occurred more than once in strains isolated from different nonhuman primates, likely indicating that the polymorphisms arose independently. In addition to amino acid replacements, other mutations gave rise to 13 nonsense codons in fabT. Their distribution was widespread across the gene (Fig. 1A, green triangles); for the 145 codons, the closest to the fabT 5′ end occurred at codon 2 and at the 3′ end at codon 138.
FIG 1.
Location of nonsynonymous substitutions within FabT. (A) Secondary-structure prediction for FabT derived from Jpred. Nonsynonymous substitutions are represented in black (mutations identified in isolates cultured from nonhuman primates) and red (mutations identified in isolates cultured from humans). Stop codons are represented by green arrowheads (mutations identified in isolates cultured from nonhuman primates). The residues in blue are conserved among members of the MarR superfamily of proteins. (B) Helical-wheel representation of the FabT amphipathic alpha helix 5 (α5). The amino acid sequence, with the mutated residues in red, is shown at the bottom; polar and hydrophobic residues are represented by yellow circles and blue squares, respectively. The resulting nonsynonymous substitutions are shown in red, and the arrows denote the corresponding changes. Primates, nonhuman primates; Humans, host from which the respective mutated GAS strain was isolated.
We recently sequenced the genomes of 3,615 pandemic serotype M1 strains isolated from human patients with invasive infections or pharyngitis (12). The fabT gene ranked 11th among genes containing polymorphisms in these 3,615 strains. After grouping the polymorphisms as a nonredundant set, we found three insertions, one deletion, and five nonsynonymous SNPs in the fabT coding sequence (Fig. 1A, red arrows and altered amino acid residues). In addition, two SNPs were found immediately upstream of fabT at positions −47 and −48, located between the putative −35 and −10 promoter regions. Similar upstream SNPs were found in the invasive GAS strains isolated from nonhuman primates in this study (data not shown). The phylogenetic distribution of the strains isolated from human patients with fabT polymorphisms indicates that most of the changes likely originated independently (see Fig. S2 in the supplemental material).
Several of the altered amino acid residues identified in the present study are highly conserved among the MarR superfamily of transcriptional regulators (Fig. 1A, blue residues). The FabT amino acid changes are primarily clustered in α-helices 2 through 5. Of note, the variant amino acids do not appear to be randomly distributed. For example, the polymorphisms mapping to α-helix 5 cluster primarily on the hydrophobic face of the amphipathic helix (Fig. 1B). This region could either be involved in dimerization (29) or provide a hydrophobic pocket for an effector molecule involved in allosteric regulation of FabT.
Growth characteristics of a fabT deletion mutant strain.
To begin to determine the role of FabT in S. pyogenes pathogenesis, we constructed the isogenic fabT deletion mutant strain MGAS2221ΔfabT. In this mutant, the entire fabT gene is replaced with the coding sequence of the cat gene, encoding chloramphenicol acetyltransferase, resulting in the intact fabT promoter driving transcription of cat and downstream genes.
We tested the hypothesis that the fabT mutant strain differed in growth characteristics from the wild-type parental organism. Two different temperatures were used for this analysis, selected to represent differences in affected anatomical sites: 35°C for pharyngitis (30) and 40°C for serious invasive infections. Both strains grew to an OD of 1.5 at both temperatures (Fig. 2). However, the fabT deletion mutant strain consistently required a longer time to reach the mid-exponential phase (OD = 0.5) at 40°C. Once this OD value was reached, the doubling times were similar for both strains, as calculated from the corresponding logarithmic graphs (data not shown). A similar growth pattern was observed at 37°C (data not shown). Thus, although the growth rates of wild-type MGAS2221 and the isogenic fabT deletion mutant were similar under the experimental conditions, the fabT mutant strain had a prolonged lag phase early in growth compared to the wild-type parental strain.
FIG 2.
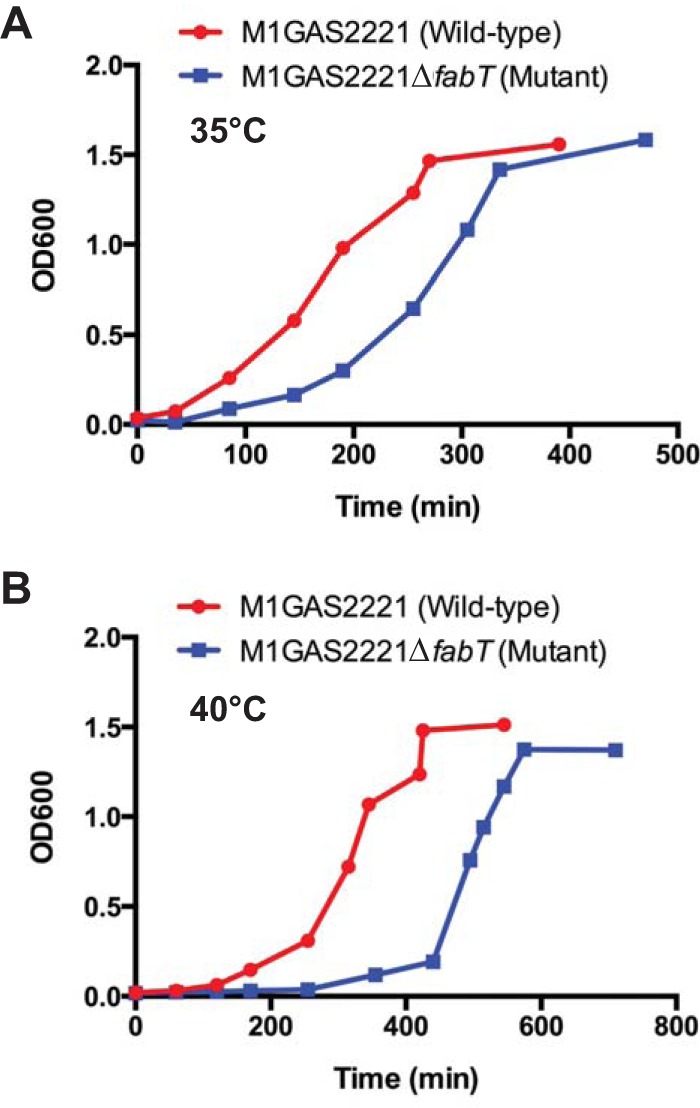
Growth curves. The wild-type strain MGAS2221 and the FabT deletion strain MGAS2221ΔfabT were grown at 35°C (A) and 40°C (B) in THY medium.
RNA-seq analysis to identify differentially expressed genes.
We next performed RNA-seq analysis to identify genes whose expression was directly or indirectly regulated by FabT. The wild-type strain (MGAS2221) and the fabT deletion mutant strain (MGAS2221ΔfabT) were grown at 35°C and 40°C, and samples acquired at the mid-exponential (OD = 1.0) and stationary (2 h after an OD of 1.4 was reached) growth phases were analyzed.
Depending on the growth temperature and phase, between 3.7 and 28.5% of the 1,841 S. pyogenes genes (excluding rRNA and tRNA genes) were differentially expressed in the mutant strain. Notably, the number of differentially expressed genes was greater during stationary phase (see Table S1 in the supplemental material). Genes involved in transport, translation, nucleotide transport and metabolism, and central metabolism were primarily affected, although most functional categories were represented. Based on the large number of differentially expressed genes, a substantial proportion are likely to be regulated indirectly rather than directly by FabT. A comprehensive list of the genes differentially expressed under each condition is presented in Tables S2 and S3 in the supplemental material.
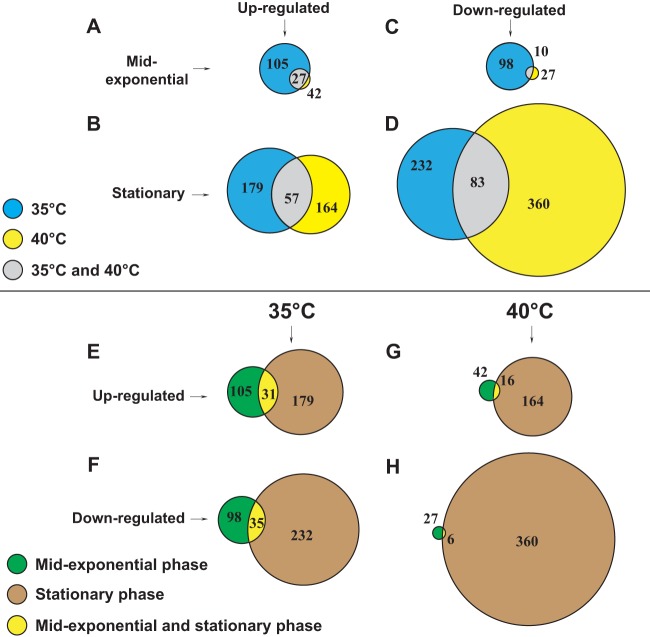
The genes differentially expressed according to either growth temperature or growth phase, including genes differentially regulated in more than one category, are shown in Fig. 3. The genes were categorized based on their differential expression at one or both growth phase time points and/or their differential expression at one or both growth temperatures. We hypothesized that the genes directly regulated by FabT would be differentially expressed under all the conditions tested. Several genes meeting these criteria are discussed below.
FIG 3.
Area-proportional Venn diagrams representing differentially expressed genes. (A to D) Genes differentially expressed at 35°C and 40°C. Common genes differentially expressed at both growth temperatures are also depicted. (A) Genes upregulated during mid-exponential phase. (B) Genes upregulated during stationary phase. (C) Genes downregulated during mid-exponential phase. (D) Genes downregulated during stationary phase. (E to H) Genes differentially expressed during mid-exponential and stationary phases. Common genes differentially expressed during both phases of growth are also depicted (E) Genes upregulated at 35°C. (F) Genes downregulated at 35°C. (G) Genes upregulated at 40°C. (H) Genes downregulated at 40°C.
Twenty-seven genes were upregulated at both 35°C and 40°C during mid-exponential phase in the FabT mutant strain, and this number increased to 57 during stationary phase (Fig. 3A and B). Conversely, 10 and 83 genes were downregulated at both temperatures during the mid-exponential and stationary phases, respectively (Fig. 3C and D). Thus, 8 to 18% of the differentially expressed genes were shared at both growth temperatures, whereas a larger group of genes were differentially expressed at only one growth temperature.
Thirty-one and 16 genes were commonly upregulated at 35°C and 40°C, respectively, in both phases of growth (Fig. 3E and G). Conversely, 35 genes were downregulated during both phases of growth at 35°C, but only 6 at 40°C (Fig. 3F and H). Thus, 8 to 10% of the genes were commonly shared between the mid-exponential and stationary phases of growth, apart from downregulated genes in the fabT deletion mutant at 40°C, in which only 1.6% of the genes were shared. Thus, a larger group of genes were differentially expressed in only one of the two growth phases.
Genes differentially expressed under all conditions tested: FA synthesis genes.
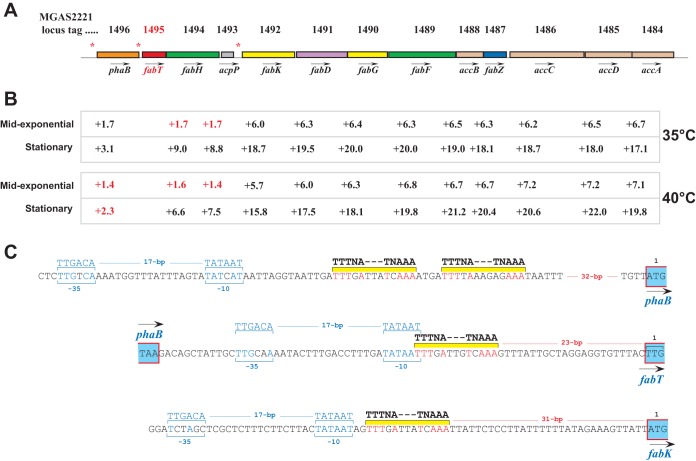
Consistent with the function of FabT as a transcriptional repressor of FA synthesis genes in other bacterial species (31–33), all genes in the fatty acid biosynthesis (Fab) gene cluster were upregulated in the isogenic fabT deletion strain under all conditions tested. The Fab cluster in strain MGAS2221 consists of 13 genes (Fig. 4A). The fold differences were more pronounced in the stationary phase of growth at both 35°C and 40°C (Fig. 4B). Based on the magnitude of the changes, and assuming no major differences in mRNA half-life, our data suggest that the Fab cluster in S. pyogenes is divided into three distinct transcriptional units. First, there is a putative monogenic transcript of Spy_1496 (phaB). A second putative transcript encompasses Spy_1495 (fabT), Spy_1494 (fabH), and Spy_1493 (acpP), consistent with data from Streptococcus pneumoniae (33). A third putative long transcript encompasses Spy_1492 (fabK) at the 5′ end, extending to the 3′ end of Spy_1484 (accA). This transcriptional organization is consistent with the presence of three putative FabT-binding sites located upstream of phaB, fabT, and fabK (Fig. 4A, asterisks). Based on homology with a consensus FabT operator site, their locations in the respective genes in relation to promoter and translation initiation codons are shown in Fig. 4C. These data are consistent with our hypothesis that genes and/or operons directly regulated by FabT are upregulated in the fabT deletion mutant under all the conditions tested.
FIG 4.
Regulation of fab genes by FabT. (A) The Fab cluster of GAS, showing the locations of three putative FabT-binding DNA sequences (asterisks) located in front of phaB, fabT, and fabK. fabT is depicted in red. (B) Fold expression of each gene at both temperatures and phases of growth. The values in red indicate either that the differential fold expression was below the allotted 1.5 or that the P value was excluded after applying the Bonferroni correction. (C) Figure representing the regulatory regions of phaB, fabT, and fabK, with putative promoter sequences and FabT-binding DNA sequences indicated. Nucleotide residues matching the promoter sequence and FabT site are indicated in blue and red, respectively.
Additional genes or operons upregulated under all the conditions tested are listed in Table 1. Matches to the FabT DNA-binding consensus sequence were detected in their upstream regulatory regions, suggesting that FabT directly regulates the expression of these genes (data not shown).
TABLE 1.
Genes upregulated in the fabT mutant, compared to the wild type under all conditions tested
| Locus taga | Gene | Fold changeb |
|||
|---|---|---|---|---|---|
| 35°C |
40°C |
||||
| ME | Stat | ME | Stat | ||
| Spy_1828 | 39.5 | 36.7 | 30.3 | 25.5 | |
| Spy_0879 | dgk | 3.5 | 9.7 | 3.2 | 5.5 |
| Spy_0880 | hlyIII | 5.3 | 12.5 | 4.4 | 4.9 |
| Spy_0881 | 6.4 | 7.5 | 5.2 | 3.1 | |
| Spy_0385 | 5.4 | 2.5 | 5.0 | 2.1 | |
| Spy_1650 | degV | 1.8 | 2.8 | 1.9 | 1.7 |
Spy_1828 encodes a membrane protein used as a phage receptor in Lactococcus lactis, Spy_0879 encodes a putative diacyl glycerol kinase, Spy_0880 encodes a hemolysin III-related protein, Spy_0881 encodes a transcriptional regulator of the MerR family, Spy_0385 encodes acute rheumatic fever myosin cross-reactive protein, and Spy_1650 encodes an uncharacterized protein containing a bound fatty acid molecule.
ME, mid-exponential growth phase; Stat, stationary growth phase.
Selected genes that were downregulated in the isogenic fabT deletion mutant compared to the wild type under at least two of the conditions studied are involved in sugar utilization and fermentation pathways (Table 2). No putative FabT-binding DNA sequences were found in the regulatory regions of these genes, suggesting that FabT indirectly regulates their expression.
TABLE 2.
Genes downregulated in the fabT mutant compared to the wild type under the tested conditions
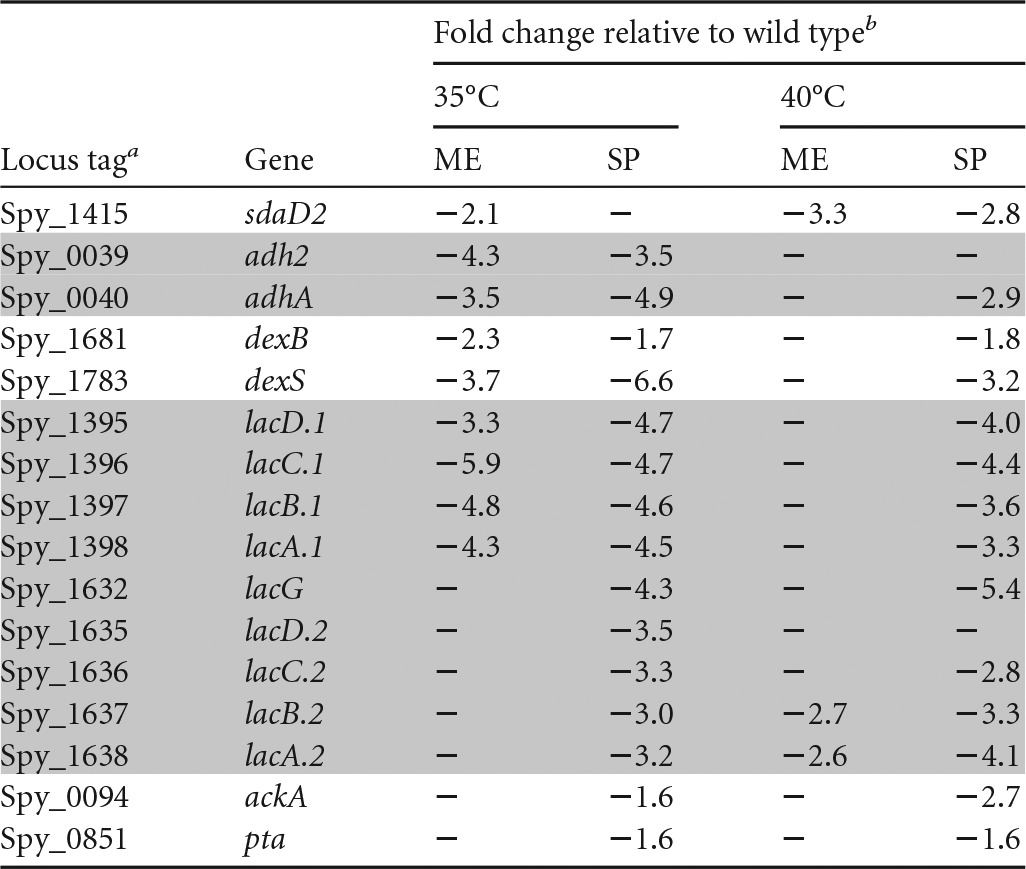
Spy_0039 and Spy_0040 encode a bifunctional acetaldehyde/alcohol dehydrogenase and an alcohol dehydrogenase, respectively; Spy_ 1681 encodes glucan 1,6-alpha-glucosidase; Spy_ 1783 encodes a putative alpha amylase/glycosidase; Spy_1395, Spy_1396, Spy_1397, and Spy_1398 encode enzymes of the tagatose pathway cluster 1; Spy_1632, Spy_1635, Spy_1636, Spy_1637, and Spy_1638 encode enzymes of the tagatose pathway cluster 2; Spy_0094 encodes acetate kinase. Spy_0851 encodes phosphotransacetylase. The shaded rows indicate genes in the same operon.
ME, mid-exponential growth phase; SP, stationary growth phase. −, the gene does not satisfy the P value (including the Bonferroni correction) and/or fold requirement.
Differential expression of genes related to metabolism.
The RNA-seq analysis identified differential expression of many genes related to metabolism, including genes encoding enzymes involved in the pyruvate hub, fermentation pathways, and entry of carbon sources into the glycolytic pathway (Table 3). Taken together, carbon flow around the pyruvate hub and fermentation of carbon sources, including the pathways and end products, may be different in the fabT deletion mutant than in the wild type.
TABLE 3.
Carbon metabolism genes differentially expressed between the isogenic fabT deletion mutant and the wild-type strain MGAS2221 grown at 35°C
| Locus tag | Gene | Fold changea | Growth phaseb | Function |
|---|---|---|---|---|
| Pyruvate hub enzymes | ||||
| M5005_Spy0751 | acoA | 2.5 | ME | Pyruvate dehydrogenase E1 component, alpha subunit |
| M5005_Spy0752 | acoB | 2.5 | ME | Pyruvate dehydrogenase E1 component, beta subunit |
| M5005_Spy0753 | acoC | 2.4 | ME | Pyruvate dehydrogenase, dihydrolipoamide acyltransferase (E2) component |
| M5005_Spy0755 | acoL | 2.4 | ME | Pyruvate dehydrogenase, dihydrolipoamide dehydrogenase (E3) component |
| M5005_Spy1569 | pfl | 1.8 | ME | Pyruvate formate lyase |
| M5005_Spy1841 | sdhB | 6.3 | ME | Serine dehydratase beta chain |
| M5005_Spy1842 | sdhA | 6.0 | ME | Serine dehydratase alpha chain |
| Genes involved in fermentation pathways | ||||
| M5005_Spy0873 | ldh | 2.3 | SP | l-Lactate dehydrogenase |
| M5005_Spy0039 | adh2 | −4.3 | ME | Acetaldehyde dehydrogenase |
| M5005_Spy0040 | adhA | −3.5 | ME | Alcohol dehydrogenase |
| M5005_Spy0039 | adh2 | −3.5 | SP | Acetaldehyde dehydrogenase |
| M5005_Spy0040 | adhA | −4.9 | SP | Alcohol dehydrogenase |
| M5005_Spy0094 | ackA | −1.6 | SP | Acetate kinase |
| M5005_Spy0851 | pta | −1.6 | SP | Phosphotransacetylase |
| Upregulated genes involved in entry of carbon sources into the Embden-Meyerhof-Parnas (glycolysis) pathway | ||||
| M5005_Spy0661 | fruB | 3.3 | SP | l-Phosphofructokinase |
| M5005_Spy0938 | pgmA | 1.6 | SP | Phosphoglucomutase |
| M5005_Spy1538 | pmi | 1.6 | ME | Mannose-6-phosphate isomerase |
| Downregulated genes involved in entry of carbon sources into the Embden-Meyerhof-Parnas (glycolysis) pathway | ||||
| M5005_Spy0903 | oadB | −2.5 | SP | Oxaloacetate decarboxylase beta subunit |
| M5005_Spy0905 | citD | −1.9 | SP | Citrate lyase subunit gamma |
| M5005_Spy0906 | citE | −1.9 | SP | Citrate lyase subunit beta/citryl-CoA lyase subunit |
| M5005_Spy0907 | citF | −2.0 | SP | Citrate lyase subunit alpha/citrate CoA-transferase |
| M5005_Spy0908 | citX | −2.4 | SP | Citrate lyase activation subunit |
| M5005_Spy1062 | malA | −2.1 | SP | Maltodextrose utilization protein |
| M5005_Spy1063 | malD | −2.1 | SP | ABC transporter transmembrane subunit |
| M5005_Spy1064 | malC | −2.0 | SP | Maltose/maltodextrin transporter membrane protein |
| M5005_Spy1067 | malX | −2.0 | SP | Maltose/maltodextrin transporter subunit |
| M5005_Spy0832 | maeP | −11.3 | SP | Putative malate transporter |
| M5005_Spy0833 | maeE | −10.0 | SP | Malic enzyme |
| M5005_Spy1379 | glpF | −4.4 | SP | Glycerol uptake facilitator |
| M5005_Spy1380 | glpO | −4.6 | SP | Glycerol-3-phosphate dehydrogenase |
| M5005_Spy1381 | glpK | −5.5 | SP | Glycerol kinase |
| M5005_Spy1542 | scrA | −2.4 | SP | Sucrose-specific transporter PTS system subunit II |
| M5005_Spy1543 | scrB | −2.4 | SP | Glycosyl hydrolase involved in sucrose utilization |
| M5005_Spy1544 | scrR | −2.4 | SP | Putative transcriptional regulator involved in sucrose utilization |
Fold change in the fabT mutant relative to the wild type.
ME, mid-exponential growth phase; SP, stationary growth phase.
Genes encoding enzymes involved in use of alternative carbon sources, other than glucose, were also differentially expressed. For example, genes encoding enzymes favoring the entrance of glucose-1-phosphate, mannose-6-phosphate, and fructose-1-phosphate into upper glycolysis were upregulated in the fabT deletion mutant compared to the wild type (Table 3). In contrast, other pathways funneling carbon into glycolysis, such as glycerol transport and degradation and citrate and malate utilization, which are abundant in host tissues, and maltose transport and its conversion into glucose, were downregulated in the fabT deletion mutant during the stationary phase of growth at 35°C. These results suggest that entry of carbon sources other than glucose into the Embden-Meyerhof-Parnas (glycolysis) pathway may occur differently in the fabT deletion mutant than in the wild type.
The two gene clusters encoding proteins and enzymes involved in lactose and galactose transport and degradation, the tagatose pathway, were also downregulated in the FabT mutant at both temperatures during the stationary phase (Table 2). Cluster I, or Lac.1, is specific for galactose (34, 35). It contains genes extending from Spy_1395 to Spy_1402. The genes present in cluster II, or Lac.2, are specific for lactose (34, 35). They extend from Spy_1632 to Spy_1639. Interestingly, LacD.1 acts as a transcriptional regulator of virulence genes (34).
Differential expression of genes related to virulence.
Differentially expressed virulence genes are shown in Table 4. Spy_1106 (grab) and Spy_1720 (mga) were significantly upregulated during the mid-exponential and stationary phases, respectively, at both growth temperatures. These genes encode GRAB, a surface-anchored protein that binds α2-macroglobulin, a major protease inhibitor present in human plasma (36), and Mga, a critical positive transcriptional regulator of virulence genes found in all GAS strains. Conversely, the gene encoding the two-component system histidine kinase CovS, Spy_0283 (covS), and the sic gene (Spy_1718), which encodes the streptococcal inhibitor of complement (SIC), were downregulated in the stationary and mid-exponential phases, respectively, at both growth temperatures.
TABLE 4.
Differentially expressed virulence genes in the isogenic fabT deletion mutant compared to wild-type MGAS2221
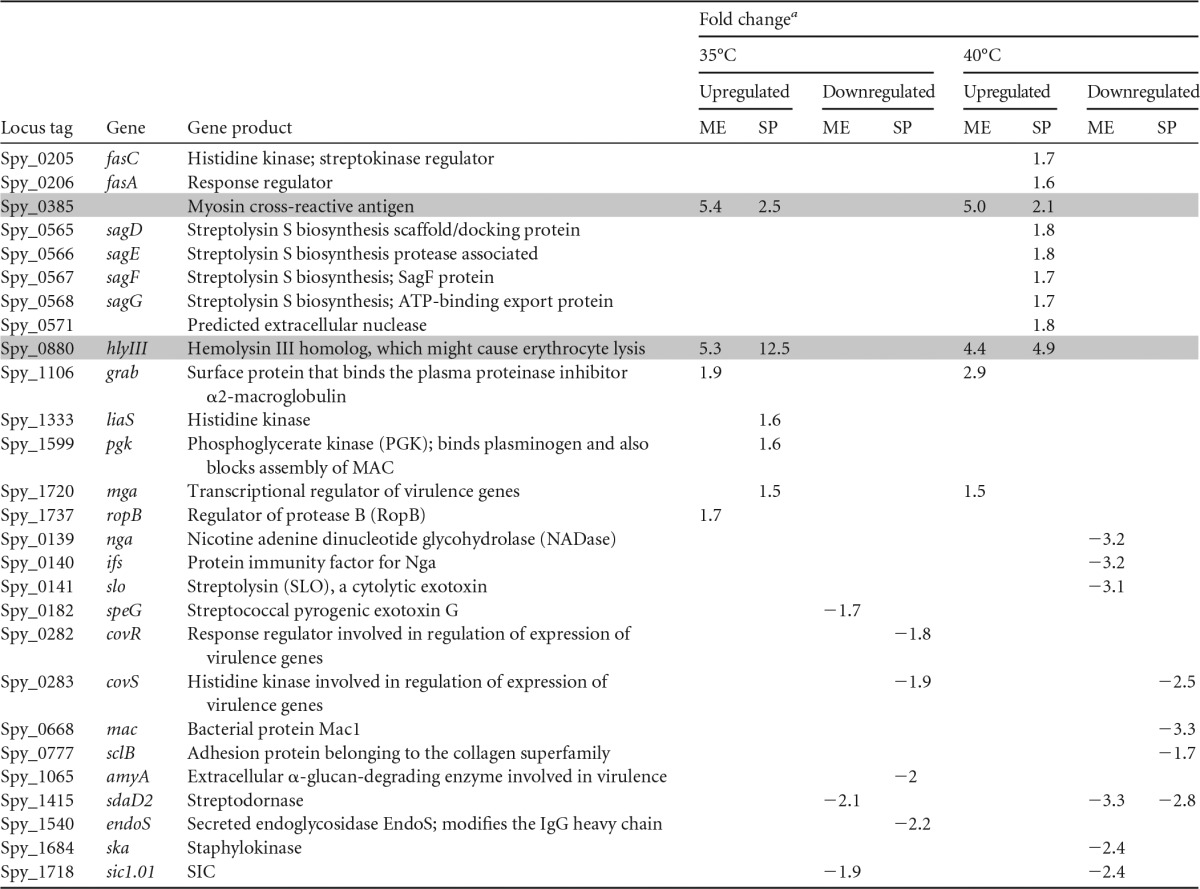
The shaded rows indicate genes that are differentially regulated in the mid-logarithmic and stationary phases and at both temperatures; ME, mid-exponential growth phase; SP, stationary growth phase.
The other genes in Table 4 were differentially regulated under only one of the assay conditions tested. The streptolysin S biosynthesis genes were upregulated in the fabT deletion mutant during the stationary growth phase at 40°C. Spy_0385, which encodes a 67-kDa myosin-cross-reactive antigen involved in fatty acid metabolism (37, 38), and Spy_0880 (hlyIII), encoding a hemolysin III-like protein that in Bacillus cereus is involved in erythrocyte lysis (39), were upregulated under all conditions tested. We identified putative FabT-binding sequences immediately upstream of Spy_0385 and upstream of Spy_0881 (see Fig. S3A in the supplemental material). In addition, Spy_1415 (sdaD2), which encodes streptodornase, was downregulated under three of the conditions tested, but not during stationary phase at 35°C.
Other notable differentially expressed genes.
Pyrimidine and purine synthesis genes were also differentially expressed. Genes involved in the de novo pyrimidine synthesis were downregulated in the fabT mutant strain during the mid-exponential phase of growth and upregulated in the stationary phase, preferentially at 35°C (see Fig. S4 in the supplemental material). Genes in the purine synthesis pathway were downregulated during the stationary phase in the fabT deletion mutant compared to the wild type (see Fig. S5 in the supplemental material). Pyrimidine and purine bases and nucleosides are either unavailable or at very low concentrations as exogenous nutrients (40, 41), especially in human plasma, where S. pyogenes increases the de novo synthesis of both pyrimidines and purines (42).
Spy_0687 (mvaS1) and Spy_0686 (mvaA), which encode HMG-coenzyme A (CoA) synthase and HMG-CoA reductase, two enzymes that control entry points to the mevalonate pathway, were upregulated in the fabT deletion mutant by >1.7-fold during the stationary phase. The mevalonate pathway is required to make undecaprenyl pyrophosphate and lipid II (43). The consequence of this upregulation in the fabT deletion mutant strain might be increased production of lipid II, potentially leading to (i) increased resistance to cationic antimicrobial peptides (CAMPs) and lantibiotics that target lipid II (44–46) and (ii) higher levels of peptidoglycan (PG) and other envelope components that also are targets for CAMPs (47, 48).
The gene Spy_0124 (sloR), encoding a regulator involved in metal homeostasis, was also significantly upregulated by 10.3-fold in the fabT deletion mutant during mid-exponential phase at 35°C. Metalloregulators have been shown to modulate virulence gene expression in S. pyogenes (49–51).
The arginine deiminase pathway genes, from Spy_1270 (arcC) to Spy_1275 (arcA), were downregulated by over 10-fold during the mid-exponential phase of growth in the fabT deletion mutant, but only at 35°C. Notably, this change in differential expression was the largest found for downregulated genes in this study.
Phenotypic analysis of the fabT deletion mutant: resistance to polymyxin B.
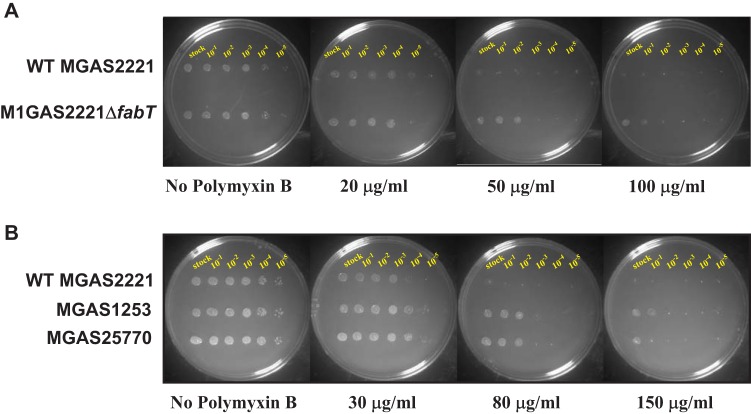
The infection protocol used in this study consisted of 24-h intramuscular exposure in the nonhuman primate hosts before bacteria were harvested. Because of the relatively short time of exposure to the bacteria, we assumed that largely innate immune processes would be operative. CAMPs made by the host are an important part of the innate immunity repertoire. CAMPs kill bacteria through insertion into and subsequent pore formation in bacterial membranes (52). We hypothesized that, compared to the wild-type parental strain, the fabT mutant would have increased resistance to polymyxin B, an antimicrobial agent that mimics endogenous CAMPs. Consistent with the hypothesis, mutant strain MGAS2221ΔfabT had significantly increased resistance to polymyxin B compared to the wild-type parental strain (Fig. 5A). Similarly, two naturally occurring representative FabT mutants isolated from humans with invasive infections (12) had significantly increased resistance to polymyxin B, as expected based on the data for strain MGAS2221ΔfabT (Fig. 5B). These two human strains have single nucleotide insertions located early in the fabT gene that produce frameshift mutations resulting in a FabT-null organism (Fig. 5B).
FIG 5.
MGAS2221ΔfabT shows increased resistance to polymyxin B. (A) Spot dilution assay of wild-type strain MGAS2221 and the fabT deletion mutant on THY plates containing either 0, 20, 50, or 100 μg/ml polymyxin B. Shown from left to right are undiluted and 10−1- to 10−5-diluted samples of a mid-logarithmic phase culture grown in THY medium. (B) Spot dilution assay of wild-type strain MGAS2221 and strains MGAS1253 and MGAS25770 on THY plates containing 0, 30, 80, or 150 μg/ml polymyxin B. The last two strains were isolated from patients with invasive disease and contain single nucleotide insertions within the fabT gene: MGAS1253, A15 insertion, SF370-like strain isolated around 1920 (12); MGAS25770, A106 insertion, 5005-like strain isolated in 2006 (12).
The fabT deletion mutant strain is less virulent in an animal model of necrotizing fasciitis.
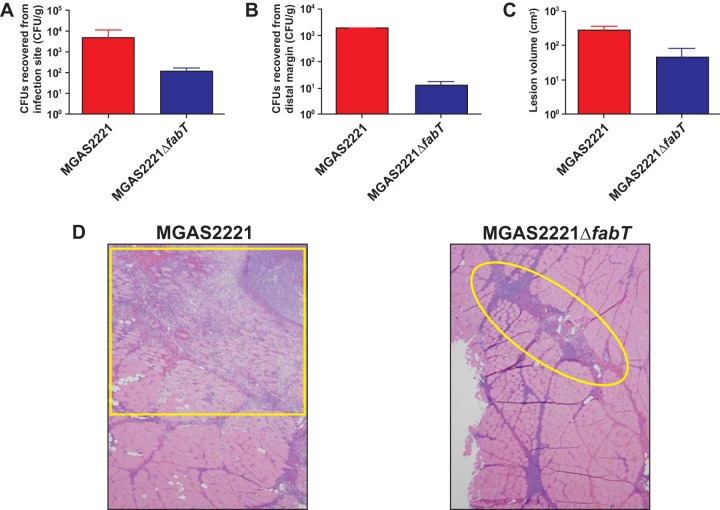
Given the number and types of genes identified by the RNA-seq analysis to be differentially regulated in the fabT mutant, we hypothesized that FabT inactivation significantly decreases S. pyogenes virulence. A nonhuman primate model of necrotizing fasciitis was used to test this hypothesis. Compared to the wild-type parental strain, the isogenic mutant strain MGAS2221ΔfabT caused significantly less dissemination (Fig. 6A and B), smaller lesions (Fig. 6C), and less tissue destruction (Fig. 6D). The wild-type strain caused extensive destruction of fascia and muscle with severe myonecrosis and hemorrhage (Fig. 6D, left), whereas the isogenic fabT mutant strain caused only limited myocyte damage (Fig. 6D, right). Together, these data demonstrate that fabT inactivation decreases virulence in this animal mimetic of human necrotizing fasciitis.
FIG 6.
The fabT deletion strain is less virulent in a nonhuman primate model of necrotizing fasciitis. Cynomolgus macaques were inoculated intramuscularly in the anterior thigh. (A) CFU recovered from the inoculation site in the thigh. (B) CFU recovered from the distal muscle margin near the hip. (C) Lesion size. The error bars indicate standard errors of the mean (SEM). (D) Histopathology. The boxed region in the left micrograph shows that the wild-type strain causes extensive fascial necrosis, muscle cell destruction, and hemorrhage at the inoculation site. The circled region in the right micrograph shows that strain MGAS2221ΔfabT causes less severe necrotizing fasciitis. Original magnification, ×4.
Significantly decreased survival of FabT mutant bacteria in human blood and during phagocytic interaction with human PMNs.
Two possibilities to account for the altered virulence of isogenic mutant strain MGAS2221ΔfabT are decreased survival in blood and increased killing by PMNs. To test these possibilities, wild-type strain MGAS2221 and isogenic mutant strain MGAS2221ΔfabT were compared for survival in whole human blood and after incubation with purified PMNs. Consistent with our idea, the ΔfabT mutant strain had significantly decreased survival in both assays compared to the wild-type parental strain (Fig. 7A and B).
FIG 7.
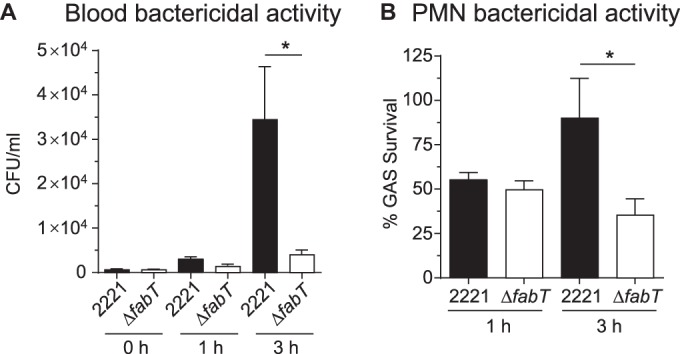
The fabT deletion strain is more susceptible to blood and PMN killing than the wild-type strain. (A) Strains were exposed to blood bactericidal activity and assayed at 0, 1, and 3 h. (B) Strains were exposed to PMNs, and bactericidal activity was assayed at 0 and 3 h. The results are means and SEM of data from 9 separate experiments. *, P < 0.05 using a paired t test.
DISCUSSION
S. pyogenes modulates gene expression to adapt to and survive conditions encountered in various host tissues (2, 53). As a continuation of our studies investigating the molecular adaptation of the pathogen to the host environment, we investigated the genomic landscape of mutational variation 24 h after infection in a model of necrotizing fasciitis. The strain used is genetically representative of serotype M1 organisms that have caused pandemic disease in the last 30 years. Our data demonstrate repeated and abundant mutational inactivation of the fabT gene encoding a regulator of fatty acid synthesis, virulence, and other genes. The findings support previous studies showing that FabT is a transcriptional regulator that directly or indirectly regulates multiple genes assigned to many different functional categories, including lipid and carbon metabolism and virulence.
We found that 21.8% of the 2,954 strains recovered from diseased host muscle had polymorphisms (primarily one per strain), with 45% of them apparently randomly distributed throughout the genome. Importantly, 55% of the polymorphisms were located in the fabT gene, which encodes a transcriptional repressor of fatty acid biosynthesis. The mutated amino acid residues preferentially occurred in predicted functional domains in the FabT protein. Several of the amino acid changes we identified in FabT are located in highly conserved domains present in the MarR superfamily of transcriptional regulators. Most of the polymorphisms are predicted to result in frameshift mutations, premature protein termination, or radical structure change. The only reasonable explanation for the great abundance of mutations in fabT, coupled with the inferred detrimental effect on FabT, is that this transcriptional regulator is under strong selective pressure during deep-seated invasive infection. Data from sequence analysis of 3,615 serotype M1 S. pyogenes genomes cultured from infected humans are fully consistent with and support this idea (12).
We propose three potential explanations for why fabT inactivation is potently selected for during invasive infection in the experimentally infected animals and diseased humans. First, it is possible that inactivation of fabT confers beneficial consequences for the pathogen, such as enhanced survival due to membrane alterations. For example, it may be that membrane alterations confer resistance to host CAMPs, which is achieved by alteration of the acyl chains in their phospholipids (54). Consistent with this idea, compared to the wild-type parental strain, we found that the fabT mutant was more resistant to treatment with polymyxin B, a cationic antimicrobial agent. Our result is consistent with recent data showing that mutations in fabT render S. pyogenes more resistant to polymyxin B (55). Lack of FabT could also confer on GAS increased tolerance for acidic conditions, such as those found in necrotic tissue.
A second possibility invokes host adaptation due to diversification of the infecting organisms into subpopulations with significantly different physiological capacities. For example, it may be that deleterious mutations in fabT result in subpopulations that provide critical or otherwise limiting nutrients to wild-type cells in the necrotic host environment. Serotype M49 GAS grown in THY broth during the stationary phase showed similar genetic diversification (56). In that study, it was proposed that the diversification of the M49 population into genetically different cell types was beneficial to control the pH of the cultures (56). In addition to GAS, other examples of metabolic diversity achieved by mutation within a single microbial species have been reported (57–59).
A third possibility is that altered virulence factor production between FabT-deficient and wild-type cells contributes to population fitness. In this context, several virulence factors with genes that are upregulated in the FabT mutant may be secreted in larger quantities and contribute to adaptation during infection. Regardless, in view of our findings that inactivation of fabT in necrotizing fasciitis apparently assists GAS survival whereas deletion of fabT does not increase strain virulence in this infection type, we believe further studies to dissect the role of FabT mutant cells in host-pathogen interactions and virulence are warranted.
The primary goal of our study was to obtain new information about S. pyogenes intrahost genetic variation and strain diversification during invasive infection. We used a nonbiased approach to address this knowledge gap by sequencing the genomes of 2,954 strains recovered from animals with experimental necrotizing fasciitis. We previously analyzed (60) intrahost strain diversity using targeted sequencing of the sic gene encoding the streptococcal inhibitor of complement, an antiphagocytic and highly polymorphic secreted virulence factor that is under positive selection in the host. We sequenced the sic gene from 2,000 serotype M1 colonies obtained in one pharyngeal swab each from 20 human patients with pharyngitis (100 colonies per patient) and discovered that 25% of the individuals had infections with two or more sic allelic variants (60). The study documented intrahost genetic variation in clonal derivatives of the infecting strain. The present study confirms the idea of intrahost genetic variation and extends it to the full-genome level.
Extensive intrahost genetic variation has been well documented in certain RNA viruses. Although less well studied in bacterial pathogens, the subject has been gaining more interest in recent years as DNA-sequencing costs decrease and technology significantly improves. For example, sequence analysis of the genomes of 95 colonies of Staphylococcus aureus cultured at one time point from a human patient with a perinephric abscess found an average pairwise distance between the genomes of 7.4 SNPs (61). Similarly, in related work (62–64), several investigators have studied bacterial genome-scale intrahost evolution over time, but in these studies, generally only a single colony at each time point has been analyzed (65–69).
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Musser laboratory for helpful discussions and Connie Cantu for technical help with animal experiments.
The research was supported in part by the Fondren Foundation and the Intramural Research Program of the NIAID, NIH. P.E.B. was supported in part by the Texas A&M Health Science Center College of Medicine M.D./Ph.D. program. P.E.B. is a Fondren Foundation clinician-scientist trainee. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
The research was supported in part by the Fondren Foundation and the Intramural Research Program of the NIAID, NIH. The funders had no role in study design, data collection and interpretation, or decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00608-16.
REFERENCES
- 1.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musser JM, Shelburne SA III. 2009. A decade of molecular pathogenomic analysis of group A Streptococcus. J Clin Invest 119:2455–2463. doi: 10.1172/JCI38095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henningham A, Barnett TC, Maamary PG, Walker MJ. 2012. Pathogenesis of group A streptococcal infections. Discov Med 13:329–342. [PubMed] [Google Scholar]
- 4.Cohen-Poradosu R, Kasper DL. 2007. Group A streptococcus epidemiology and vaccine implications. Clin Infect Dis 45:863–865. doi: 10.1086/521263. [DOI] [PubMed] [Google Scholar]
- 5.Bessen DE. 2009. Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect Genet Evol 9:581–593. doi: 10.1016/j.meegid.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores AR, Jewell BE, Olsen RJ, Shelburne SA III, Fittipaldi N, Beres SB, Musser JM. 2014. Asymptomatic carriage of group A Streptococcus is associated with elimination of capsule production. Infect Immun 82:3958–3967. doi: 10.1128/IAI.01788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM. 2006. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci U S A 103:7059–7064. doi: 10.1073/pnas.0510279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll RK, Shelburne SA III, Olsen RJ, Suber B, Sahasrabhojane P, Kumaraswami M, Beres SB, Shea PR, Flores AR, Musser JM. 2011. Naturally occurring single amino acid replacements in a regulatory protein alter streptococcal gene expression and virulence in mice. J Clin Invest 121:1956–1968. doi: 10.1172/JCI45169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores AR, Olsen RJ, Wunsche A, Kumaraswami M, Shelburne SA III, Carroll RK, Musser JM. 2013. Natural variation in the promoter of the gene encoding the Mga regulator alters host-pathogen interactions in group A Streptococcus carrier strains. Infect Immun 81:4128–4138. doi: 10.1128/IAI.00405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanson M, Makthal N, Gavagan M, Cantu C, Olsen RJ, Musser JM, Kumaraswami M. 2015. Phosphorylation events in the multiple gene regulator of group A Streptococcus (Mga) significantly influences global gene expression and virulence. Infect Immun 83:2382–2395. doi: 10.1128/IAI.03023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beres SB, Kachroo P, Nasser W, Olsen RJ, Zhu L, Flores AR, de la Riva I, Paez-Mayorga J, Jimenez FE, Cantu C, Vuopio J, Jalava J, Kristinsson KG, Gottfredsson M, Corander J, Fittipaldi N, Di Luca MC, Petrelli D, Vitali LA, Raiford A, Jenkins L, Musser JM. 2016. Transcriptome remodeling contributes to epidemic disease caused by the human pathogen Streptococcus pyogenes. mBio 7:e00403–16. doi: 10.1128/mBio.00403-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, Kristinsson KG, Gottfredsson M, Vuopio J, Raisanen K, Caugant DA, Steinbakk M, Low DE, McGeer A, Darenberg J, Henriques-Normark B, Van Beneden CA, Hoffmann S, Musser JM. 2014. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci U S A 111:E1768–E1776. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L, Olsen RJ, Nasser W, Beres SB, Vuopio J, Kristinsson KG, Gottfredsson M, Porter AR, DeLeo FR, Musser JM. 2015. A molecular trigger for intercontinental epidemics of group A Streptococcus. J Clin Invest 125:3545–3559. doi: 10.1172/JCI82478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, Beres SB, Green NM, Lei B, Humbird T, Greaver J, Chang E, Ragasa WP, Montgomery CA, Cartwright J Jr, McGeer A, Low DE, Whitney AR, Cagle PT, Blasdel TL, DeLeo FR, Musser JM. 2010. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A 107:888–893. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fittipaldi N, Beres SB, Olsen RJ, Kapur V, Shea PR, Watkins ME, Cantu CC, Laucirica DR, Jenkins L, Flores AR, Lovgren M, Ardanuy C, Linares J, Low DE, Tyrrell GJ, Musser JM. 2012. Full-genome dissection of an epidemic of severe invasive disease caused by a hypervirulent, recently emerged clone of group A Streptococcus. Am J Pathol 180:1522–1534. doi: 10.1016/j.ajpath.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Schroder J, Schmidt B. 2013. Musket: a multistage k-mer spectrum-based error corrector for Illumina sequence data. Bioinformatics 29:308–315. doi: 10.1093/bioinformatics/bts690. [DOI] [PubMed] [Google Scholar]
- 17.Olsen RJ, Fittipaldi N, Kachroo P, Sanson MA, Long SW, Como-Sabetti KJ, Valson C, Cantu C, Lynfield R, Van Beneden C, Beres SB, Musser JM. 2014. Clinical laboratory response to a mock outbreak of invasive bacterial infections: a preparedness study. J Clin Microbiol 52:4210–4216. doi: 10.1128/JCM.02164-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai CY, Cronan JE. 2003. Beta-ketoacyl-acyl carrier protein synthase III (FabH) is essential for bacterial fatty acid synthesis. J Biol Chem 278:51494–51503. doi: 10.1074/jbc.M308638200. [DOI] [PubMed] [Google Scholar]
- 19.Eraso JM, Kaplan S. 2002. Redox flow as an instrument of gene regulation. Methods Enzymol 348:216–229. doi: 10.1016/S0076-6879(02)48640-5. [DOI] [PubMed] [Google Scholar]
- 20.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu T, Porter AR, Kennedy AD, Kobayashi SD, DeLeo FR. 2014. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun 6:639–649. doi: 10.1159/000360478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. 2002. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci U S A 99:6901–6906. doi: 10.1073/pnas.092148299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner JM, Caro-Aguilar IC, Payne AM, Indrawati L, Fontenot J, Heinrichs JH. 2011. Comparison of rhesus and cynomolgus macaques in a Streptococcus pyogenes infection model for vaccine evaluation. Microb Pathog 50:39–47. doi: 10.1016/j.micpath.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Sumby P, Tart AH, Musser JM. 2008. A non-human primate model of acute group a Streptococcus pharyngitis. Methods Mol Biol 431:255–267. [DOI] [PubMed] [Google Scholar]
- 25.Olsen RJ, Musser JM. 2010. Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol 5:1–31. doi: 10.1146/annurev-pathol-121808-102135. [DOI] [PubMed] [Google Scholar]
- 26.Sanson M, O'Neill BE, Kachroo P, Anderson JR, Flores AR, Valson C, Cantu CC, Makthal N, Karmonik C, Fittipaldi N, Kumaraswami M, Musser JM, Olsen RJ. 2015. A naturally occurring single amino acid replacement in multiple gene regulator of group A Streptococcus significantly increases virulence. Am J Pathol 185:462–471. doi: 10.1016/j.ajpath.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog 2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor FB Jr, Bryant AE, Blick KE, Hack E, Jansen PM, Kosanke SD, Stevens DL. 1999. Staging of the baboon response to group A streptococci administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response patterns. Clin Infect Dis 29:167–177. doi: 10.1086/520147. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson SP, Grove A. 2006. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr Issues Mol Biol 8:51–62. [PubMed] [Google Scholar]
- 30.Sund-Levander M, Forsberg C, Wahren LK. 2002. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci 16:122–128. doi: 10.1046/j.1471-6712.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 31.Eckhardt TH, Skotnicka D, Kok J, Kuipers OP. 2013. Transcriptional regulation of fatty acid biosynthesis in Lactococcus lactis. J Bacteriol 195:1081–1089. doi: 10.1128/JB.02043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faustoferri RC, Hubbard CJ, Santiago B, Buckley AA, Seifert TB, Quivey RG Jr. 2015. Regulation of fatty acid biosynthesis by the global regulator CcpA and the local regulator FabT in Streptococcus mutans. Mol Oral Microbiol 30:128–146. doi: 10.1111/omi.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu YJ, Rock CO. 2006. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol Microbiol 59:551–566. doi: 10.1111/j.1365-2958.2005.04951.x. [DOI] [PubMed] [Google Scholar]
- 34.Loughman JA, Caparon MG. 2006. A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes. EMBO J 25:5414–5422. doi: 10.1038/sj.emboj.7601393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng L, Martino NC, Burne RA. 2012. Two gene clusters coordinate galactose and lactose metabolism in Streptococcus gordonii. Appl Environ Microbiol 78:5597–5605. doi: 10.1128/AEM.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyberg P, Rasmussen M, Bjorck L. 2004. Alpha2-macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J Biol Chem 279:52820–52823. doi: 10.1074/jbc.C400485200. [DOI] [PubMed] [Google Scholar]
- 37.Kil KS, Cunningham MW, Barnett LA. 1994. Cloning and sequence analysis of a gene encoding a 67-kilodalton myosin-cross-reactive antigen of Streptococcus pyogenes reveals its similarity with class II major histocompatibility antigens. Infect Immun 62:2440–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkov A, Liavonchanka A, Kamneva O, Fiedler T, Goebel C, Kreikemeyer B, Feussner I. 2010. Myosin cross-reactive antigen of Streptococcus pyogenes M49 encodes a fatty acid double bond hydratase that plays a role in oleic acid detoxification and bacterial virulence. J Biol Chem 285:10353–10361. doi: 10.1074/jbc.M109.081851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baida GE, Kuzmin NP. 1996. Mechanism of action of hemolysin III from Bacillus cereus. Biochim Biophys Acta 1284:122–124. doi: 10.1016/S0005-2736(96)00168-X. [DOI] [PubMed] [Google Scholar]
- 40.Samant S, Lee H, Ghassemi M, Chen J, Cook JL, Mankin AS, Neyfakh AA. 2008. Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog 4:e37. doi: 10.1371/journal.ppat.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turnbough CL Jr, Switzer RL. 2008. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol Mol Biol Rev 72:266–300. doi: 10.1128/MMBR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malmstrom J, Karlsson C, Nordenfelt P, Ossola R, Weisser H, Quandt A, Hansson K, Aebersold R, Malmstrom L, Bjorck L. 2012. Streptococcus pyogenes in human plasma: adaptive mechanisms analyzed by mass spectrometry-based proteomics. J Biol Chem 287:1415–1425. doi: 10.1074/jbc.M111.267674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilding EI, Brown JR, Bryant AP, Chalker AF, Holmes DJ, Ingraham KA, Iordanescu S, So CY, Rosenberg M, Gwynn MN. 2000. Identification, evolution, and essentiality of the mevalonate pathway for isopentenyl diphosphate biosynthesis in gram-positive cocci. J Bacteriol 182:4319–4327. doi: 10.1128/JB.182.15.4319-4327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Leeuw E, Li C, Zeng P, Li C, Diepeveen-de Buin M, Lu WY, Breukink E, Lu W. 2010. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett 584:1543–1548. doi: 10.1016/j.febslet.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin NI, Breukink E. 2007. Expanding role of lipid II as a target for lantibiotics. Future Microbiol 2:513–525. doi: 10.2217/17460913.2.5.513. [DOI] [PubMed] [Google Scholar]
- 46.Wenzel M, Chiriac AI, Otto A, Zweytick D, May C, Schumacher C, Gust R, Albada HB, Penkova M, Kramer U, Erdmann R, Metzler-Nolte N, Straus SK, Bremer E, Becher D, Brotz-Oesterhelt H, Sahl HG, Bandow JE. 2014. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc Natl Acad Sci U S A 111:E1409–E1418. doi: 10.1073/pnas.1319900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sass V, Schneider T, Wilmes M, Korner C, Tossi A, Novikova N, Shamova O, Sahl HG. 2010. Human beta-defensin 3 inhibits cell wall biosynthesis in staphylococci. Infect Immun 78:2793–2800. doi: 10.1128/IAI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yount NY, Yeaman MR. 2013. Peptide antimicrobials: cell wall as a bacterial target. Ann N Y Acad Sci 1277:127–138. doi: 10.1111/nyas.12005. [DOI] [PubMed] [Google Scholar]
- 49.Rolerson E, Swick A, Newlon L, Palmer C, Pan Y, Keeshan B, Spatafora G. 2006. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. J Bacteriol 188:5033–5044. doi: 10.1128/JB.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanson M, Makthal N, Flores AR, Olsen RJ, Musser JM, Kumaraswami M. 2015. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res 43:418–432. doi: 10.1093/nar/gku1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ. 2015. Manganese homeostasis in group A streptococcus is critical for resistance to oxidative stress and virulence. mBio 6:e00278-15. doi: 10.1128/mBio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaRock CN, Nizet V. 2015. Cationic antimicrobial peptide resistance mechanisms of streptococcal pathogens. Biochim Biophys Acta 1848:3047–3054. doi: 10.1016/j.bbamem.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shelburne SA, Davenport MT, Keith DB, Musser JM. 2008. The role of complex carbohydrate catabolism in the pathogenesis of invasive streptococci. Trends Microbiol 16:318–325. doi: 10.1016/j.tim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YM, Rock CO. 2008. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 55.Port GC, Vega LA, Nylander AB, Caparon MG. 2014. Streptococcus pyogenes polymyxin B-resistant mutants display enhanced ExPortal integrity. J Bacteriol 196:2563–2577. doi: 10.1128/JB.01596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood DN, Weinstein KE, Podbielski A, Kreikemeyer B, Gaughan JP, Valentine S, Buttaro BA. 2009. Generation of metabolically diverse strains of Streptococcus pyogenes during survival in stationary phase. J Bacteriol 191:6242–6252. doi: 10.1128/JB.00440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boles BR, Thoendel M, Singh PK. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc Natl Acad Sci U S A 101:16630–16635. doi: 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brazelton WJ, Mehta MP, Kelley DS, Baross JA. 2011. Physiological differentiation within a single-species biofilm fueled by serpentinization. mBio 2:e00127-11. doi: 10.1128/mBio.00127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenzweig RF, Sharp RR, Treves DS, Adams J. 1994. Microbial evolution in a simple unstructured environment: genetic differentiation in Escherichia coli. Genetics 137:903–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto M, Hoe NP, Liu M, Beres SB, Sylva GL, Brandt CM, Haase G, Musser JM. 2003. Intrahost sequence variation in the streptococcal inhibitor of complement gene in patients with human pharyngitis. J Infect Dis 187:604–612. doi: 10.1086/367993. [DOI] [PubMed] [Google Scholar]
- 61.Long SW, Beres SB, Olsen RJ, Musser JM. 2014. Absence of patient-to-patient intrahospital transmission of Staphylococcus aureus as determined by whole-genome sequencing. mBio 5:e01692-14. doi: 10.1128/mBio.01692-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das S, Lindemann C, Young BC, Muller J, Osterreich B, Ternette N, Winkler AC, Paprotka K, Reinhardt R, Forstner KU, Allen E, Flaxman A, Yamaguchi Y, Rollier CS, van Diemen P, Blattner S, Remmele CW, Selle M, Dittrich M, Muller T, Vogel J, Ohlsen K, Crook DW, Massey R, Wilson DJ, Rudel T, Wyllie DH, Fraunholz MJ. 2016. Natural mutations in a Staphylococcus aureus virulence regulator attenuate cytotoxicity but permit bacteremia and abscess formation. Proc Natl Acad Sci U S A 113:E3101–E3110. doi: 10.1073/pnas.1520255113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. 2011. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A 108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris SR, Feil EJ, Holden MT, Quail MA, Nickerson EK, Chantratita N, Gardete S, Tavares A, Day N, Lindsay JA, Edgeworth JD, de Lencastre H, Parkhill J, Peacock SJ, Bentley SD. 2010. Evolution of MRSA during hospital transmission and intercontinental spread. Science 327:469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Betancor L, Yim L, Fookes M, Martinez A, Thomson NR, Ivens A, Peters S, Bryant C, Algorta G, Kariuki S, Schelotto F, Maskell D, Dougan G, Chabalgoity JA. 2009. Genomic and phenotypic variation in epidemic-spanning Salmonella enterica serovar Enteritidis isolates. BMC Microbiol 9:237. doi: 10.1186/1471-2180-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diggle MA, Clarke SC. 2005. Increased genetic diversity of Neisseria meningitidis isolates after the introduction of meningococcal serogroup C polysaccharide conjugate vaccines. J Clin Microbiol 43:4649–4653. doi: 10.1128/JCM.43.9.4649-4653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko KS, Miyamoto H, Lee HK, Park MY, Fukuda K, Park BJ, Kook YH. 2006. Genetic diversity of Legionella pneumophila inferred from rpoB and dotA sequences. Clin Microbiol Infect 12:254–261. doi: 10.1111/j.1469-0691.2005.01338.x. [DOI] [PubMed] [Google Scholar]
- 68.Mayfield JA, Liang Z, Agrahari G, Lee SW, Donahue DL, Ploplis VA, Castellino FJ. 2014. Mutations in the control of virulence sensor gene from Streptococcus pyogenes after infection in mice lead to clonal bacterial variants with altered gene regulatory activity and virulence. PLoS One 9:e100698. doi: 10.1371/journal.pone.0100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L, Jelsbak L, Marvig RL, Damkiaer S, Workman CT, Rau MH, Hansen SK, Folkesson A, Johansen HK, Ciofu O, Hoiby N, Sommer MO, Molin S. 2011. Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci U S A 108:7481–7486. doi: 10.1073/pnas.1018249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.