Abstract
Leptospirosis, caused by pathogenic spirochetes, is a zoonotic disease of global importance. The detailed pathogenesis of leptospirosis is still unclear, which limits the ideal treatment of leptospirosis. In this study, we analyzed the expression of Toll-like receptor 2 (TLR2) and TLR4 in target organs of both resistant mice and susceptible hamsters after Leptospira interrogans serovar Autumnalis infection. TLR2 but not TLR4 transcripts in mouse organs contrasted with delayed induction and overexpression in hamster organs. Coinjection of leptospires and the TLR2 agonist Pam3CSK4 into hamsters improved their survival rate, alleviated tissue injury, and decreased the abundance of leptospires in target organs. The production of interleukin-10 (IL-10) from tissues was enhanced in hamsters of the group coinjected with leptospires and Pam3CSK4 compared with the leptospira-injected group. Similarly, IL-10 levels in TLR2-deficient mice were lower than those in wild-type mice. A high ratio of IL-10/tumor necrosis factor alpha (TNF-α) levels was found in both infected wild-type mice and hamsters coinjected with leptospires and Pam3CSK4. Moreover, TLR2-dependent IL-10 expression was detected in peritoneal macrophages after leptospira infection. Our data demonstrate that coinjection of leptospires and Pam3CSK4 alleviates the pathology of leptospirosis in hamsters; this effect may result from the enhanced expression of TLR2-dependent IL-10.
INTRODUCTION
Leptospirosis, caused by pathogenic spirochetes, is responsible for a worldwide zoonotic disease. Infected hosts present a diverse array of clinical manifestations ranging from asymptomatic forms to jaundice, renal failure, and even death (1). After infection of maintenance hosts, leptospires rapidly disseminate to almost all tissues during early stages, followed by clearance, except from the kidney, causing chronic infection (2). As the main maintenance hosts, rodents can shed pathogenic leptospires in their urine, which contaminate water and soil. Humans and animals may acquire this disease by direct contact with urine or indirectly from contaminated water (3). Thus, the renal carrier state constitutes an important aspect of the persistence and epidemiology of leptospirosis (3). Although the mechanisms of leptospira-induced immunoreaction have been noted in several studies, the role of innate immune responses in protection against leptospirosis is poorly understood (4).
Toll-like receptors (TLRs) acting as pattern recognition receptors (PRRs) can recognize a variety of pathogen-associated molecular patterns (PAMPs) (5). Genetic data from mouse studies have demonstrated that the sole receptor for classical enterobacterial lipopolysaccharide (LPS) is indeed TLR4 (6), but highly purified leptospiral LPS utilizes both TLR2 and TLR4 in mice and only TLR2 in humans (7). Previous studies have shown that TLR4 is vital for the control of the leptospiral burden in vivo, whereas both TLR2 and TLR4 control the leptospiral burden in the kidney, and tissue differences in TLR signaling may exist (8, 9). In vitro, many hemolysins of Leptospira induce proinflammatory cytokines through both the TLR2- and TLR4-dependent c-Jun N-terminal kinase (JNK) and nuclear factor κB (NF-κB) pathways (10), and leptospiral membrane proteins stimulate proinflammatory chemokines by TLR2 in renal proximal tubule cells (11). All those studies indicate that the TLRs, particularly TLR2 and TLR4, can play a crucial role in the development of leptospirosis. However, the models of leptospirosis used in those studies are mostly mice, which are resistant to leptospiral infection. Hamsters, which are susceptible animals, also have TLR2 and TLR4. Are the different outcomes of leptospirosis in mice and hamsters due to different TLR inductions? Leishmania major-infected bone marrow-derived dendritic cells upregulate TLR2 expression only in C57BL/6 mice, which are resistant to Leishmania major infection (12). Thus, the investigation of dynamic mRNA expression profiles of TLR2 and TLR4 between mice and hamsters after leptospiral infection may contribute to a better understanding of the pathogenic mechanism of leptospirosis.
It has been reported that the development of severe lesions in target organs of susceptible individuals can be correlated with the sustained overexpression of cytokines and chemokines and Leptospira burden compared to those in asymptomatic or less susceptible animals (13). Both clinical studies and animal experiments indicate that the levels of interleukin-10 (IL-10)/tumor necrosis factor alpha (TNF-α) are connected with the outcome of leptospirosis (13, 14). Therefore, these cytokines and chemokines are correlated with the progression of leptospirosis. Although studies of the leptospira-induced inflammatory response through activation of TLRs have been reported (8, 9, 15), there is little information demonstrating a relationship of TLRs with an anti-inflammatory cytokine, for example, IL-10, after leptospiral infection. The activation of TLRs can induce cytokine secretion (16). Therefore, determination of the relevant TLR causing the changes in IL-10/TNF-α levels will contribute to new treatment strategies against leptospirosis.
As the animal models most frequently used for leptospirosis, hamsters and mice recapitulate its acute and chronic phases, respectively (17, 18). Studying any differences in innate immune responses to Leptospira between these two animal models will help illustrate the pathogenesis of leptospirosis and find new treatment strategies. In this study, we characterized the mRNA expression levels of TLR2 and TLR4 in susceptible hamsters and resistant mice after Leptospira infection, and we evaluated the efficacy of Pam3CSK4 against leptospirosis in hamsters. Using primary peritoneal macrophages from mice and hamsters, we showed that the expression of IL-10 was TLR2 dependent.
MATERIALS AND METHODS
Ethics statement.
All animals were maintained on standard rodent chow with water supplied ad libitum and with a 12-h light/12-h dark cycle during the experimental period. All animal experiments were performed according to regulations of the Administration of Affairs Concerning Experimental Animals in China. The protocol was approved by the Committee on the Ethics of Animal Experiments of the First Norman Bethune Hospital of Jilin University, China (2013 clinical trial [2013-121]).
Bacterial strains and animals.
Pathogenic Leptospira interrogans serovar Autumnalis (56606) was kindly provided by Xiaokui Guo. The strain was cultivated at 29°C in Ellinghausen-McCullough-Johnson-Harris (EMJH) liquid medium, and pathogenicity was maintained by passage in hamsters. The concentration of bacterial cells was determined by using a Petroff-Hausser counting chamber and dark-field microscopy. The strain had undergone <5 in vitro passages before being used to infect mice. BALB/c mice and Syrian golden hamsters (Mesocricetus auratus) were provided by the Baiqiuen Medical College of Jilin University (Jilin, China). C57BL/6J wild-type (WT), TLR2-deficient (TLR2−/−), and TLR4−/− mice were provided by the Model Animal Research Center of Nanjing University.
Experimental infections.
Just before infection, bacteria in early stationary phase were centrifuged, resuspended in endotoxin-free phosphate-buffered saline (PBS), and counted by using a Petroff-Hausser chamber. To detect the expression of TLR2 and TLR4 in hamsters and mice, 4- to 6-week-old female BALB/c mice and hamsters were inoculated intraperitoneally with 0.5 ml of PBS containing 106 leptospires. For TLR2 agonist testing, hamsters were inoculated with 106 leptospires either alone or mixed with 100 μg of a single TLR2 agonist, the synthetic triacylated lipopeptide Pam3CSK4 (InvivoGen, San Diego, CA) (12, 19). Hamsters treated with Pam3CSK4 after 6 h of infection were also studied. To test the role of TLR2 in mice, WT and TLR2−/− mice were inoculated with 106 leptospires. Kinetic analyses of gene expression and target organ histology were conducted postinfection (p.i.). For leptospiral infection in hamsters, except for cytokine detection at 24 h p.i., other detections were conducted during the 21-day experimental period. Surviving hamsters were humanely euthanized after 21 days by using CO2.
Effect of Pam3CSK4 on leptospiral growth.
To analyze the influence of Pam3CSK4 on leptospiral growth, 106 leptospires in 0.5 ml of EMJH medium supplemented with or without 100 μg of Pam3CSK4, which was dissolved in sterile water, were cultured at 29°C. Growth was analyzed for 4 days by using a Petroff-Hausser chamber and a dark-field microscope.
Bacterial load and PCR assay.
The leptospiral burdens in infected organs were determined by quantitative PCR (qPCR) using an Applied Bioscience 7500 thermocycler and FastStart Universal SYBR green Master (Roche Applied Science, Germany). The samples used for DNA extraction were treated as previously described (19). Specimens (0.09 to 0.15 g) of liver, lung, and kidney tissues were homogenized and centrifuged, after which the supernatant was subjected to DNA extraction using the TIANamp Bacteria DNA kit (Tiangen, China) according to the manufacturer's instructions. The concentration of DNA was measured by spectrometry. The genomic DNA of a counted number of leptospires was used as a calibrator for qPCR. Specific primers of the lipL32 gene were used as previously described (20). The results were expressed as the number of genome equivalents per milligram of organ DNA.
Culture of primary mouse macrophages and infection by Leptospira.
Hamsters and WT, TLR2−/−, and TLR4−/− mice were injected with 2 ml of 3% thioglycolate medium (BD Biosciences, Sparks, MD). Three days after the injection, peritoneal macrophages were isolated by washing the peritoneal cavity with PBS. The cells were cultured at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum. A total of 1 × 106 cells/well were seeded onto 6-well culture plates in 2 ml of fresh culture medium. After incubation for 6 h at 37°C in 5% CO2, the cells cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum were challenged with leptospires at a multiplicity of infection (MOI) of 100 leptospires per cell with or without Pam3CSK4. The cells were then incubated for 24 h; thereafter, the cells were harvested, and total RNA extraction was performed.
Real-time and reverse transcription-qPCR (RT-qPCR).
Total RNA was extracted from cells or 0.05 to 0.1 g of organs by using TRIzol (Invitrogen, USA) according to the manufacturer's instructions. RNA was reverse transcribed into cDNA by using random primers from a TransScript One-Step gDNA Removal kit and cDNA Synthesis SuperMix (TransGen Biotech, China). Quantification of relative mRNA concentrations was conducted by using an Applied Bioscience 7500 thermocycler and FastStart Universal SYBR green Master (Roche Applied Science, Germany). PCR conditions were as follows: 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of amplification at 95°C for 15 s and 60°C for 60 s. The primers selected for this study are listed in Table 1. By using the EQUATION method, the level of expression of the target gene was normalized to the level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
TABLE 1.
Sequences of primers used for qPCR assays
| Gene | Primer type | Sequence (5′–3′) |
|---|---|---|
| Mouse GAPDH | Sense | AGGTCGGTGTGAACGGATTTG |
| Antisense | GGGGTCGTTGATGGCAACA | |
| Hamster GAPDH | Sense | GATGCTGGTGCCGAGTATGT |
| Antisense | GCAGAAGGTGCGGAGATGA | |
| Mouse TLR2 | Sense | TTTGCTCCTGCGAACTCC |
| Antisense | GCCACGCCCACATCATTC | |
| Hamster TLR2 | Sense | TGTTTCCCGTGTTACTGGTCAT |
| Antisense | CACCTGCTTCCAGACTCACC | |
| Mouse TLR4 | Sense | TTCAGAGCCGTTGGTGTATC |
| Antisense | CTCCCATTCCAGGTAGGTGT | |
| Hamster TLR4 | Sense | ACGACGAGGACTGGGTGAGA |
| Antisense | GCCTTCCTGGATGATGTTGG | |
| Mouse TNF-α | Sense | CCTATGTCTCAGCCTCTTCTCAT |
| Antisense | CACTTGGTGGTTTGCTACGA | |
| Hamster TNF-α | Sense | GGTGATACCAGCAGACGG |
| Antisense | CTTGATGGCGGACAGGA | |
| Mouse IL-10 | Sense | GACAACATACTGCTAACCGACTCC |
| Antisense | ATGCTCCTTGATTTCTGGGC | |
| Hamster IL-10 | Sense | AAGGGTTACTTGGGTTGCC |
| Antisense | AATGCTCCTTGATTTCTGGC |
Histopathological examination.
Animals were humanely euthanized with CO2, and primary organs (liver, kidney, and lung) were immediately removed. To evaluate changes in the target organs, livers, kidneys, and lungs were fixed in 10% neutral buffered formalin; dehydrated, paraffin embedded, and sliced, followed by staining with hematoxylin and eosin (H&E). Pathological changes were examined by using a microscope (Olympus, Japan). The severity of leptospire-induced lesions was graded as described previously (21). Briefly, tubulointerstitial nephritis was graded as follows: 0 for normal, 1 for mild, 2 for moderate, and 3 for severe. Liver pathology was graded based on the average number of inflammatory foci in 10-by-10 fields as follows: 0 for normal, 1 for 1 to 3 foci, 2 for 4 to 7 foci, and 3 for >7 foci. The extent of pulmonary hemorrhage was graded as follows: 0 for no hemorrhage, 1 for a single focus, 2 for multiple foci, and 3 for locally extensive hemorrhage.
Data analysis.
Comparisons between groups were performed by using one-way analysis of variance (ANOVA) followed by the Newman-Keuls test. Survival differences between the study groups were compared by using the Kaplan-Meier log rank test. P values of <0.05 were considered significant.
RESULTS
TLR2 but not TLR4 transcript levels in mice contrast with delayed induction in hamsters.
The expression of TLR2 in all of the organs of mice increased at 6 h p.i., reaching a peak at 24 h p.i. for kidney, 12 h p.i. for liver, and day 2 p.i. for lung. In contrast, induction was delayed in hamsters (Fig. 1). The expression of TLR4 was suppressed or less induced during early infection in both mice and hamsters, except for the livers of mice, where it was overexpressed and reached a peak at 12 h p.i. (Fig. 2). In hamsters, TLR4 was highly induced at day 4 p.i., similarly to the expression of TLR2. The induction of both TLR2 and TLR4 was delayed in hamsters (Fig. 1 and 2). Throughout the time course, the levels of TLR2 and TLR4 in the kidneys of mice returned to normal after day 11 p.i. Only transcript levels, not protein levels, of TLR2 and TLR4 were measured. These results indicated that the expression levels of TLR2 and TLR4 were different between resistant mice and susceptible hamsters. In consideration of the early regulation of TLR2 in mice but not hamsters, TLR2 might play an important role in the development of leptospirosis.
FIG 1.
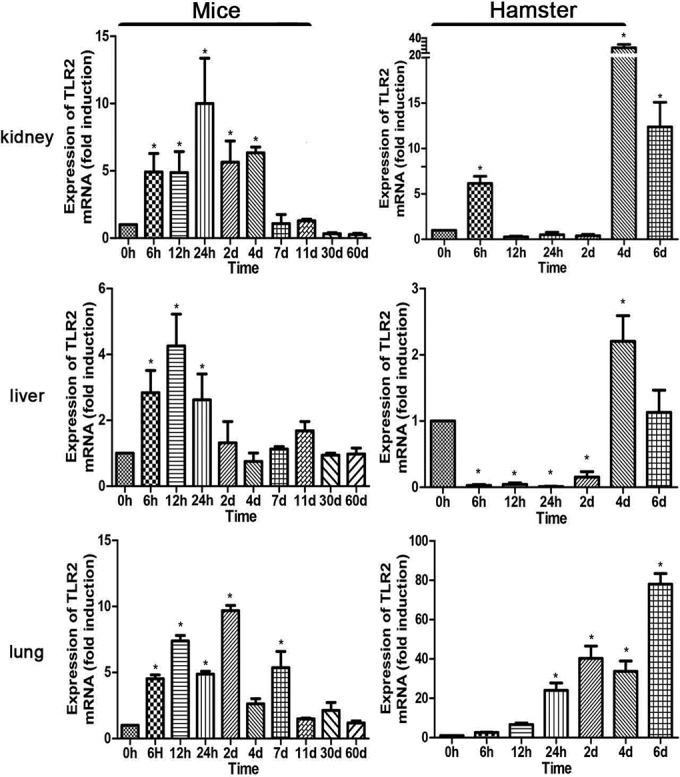
Modulation of TLR2 mRNA expression in tissues after injection of leptospires. TLR2 mRNA levels in kidneys, livers, and lungs from BALB/c mice and hamsters were quantified by RT-qPCR at a series of time points. The results were normalized to the expression level of the housekeeping gene GAPDH. Bars show the levels of TLR2 (means ± standard deviations) in tissues of mice and hamsters at each time point (n = 3). The levels of TLR2 in different tissues from three healthy individuals at 0 h were given a value of 1.0. Different mRNA expression levels between the infected group and the healthy group (0 h) were compared by one-way ANOVA. *, P < 0.05.
FIG 2.
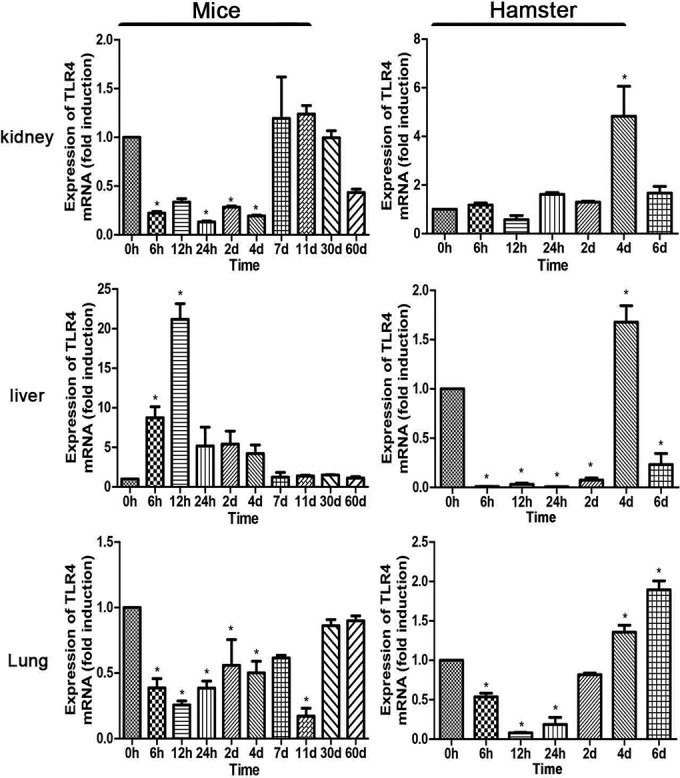
Modulation of TLR4 mRNA expression in tissues after injection of leptospires. TLR4 mRNA levels in kidneys, livers, and lungs from BALB/c mice and hamsters were quantified by RT-qPCR at a series of time points. The results were normalized to the expression levels of the housekeeping gene GAPDH. Bars show levels of TLR4 (means ± standard deviations) in tissues of mice and hamsters at each time point (n = 3). The levels of TLR4 in different tissues from three healthy individuals at 0 h were given a value of 1.0. Different mRNA expression levels between the infected group and the healthy group (0 h) were compared by one-way ANOVA. *, P < 0.05.
The TLR2 agonist Pam3CSK4 improves the survival rate, alleviates the pathology of leptospirosis, and decreases the abundance of leptospires in hamster.
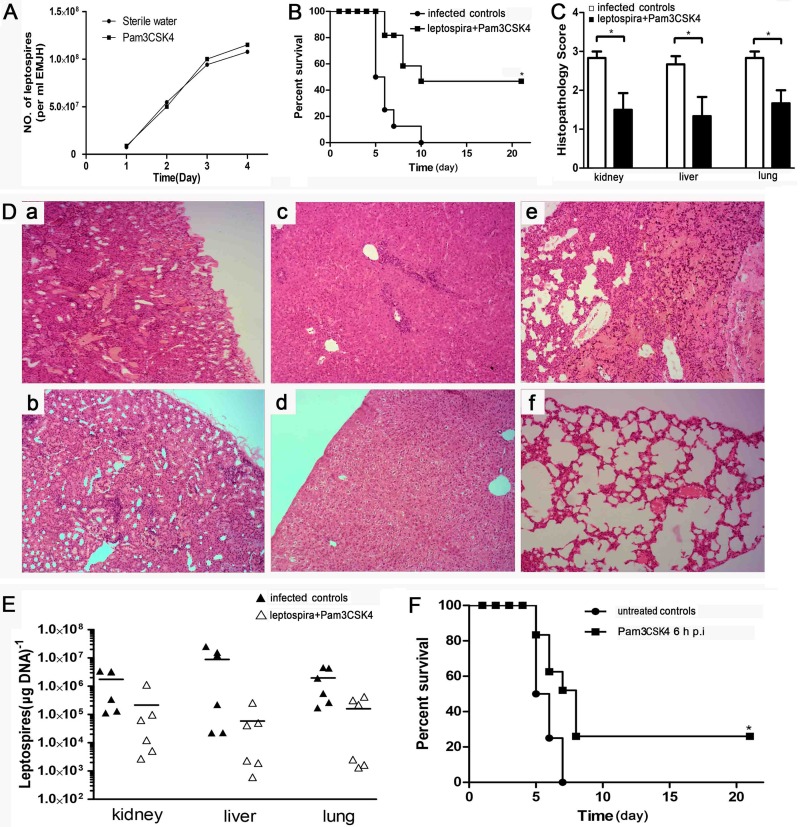
To test the role of the induction of TLR2 against leptospirosis in hamsters, the TLR2 agonist Pam3CSK4 was used as a coinjection with leptospires. We analyzed the effect of Pam3CSK4 on leptospiral growth. The data revealed that Pam3CSK4 had nothing to do with leptospiral growth during 4 days (Fig. 3A). The hamster group injected with a mix of leptospires and Pam3CSK4 showed a higher survival rate than that of the group receiving only leptospires (infected controls) (Fig. 3B). Kidney, lung, and liver lesion grades were lower for the group coinjected with Pam3CSK and leptospires than for the infected controls (Fig. 3C). Representative photographs of hamster kidneys and lungs were selected from the group coinjected with Pam3CSK and leptospires and the infected controls. Dramatic lesions with hemorrhage were found in renal tissues of the infected controls (Fig. 3Da). In contrast, the kidneys of the group coinjected with Pam3CSK and leptospires showed little evidence of hemorrhage (Fig. 3Db). The livers of infected control hamsters showed more inflammatory foci and a wider intercellular space than did those of the group coinjected with Pam3CSK and leptospires (Fig. 3Dc and d). Severe pulmonary hemorrhages were found in the lungs of infected controls (Fig. 3De), whereas no hemorrhagic foci were found in the group coinjected with Pam3CSK and leptospires (Fig. 3Df).
FIG 3.
Influence of TLR2 agonist Pam3CSK4 on pathology of hamsters with leptospirosis. (A) Effect of Pam3CSK4 on leptospiral growth. A total 106 leptospires in 0.5 ml of EMJH medium supplemented with or without 100 μg of Pam3CSK4 were cultured at 29°C. Growth was analyzed for 4 days by using a Petroff-Hausser chamber and a dark-field microscope. Each data point exhibits the means from triplicates ± standard deviations for three independent experiments. P values of <0.05 were considered significant (*, P < 0.05). (B) Survival curves of hamsters in the infected control group (n = 6) and the group coinjected with leptospires and Pam3CSK4 (n = 6). Hamsters were injected with leptospires or coinjected with leptospires and Pam3CSK4. *, P < 0.05 versus untreated controls as determined by a by Kaplan-Meier log rank test. (C) Histopathology scores for kidneys, livers, and lungs of hamsters. The data represent the mean histopathology scores for the two groups of hamsters. Statistical analysis of the results for infected controls (n = 6) and the group coinjected with leptospires and Pam3CSK4 (n = 6) was performed by using the Wilcoxon rank sum test. *, P < 0.05. (D) Histopathology of kidneys (a and b), livers (c and d), and lungs (e and f) of hamsters in the infected control group (a, c, and e) and the group coinjected with leptospires and Pam3CSK4 (b, d, and f). Magnification, ×200. Samples were collected over a 21-day experimental period, and representative photographs are presented. (E) Leptospiral burdens in the kidneys, livers, and lungs of hamsters in the infected control group (n = 6) and the group coinjected with leptospires and Pam3CSK4 (n = 6) as determined by qPCR. Samples were collected over a 21-day experimental period. The results are presented as numbers of genome equivalents per microgram of tissue DNA, and the differences were compared by one-way ANOVA. *, P < 0.05. (F) Survival curves of hamsters treated with Pam3CSK4 6 h after infection with leptospires (n = 8) and untreated controls (n = 8). Hamsters were injected with leptospires. Six hours later, Pam3CSK4 (100 μg) was intraperitoneally injected into hamsters. *, P < 0.05 versus untreated controls as determined by a Kaplan-Meier log rank test.
The leptospiral burdens in the kidneys, livers, and lungs of hamsters from the group coinjected with Pam3CSK and leptospires and the infected control group were measured by qPCR. A lower leptospiral burden was found in the group coinjected with Pam3CSK and leptospires than in the infected controls (Fig. 3E). The differences are statistically not significant and represent trends. To test the efficacy of Pam3CSK injected 6 h after infection with leptospires, we studied the survival rate compared to that of the group coinjected with leptospires and normal saline (untreated controls). Injection of the TLR2 agonist Pam3CSK4 6 h after infection with leptospires also improved the survival rate of the hamsters (Fig. 3F).
TLR2 contributes to the control of leptospiral burden in kidney and alleviates pathology in mice.
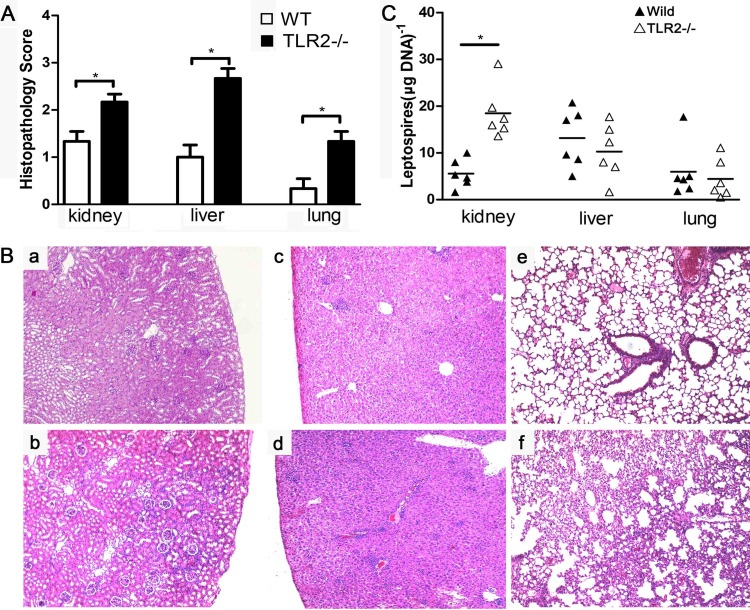
To test the role of the induction of TLR2 against leptospirosis in mice, C57BL/6J WT and TLR2−/− mice were infected with 106 leptospires. Leptospiral burden and histopathology of the kidney, liver, and lung were studied at 4 days p.i. The lesion grades for kidneys, lungs, and livers were higher for TLR2−/− mice than for WT mice (Fig. 4A). Although the TLR2−/− mice did not die from leptospirosis (data not shown), aggravated inflammatory cell infiltration was found in TLR2−/− mice (Fig. 4Bb) compared to WT mice (Fig. 4Ba). The livers of WT mice showed moderate inflammation (Fig. 4Bc). However, severe inflammation with a mass of inflammatory foci was found in TLR2−/− mice (Fig. 4Bd). The lungs of WT mice were almost normal (Fig. 4Be), in contrast to the pulmonary hemorrhage and interstitial pneumonia in TLR2−/− mice (Fig. 4Bf).
FIG 4.
TLR2 contributes to the control of leptospiral burden in kidney and alleviates pathology in mice. (A) Histopathology scores for the kidneys, livers, and lungs of mice. The data represent the mean histopathology scores for the two groups of mice. Statistical analysis of results for WT mice (n = 6) and TLR2−/− mice (n = 6) was performed by using the Wilcoxon rank sum test. *, P < 0.05. (B) Histopathology of kidneys (a and b), livers (c and d), and lungs (e and f) of WT mice (a, c, and e) and TLR2−/− mice (b, d, and f) after infection with leptospires at 4 days p.i. Magnification, ×200. Representative photographs are presented. (C) Leptospiral burdens in kidneys, livers, and lungs of WT mice (n = 6) and TLR2−/− mice (n = 6) as determined by qPCR at 4 days p.i. The results are presented as numbers of genome equivalents per microgram of tissue DNA, and the differences were compared by one-way ANOVA. *, P < 0.05.
The leptospiral burdens in the kidneys, livers, and lungs of WT and TLR2−/− mice were measured by qPCR. Interestingly, the leptospiral burdens in livers and lungs of WT and TLR2−/− mice were comparable, whereas the leptospiral burden in the kidney was higher for TLR2−/− mice than for WT mice (Fig. 4C).
The TLR2 agonist Pam3CSK4 improves the IL-10/TNF-α ratio.
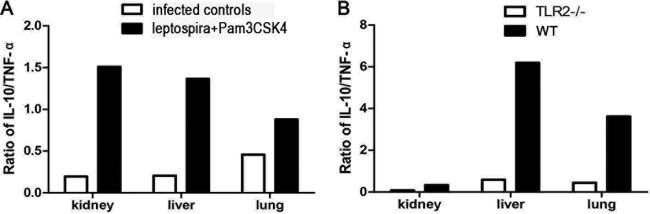
Only transcript levels, and not protein levels, were measured. The expression levels of IL-10 and TNF-α in hamsters and mice were measured by RT-qPCR. Hamsters were injected with a mix of the TLR2 agonist Pam3CSK4 and leptospires. Samples were collected 24 h after infection with leptospires. IL-10 expression in kidney, liver, and lung was significantly improved in hamsters of the group coinjected with Pam3CSK4 and leptospires than in the infected controls, whereas TNF-α expression was improved only in the lungs, as shown in Table 2. The IL-10/TNF-α ratio was improved in the kidneys, livers, and lungs of hamsters in the group coinjected with Pam3CSK4 and leptospires compared with infected controls (Fig. 5A).
TABLE 2.
Relative normalized expression levels of cytokinesa
| Organ | Presence of Pam3CSK4 | Mean expression level (fold induction) ± SE (n = 3) |
|
|---|---|---|---|
| IL-10 | TNF-α | ||
| Kidney | − | 0.0343 ± 0.0272 | 0.1668 ± 0.1916 |
| + | 0.2349 ± 0.2104* | 0.1719 ± 0.0626 | |
| Liver | − | 1.6743 ± 0.4519 | 8.5212 ± 1.9813 |
| + | 12.8796 ± 7.0677* | 8.5382 ± 1.7210 | |
| Lung | − | 1.3286 ± 0.5489 | 2.9089 ± 0.8059 |
| + | 14.2053 ± 10.9635* | 16.1554 ± 6.2201* | |
Hamsters were intraperitoneally infected with leptospires either alone or mixed with Pam3CSK4. The expression levels of IL-10 and TNF-α in each tissue were quantified by RT-qPCR at 24 h p.i. *, P < 0.05 for the group coinjected with leptospires and Pam3CSK4 versus infected controls as determined by one-way ANOVA followed by the Newman-Keuls test.
FIG 5.
IL-10/TNF-α ratios in mice and hamsters after injection of leptospires. (A) IL-10/TNF-α ratios in hamsters in the infected control group and the group coinjected with leptospires and Pam3CSK4 at 24 h p.i. based on the data shown in Table 2. (B) IL-10/TNF-α ratios in WT and TLR2−/− mice at 4 days p.i. based on the data shown in Table 3.
The expression levels of IL-10 and TNF-α in tissues of WT and TLR2−/− mice were also quantified by RT-qPCR 4 days after infection with leptospires. IL-10 expression was dramatically inhibited in the lungs of TLR2−/− mice compared to those of WT mice. Interestingly, upregulated TNF-α was found in the kidneys and lungs of TLR2−/− mice, as shown in Table 3. The IL-10/TNF-α ratio was reduced in the kidneys, livers, and lungs of TLR2−/− mice compared to that in WT mice (Fig. 5B).
TABLE 3.
Relative normalized expression levels of cytokines in micea
| Organ | Mouse type | Mean expression level (fold induction) ± SE (n = 3) |
|
|---|---|---|---|
| IL-10 | TNF-α | ||
| Kidney | TLR2−/− | 4.3941 ± 1.8864 | 53.4172 ± 18.3277* |
| WT | 6.5701 ± 3.2300 | 19.8598 ± 11.9571 | |
| Liver | TLR2−/− | 3.9898 ± 1.6002 | 6.7567 ± 5.3288 |
| WT | 6.8564 ± 3.7698 | 1.1072 ± 0.1316 | |
| Lung | TLR2−/− | 1.4039 ± 0.4742* | 3.1589 ± 1.3706* |
| WT | 3.1605 ± 0.8553 | 0.8719 ± 0.2210 | |
Wild-type and TLR2-deficient mice were intraperitoneally infected with leptospires. The expression levels of IL-10 and TNF-α in each tissue were quantified by RT-qPCR at 4 days p.i. *, P < 0.05 for TLR2−/− mice versus WT mice by one-way ANOVA followed by the Newman-Keuls test.
IL-10 expression after leptospiral infection is TLR2 dependent in peritoneal macrophages.
The IL-10 expression level in mice and hamsters was connected with the induction of TLR2. Peritoneal macrophages from hamsters and WT, TLR2−/−, and TLR4−/− mice were used. For peritoneal macrophages from hamsters, cells were challenged with leptospires or with leptospires and Pam3CSK4 for 24 h, followed by RNA extraction from cells and RT-qPCR to measure IL-10 and TNF-α expression levels. The IL-10 level was dramatically lower in TLR2−/− but not TLR4−/− macrophages (Fig. 6A). TLR2 and TLR4 had no influence on TNF-α levels (Fig. 6B). The TLR2 agonist Pam3CSK4 improved IL-10 but not TNF-α levels in peritoneal macrophages from hamsters (Fig. 6C). This result explains the improved IL-10/TNF-α ratio in hamsters treated with Pam3CSK4.
FIG 6.
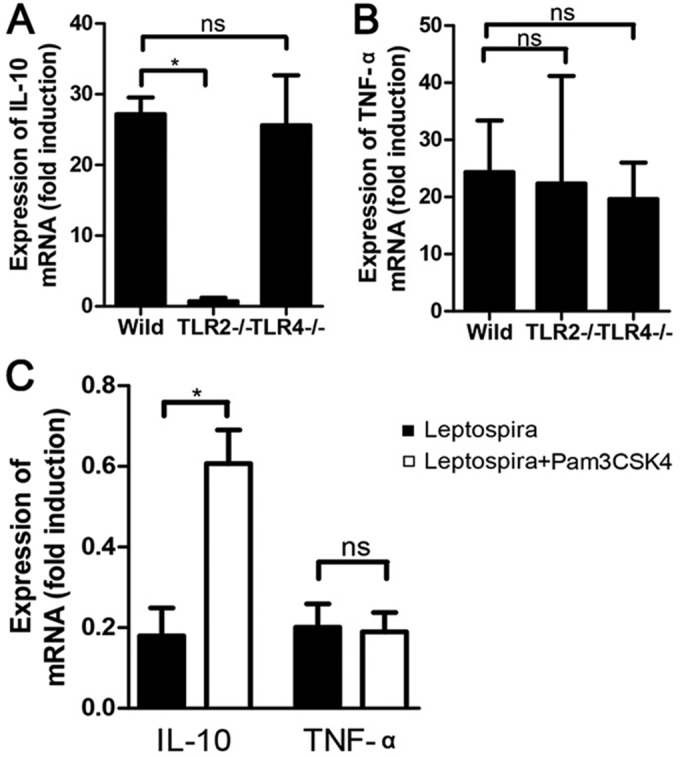
IL-10 and TNF-α expression levels in peritoneal macrophages of hamsters and mice. Peritoneal macrophages of hamsters were challenged with leptospires or with leptospires plus Pam3CSK4 for 24 h. Peritoneal macrophages from WT, TLR2−/−, and TLR4−/− mice were challenged with leptospires for 24 h, and the mRNA levels of IL-10 and TNF-α were quantified by RT-qPCR. (A and B) IL-10 (A) and TNF-α (B) mRNA levels in peritoneal macrophages of WT, TLR2−/−, and TLR4−/− mice. (C) IL-10 and TNF-α mRNA levels in peritoneal macrophages of hamsters. The results were normalized to the expression level of the housekeeping gene GAPDH. Bars show mean levels of cytokines ± standard deviations, and differences were analyzed by one-way ANOVA. *, P < 0.05; ns, none significant.
DISCUSSION
Leptospirosis, a reemerging infectious disease, has a variety of animal hosts for pathogenic leptospires in nature (22). The detailed pathogenesis of leptospirosis is still unclear. TLR4 was recognized as playing a key role in protecting against leptospiral infection and contributing to the control of the leptospiral burden in vivo (8). In our study, the expression level of TLR4 did not increase rapidly in either mice or hamsters, except for the livers of mice. The poorer outcome and greater leptospiral burden in hamsters than in mice were not attributable simply to the expression of TLR4, although TLR4 could control the leptospiral burden.
We found that the induction of TLR2 was different between mice and hamsters. Furthermore, our results revealed that the expression of the anti-inflammatory cytokine IL-10 was dependent on TLR2 in peritoneal macrophages of mice and hamsters during leptospiral infection. TLR2-dependent IL-10 production has been studied for other pathogens such as Helicobacter pylori and Porphyromonas gingivalis (23, 24). This work is the first to illustrate the connection between TLR2 and IL-10 during L. interrogans infection. The different TLR2-dependent IL-10 expression levels in mice and hamsters may help explain the pathogenesis of leptospirosis. A higher leptospiral burden was found in the kidneys of TLR2−/− mice, showing that TLR2 controls bacterial growth in the kidney, which is consistent with results of previous studies (9).
Some studies have reported that the TLR2 agonist Pam3CSK4 could control Staphylococcus aureus and Leishmania major infections (12, 25). However, little is known about the activation of TLR2 using Pam3CSK4 against leptospiral infection. In our study, treatment with Pam3CSK4 6 h after infection with leptospires also improved the survival of hamsters, although the rate of survival was lower than that with the coinjection. This result indicated that TLR2 activation during early leptospiral infection protected against leptospirosis in hamsters. TLR2 induction occurred earlier in mice than in hamsters, and mice presented with sublethal infection. Tissue injury was severe, and the leptospiral burden in kidney was heavy in TLR2−/− mice compared with that in WT mice. This result indicated that TLR2 plays a role during early infection but is not sufficient to prevent disease or its outcomes.
The association between the levels of cytokines and the clinical outcome of leptospirosis has been controversial. One group showed that a low ratio of IL-10/TNF-α levels was associated with a poor outcome for leptospirosis (13, 14). However, another group reported the identification of a positive correlation between the IL-10/TNF-α ratio and fatal outcomes (26). Our results could not demonstrate a precise association between the levels of cytokines and clinical outcomes for leptospirosis. However, a higher ratio of IL-10/TNF-α in hamsters treated with Pam3CSK4 was associated with improved survival, and a lower ratio of IL-10/TNF-α in TLR2−/− mice was associated with severe tissue injury and increased leptospiral burdens in the kidney, liver, and lung compared with those in WT mice. Further study will consider whether there is synergistic action between the TLR2 agonist Pam3CSK4 and antibiotics against leptospirosis in clinical applications.
In conclusion, our work is the first to show evidence of differences in the expression profiles of TLR2 and TLR4 depending on host susceptibility to leptospirosis. These differences may influence the downstream expression of IL-10 and the IL-10/TNF-α ratio. TLR2 activation using Pam3CSK4 improved survival, alleviated the pathology of leptospirosis, and decreased the abundance of leptospires in hamsters. TLR2 activation also had a protective role against sublethal infection in mice. These findings will contribute to a better understanding of the pathogenic mechanism of leptospirosis and reveal new treatment strategies.
ACKNOWLEDGMENTS
We thank Xiaogui Guo for providing Leptospira interrogans serovar Autumnalis.
This work was supported by the National Natural Science Foundation of China (grant no. 31572582 and 81301404), the Special Fund for Agro-Scientific Research in the Public Interest of the People's Republic of China (grant no. 201303042), project 2015142 supported by the Graduate Innovation Fund of Jilin University, and the Beijing Natural Science Foundation (grant no. 7144196).
We declare that we have no conflicts of interest.
REFERENCES
- 1.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM, Peru-United States Leptospirosis Consortium . 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Monahan AM, Callanan JJ, Nally JE. 2009. Review paper: host-pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol 46:792–799. doi: 10.1354/vp.08-VP-0265-N-REV. [DOI] [PubMed] [Google Scholar]
- 3.Adler B, de la Pena Moctezuma A. 2010. Leptospira and leptospirosis. Vet Microbiol 140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 4.da Silva JB, Carvalho E, Covarrubias AE, Ching AT, Mattaraia VG, Paiva D, de Franco M, Favaro RD, Pereira MM, Vasconcellos S, Zorn TT, Ho PL, Martins EA. 2012. Induction of TNF-alfa and CXCL-2 mRNAs in different organs of mice infected with pathogenic Leptospira. Microb Pathog 52:206–216. doi: 10.1016/j.micpath.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Hoebe K, Du X, Ulevitch RJ. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol 74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 6.Poltorak A, Smirnova I, He X, Liu MY, Van Huffel C, McNally O, Birdwell D, Alejos E, Silva M, Du X, Thompson P, Chan EK, Ledesma J, Roe B, Clifton S, Vogel SN, Beutler B. 1998. Genetic and physical mapping of the Lps locus: identification of the Toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis 24:340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 7.Nahori MA, Fournie-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CR, Saint Girons I, Werts C. 2005. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol 175:6022–6031. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 8.Viriyakosol S, Matthias MA, Swancutt MA, Kirkland TN, Vinetz JM. 2006. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar Icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect Immun 74:887–895. doi: 10.1128/IAI.74.2.887-895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassin C, Picardeau M, Goujon JM, Bourhy P, Quellard N, Darche S, Badell E, d'Andon MF, Winter N, Lacroix-Lamande S, Buzoni-Gatel D, Vandewalle A, Werts C. 2009. TLR4- and TLR2-mediated B cell responses control the clearance of the bacterial pathogen, Leptospira interrogans. J Immunol 183:2669–2677. doi: 10.4049/jimmunol.0900506. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Wu Y, Ojcius DM, Yang XF, Zhang C, Ding S, Lin X, Yan J. 2012. Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2- and 4-mediated JNK and NF-kappaB signaling pathways. PLoS One 7:e42266. doi: 10.1371/journal.pone.0042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung CC, Chang CT, Tian YC, Wu MS, Yu CC, Pan MJ, Vandewalle A, Yang CW. 2006. Leptospiral membrane proteins stimulate pro-inflammatory chemokines secretion by renal tubule epithelial cells through Toll-like receptor 2 and p38 mitogen activated protein kinase. Nephrol Dial Transplant 21:898–910. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Hinchman M, Mendez S. 2015. Coinjection with TLR2 agonist Pam3CSK4 reduces the pathology of leishmanization in mice. PLoS Negl Trop Dis 9:e0003546. doi: 10.1371/journal.pntd.0003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui M, Rouleau V, Bruyère-Ostells L, Goarant C. 2011. Gene expression profiles of immune mediators and histopathological findings in animal models of leptospirosis: comparison between susceptible hamsters and resistant mice. Infect Immun 79:4480–4492. doi: 10.1128/IAI.05727-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tajiki MH, Nakama AS, Salomäo R. 1997. The ratio of plasma levels of IL-10/TNF-a and its relationship to disease severity and survival in patients with leptospirosis. Braz J Infect Dis 1:138–141. [PubMed] [Google Scholar]
- 15.Lesur I, Textoris J, Loriod B, Courbon C, Garcia S, Leone M, Nguyen C. 2010. Gene expression profiles characterize inflammation stages in the acute lung injury in mice. PLoS One 5:e11485. doi: 10.1371/journal.pone.0011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. 2000. Bacterial lipopolysaccharide activates NF-kappa B through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells—differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem 275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 17.Silva EF, Santos CS, Athanazio DA, Seyffert N, Seixas FK, Cerqueira GM, Fagundes MQ, Brod CS, Reis MG, Dellagostin OA, Ko AI. 2008. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine 26:3892–3896. doi: 10.1016/j.vaccine.2008.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos CS, Macedo JO, Bandeira MS, Chagas-Junior AD, McBride AJ, McBride FW, Reis MG, Athanazio DA. 2010. Different outcomes of experimental leptospiral infection in mouse strains with distinct genotypes. J Med Microbiol 59:1101–1106. doi: 10.1099/jmm.0.021089-0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang WL, Zhang NS, Wang W, Wang F, Gong Y, Jiang HC, Zhang ZC, Liu XF, Song XJ, Wang TC, Ding Z, Cao YG. 2014. Efficacy of cefepime, ertapenem and norfloxacin against leptospirosis and for the clearance of pathogens in a hamster model. Microb Pathog 77:78–83. doi: 10.1016/j.micpath.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Rojas P, Monahan AM, Schuller S, Miller IS, Markey BK, Nally JE. 2010. Detection and quantification of leptospires in urine of dogs: a maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis 29:1305–1309. doi: 10.1007/s10096-010-0991-2. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Faisal SM, Yan W, Chang YC, McDonough SP, Zhang N, Akey BL, Chang YF. 2011. Evaluation of novel fusion proteins derived from extracellular matrix binding domains of LigB as vaccine candidates against leptospirosis in a hamster model. Vaccine 29:7379–7386. doi: 10.1016/j.vaccine.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 22.Zhang CL, Wang H, Yan J. 2012. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes Infect 14:317–323. doi: 10.1016/j.micinf.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Zhang M, El-Zataari M, Owyang SY, Eaton KA, Liu M, Chang YM, Zou W, Kao JY. 2013. TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice. PLoS One 8:e74595. doi: 10.1371/journal.pone.0074595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaddis DE, Maynard CL, Weaver CT, Michalek SM, Katz J. 2013. Role of TLR2-dependent IL-10 production in the inhibition of the initial IFN-gamma T cell response to Porphyromonas gingivalis. J Leukoc Biol 93:21–31. doi: 10.1189/jlb.0512220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YG, Zhang Y, Deng LQ, Chen H, Zhang YJ, Zhou NJ, Yuan K, Yu LZ, Xiong ZH, Gui XM, Yu YR, Wu XM, Min WP. 2016. Control of methicillin-resistant Staphylococcus aureus pneumonia utilizing TLR2 agonist Pam3CSK4. PLoS One 11:e0149233. doi: 10.1371/journal.pone.0149233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyriakidis I, Samara P, Papa A. 2011. Serum TNF-alpha, sTNFR1, IL-6, IL-8 and IL-10 levels in Weil's syndrome. Cytokine 54:117–120. doi: 10.1016/j.cyto.2011.01.014. [DOI] [PubMed] [Google Scholar]