Abstract
Infection with parasitic nematodes, especially gastrointestinal geohelminths, affects hundreds of millions of people worldwide and thus poses a major risk to global health. The host mechanism of defense against enteric nematode infection remains to be fully understood, but it involves a polarized type 2 immunity leading to alterations in intestinal function that facilitate worm expulsion. We investigated the role of interleukin-25 (IL-25) in host protection against Heligmosomoides polygyrus bakeri infection in mice. Our results showed that Il25 and its receptor subunit, Il17rb, were upregulated during a primary infection and a secondary challenge infection with H. polygyrus bakeri. Genetic deletion of IL-25 (IL-25−/−) led to an attenuated type 2 cytokine response and increased worm fecundity in mice with a primary H. polygyrus bakeri infection. In addition, the full spectrum of the host memory response against a secondary infection with H. polygyrus bakeri was severely impaired in IL-25−/− mice, including delayed type 2 cytokine responses, an attenuated functional response of the intestinal smooth muscle and epithelium, diminished intestinal smooth muscle hypertrophy/hyperplasia, and impaired worm expulsion. Furthermore, exogenous administration of IL-25 restored the host protective memory response against H. polygyrus bakeri infection in IL-25−/− mice. These data demonstrate that IL-25 is critical for host protective immunity against H. polygyrus bakeri infection, highlighting its potential application as a therapeutic agent against parasitic nematode infection worldwide.
INTRODUCTION
Although studies using mouse models have advanced our understanding of the molecular and cellular mechanisms underlying host protection against nematode infection, many of the details remain to be fully elucidated. Infection with gastrointestinal nematode parasites induces a polarized Th2 immune response featuring elevated levels of production of interleukin-4 (IL-4), IL-5, and IL-13. IL-4 and IL-13 activate STAT6 signaling pathways, leading to characteristic alterations in intestinal function that facilitate worm expulsion. IL-25, also called IL-17E, is a cytokine member of the IL-17 family that includes IL-17A through IL-17F. Unlike other members of the IL-17 family that are involved in various inflammatory pathologies, IL-25 possesses immune-modulating properties that inhibit Th1/Th17-associated inflammation.
It has been observed that intestinal epithelium-derived IL-25 plays a pivotal role in the initiation of the host protective immune cascade against nematode infection. In particular, intestinal epithelial tuft cells produce IL-25 (1, 2) in response to early-stage worm infection, leading to the expansion and activation of type 2 innate lymphoid cells (ILC2), a recently identified noncytotoxic innate lymphoid cell (ILC) family member that has a classic lymphoid cell morphology but that lacks the expression of cell surface markers of other known immune lymphocytes (3, 4). The activated ILC2 then release Th2-associated cytokines IL-5 and IL-13. It is the IL-13 activation of STAT6 pathways that coordinates the upregulation of downstream effector molecules, such as RELMβ and MUC5AC, as well as stereotypic changes in intestinal function, including smooth muscle hypercontractility, epithelial cell hyposecretion, and increased mucosal permeability.
Previous studies have demonstrated a critical role for IL-25 in the host defense against gastrointestinal nematodes, like Nippostrongylus brasiliensis (4, 5), Trichinella spiralis (6), and Trichuris muris (7). Unlike N. brasiliensis, which colonizes the small intestine via the skin-lung route, leading to an acute and transient infection, Heligmosomoides polygyrus bakeri causes a strictly enteral infection, with larvae first developing in the submucosa of the duodenum and then with adult worms being released into the intestinal lumen at about day 8 after inoculation. Importantly, mice develop chronic infection after primary inoculation with H. polygyrus bakeri but are protected from a secondary challenge infection due to a potent Th2 memory response.
Whether IL-25 is involved in host protective immunity against H. polygyrus bakeri infection has not been investigated. Therefore, the present study was designed to (i) determine the time-dependent alterations in the expression of IL-25 and its receptor subunits in response to H. polygyrus bakeri infection, (ii) investigate the role of IL-25 in host protective type 2 immunity against primary infection as well as secondary challenge infection with H. polygyrus bakeri, and (iii) elucidate the contribution of IL-25 to the characteristic changes in intestinal smooth muscle and mucosal function induced by a secondary challenge infection with H. polygyrus bakeri. This study demonstrates that IL-25 and its receptor subunit, the IL-17B receptor (IL-17RB), are upregulated during the early stage of H. polygyrus bakeri infection and that endogenous IL-25 plays a critical role in host protective immunity against a secondary H. polygyrus bakeri infection.
MATERIALS AND METHODS
Mice.
Wild-type (WT) C57BL/6 mice were purchased from Charles River Laboratories (Frederick, MD) and bred in the USDA, Beltsville Agricultural Research Center, animal facility. Mice deficient in IL-25 (IL-25−/−) were generated by Regeneron Pharmaceuticals (Tarrytown, NY) and were backcrossed to mice of the C57BL/6 mouse background for 10 generations and also bred in the USDA, Beltsville Agricultural Research Center, animal facility. All studies were conducted with institutional approval from both the University of Maryland, Baltimore, and the USDA, Beltsville Area Agricultural Research Service, Institutional Animal Care and Use Committees, in accordance with the principles set forth in the Guide for the Care and Use of Laboratory Animals (8).
Enteric nematode infection and worm expulsion.
Infective, ensheathed, third-stage larvae (L3) of H. polygyrus bakeri were propagated and stored at 4°C as previously described (9). For primary infection, groups of mice were inoculated orally with 200 L3 using a ball-tipped feeding needle. For secondary challenge infection, mice were cured with the anthelmintic drug pyrantel tartrate 2 weeks after the primary inoculation and were reinoculated orally with 200 L3 4 or more weeks later. Studies with appropriate age-matched controls were performed for each infection. Determination of adult worm numbers and egg production in feces was performed as described previously (9). Adult worms were detected quantitatively by scanning the intestinal surface with a dissecting scope.
Administration of IL-25.
Mice were injected intraperitoneally with 1 μg of recombinant IL-25 (R&D Systems, Minneapolis, MN) in 100 μl of phosphate-buffered saline (PBS) containing 35 μg bovine serum albumin (BSA) as a carrier protein every other day starting at day 5 postinoculation (p.i.) with H. polygyrus bakeri L3. Control mice were given injections of 35 μg BSA in PBS. The amount of cytokine administered was based on the results of a previous study showing that this dose of IL-25 induces a prominent Th2 immune response (5).
In vitro smooth muscle contractility in organ baths and smooth muscle thickness.
In vitro smooth muscle contractility was measured as described previously (10). Frequency- or concentration-dependent smooth muscle responses to electric field stimulation (EFS; 1 to 20 Hz, 100 V) or acetylcholine (10 nM to 0.1 mM) were determined. Tension was expressed as the force per cross-sectional area (11). Segments of jejunum were fixed in 4% paraformaldehyde for 4 h. Sections (4 μm) of jejunum tissue were cut from paraffin-embedded blocks and stained with hematoxylin and eosin (H&E). The smooth muscle thickness of H&E-stained sections of the jejunum was determined for each treatment group.
In vitro epithelial cell ion transport in Ussing chambers.
Muscle-free segments of small intestine were mounted in Ussing chambers as described previously (12). After a 15-min period, concentration-dependent changes in the short-circuit current (Isc) in response to the cumulative addition of acetylcholine (10 nM to 1 mM) to the serosal side were determined. Responses from all acetylcholine-exposed tissue segments from an individual animal were averaged to yield a mean response per animal.
Microsnap well assay for mucosal TEER.
The modified microsnap well system used in the present study was a miniaturized version of the standard Ussing chamber that has been engineered to measure the transepithelial electrical resistance (TEER) of intestinal fragments exposed to various stimuli (13). A decrease in TEER reflects increased tissue permeability. Briefly, segments of mouse intestine stripped of both muscle and serosal layers were placed in the microsnap well system. Two hundred fifty microliters of Dulbecco modified Eagle medium containing 4.5 g/liter glucose, 4 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and minimal essential medium with 1 mM nonessential amino acids was added to the mucosal side. Three milliliters of the same medium was added to the serosal side. The system was incubated at 37°C with 5% CO2 in air for 30 min to stabilize the pH, and the baseline TEER was measured.
RNA extraction, cDNA synthesis, and real-time qPCR.
Total RNA was extracted from intestine whole tissue as described previously (14). RNA samples (2 μg) were reverse transcribed to cDNA using a first-strand cDNA synthase kit (MBI Fermentas, Hanover, MD) with a random hexamer primer. Real-time quantitative PCR (qPCR) was performed on an iCycler detection system (Bio-Rad, CA). PCR was performed in a 25-μl volume using SYBR green Supermix (Bio-Rad, Hercules, CA). Amplification conditions were 95°C for 3 min and 50 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 20 s. The fold changes in the levels of expression of mRNA for targeted genes were relative to the levels of expression for the respective vehicle-treated groups of mice after normalization to the level of 18S rRNA expression. Primer sequences (listed in Table 1) were designed by using Beacon Designer (version 7.0) software (Premier Biosoft International, Palo Alto, CA) and synthesized by Sigma.
TABLE 1.
Primer sequences for real-time qPCR
| Gene | Orientation | Primer sequence (5′ to 3′) |
|---|---|---|
| Il25 | Forward | CAGCAAAGAGCAAGAACC |
| Reverse | CCCTGTCCAACTCATAGC | |
| Il17rb | Forward | ACCGTCTGTCGCTTCACTG |
| Reverse | C CCACTTTATCTGCCGCTTGC | |
| Il17ra | Forward | ACTGTGGAGACCTTGGAC |
| Reverse | CTTGCTTAGAGTGAATGTGAC | |
| Il4 | Forward | AAAATCACTTGAGAGAGATCAT |
| Reverse | GTTTGGCACATCCATCTC | |
| Il5 | Forward | GACAAGCAATGAGACGATGAGG |
| Reverse | CCCACGGACAGTTTGATTCTTC | |
| Il13 | Forward | GACCAGACTCCCCTGTGCAA |
| Reverse | TGGGTCCTGTAGATGGCATTG | |
| Arg1 | Forward | CTGGCAGTTGGAAGCATCTCT |
| Reverse | GTGAGCATCCACCCAAATGAC | |
| Chil3 | Forward | ATCTATGCCTTTGCTGGAATGC |
| Reverse | TGAATGAATATCTGACGGTTCTGAG | |
| Retnla | Forward | CCTCCACTGTAACGAAGACTCTC |
| Reverse | GCAAAGCCACAAGCACACC | |
| Adgre1 | Forward | AAAGACTGGATTCTGGGAAGTTTGG |
| Reverse | CGAGAGTGTTGTGGCAGGTTG | |
| Retnlb | Forward | TCTCCCTTTTCCCACTGATAG |
| Reverse | TCTTAGGCTCTTGACGACTG | |
| Muc5ac | Forward | AGGACGACTAATTTGGATAA |
| Reverse | AACTGTACTGCTGTATGG |
Data analysis.
Agonist responses were fitted to sigmoid curves (GraphPad Software, San Diego, CA). Statistical analysis was performed using one-way analysis of variance followed by the Neumann-Keuls test to compare the differences among three or more treatment groups or the Student t test to compare the differences between two groups. The data are presented as the mean ± standard error of the mean and are representative of those from at least two independent experiments with five to eight mice per group. P values of <0.05 were considered significant.
RESULTS
Upregulation of Il25 and Il17rb in the intestine in response to infection with H. polygyrus bakeri.
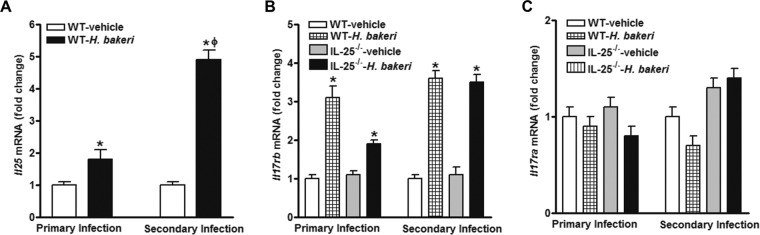
Upregulation of IL-25 and IL-17RB in the small intestines of mice infected with N. brasiliensis was reported previously (5). Heligmosomoides polygyrus bakeri is also a gastrointestinal nematode parasite, but unlike N. brasiliensis, it develops into a chronic infection lasting for weeks to months, depending on the mouse strain. However, clearance of the primary infection using an anthelmintic drug followed by a secondary challenge infection induces a potent Th2 memory response that is host protective (15). We examined the effects of H. polygyrus bakeri infection on the expression of Il25 and its receptor. Both primary infection and secondary challenge infection of mice upregulated the expression of Il25 in the small intestine, although a more potent effect was observed in mice receiving the secondary infection (Fig. 1A). The IL-25 receptor is composed of the IL-17RA and IL-17RB subunits, with the IL-17RA subunit being shared by other members of the IL-17 cytokine family. Both primary infection and secondary infection induced comparable levels of upregulation of Il17rb in the intestine of WT as well as IL-25−/− mice (Fig. 1B). The infection did not, however, alter the expression of Il17ra (Fig. 1C).
FIG 1.
Upregulation of Il25 and its receptor in the intestines of mice infected with H. polygyrus bakeri. Mice received a primary infection or a secondary challenge infection with H. polygyrus bakeri and were studied at day 14 postinfection. qPCR was performed to measure the levels of mRNA expression in the small intestine. The fold changes were relative to the level of expression for the individual vehicle groups after normalization to the level of 18S rRNA expression. *, P < 0.05 versus the respective vehicle group; ϕ, P < 0.05 versus the respective primary infection group (n ≥ 5 for each group).
Impaired type 2 cytokine response to primary infection with H. polygyrus bakeri in mice deficient in IL-25.
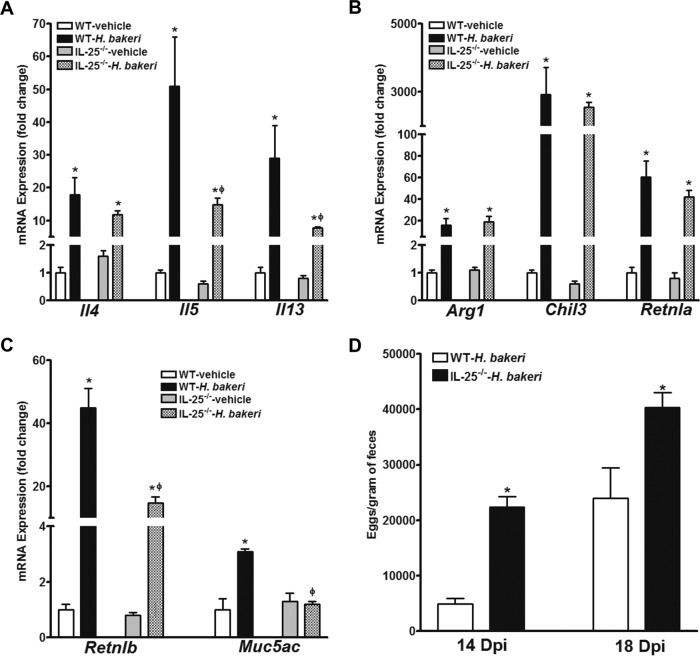
The host defense against nematode infection features polarized type 2 immunity. As expected, a primary H. polygyrus bakeri infection of mice increased the intestinal expression of type 2 cytokines when expression was examined at day 14 postinoculation (Fig. 2A). Notably, the upregulation of Il5 or Il13, but not that of Il4, was significantly less in IL-25−/− mice than WT mice (Fig. 2A). The infection also upregulated the expression of alternatively activated macrophage (M2) markers (Arg1, Chil3, and Retnla) as well as Retnlb and Muc5ac, two major effector molecules critical for the host defense against nematode infection in WT mice (16, 17). The upregulation of M2 markers was comparable in WT and IL-25−/− mice (Fig. 2B); however, the upregulation of Retnlb and Muc5ac was significantly less in IL-25−/− mice (Fig. 2C). Finally, IL-25−/− mice did not have an exaggerated Th1 or Th17 cytokine response since no significant differences in the levels of expression of Tnf, Ifng, Il17a, or nitric oxide synthase-2 were detected between WT and IL-25−/− mice before or after the infection (data not shown). Worm fecundity (measured by determination of the number of eggs per gram of feces) was significantly higher during primary infection of IL-25−/− mice than primary infection of WT mice at day 14 as well as day 18 postinoculation (Fig. 2D). A primary infection with H. polygyrus bakeri was chronic, with many adult worms being observed microscopically in both WT and IL-25−/− mice at 18 days after inoculation.
FIG 2.
Impaired type 2 cytokine response to primary infection with H. polygyrus bakeri in mice deficient in IL-25. Mice received a primary infection with H. polygyrus bakeri. Segments of jejunum were collected at day 14 postinfection and analyzed by qPCR for the levels of expression of mRNA for type 2 cytokines (A), molecular markers for alternatively activated macrophages (B), and host defense effector molecules (C). The fold changes in levels of expression were relative to the levels of expression for the respective WT-vehicle groups after normalization to the level of 18S rRNA expression. *, P < 0.05 versus the respective vehicle group; ϕ, P < 0.05 versus the respective WT group. (D) The numbers of worm eggs were determined at 14 and 18 days postinfection (Dpi). *, P < 0.05 versus WT mice infected with H. polygyrus bakeri (WT-H. bakeri) (n ≥ 5 for each group).
Defective memory response against a secondary challenge infection with H. polygyrus bakeri in IL-25−/− mice.
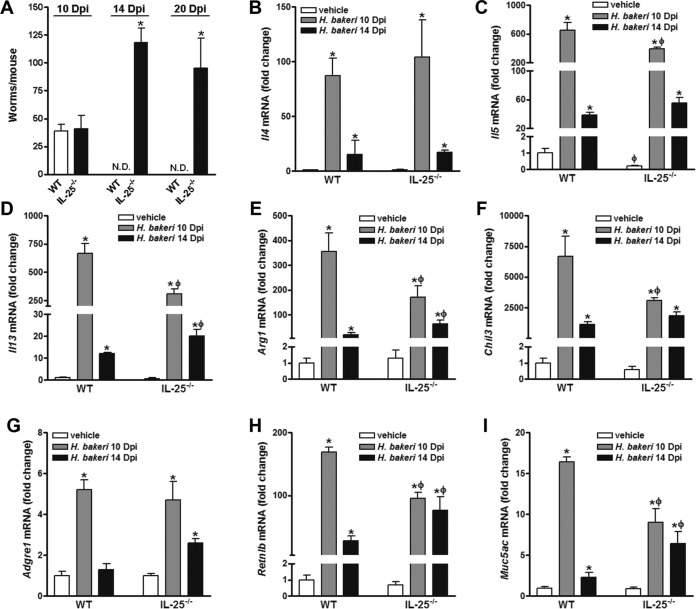
To further investigate whether IL-25 is required for the host memory response against infection with H. polygyrus bakeri, mice with primary infection were cured with an anthelminthic drug and rechallenged after at least a 4-week rest to allow development of the secondary response. Mice were euthanized at days 10, 14, and 20 postinoculation (p.i.) to evaluate worm expulsion as well as molecular and functional alterations in the intestine. As shown in Fig. 3A, both WT and IL-25−/− mice harbored similar numbers of adult worms at day 10 p.i., indicating equivalent levels of infection between the two mouse strains. In contrast, WT mice cleared the adult worms by day 14 p.i., whereas IL-25−/− mice still harbored a significant number of worms in the gut lumen even at day 20 p.i. (Fig. 3A).
FIG 3.
Impaired host defense against a secondary challenge infection with H. polygyrus bakeri in mice deficient in IL-25. Mice were infected with H. polygyrus bakeri, cured with an anthelmintic drug, and reinfected with H. polygyrus bakeri infective larvae. (A) Numbers of adult worms in the intestines of mice euthanized at 10, 14, and 20 days postinfection (Dpi). *, P < 0.05 versus the WT group. N.D., not detected. (B to I) Segments of jejunum were collected at 10 and 14 days postinfection and analyzed by qPCR for the levels of expression of mRNA for the type 2 cytokines Il4 (B), Il5 (C), Il13 (D), alternatively activated macrophage markers Arg1 (E) and Chil3 (F), the general macrophage marker Adgre1 (G), and host defense effector molecules Retnlb (H) and Muc5ac (I). The fold changes in levels of expression were relative to the levels of expression for the respective WT-vehicle groups after normalization to the level of 18S rRNA expression. *, P < 0.05 versus the respective vehicle group; ϕ, P < 0.05 versus the respective WT group (n ≥ 5 for each group).
Type 2-associated cytokines/immune mediators play a prominent role in the protective memory response against nematode infection. We investigated whether impaired host protection was associated with defective intestinal cytokine gene expression at day 10 p.i., when the immune response in WT mice peaked, and at day 14 p.i., when worms were cleared from WT mice (18). As expected, a secondary challenge infection with H. polygyrus bakeri in WT mice induced a robust type 2 immunity characterized by significantly increased expression of Il4, Il5, and Il13 on days 10 and 14 p.i., with higher levels being observed at day 10 p.i. (Fig. 3B to D). In comparison, at day 10 p.i. infection-induced upregulation of type 2 cytokines (Il5 and Il13) in IL-25−/− mice was significantly less than that in WT mice, with the exception of Il4. By day 14 p.i., when cytokine gene expression levels in the infected WT mice declined, those in the infected IL-25−/− mice, particularly the levels of Il13 expression, turned higher, likely due to the continuous presence of worms in the intestine (Fig. 3B to D). Following a similar pattern, upregulation of the M2 markers Arg1 and Chil3 was less in IL-25−/− mice than in WT mice at day 10 p.i. (Fig. 3E and F), while the expression levels of Adgre1 (F4/80), a general macrophage marker, were comparable between the two groups of infected mice at day 10 p.i. (Fig. 3G). Retnlb and Muc5ac were significantly induced by the infection in WT mice, with their levels of expression peaking at day 10 p.i. and declining at day 14 p.i. (Fig. 3H and I). In IL-25−/− mice, the infection-induced upregulation of Retnlb and Muc5ac was less pronounced at day 10 but was more pronounced at day 14 p.i. (Fig. 3H and I), which followed the pattern of Il13 expression (Fig. 3D).
IL-25 deficiency impaired the functional responses of intestinal smooth muscle and epithelium to H. polygyrus bakeri infection.
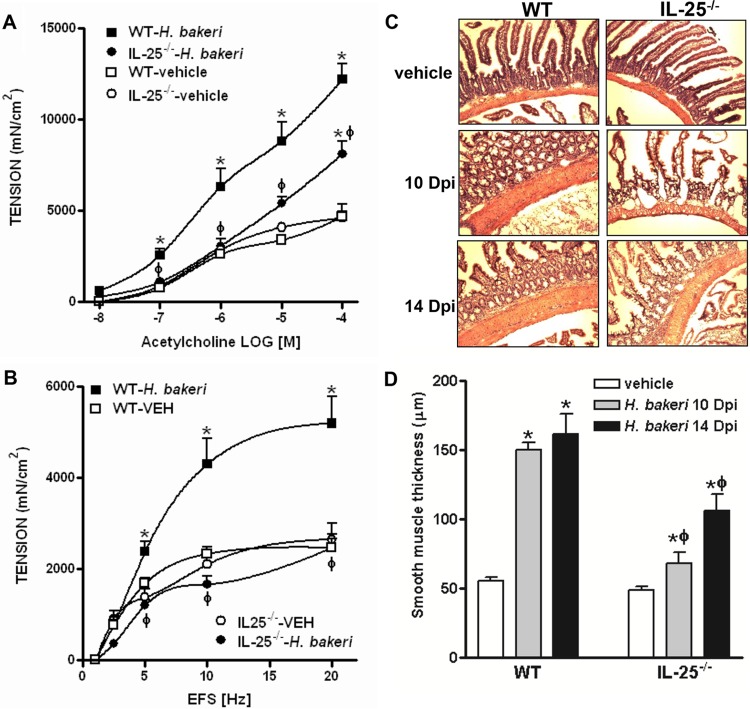
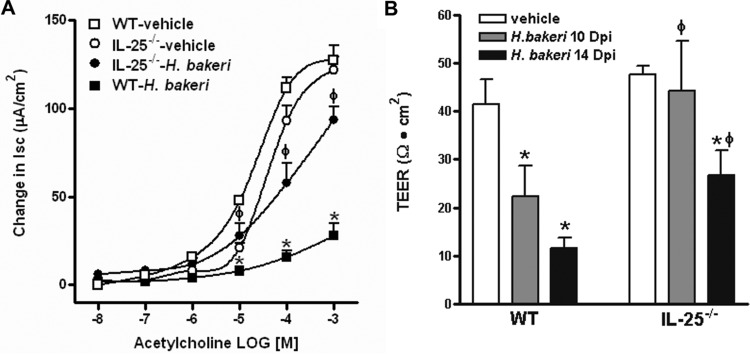
Enteric nematode infections induce characteristic alterations in gut function that peak at day 14 of a primary infection with H. polygyrus bakeri (18, 19). We next evaluated gut function in mice receiving a secondary challenge infection with H. polygyrus bakeri. Indeed, the infected WT mice had an intestinal smooth muscle hypercontractile response to acetylcholine as well as electric field stimulation (EFS) (Fig. 4A and B) consistent with that shown previously (10, 20–22). However, this infection-induced hypercontractility was either significantly attenuated (acetylcholine) or absent (EFS) in IL-25−/− mice (Fig. 4A and B). In addition, the infection drastically increased the thickness of the intestinal smooth muscle layer in WT mice at both day 10 and day 14 p.i., and infection-induced smooth muscle hypertrophy/hyperplasia was much less evident in IL-25−/− mice, and only marginal effects were observed at day 10 p.i. (Fig. 4C and D).
FIG 4.
Attenuated intestinal smooth muscle hypercontractile responses to H. polygyrus bakeri infection in mice deficient in IL-25. Mice were infected with H. polygyrus bakeri, cured with an anthelmintic drug, and reinfected with H. polygyrus bakeri infective larvae. Mice were euthanized at day 14 postinfection, and the intestinal strips were suspended longitudinally in organ baths for in vitro contractility studies in response to acetylcholine (10 nM to 0.1 mM) (A) and EFS (1 to 20 Hz, 100 V) (B). VEH, vehicle. (C) Representative H&E-stained intestinal sections from mice euthanized at 10 or 14 days postinfection (Dpi). Magnification, ×100. (D) The thickness of the smooth muscle layer was measured by microscopic examination of the H&E-stained intestinal sections. *, P < 0.05 versus the respective vehicle group; ϕ, P < 0.05 versus the respective WT group (n ≥ 5 for each group).
A deficiency in IL-25 had a significant impact on H. polygyrus bakeri infection-induced changes in mucosal epithelial function. As shown in Fig. 5A, the infection-induced stereotypic reductions in epithelial secretion in response to acetylcholine (a decrease in Isc) was significantly less in IL-25−/− mice than in WT mice when the mice were examined at day 14 p.i. At day 10 p.i., mucosal permeability was increased (there was a decrease in TEER) in infected WT mice but not in IL-25−/− mice (Fig. 5B). At day 14 p.i., the permeability was decreased in both strains of mice, but the decrease was significantly less in IL-25−/− mice than in WT mice (Fig. 5B).
FIG 5.
Attenuated intestinal epithelial hyposecretion and delayed mucosal permeability increase in mice deficient in IL-25 in response to infection with H. polygyrus bakeri. Mice were infected with H. polygyrus bakeri, cured with an anthelmintic drug, reinfected with H. polygyrus bakeri infective larvae, and euthanized at day 10 or 14 postinfection (Dpi). Muscle-free mucosa was mounted in Ussing chambers for the epithelial secretory response to acetylcholine (A) or in a microsnap well system for the measurement of TEER (B). *, P < 0.05 versus the respective vehicle group; ϕ, P < 0.05 versus the respective WT group (n ≥ 5 for each group).
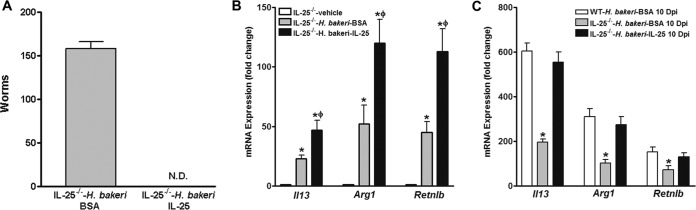
Exogenous administration of IL-25 restores host protection against H. polygyrus bakeri infection in IL-25−/− mice.
Recombinant IL-25 was administered to infected IL-25−/− mice every other day starting at day 5 p.i. using a dose known to promote type 2 immunity on the basis of the findings of our previous studies (5). IL-25−/− mice receiving IL-25 cleared the worm by day 14 p.i., similar to the findings for WT mice, while mice receiving BSA still harbored >150 worms in the lumen (Fig. 6A). To determine whether exogenous IL-25 rescued the immune response defect in IL-25−/− mice, we selectively analyzed the gene expression of three key molecules (IL-13, arginase 1, and RELMβ) as representatives of host protective immunity. Indeed, exogenous IL-25-induced worm expulsion was associated with significantly increased expression of Il13, the M2 marker Arg1, as well as effector molecule Retnlb in intestines that were collected at day 14 p.i. (Fig. 6B). Furthermore, exogenous administration of IL-25 led to a nearly full restoration of the host type 2 immune response of IL-25−/− mice to the rechallenge infection with H. polygyrus bakeri, as similar levels of intestinal expression of Il13, Arg1, and Retnlb were detected between infected WT mice and IL-25−/− mice that received exogenous IL-25 when they were assayed at day 10 postinfection (Fig. 6C), a time point when the host type 2 immune response peaked (Fig. 3).
FIG 6.
Exogenous IL-25 restores the protective memory response against H. polygyrus bakeri infection in mice deficient in IL-25. WT or IL-25−/− mice were infected with H. polygyrus bakeri, cured with an anthelmintic drug, and reinfected with H. polygyrus bakeri infective larvae. IL-25 or BSA, as a control, was injected into mice every other day starting at 5 days post-secondary infection, and the mice were euthanized at 10 days post-secondary infection (10 Dpi) (C) or 14 days post-secondary infection (A, B). (A) Numbers of adult worms in the intestines of mice at 14 days postinfection. Segments of jejunum collected at 10 days postinfection (C) and 14 days postinfection (B) were analyzed by qPCR for expression of mRNA for Il13, Arg1, and Retnlb. The fold changes in the levels of expression were relative to the levels of expression for the respective WT-vehicle groups after normalization to the levels of 18S rRNA expression. *, P < 0.05 versus the respective vehicle group (B) or WT mice infected with H. polygyrus bakeri and treated with BSA (WT-H. bakeri-BSA) at 10 days postinfection (C); ϕ, P < 0.05 versus the respective BSA group (n ≥ 5 for each group).
DISCUSSION
The present study demonstrated that IL-25, a primarily epithelium-derived cytokine, contributes to protective immunity against infection with H. polygyrus bakeri during a primary infection and following a secondary challenge infection. Heligmosomoides polygyrus bakeri has a strictly enteral life cycle that establishes a chronic infection and activates multiple lymphoid and myeloid cell interactions (23). Thus, H. polygyrus bakeri infection of mice mimics many aspects of human hookworm infection and thus is commonly used for studying host protective immunity (24, 25) as well as helminth-associated immune-modulating mechanisms (26).
Although primary infections in mice are chronic, they induce no apparent morbidity; however, a secondary challenge infection activates a potent host memory response that leads to worm expulsion within 2 weeks or less. The immune response to H. polygyrus bakeri features a robust type 2 immunity characterized by increased expression of IL-4, IL-5, IL-9, and IL-13 (27–30). Although epithelium-derived cytokines/mediators, particularly IL-25, play a pivotal role in initiating type 2 immunity in general, the role of IL-25 in the host defense against H. polygyrus bakeri was not known. Previous work showed that IL-25 was indispensable for host protective immunity against N. brasiliensis, T. muris, and T. spiralis in mice. In particular, mice deficient in IL-25 had impaired cytokine responses to infection with N. brasiliensis and were unable to efficiently expel adult worms from the intestine (4, 5). Injection of IL-25 into genetically susceptible mice promoted a type 2 cytokine response to T. muris, whereas IL-25-deficient mice on a genetically resistant background failed to eliminate the infection (7). Angkasekwinai et al. (6) showed that T. spiralis-infected mice treated with IL-25 exhibited a lower adult worm burden and fewer muscle larvae, which were associated with an antigen-specific IL-9 response, while mice treated with neutralizing anti-IL-25 antibody failed to effectively expel T. spiralis adults.
Extending our previous findings from studies with mice infected with N. brasiliensis, the present study showed that both a primary response and a secondary memory immune response to H. polygyrus bakeri included the upregulation of Il25 similar to that induced by other parasitic nematodes, with a higher response being observed in the secondary challenge infection, consistent with a more potent type 2 memory response. In mice with a primary infection with H. polygyrus bakeri, IL-25 deficiency had a moderate effect on the upregulation of type 2 cytokines or effector molecules and did not influence the gene expression of characteristic M2 markers. The moderate effect of IL-25 deficiency on some but not all key immune mediators may reflect the fact that primary infection of mice with H. polygyrus bakeri is chronic and the host immune response elicited is not very potent. Nonetheless, the host protective response was impaired because adult worm egg production, an indicator of worm fecundity and vigor, was increased in IL-25−/− mice. The presence of robust adult worms could also explain the modest gene expression of some immune mediators that are not strictly IL-25 dependent. The impact of IL-25 deficiency on the host memory response to a secondary challenge infection with H. polygyrus bakeri was more profound, as the expression of type 2 cytokines, effector molecules for host defense, and molecular markers of M2 development were all negatively affected.
Of note, our current results indicate that the upregulation of Il4 induced by either primary or secondary infection of H. polygyrus bakeri was not affected by IL-25 deficiency. IL-4 is an important cytokine with multiple immunoregulatory functions, including differentiation of Th2 cells. The cellular source of IL-4 following nematode infection includes T cells, basophils, and eosinophils (31). Exogenous IL-4 can cure established H. polygyrus bakeri infection (32), and anti-IL-4 treatment only partially blocked the protective immunity against secondary H. polygyrus bakeri infection in mice (33). However, a definite role of IL-4 in the protective response to H. polygyrus bakeri remains to be fully established. A very recent study reported that ILC2 are the major source of IL-4 and that IL-4-producing ILC2 are required for the differentiation of Th2 cells following primary H. polygyrus bakeri infection (34). That study further reported that IL-25 is incapable of inducing IL-4 secretion from ILC2, a finding which is consistent with data from our current study that no defect in Il4 expression was detected in IL-25−/− mice. While it was not investigated in the current study, it is possible that IL-25 deficiency did not affect IL-4 production from ILC2 stimulated by mediators, such as leukotriene D4 (34). Whether defective IL-25 signaling affects Th2 development during H. polygyrus bakeri infection remains to be determined. However, we have recently shown that resistance to Nippostrongylus brasiliensis and other parasitic nematode species is dependent on the relative abundance of ILC2 and Th2 cells producing IL-13 (35), which may suggest a critical role for the relative abundance of these cells in the protective response to a secondary infection with H. polygyrus bakeri. These IL-4- and IL-13-producing cells may not expand optimally during infection in the absence of IL-25. Further studies will continue to explore the redundancy of the response to infection.
IL-25 in the intestine is mainly produced by epithelial cells, more specifically, by tuft cells in the epithelium, which then activate ILC2 to release the type 2 cytokines IL-5 and IL-13 (1, 2). The receptor for IL-25 consists of IL-17RB, a 56-kDa single transmembrane protein that binds IL-25, and IL-17RA, a ubiquitously expressed receptor subunit also shared by IL-17A, IL-17C, and IL-17F (36). Infection of mice with H. polygyrus bakeri increased the level of the Il17rb transcript in the intestine independently of IL-25. Although both transcriptional upregulation and expansion of the IL-25-responsive cells, particularly ILC2, could contribute to that increase, the fact that the infection did not alter Il17ra expression suggested that transcriptional upregulation is likely the case. The mechanism underlying the upregulation of Il17rb could be similar to that utilized by N. brasiliensis, which involves IL-4/IL-13 and STAT6 (5). The biological significance of the divergent effect of H. polygyrus bakeri on the two receptor subunits of IL-25 is not understood but may reflect the ability of the host to maintain a potent type 2 immunity while avoiding an exaggerated Th17 response that would be detrimental for defending against the parasite.
Enteric nematode infection induces characteristic changes in intestinal function and morphology featuring smooth muscle hypercontractility, smooth muscle hypotrophy/hyperplasia, epithelial hyposecretion, as well as increases in mucosal permeability (22, 37, 38). The gut functional responses depend on host type 2 immunity, which is induced in particular by IL-13, which binds to the type 2 IL-4 receptor consisting of IL-4Rα and IL-13Rα1 and activates STAT6 signaling pathways. Changes in gut function facilitate worm expulsion, thereby constituting an integral part of the host defense against nematode infection. During enteric nematode infection, various types of innate and adaptive immune cells are recruited to the site of infection. Among those first responders, macrophages accumulate in the mucosa as well as in the smooth muscle of the intestine. More importantly, the type 2 cytokines IL-4 and IL-13 induce alternative activation of macrophages into the M2 phenotype that is indispensable to the morphological and functional alterations of intestinal smooth muscle and epithelial cells (22, 39). The absence of IL-25 resulted in a delayed type 2 immune response leading to defective M2 development. Consequently, the infection-induced alternations in intestinal smooth muscle function, epithelial secretion, as well as mucosal permeability were attenuated in mice deficient in IL-25, which in turn led to impaired worm expulsion.
Of interest was the ability of exogenous IL-25 to restore the host defense against H. polygyrus bakeri. Indeed, even when IL-25 was given only during the secondary challenge infection, a full spectrum of features of the host protective response resumed, including worm expulsion, type 2 cytokine responses, M2 development, and the expression of host defense effector molecules. Our current study did not examine how exogenous IL-25 affected the intestinal function of the mice. However, it is well established that host protection against H. polygyrus bakeri infection is accompanied by characteristic changes in intestinal smooth muscle and epithelial function that contribute to worm expulsion (10, 12, 38). Our previous study also showed that exogenous IL-25 induced similar changes in WT mice (5). Thus, it is conceivable that the characteristic alterations in intestinal function also occurred in the mice that received exogenous IL-25 in the current study.
In conclusion, infection with a strictly enteral parasite, H. polygyrus bakeri, upregulated the expression of Il25 and Il17rb in the intestine. A genetic deficiency in IL-25 negatively affected the host defense against both a primary infection and a secondary challenge infection with H. polygyrus bakeri. In concert with previous findings, IL-25 appeared to be critical to the host defense against parasitic nematodes through the regulation of type 2 cytokines that activate protective mechanisms, highlighting the potential of IL-25 as a therapeutic agent for the control of parasitic nematode infections worldwide.
ACKNOWLEDGMENTS
We thank Tomas A. Wynn and Thirumalai R. Ramalingam, NIAID, NIH, for providing the IL-25-deficient mice.
This work was supported by NIH grants R01-DK083418 (to A.Z.), R01-AI/DK49316 (to T.S.-D.), USDA CRIS project no. 8040-51000-058 (to J.F.U.), NSFC grants 81370945 and 81570764 (to Z.Y.), and Guangdong NSF grant 2015A030313029 (to Z.Y.).
We have no conflicts of interest to disclose.
This article was prepared while Aiping Zhao was employed at the University of Maryland School of Medicine. The opinions expressed in this article are the authors' own and do not reflect the view of the National Institutes of Health, the U.S. Department of Health and Human Services, or the United States government. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Moltke J, Ji M, Liang HE, Locksley RM. 2016. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A, Artis D. 2013. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med 210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. 2006. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao A, Urban JF, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. 2010. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol 185:6921–6929. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angkasekwinai P, Srimanote P, Wang YH, Pootong A, Sakolvaree Y, Pattanapanyasat K, Chaicumpa W, Chaiyaroj S, Dong C. 2013. Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect Immun 81:3731–3741. doi: 10.1128/IAI.00646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 9.Yang Z, Grinchuk V, Urban JF, Bohl J, Sun R, Notari L, Yan S, Ramalingam T, Keegan AD, Wynn TA, Shea-Donohue T, Zhao A. 2013. Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS One 8:e59441. doi: 10.1371/journal.pone.0059441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao A, McDermott J, Urban JF, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol 171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 11.Zhao A, Bossone C, Piñeiro-Carrero V, Shea-Donohue T. 2001. Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J Pharmacol Exp Ther 299:768–774. [PubMed] [Google Scholar]
- 12.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Piñeiro-Carrero V, Urban JF. 2001. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol 167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 13.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. 2002. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto M, Zhao A, Sun R, Stiltz J, Madden KB, Mentink-Kane M, Ramalingam T, Wynn TA, Urban JF, Shea-Donohue T. 2009. IL-13 receptor alpha2 regulates the immune and functional response to Nippostrongylus brasiliensis infection. J Immunol 183:1934–1939. doi: 10.4049/jimmunol.0804299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol 15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 16.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. 2004. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A 101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. 2011. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao A, Yang Z, Sun R, Grinchuk V, Netzel-Arnett S, Anglin IE, Driesbaugh KH, Notari L, Bohl JA, Madden KB, Urban JF, Antalis TM, Shea-Donohue T. 2013. SerpinB2 is critical to Th2 immunity against enteric nematode infection. J Immunol 190:5779–5787. doi: 10.4049/jimmunol.1200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Rothenberg ME, Finkelman FD. 2009. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med 206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao A, Urban JF, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. 2006. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology 131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Shea-Donohue T. 2005. Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J Immunol 175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao A, Urban JF, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. 2008. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135:217–225.e1. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filbey KJ, Grainger JR, Smith KA, Boon L, van Rooijen N, Harcus Y, Jenkins S, Hewitson JP, Maizels RM. 2014. Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol Cell Biol 92:436–448. doi: 10.1038/icb.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizels RM, Hewitson JP, Smith KA. 2012. Susceptibility and immunity to helminth parasites. Curr Opin Immunol 24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maizels RM, Hewitson JP, Murray J, Harcus YM, Dayer B, Filbey KJ, Grainger JR, McSorley HJ, Reynolds LA, Smith KA. 2012. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol 132:76–89. doi: 10.1016/j.exppara.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monroy FG, Enriquez FJ. 1992. Heligmosomoides polygyrus: a model for chronic gastrointestinal helminthiasis. Parasitol Today 8:49–54. doi: 10.1016/0169-4758(92)90084-F. [DOI] [PubMed] [Google Scholar]
- 27.Rzepecka J, Rausch S, Klotz C, Schnöller C, Kornprobst T, Hagen J, Ignatius R, Lucius R, Hartmann S. 2009. Calreticulin from the intestinal nematode Heligmosomoides polygyrus is a Th2-skewing protein and interacts with murine scavenger receptor-A. Mol Immunol 46:1109–1119. doi: 10.1016/j.molimm.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Gause WC, Lu P, Zhou XD, Chen SJ, Madden KB, Morris SC, Linsley PS, Finkelman FD, Urban JF. 1996. H. polygyrus: B7-independence of the secondary type 2 response. Exp Parasitol 84:264–273. doi: 10.1006/expr.1996.0112. [DOI] [PubMed] [Google Scholar]
- 29.Behnke JM, Wahid FN, Grencis RK, Else KJ, Ben-Smith AW, Goyal PK. 1993. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): downregulation of specific cytokine secretion (IL-9 and IL-10) correlates with poor mastocytosis and chronic survival of adult worms. Parasite Immunol 15:415–421. doi: 10.1111/j.1365-3024.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 30.Grencis RK. 2015. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu Rev Immunol 33:201–225. doi: 10.1146/annurev-immunol-032713-120218. [DOI] [PubMed] [Google Scholar]
- 31.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. 2005. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban JF, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. 1995. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol 154:4675–4684. [PubMed] [Google Scholar]
- 33.Urban JF, Katona IM, Paul WE, Finkelman FD. 1991. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A 88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Rückerl D, Seddon B, MacDonald AS, McKenzie A, Wilson MS. 17 February 2016. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Paul WE. 2015. Innate immunological function of TH2 cells in vivo. Nat Immunol 16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu C, Wu L, Li X. 2013. IL-17 family: cytokines, receptors and signaling. Cytokine 64:477–485. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Katona IM, Finkelman FD, Shea-Donohue T. 2002. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol 169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 38.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Shea-Donohue T. 2004. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol 172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 39.Notari L, Riera DC, Sun R, Bohl JA, McLean LP, Madden KB, van Rooijen N, Vanuytsel T, Urban JF, Zhao A, Shea-Donohue T. 2014. Role of macrophages in the altered epithelial function during a type 2 immune response induced by enteric nematode infection. PLoS One 9:e84763. doi: 10.1371/journal.pone.0084763. [DOI] [PMC free article] [PubMed] [Google Scholar]