Abstract
Neutrophils are essential components of immunity and are rapidly recruited to infected or injured tissue. Upon their activation, neutrophils release granules to the cell's exterior, through a process called degranulation. These granules contain proteins with antimicrobial properties that help combat infection. The enteropathogenic bacterium Yersinia pseudotuberculosis successfully persists as an extracellular bacterium during infection by virtue of its translocation of virulence effectors (Yersinia outer proteins [Yops]) that act in the cytosol of host immune cells to subvert phagocytosis and proinflammatory responses. Here, we investigated the effect of Y. pseudotuberculosis on neutrophil degranulation upon cell contact. We found that virulent Y. pseudotuberculosis was able to prevent secondary granule release. The blocking effect was general, as the release of primary and tertiary granules was also reduced. Degranulation of secondary granules was also blocked in primed neutrophils, suggesting that this mechanism could be an important element of immune evasion. Further, wild-type bacteria conferred a transient block on neutrophils that prevented their degranulation upon contact with plasmid-cured, avirulent Y. pseudotuberculosis and Escherichia coli. Detailed analyses showed that the block was strictly dependent on the cooperative actions of the two antiphagocytic effectors, YopE and YopH, suggesting that the neutrophil target structures constituting signaling molecules needed to initiate both phagocytosis and general degranulation. Thus, via these virulence effectors, Yersinia can impair several mechanisms of the neutrophil's antimicrobial arsenal, which underscores the power of its virulence effector machinery.
INTRODUCTION
Neutrophils are an important element of the innate immune response. Circulating neutrophils are recruited into tissues upon injury or infection in order to play key roles in the defense against bacterial or fungal pathogens. Several mechanisms are used to eliminate microbial invaders such as phagocytosis, neutrophil extracellular trap (NET) formation, and degranulation. Degranulation is triggered upon the activation of neutrophils by microbial or inflammatory stimuli and leads to the release of granule contents. Neutrophils contain four types of granules and are categorized as peroxidase-positive (azurophilic or primary) granules, peroxidase-negative (specific or secondary, and gelatinase or tertiary) granules, and secretory vesicles (1, 2). Secretory vesicles and tertiary granules are released during adhesion and transmigration through the endothelium, respectively, whereas the primary and secondary granules hold the majority of the cell's antimicrobial activity and are released at the infectious site (1–3). Primary granules contain high quantities of myeloperoxidase (MPO), as well as defensins, elastase, heparin binding proteins, and proteinases. Secondary granules are rich in lactoferrin and enzymes such as collagenase and gelatinase, as well as the LL-37 precursor hCAP-18. However, these granules can be subdivided further, and instead of representing strict granule types, the granule heterogeneity depends on controlled synthesis of granule proteins during differentiation in the bone marrow (1, 4). Granule contents are released by exocytosis to the exterior of the cell, or into the phagosome (primary granules), in four separate steps: (i) translocation of granules from the cytoplasm to the target membrane; (ii) granule binding resulting in contact between the outer surfaces of the granule membrane and the inner surface of the target membrane; (iii) development of a fusion pore between granules and target membranes; and (iv) granule fusion with target membranes resulting in the release of their contents (5–7).
The degranulation process involves different signaling pathways, including calcium and phospholipid signaling (5, 8). Increased cytosolic calcium is required for degranulation, as calcium is involved in the fusion of granule subsets with the plasma or phagosomal membrane, with the highest dependence shown for primary granules and the lowest for secretory vesicles (9–11). Several studies have indicated a role for phospholipids, mainly polyphosphoinositides such as phosphatidylinositol 4,5-bisphosphate, also known as PIP2, in the regulation of degranulation (5). Neutrophil degranulation is also tightly regulated by the cytoskeleton; granules are transported to membranes on microtubules in an ATP-dependent manner (12), and actin remodeling has been shown to be important for the release of granules to the exterior (5).
Yersinia pseudotuberculosis is a Gram-negative, rod-shaped extracellular enteropathogen, closely related to Yersinia pestis. Y. pseudotuberculosis is transmitted by the oral route and gives rise to a self-limiting, mesenteric lymphadenitis in humans (13). This pathogen survives extracellularly in lymphoid aggregates, where it efficiently resists the innate immune system by avoiding phagocytosis and interferes with the production of proinflammatory cytokines (14–16). This trait is dependent on a 70-kb virulence plasmid encoding a type III secretion system (T3SS), which includes the Yop effector proteins YopH, YopE, YopM, YopK, YopJ, and YpkA (17–19). In vivo, Yop secretion is triggered by the close interaction between Yersinia and target cells, followed by translocation of virulence effectors into the cytoplasm of the host cell via T3SS (20, 21). Mice are a commonly used virulence infection model for Y. pseudotuberculosis, with targeting of the gastrointestinal tract, where the pathogen infects the Peyer's patches of the small intestine and cecal lymphoid aggregates (22–24). The neutrophils that are subsequently recruited in large numbers to the site of infection have been shown to be the main target for Yop delivery during infection (23). Two essential virulence effectors, YopH and YopE, impair the signaling machinery needed for phagocytosis, an important mechanism in this pathogen's ability to survive extracellularly in the lymph node (25). YopH is a protein tyrosine phosphatase (PTPase) that inhibits phagocytosis by interfering with signaling from the target cell receptor abrogating phagocytosis (26, 27). Moreover, YopH blocks immediate early Ca2+ signaling in neutrophils (28, 29). YopE is a GTPase-activating protein that targets small RhoA family G proteins, resulting in the impairment of actin dynamics required for phagocytosis (30, 31).
Previously we have shown that Y. pseudotuberculosis efficiently evades neutrophilic attack in the early stages of infection and that mice with reduced numbers of neutrophils are more sensitive to initial bacterial colonization (32), indicating that neutrophil escape is an important feature of virulent Yersinia species. While it is well appreciated that Y. pseudotuberculosis resists phagocytic engulfment by both neutrophils and macrophages (33, 34), less is known about how this pathogen faces the challenge posed by neutrophil degranulation. Here, we investigated the effects of Y. pseudotuberculosis on degranulation by human neutrophils. We show that degranulation is blocked by wild-type Y. pseudotuberculosis. Further, detailed analysis of the effects on secondary granules showed that similarly to phagocytosis blockade, this immune evasion mechanism results from the coordinated action of YopH and YopE. Hence, collectively, these two virulence effectors play a crucial role in enabling this extracellular pathogen to resist neutrophils.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Y. pseudotuberculosis strains used in this study are listed in Table 1. Bacteria were cultured in Luria Bertani (LB) broth overnight at 26°C with agitation. Overnight cultures were diluted 1:100 in LB or brain heart infusion (BHI) broth depleted of calcium by the addition of 5 mM EGTA and 20 mM MgCl2 and then incubated for 1 h at 26°C, followed by a temperature shift to 37°C with agitation at 150 rpm for 2 h to induce Yersinia outer protein expression. Escherichia coli strain K-12 was grown overnight at 37°C, diluted 1:100 in BHI, and then incubated for 3 h at 37°C with agitation.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Reference |
|---|---|---|
| E. coli K-12 MG1655 | ||
| Y. pseudotuberculosis | ||
| YPIII(pIB102) | Wild-type with deletion of yadA, Kmr | 49 |
| YPIII | Plasmid-cured with deletion of yadA | 50 |
| YPIII ΔyopH | Deletion of yopH | 32 |
| YPIII ΔyopE | Deletion of yopE | 51 |
| YPIII ΔyopHE | Deletion of yopH and yopE | 22 |
| YPIII ΔyopJ | Deletion of yopJ | 52 |
| YPIII ΔyopK | Deletion of yopK | 53 |
| YPIII ΔyopM | Deletion of yopM | 54 |
| YPIII ΔypkA | Deletion of ypkA | 55 |
| YPIII(pIB1) | Wild-type, yadA+ | 50 |
Isolation of human neutrophils.
Neutrophils were isolated from heparinized blood (whole blood) from healthy donors using Polymorphprep (Axis-Shield, Oslo, Norway), according to the manufacturer's protocol. In experiments with multiple repeats, blood from different donors (26 in total) was used. Neutrophils were resuspended in Krebs-Ringer phosphate buffer (KRG) containing 120 mM NaCl, 8.3 mM Na2HPO4, 1.7 mM KH2PO4, 4.9 mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, and 5 mM glucose (pH 7.4) and then kept at room temperature (RT) until use. Cells were counted, and viability was confirmed as being >95%, as determined by trypan blue exclusion. All experiments were performed within 3 to 4 h of neutrophil isolation.
Neutrophil degranulation.
Bacteria were washed and resuspended to a concentration of 4 × 107 bacteria/ml and then incubated with neutrophils at a multiplicity of infection (MOI) of 10 for different periods of time at 37°C. As positive controls for degranulation, neutrophils were stimulated with 1 μg/ml phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C. Samples were centrifuged at 500 × g for 5 min, with supernatants collected into Protein LoBind tubes (Eppendorf AG, Hamburg, Germany), boiled for 5 min at 95°C in sample buffer, and then loaded (40 μl) into the wells of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. In coinfection experiments, wild-type bacteria were removed by centrifugation at 500 × g after 15 min, followed by the addition of 100 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO, USA). For preactivation, neutrophils in KRG were treated with 1 μg/ml lipopolysaccharide (LPS) from E. coli serotype O111:B4 (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C prior to infection.
Flow cytometry.
Cell surface expression of CD66b was monitored for assessing the degranulation of specific granules by flow cytometry. Neutrophils were seeded in plates coated with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 h and then infected with plasmid-cured or wild-type Y. pseudotuberculosis at an MOI of 50 for 10 min at 37°C. Cells were centrifuged at 500 × g for 10 min, washed with prewarmed fluorescence-activated cell sorter (FACS) buffer (5% fetal calf serum [FCS] and 0.02% NaN3 in PBS), and fixed in 4% paraformaldehyde (PFA) in PBS for 5 min. Nonspecific binding of the antibodies was prevented by incubating the neutrophils with Fc Receptor Blocker (Innovex Biosciences Inc., Richmond, CA, USA) for 15 min on ice. Fc Receptor Blocker was removed by centrifugation at 500 × g for 5 min followed by one wash with FACS buffer. Neutrophils were incubated with fluorescein isothiocyanate (FITC) anti-human CD66b antibody (1:50; clone G10F5; BioLegend, San Diego, CA, USA) or FITC IgM isotype control antibody (1:80; clone 11E10; eBioscience Inc., San Diego, CA, USA) in FACS buffer for 30 min on ice with gentle agitation. Cells were washed, resuspended in FACS buffer, and analyzed by the FACSCalibur Analyzer (BD Biosciences, San Jose, CA, USA) using CellQuest software.
To determine cell viability after 30 min of infection, neutrophils were washed in FACS buffer and propidium iodide (0.5 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) added (see Fig. S1B in the supplemental material). Fluorescence intensity was analyzed by Guava (Merck Millipore, Darmstadt, Germany), using InCyte software.
Extracellular bacterial survival.
For extracellular bacterial survival determination, neutrophils (2 × 106 cells/ml) were left untreated or treated with 1 μg/ml PMA for 30 min at 37°C and the extracellular supernatants containing degranulated proteins were collected. Eight thousand CFU of wild-type Y. pseudotuberculosis were incubated for 1 h at 37°C with supernatants from cells treated with PMA or left untreated, and bacterial survival was determined by culturing serially diluted bacteria on LB agar plates.
Treatment of neutrophils with inhibitors.
Neutrophils were pretreated with inhibitors (unless otherwise indicated, all inhibitors were purchased from Enzo Life Sciences, Lausen, Switzerland) for 30 min at 37°C prior to infection at the following concentrations: 50 μM D609, 1 μM edelfosine (sn-Et-18-OCH3), 50 μM genistein, 25 μM LY294002, 2 μM Ro 318220, 10 μM BAPTA-AM (Invitrogen, Eugene, OR, USA), 20 μM cytochalasin D (Sigma-Aldrich, St. Louis, MO, USA). Inhibitors were titrated to avoid cytotoxic effects on neutrophils.
Western blot analyses.
Protein samples were resolved by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) at a constant current of 0.8 mA/cm2 of membrane area for 75 min by semidry electroblotting. Nonspecific binding was blocked by incubation of the membranes in 5% milk powder in Tris-buffered saline (pH 8) containing 0.1% Tween 20 (TBST) for 1 h at RT. Membranes were incubated with a rabbit anti-human lactoferrin antibody (1:10,000; Sigma-Aldrich, St. Louis, MO, USA), rabbit anti-human myeloperoxidase antibody (1:5,000; Dako, Glostrup, Denmark), rabbit anti-human matrix metalloproteinase-9 antibody (1:2,000; Millipore, Darmstadt, Germany) in TBST, or rabbit anti-human ERK1/2 antibody (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) in 5% BSA in TBST for 1 h at RT or overnight at 4°C followed by washing in TBST and incubation with horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:5,000; GE Healthcare, Buckinghamshire, United Kingdom) for 1 h at RT. Amersham ECL (GE Healthcare, Buckinghamshire, United Kingdom) was used for detection.
Immunochemistry.
Glass coverslips (11 mm; Thermo Scientific, Bremen, Germany) were coated with 100 μg/ml poly-l-lysine (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at RT, washed in water, and then dried. Freshly prepared neutrophils in KRG were allowed to adhere to poly-l-lysine-coated coverslips (50,000 cells/coverslip) for 1 h at RT. Bacterial suspensions of Y. pseudotuberculosis strains were washed and resuspended to 2 × 107 bacteria/ml in KRG and then incubated with neutrophils at an MOI of 30 and different incubation times at RT. Internalization of bacteria was inhibited by pretreatment of neutrophils with 1 μM cytochalasin D for 15 min at RT prior to infection (35). Nonadherent bacteria were removed by PBS washes, and then cells were fixed in 4% paraformaldehyde in PBS for 10 min at RT. After 3 washes with PBS, cells were permeabilized with 0.5% Triton X-100 in PBS for 3 min, followed by PBS washes and then incubation in 0.1 M glycine in PBS for 10 min at RT. After three rinses, nonspecific protein binding was blocked by 1% BSA in PBS supplemented with 5% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 30 min at RT. Coverslips were incubated with primary antibodies for 1 h at RT and then washed 3 or 4 times in PBS. To detect secondary granules, mouse anti-human CD66b antibody was used (1:200; clone 80H3; AbD SeroTec, Oxford, United Kingdom); for detection of primary granules, mouse anti-human CD63 antibody (1:300; clone 2Q1430, Nordic BioSite AB, Täby, Sweden) was used. As negative controls, coverslips were incubated without the primary antibody. Alexa Fluor 488-conjugated donkey anti-mouse IgG (1:500; Invitrogen, Paisley, United Kingdom) was used as a secondary antibody. After incubation for 30 min at RT and then 3 or 4 washes with PBS, coverslips were blocked with 5% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in PBS containing 1% BSA to stain bacteria. Coverslips were then incubated with rabbit anti-Yersinia antiserum (1:500; AgriSera, Vännäs, Sweden) for 1 h at RT, followed by 3 or 4 washes in PBS and incubation with an Alexa Fluor 555-conjugated goat anti-rabbit IgG (1:500; Invitrogen, Paisley, United Kingdom) for 30 min at RT. After 3 or 4 washes in PBS, nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; 1:5,000; Invitrogen, Paisley, United Kingdom). After a further 3 or 4 rinses in PBS and 1 rinse in water, coverslips were mounted on glass slides and examined using a Nikon 90i laser scanning confocal microscope (Nikon, Japan) equipped with a Nikon camera. Imaging was performed with a 100×/1.4 Plan Apochromat oil immersion objective. Only cells with adhered bacteria were analyzed for accumulation. For quantification, after background subtraction, only distinct CD66b- or CD63-positive granules around adhered bacteria were scored as positive for accumulation.
Statistical analyses of data.
All graphs were created using Graph Pad PRISM V6.05. Comparisons were made by analysis of variance (ANOVA; Tukey's multiple-comparison test) or the Student t test. All densitometric analyses of Western blots were performed with ImageJ 1.48v (NIH), and the numbers were provided at the bottom of each Western blot. Unless otherwise indicated, numbers under the bands indicate the relative fold increases or decreases in the protein levels released by infected cells compared to uninfected cells, in the latter of which the protein levels were set as 1.0.
RESULTS
Y. pseudotuberculosis blocks the release of neutrophil granules, which increases its survival.
Neutrophil granule content is expected to be released to the cell exterior at the infection site and would ordinarily be expected to adversely affect extracellular bacteria, such as Y. pseudotuberculosis. This includes secondary granules that are released externally as well as primary granules that are mainly released into the phagosome but also expected to be exposed to extracellular bacteria at the infection site by being released externally during the phagocytic process. Tertiary granules with extracellular matrix (ECM)-degrading enzymes, on the other hand, are released at earlier stages (36). First, we investigated the effect of Y. pseudotuberculosis on neutrophil degranulation of secondary granules. This was determined by detection of lactoferrin in supernatants of infected neutrophils, these having been isolated from human blood and infected with the Y. pseudotuberculosis wild-type strain or the nonvirulent YPIII plasmid-cured strain for 30 min. Supernatants of PMA-stimulated neutrophils were included as positive controls. Western blot analyses revealed the presence of lactoferrin in cell-free supernatants derived from neutrophils incubated with the plasmid-cured bacteria; only very low levels of lactoferrin were found in the supernatants of neutrophils incubated with the wild-type strain (Fig. 1A). Western blotting using anti-ERK1/2 antibodies confirmed that the observed release of lactoferrin was not due to cell lysis (Fig. 1A). These data suggest that virulent wild-type Y. pseudotuberculosis blocks the release of secondary granules. To verify this further, we employed flow cytometry to determine the expression of the membrane-bound secondary granule marker CD66b on neutrophil cell surface 10 min after infection. Also with this method the results indicated a blocking effect by the wild-type strain, where neutrophils infected with the plasmid-cured strain had increased surface expression of CD66b compared to neutrophils infected with the wild-type strain (Fig. 1B).
FIG 1.
Y. pseudotuberculosis blocks the release of neutrophil granules, which increases its survival. (A) Neutrophils left untreated or infected with plasmid-cured (pc) or wild-type (wt) Y. pseudotuberculosis at an MOI of 10 for 30 min. Cell-free supernatants from equivalent cell numbers were analyzed by Western blotting using lactoferrin (LF), myeloperoxidase (MPO), and matrix metalloproteinase-9 (MMP9) as markers of secondary, primary, and tertiary granules, respectively. ERK1/2 was used as a marker for cell lysis. Supernatants from PMA-treated neutrophils were used as positive controls. Cell lysates were immunoblotted for levels of remaining lactoferrin and ERK1/2 as loading control. The blots shown are representative of 3 to 10 independent experiments done on cells from different donors. (B) Cell surface expression of secondary granules determined by flow cytometry using CD66b as a marker. Neutrophils were left untreated or infected with pc or wt Y. pseudotuberculosis for 10 min. Data represent the means ± standard errors of the means (SEM) from three independent experiments done on cells from different donors (differences between conditions were analyzed by Tukey's multiple-comparison test; **, P ≤ 0.01). (C) Survival of wt Y. pseudotuberculosis was checked after treatment for 60 min with cell-free supernatant from neutrophils treated with or without PMA for 30 min. Data are presented as percentages of surviving bacteria of the original number at time zero and means ± SEM for four independent experiments done on cells from different donors (differences between conditions were analyzed by the Student t test; **, P ≤ 0.01).
The release of primary granules was determined by detection of myeloperoxidase (MPO) in cell-free supernatants. Plasmid-cured bacteria induced the release also of this granule type, and similar to what was seen for the secondary granule marker, there was less MPO in supernatants from neutrophils infected with the wild-type strain (Fig. 1A). At earlier time points (15 min after infection), only lactoferrin and no MPO was detected in the supernatant of neutrophils infected with the plasmid-cured strain, suggesting that part of the detected MPO is a result of phagocytic activity, which is expected to require stronger stimulation (see Fig. S1A in the supplemental material). A similar tendency was seen for the release of tertiary granules that was determined by the detection of matrix metalloproteinase-9 (MMP9), with a low level of release as response to infection with the plasmid-cured strain and no release from cells infected with the wild-type strain (Fig. 1A). The levels of MMP-9 in the supernatant were, however, very low, which likely reflects that these granules had been partly released prior to infection, as a result of handling of the cells. Nevertheless, our data suggest a general blocking effect by Y. pseudotuberculosis on neutrophil degranulation.
Next, we evaluated the potentially detrimental effect of neutrophil degranulated products on Y. pseudotuberculosis. For this, neutrophil degranulation was induced with PMA, which causes release of primary, secondary, and tertiary granules. The supernatants were collected and incubated with bacteria for 60 min before plating bacteria for viable count determination. The resulting data showed that supernatants from PMA-treated neutrophils markedly reduced bacterial viability (Fig. 1C). Hence, this highlights the potential importance for Y. pseudotuberculosis to block neutrophil degranulation for its survival and thereby a successful infection.
Y. pseudotuberculosis blocks primed neutrophils and impairs the ability to respond to other bacteria.
Neutrophils, at sites of infection, are expected to be primed due to prestimulation by low concentrations of bacterial products and inflammatory factors, making them highly responsive to an activating stimulus (37). Therefore, we next investigated whether bacterially induced degranulation of primed neutrophils would also be blocked by the wild-type strain. To accomplish this, neutrophils were prestimulated with E. coli LPS prior to infection with plasmid-cured or wild-type bacteria. The LPS priming did not affect PMA-induced release (see Fig. S2A in the supplemental material). It was, however, clear that the priming increased the release of secondary granules following infection by both wild-type and plasmid-cured bacteria, but the level of release was still considerably lower for cells infected with the wild-type strain than with the plasmid-cured bacteria (Fig. 2A). Thus, wild-type Y. pseudotuberculosis is also able to block secondary granule degranulation by primed neutrophils.
FIG 2.
Y. pseudotuberculosis blocks primed neutrophils and affects the ability to respond to other bacteria. (A) Neutrophils left untreated or preactivated with LPS for 30 min prior to infection with plasmid-cured (pc) or wild-type (wt) Y. pseudotuberculosis at an MOI of 10 for 30 min. (B) Neutrophils left untreated or pretreated with wt Y. pseudotuberculosis (wt-pt) for 15 min prior to infection with either pc Y. pseudotuberculosis or E. coli for 30 min. (C) Neutrophils left untreated or pretreated with wt Y. pseudotuberculosis (wt-pt) for 15 min followed by the removal of residual bacteria by gentle centrifugation. Neutrophils were then left resting for 0 or 30 min in the presence of gentamicin prior to infection with pc Y. pseudotuberculosis or E. coli. All blots shown are representative of three independent experiments done on cells from different donors.
To further investigate this phenomenon, we evaluated whether virulent Y. pseudotuberculosis could influence the ability of neutrophils to respond to other bacteria. First, we analyzed the ability of wild-type bacteria to block degranulation in the presence of plasmid-cured Y. pseudotuberculosis or E. coli. For this, neutrophils were left uninfected or preinfected with wild-type Y. pseudotuberculosis, followed by infection with plasmid-cured Y. pseudotuberculosis or E. coli. Interestingly, preincubation with wild-type Y. pseudotuberculosis was sufficient to efficiently prevent the release of lactoferrin both by the plasmid-cured strain and by E. coli (Fig. 2B). PMA-induced release was, however, unaffected. Next, we addressed if this unresponsive state of neutrophils required the presence of wild-type bacteria. For this, neutrophils were left uninfected or preinfected with wild-type Y. pseudotuberculosis, followed by the removal of bacteria with gentle centrifugation and then gentamicin treatment. Subsequently, both uninfected neutrophils and neutrophils preinfected with wild-type bacteria were infected with plasmid-cured Y. pseudotuberculosis or E. coli at 0, 30, and 120 min after the removal of residual wild-type bacteria and gentamicin. It was clear that the neutrophils that were preincubated with wild-type bacteria were rendered unresponsive to plasmid-cured bacteria and E. coli 30 min after removal of the wild-type bacteria (Fig. 2C). This unresponsive state was transient and declined until rendered undetectable after 120 min (see Fig. S2B in the supplemental material). Together, these data suggest that neutrophils that are preexposed to wild-type Y. pseudotuberculosis have a reduced ability to combat other bacteria.
Y. pseudotuberculosis blocks the accumulation of secondary granules in neutrophils.
For degranulation to occur, a prior translocation of the granules to phagosomes or the plasma membrane is required (6, 38). To examine the effect of Y. pseudotuberculosis on the accumulation of secondary granules within neutrophils upon infection, neutrophils were adhered to poly-l-lysine-coated coverslips and then incubated with Y. pseudotuberculosis plasmid-cured or wild-type bacteria for 5, 10, and 15 min. This was followed by double immunostainings for bacteria and the secondary granule marker CD66b. Only cells with adhered bacteria were analyzed for accumulation of secondary granules. We noted that CD66b-positive secondary granules accumulated close to the bacterial contact site in neutrophils infected with the plasmid-cured strain while this accumulation was absent in cells incubated with the wild-type strain (Fig. 3A). Granule accumulation at the bacterial contact site was rapid, with 27% of neutrophils infected with the plasmid-cured strain demonstrating granule recruitment after 5 min, and 38% after 10 min (see Fig. S3A in the supplemental material). No granule accumulation was seen for neutrophils infected with wild-type bacteria at any of the analyzed time points. The observed granule accumulation was not obvious for primary granules, as there was no detected accumulation of the primary granule marker CD63 after 10 min of infection (see Fig. S3B in the supplemental material). However, after 30 min in the absence of cytochalasin D, accumulation of CD63 at bacterial contact sites in neutrophils infected with the plasmid-cured strain was detected and was higher than that in wild-type-infected cells (see Fig. S3C and D in the supplemental material). As this was apparent only in the absence of cytochalasin D, this likely reflects the coupling to bacterial internalization by the neutrophils. Together, these data suggest that wild-type Y. pseudotuberculosis blocks the degranulation of granules by preventing their recruitment to sites of adhered bacteria.
FIG 3.

Y. pseudotuberculosis blocks the accumulation of secondary granules in neutrophils. (A) Neutrophils left untreated or infected with plasmid-cured (pc) or wild-type (wt) Y. pseudotuberculosis at an MOI of 30 for 10 min and stained for secondary granules with anti-CD66b antibody (green), for Y. pseudotuberculosis with an anti-Yersinia antibody (red), and for DNA counterstained with DAPI (blue). (B) Adherence of IgG-opsonized (Ops) and nonopsonized (Non-ops) pc and wt Y. pseudotuberculosis to neutrophils after 10-min infection. Data are expressed as percentages of cells with adhered bacteria and presented as means ± SEM from four independent experiments done on cells from different donors (differences between conditions were analyzed by Tukey's multiple-comparison test; n.s., not significant; ***, P ≤ 0.001). (C) Accumulation of CD66b-positive granules at the sites of bacterial attachment to neutrophils infected with IgG-opsonized (Ops) and nonopsonized (Non-ops) pc and wt Y. pseudotuberculosis for 10 min. Data are expressed as percentages of cells with bound bacteria showing accumulation of CD66b-positive granules (number of analyzed cells for opsonized wt, n = 135; for nonopsonized wt, n = 44; for opsonized pc, n = 214; for nonopsonized pc, n = 185) and presented as means ± SEM for four independent experiments done on cells from different donors (differences between conditions were analyzed by Tukey's multiple-comparison test; n.s., not significant). (D) Release of secondary granules by neutrophils infected for 30 min with opsonized or nonopsonized pc and wt Y. pseudotuberculosis. The blot shown is representative of three independent experiments done on cells from different donors.
Given that plasmid-cured Y. pseudotuberculosis adheres to cells more efficiently than the virulent wild-type strain (39), it was necessary to rule out if this had influenced the accumulation of secondary granules. First, we determined an eventual role for YadA, an adhesion protein that is encoded by the virulence plasmid and is lacking in the YPIII/pIB102 strain used in this study. No influence of this adhesin was found when comparing levels of release of lactoferrin by neutrophils infected with Y. pseudotuberculosis wild-type strains expressing yadA, YPIII/pIB1, and YPIII/pIB102 (see Fig. S3E in the supplemental material). Second, we aimed at increasing the neutrophil adhesion of the wild-type strain to certify that the blocking effect is independent of attachment efficiency. To achieve this, bacteria were opsonized with anti-Yersinia antisera prior to infection in order to increase their adherence to neutrophil Fc receptors. Immunostaining of neutrophils infected with nonopsonized and opsonized bacteria showed that opsonization increased their adherence to cells (Fig. 3B). This was particularly apparent for the wild-type strain, for which the number of cells carrying bound bacteria increased 3-fold compared to that of nonopsonized wild-type bacteria. However, despite their increased adherence to neutrophils, no granule accumulation was detected in neutrophils in contact with opsonized wild-type bacteria (Fig. 3C; see also Fig. S3F in the supplemental material). Furthermore, the level of lactoferrin was still very low in supernatants from neutrophils infected with opsonized wild-type Y. pseudotuberculosis 30 min after infection (Fig. 3D). These data indicate that the wild-type strain efficiently modulates the degranulation machinery in order to prevent the exocytosis of secondary granules.
YopH and YopE cooperate to block degranulation in neutrophils.
Given that the observed blockage of secondary granule release by Y. pseudotuberculosis required the presence of the virulence plasmid, we then investigated the role of individual virulence effectors encoded by this virulence plasmid. For this purpose, neutrophils were infected with yopH, yopE, yopM, yopJ, yopK, and ypkA mutant strains; supernatants were then analyzed by Western blotting for the release of lactoferrin. While the release of lactoferrin by cells infected with yopM, yopJ, yopK, and ypkA was similar to that seen for the wild-type strain (see Fig. S4A in the supplemental material), it was increased in the supernatants of cells infected with the yopH and yopE mutant strains (Fig. 4A). Strikingly, infection with a yopHE double mutant strain recovered lactoferrin release to levels comparable to that achieved for cells infected with the plasmid-cured strain (Fig. 4A). This was also true for the release of MPO and MMP9 (see Fig. S4B in the supplemental material). Further, immunostaining results for CD66b and Y. pseudotuberculosis using neutrophils infected with the yopH, yopE, and yopHE mutant strains were consistent with the Western blot data collected for lactoferrin release insofar as the blockade of CD66b accumulation to bacterial contact sites was reduced for both single yop mutants compared to the wild-type strain. However, the yopHE double mutant strain induced accumulation of CD66b to the same extent as that seen in the plasmid-cured strain (Fig. 4B). Hence, the additive effect for YopE and YopH seen for both granule accumulation and release suggests their cooperation in efficiently blocking degranulation.
FIG 4.
YopH and YopE cooperate to block degranulation in neutrophils. (A) Neutrophils were left untreated or infected for 30 min with the Y. pseudotuberculosis strains indicated. The upper panel shows densitometric evaluation of lactoferrin release presented as relative density normalized to control cells and means ± SEM for six independent experiments done on cells from different donors (differences between conditions were analyzed by Tukey's multiple-comparison test; **, P ≤ 0.01). The lower panel shows a representative Western blot for the same six independent experiments. (B) Accumulation of CD66b-positive granules at bacterial contact sites after 10 min of infection with the indicated Y. pseudotuberculosis strains, as revealed by confocal microscopy. Data presented as means ± SEM for three independent experiments done on cells from different donors (differences between conditions were analyzed by Tukey's multiple-comparison test; **, P ≤ 0.01; ***, P ≤ 0.001).
Mechanisms involved in Y. pseudotuberculosis-induced secondary granule accumulation and release.
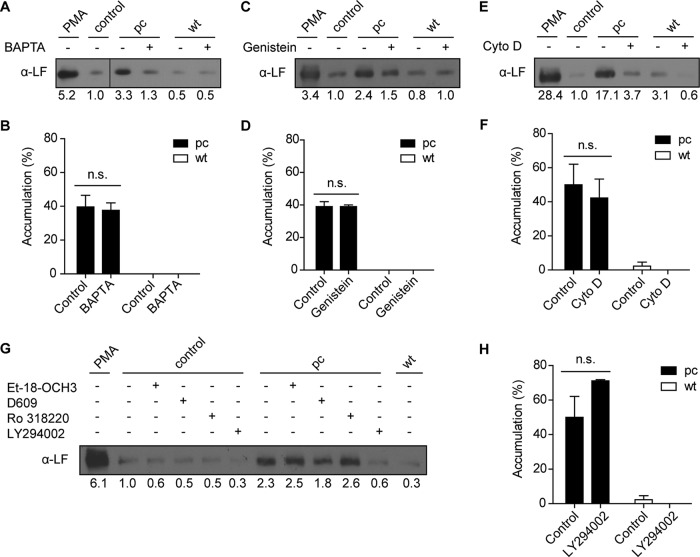
Neutrophils are major target cells for Yop translocation during infection, and YopH and YopE are expected to act on early signaling events provoked upon bacterial binding to the neutrophil surface. One of YopH's distinct effects on neutrophils is to inhibit immediate early calcium responses (28, 29). Since neutrophil degranulation is known to involve Ca2+ signaling, we analyzed whether inhibition of the Ca2+ response could be one of the underlying mechanisms behind the degranulation block instigated by wild-type Y. pseudotuberculosis. To assess this, neutrophils were pretreated with the calcium chelator BAPTA-AM to clamp the level of intracellular Ca2+. Release of the secondary granule content was largely quenched in neutrophils exposed to BAPTA-AM and subsequently infected by plasmid-cured Y. pseudotuberculosis (Fig. 5A). Surprisingly, the accumulation of CD66b-positive granules at the bacterial contact site was unaffected by the BAPTA-AM treatment (Fig. 5B; see also Fig. S5A in the supplemental material). Furthermore, since YopH is a potent tyrosine phosphatase, we investigated the effects of tyrosine kinase inhibitors on degranulation using the general tyrosine kinase inhibitor genistein. Lactoferrin release following infection with the plasmid-cured strain was clearly lower in genistein-treated cells than in untreated cells (Fig. 5C). Similar to what was seen for BAPTA-AM, genistein did not inhibit the accumulation of CD66b-positive granules to the bacterial contact site (Fig. 5D; see also Fig. S5A in the supplemental material). YopE impairs actin dynamics via its GTPase activity and in this fashion contributes to the inhibition of phagocytosis (40). To reveal the contribution of actin dynamics to granule release, neutrophils were pretreated with 20 μM cytochalasin D, which blocked lactoferrin release by the plasmid-cured strain without affecting granule accumulation (Fig. 5E and F; see also Fig. S5A in the supplemental material).
FIG 5.
Mechanisms involved in Y. pseudotuberculosis-induced secondary granule accumulation and release. Neutrophils in suspension (A, C, E) or adhered to coverslips (B, D, F) were treated with BAPTA-AM (A, B), genistein (C, D), or cytochalasin D (E, F) for 30 min prior to infection with plasmid-cured (pc) or wild-type (wt) Y. pseudotuberculosis. The presence of lactoferrin in supernatants from equivalent numbers of cells was analyzed by Western blotting (A, C, E). The blot in panel A was spliced from the same blot, shown as a thin line between lanes 2 and 3. Accumulation of CD66b-positive granules to the bacteria contact sites was analyzed by confocal microscopy (B, D, F) after staining of secondary granules with anti-CD66b antibody (green), of bacteria with anti-Yersinia antibody (red), and of DNA with DAPI (blue). The number of cells with adhered bacteria was determined for each coverslip, and the percentage of cells showing accumulation of CD66b-positive granules to the bacterial contact site was calculated. Data are presented as means ± SEM for two or three independent experiments done on cells from different donors (differences between conditions were analyzed by the Student t test; n.s., not significant). (G) Release of lactoferrin in supernatants from neutrophils treated with Et-18-OCH3, D609, LY294002, or Ro 318220 for 30 min prior to infection for 30 min with pc or wt Y. pseudotuberculosis. (H) Neutrophils were adhered to coverslips, treated with LY294002 for 30 min, and then infected with pc or wt Y. pseudotuberculosis for 10 min, before staining for secondary granules, bacteria, and DNA. The accumulation of CD66b-positive granules to bacterial contact sites was analyzed by confocal microscopy. Data are presented as means ± SEM for three independent experiments done on cells from different donors (differences between conditions were analyzed by the Student t test; n.s., not significant). All blots shown are representative of three or four independent experiments done on cells from different donors.
We next investigated the contribution of different signaling pathways to degranulation stimulated by plasmid-cured Y. pseudotuberculosis by analyzing the phospholipase C (PLC), protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K) pathways. As genetic manipulation of neutrophils is not possible, we employed different pharmacologic inhibitors to target each pathway (indicated in parentheses): Et-18-OCH3 (PI-PLC), D609 (PC-PLC), LY294002 (PI3K), and Ro 318220 (PKC). Only treatment with the PI3K inhibitor resulted in decreased degranulation (Fig. 5G), with no effect on granule accumulation (Fig. 5H; see also Fig. S5A in the supplemental material). Similar effects were seen using the yopHE double mutant (see Fig. S5B and C in the supplemental material), and none of the inhibitors affected the PMA-induced release of lactoferrin (see Fig. S5D in the supplemental material). A combination of Et-18-OCH3 (PI-PLC), D609 (PC-PLC), and Ro 318220 (PKC) was also tested but showed no obvious inhibitory effect on granule release stimulated by the plasmid-cured strain (see Fig. S5E in the supplemental material). Taken together, these results indicate that the signal transduction involved in Y. pseudotuberculosis-induced degranulation of secondary granules requires Ca2+, actin dynamics, and tyrosine kinase, as well as PI3K activity.
DISCUSSION
The antimicrobial products released by degranulating neutrophils represent a potent and immediate threat to bacteria. Thus, mechanisms to avoid bacterial exposure to granule content represent an efficient evasion strategy for those microbes that colonize those extracellular niches to which large number of neutrophils can be readily recruited. Here we investigated the effect of the enteropathogen Y. pseudotuberculosis on neutrophil degranulation. We show that wild-type Y. pseudotuberculosis efficiently suppresses the release of the secondary granule marker lactoferrin from human neutrophils and that this block is a consequence of inhibited granule accumulation at the bacterium-neutrophil contact site. Our findings indicate that Y. pseudotuberculosis mediates a general inhibitory effect on granule release, including release of primary, secondary, and tertiary granules. This is of particular interest, since precursor proteins such as hCAP-18 from secondary granules are expected to be activated by the contents from peroxidase-positive primary granules (4). This type of immune evasion trait would enable this pathogen to efficiently avoid exposure to granule components upon their initial encounter with neutrophils. Hence, together with its known antiphagocytic trait (14, 39) and the ability to block the release of neutrophil extracellular traps (41), degranulation inhibition renders Y. pseudotuberculosis superbly well equipped to derail multiple defensive strategies employed by neutrophils.
It was clear that the virulence plasmid is a prerequisite for the observed block of degranulation by the Y. pseudotuberculosis wild-type strain, as plasmid-cured bacteria could still induce degranulation. The inhibitory effect was partially lost in neutrophils exposed to yopH and yopE mutant strains and completely lost in cells infected with the yopHE double mutant, indicating that these virulence effectors are responsible for the blocking effect. Likewise, another type of degranulation is impaired by similar effector activities; Salmonella enterica serovar Typhimurium utilizes its YopH-YopE homologue SptP to suppress the degranulation of mast cells (42). The translocation and subsequent activities of YopH and YopE are expected to act immediately upon contact of the bacterium with the host cell (43). It is therefore likely that the virulence effectors translocated by the wild-type strain act on immediate early signals emanating from receptors that have bound bacteria at the neutrophil surface. This is a situation analogous to that shown previously for the YopH-mediated blockade of macrophage and neutrophil internalization (28, 29, 35, 44). Both YopH and YopE are major effectors responsible for blocking phagocytic uptake (28, 45, 46). It is likely that these effectors target neutrophil signaling molecules that are important for the initiation of both phagocytosis and degranulation, ensuring that both of these antimicrobial functions, and maybe others, are disabled. Thus, Yersinia uses one strategy to instantaneously neutralize several mechanisms of the neutrophil's antimicrobial arsenal, underscoring the power of the virulence effector machinery.
Currently, the exact molecular mechanisms involved in the blockade of secondary granule accumulation by Y. pseudotuberculosis remain unclear. Inhibition of those pathways known to be targeted by effectors (Ca2+, tyrosine kinase signaling, and actin dynamics), together with a blockage of PI3K signaling, resulted in the prevention of secondary granule release but not accumulation. Since wild-type Y. pseudotuberculosis blocks secondary granule accumulation via YopH and YopE, these data suggest that there might be other, as-yet-unknown targets for these effectors, which are specifically involved in granule accumulation. Another explanation for these results might be the various experimental conditions used; suspension cells were used to assay lactoferrin release, while neutrophils were allowed to adhere to coverslips prior to bacterial exposure for the determination of granule accumulation. In contrast to secondary granule accumulation, we noted an effect by plasmid-cured bacteria on primary granule accumulation in the absence, but not in the presence, of cytochalasin D, thus suggesting that recruitment of these granules is induced by phagocytosis. This was in contrast to secondary granules for which accumulation induced by the plasmid-cured strain was not blocked by pretreatment by cytochalasin D, suggesting that the bacterial contact itself induces the movement of these granules.
Interestingly, the block of degranulation by Yersinia appears to be very prominent, as even primed neutrophils are efficiently targeted. Primed neutrophils are sensitive to even weak external stimuli in vitro (37, 47). This reflects the in vivo scenario whereby neutrophils in the bloodstream become preactivated by activated endothelia and can sense and respond to extremely low concentrations of, for example, tissue chemoattractants. Neutrophils recruited to the infection site are expected to arrive primed and ready to tackle invading bacteria (37). Hence, the ability to block primed neutrophils illustrates a necessary aspect of Yersinia's ability to evade neutrophil defenses. In addition, we show that Yersinia can influence the neutrophil's antimicrobial activities even after its initial contact with the neutrophil surface. Preexposure of neutrophils to wild-type Y. pseudotuberculosis rendered neutrophils unresponsive to both E. coli and plasmid-cured Y. pseudotuberculosis. Although this “stunned” effect was only transient and seemed to require the presence of wild-type bacteria, it should still impact the ability of neutrophils to fight other bacteria, thereby influencing the infectious outcome.
We have previously shown that Y. pseudotuberculosis has an antiapoptotic effect on neutrophils that is independent of its Yop effectors (48). The increased survival of neutrophils induced by the bacteria is assumed to benefit Yersinia pathogenicity by preventing cell death, thereby promoting the retention of danger signals that would otherwise signal immune responses. Our present finding that Yersinia uses both YopH and YopE to inhibit degranulation, together with their previously shown inhibitory effects on phagocytosis, suggests that Yersinia uses its pathogenic effectors to specifically target antimicrobial functions. Hence, Yersinia manipulates the neutrophil in a highly sophisticated fashion, affecting neutrophil functions in manners both positive and negative. Such dual effects might allow a direct avoidance of antimicrobial effects while indirectly preventing any amplification of the innate immune response by stifling the release of danger signals.
Supplementary Material
ACKNOWLEDGMENTS
We thank Saskia Erttmann and Kristina Ruuth for technical advice, Constantin Urban for technical advice and critical reading of the manuscript, Denise Rawcliffe and Alain Damba for technical assistance, and the blood donors.
Funding Statement
This work was supported by grants from the Swedish Research Council (K2014-56X-11222-20-5) and Insamlingsstiftelsen at Umeå University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00760-16.
REFERENCES
- 1.Borregaard N. 2010. Neutrophils, from marrow to microbes. Immunity 33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Borregaard N, Sorensen OE, Theilgaard-Wnchl K. 2007. Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Soehnlein O. 2009. Direct and alternative antimicrobial mechanisms of neutrophil-derived granule proteins. J Mol Med 87:1157–1164. doi: 10.1007/s00109-009-0508-6. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796–2803. [PubMed] [Google Scholar]
- 5.Lacy P. 2006. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2:98–108. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. 2008. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol 295:C1354–C1365. doi: 10.1152/ajpcell.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollinedo F. 2003. Human neutrophil granules and exocytosis molecular control. Inmunologia 22:340–358. [Google Scholar]
- 8.Futosi K, Fodor S, Mocsai A. 2013. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Nordenfelt P, Tapper H. 2010. The role of calcium in neutrophil granule-phagosome fusion. Commun Integr Biol 3:224–226. doi: 10.4161/cib.3.3.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lew PD, Monod A, Waldvogel FA, Dewald B, Baggiolini M, Pozzan T. 1986. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol 102:2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sengeløv H, Kjeldsen L, Borregaard N. 1993. Control of exocytosis in early neutrophil activation. J Immunol 150:1535–1543. [PubMed] [Google Scholar]
- 12.Rothwell SW, Nath J, Wright DG. 1989. Interactions of cytoplasmic granules with microtubules in human neutrophils. J Cell Biol 108:2313–2326. doi: 10.1083/jcb.108.6.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smego RA, Frean J, Koornhof HJ. 1999. Yersiniosis I: microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis 18:1–15. doi: 10.1007/s100960050219. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann R, van Erp K, Trulzsch K, Heesemann J. 2004. Transcriptional responses of murine macrophages to infection with Yersinia enterocolitica. Cell Microbiol 6:377–390. doi: 10.1111/j.1462-5822.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 15.Schesser K, Spiik AK, Dukuzumuremyi JM, Neurath MF, Pettersson S, Wolf-Watz H. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol 28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 16.Brubaker RR. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect Immun 71:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis GR, Wolf-Watz H. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol 23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto H, Young GM. 2009. Translocated effectors of Yersinia. Curr Opin Microbiol 12:94–100. doi: 10.1016/j.mib.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viboud GI, Bliska JB. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 20.Akopyan K, Edgren T, Wang-Edgren H, Rosqvist R, Fahlgren A, Wolf-Watz H, Fallman M. 2011. Translocation of surface-localized effectors in type III secretion. Proc Natl Acad Sci U S A 108:1639–1644. doi: 10.1073/pnas.1013888108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson KE, Wolf-Watz H. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 22.Fahlgren A, Avican K, Westermark L, Nordfelth R, Fallman M. 2014. Colonization of cecum is important for development of persistent infection by Yersinia pseudotuberculosis. Infect Immun 82:3471–3482. doi: 10.1128/IAI.01793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durand EA, Maldonado-Arocho FJ, Castillo C, Walsh RL, Mecsas J. 2010. The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell Microbiol 12:1064–1082. doi: 10.1111/j.1462-5822.2010.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mecsas J, Bilis I, Falkow S. 2001. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infect Immun 69:2779–2787. doi: 10.1128/IAI.67.5.2779-2787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallman M, Gustavsson A. 2005. Cellular mechanisms of bacterial internalization counteracted by Yersinia. Int Rev Cytol 246:135–188. doi: 10.1016/S0074-7696(05)46004-0. [DOI] [PubMed] [Google Scholar]
- 26.Persson C, Carballeira N, WolfWatz H, Fallman M. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130(Cas) and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J 16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black DS, Bliska JB. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J 16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson K, Magnusson KE, Majeed M, Stendahl O, Fallman M. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect Immun 67:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolán HG, Durand EA, Mecsas J. 2013. Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe 14:306–317. doi: 10.1016/j.chom.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black DS, Bliska JB. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol 37:515–527. [DOI] [PubMed] [Google Scholar]
- 31.von Pawel-Rammingen U, Telepnev MV, Schmidt G, Aktories K, Wolf-Watz H, Rosqvist R. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol Microbiol 36:737–748. [DOI] [PubMed] [Google Scholar]
- 32.Westermark L, Fahlgren A, Fallman M. 2014. Yersinia pseudotuberculosis efficiently escapes polymorphonuclear neutrophils during early infection. Infect Immun 82:1181–1191. doi: 10.1128/IAI.01634-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fallman M, Andersson K, Hakansson S, Magnusson KE, Stendahl O, Wolfwatz H. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun 63:3117–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser LG, Annema A, van Furth R. 1995. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect Immun 63:2570–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahlgren A, Westermark L, Akopyan K, Fallman M. 2009. Cell type-specific effects of Yersinia pseudotuberculosis virulence effectors. Cell Microbiol 11:1750–1767. doi: 10.1111/j.1462-5822.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 36.Soehnlein O, Zernecke A, Weber C. 2009. Neutrophils launch monocyte extravasation by release of granule proteins. Thromb Haemost 102:198–205. [DOI] [PubMed] [Google Scholar]
- 37.Condliffe AM, Kitchen E, Chilvers ER. 1998. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 38.Toonen RFG, Verhage M. 2003. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol 13:177–186. doi: 10.1016/S0962-8924(03)00031-X. [DOI] [PubMed] [Google Scholar]
- 39.Rosqvist R, Bolin I, Wolfwatz H. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis—a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun 56:2139–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosqvist R, Forsberg A, Wolfwatz H. 1991. Intracellular targeting of the Yersinia Yope cytotoxin in mammalian-cells induces actin microfilament disruption. Infect Immun 59:4562–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillenius E, Urban CF. 2015. The adhesive protein invasin of Yersinia pseudotuberculosis induces neutrophil extracellular traps via beta1 integrins. Microbes Infect 17:327–336. doi: 10.1016/j.micinf.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Choi HW, Brooking-Dixon R, Neupane S, Lee CJ, Miao EA, Staats HF, Abraham SN. 2013. Salmonella typhimurium impedes innate immunity with a mast-cell-suppressing protein tyrosine phosphatase, SptP. Immunity 39:1108–1120. doi: 10.1016/j.immuni.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosqvist R, Magnusson KE, Wolfwatz H. 1994. Target-cell contact triggers expression and polarized transfer of Yersinia Yope cytotoxin into mammalian cells. EMBO J 13:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson K, Carballeira N, Magnusson KE, Persson C, Stendahl O, WolfWatz H, Fallman M. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol Microbiol 20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 45.Guan KL, Dixon JE. 1990. Protein tyrosine phosphatase-activity of an essential virulence determinant in Yersinia. Science 249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 46.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolfwatz H. 1990. The cytotoxic protein yope of yersinia obstructs the primary host defense. Mol Microbiol 4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 47.Dahinden C, Galanos C, Fehr J. 1983. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol 130:857–862. [PubMed] [Google Scholar]
- 48.Erttmann SF, Gekara NO, Fallman M. 2014. Bacteria induce prolonged PMN survival via a phosphatidylcholine-specific phospholipase C- and protein kinase C-dependent mechanism. PLoS One 9:e87859. doi: 10.1371/journal.pone.0087859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolin I, Wolf-Watz H. 1984. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun 43:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolin I, Norlander L, Wolf-Watz H. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun 37:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isaksson EL, Aili M, Fahlgren A, Carlsson SE, Rosqvist R, Wolf-Watz H. 2009. The membrane localization domain is required for intracellular localization and autoregulation of YopE in Yersinia pseudotuberculosis. Infect Immun 77:4740–4749. doi: 10.1128/IAI.00333-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alli M, Isaksson EL, Carlsson SE, Wolf-Watz H, Rosqvist R, Francis MS. 2008. Regulation of Yersinia Yop-effector delivery by translocated YopE. Int J Med Microbiol 298:183–192. doi: 10.1016/j.ijmm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Thorslund SE, Edgren T, Pettersson J, Nordfelth R, Sellin ME, Ivanova E, Francis MS, Isaksson EL, Wolf-Watz H, Fallman M. 2011. The RACK1 signaling scaffold protein selectively interacts with Yersinia pseudotuberculosis virulence function. PLoS One 6:0016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmstrom A, Petterson J, Rosqvist R, Hakansson S, Tafazoli F, Fallman M, Magnusson KE, Wolf-Watz H, Forsberg A. 1997. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol 24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 55.Galyov EE, Hakansson S, Forsberg A, Wolf-Watz H. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.