Abstract
Scrub typhus is a potentially lethal infection that is caused by the obligate intracellular bacterium Orientia tsutsugamushi. The roles of Toll-like receptor 2 (TLR2) and TLR4 in innate recognition of O. tsutsugamushi have not been elucidated. By overexpression of TLR2 or TLR4 in HEK293 cells, we demonstrated that TLR2, but not TLR4, recognizes heat-stable compounds of O. tsutsugamushi that were sensitive to treatment with sodium hydroxide, hydrogen peroxide, and proteinase K. TLR2 was required for the secretion of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) by dendritic cells. In an intradermal mouse infection model, TLR2-deficient mice did not show impaired control of bacterial growth or reduced survival. Moreover, after intraperitoneal infection, TLR2-deficient mice were even more resistant to lethal infection than C57BL/6 wild-type mice, which showed stronger symptoms and lower survival rates during the convalescent phase. Compared to the time of reduction of bacterial loads in TLR2-deficient mice, the reduction of bacterial loads in infected organs was accelerated in wild-type mice. The higher mortality of wild-type mice was associated with increased concentrations of serum alkaline phosphatase but not aspartate aminotransferase. The transcription of mRNA for TNF-α and IL-6 decreased more rapidly in peritoneum samples from wild-type mice than in those from TLR2-deficient mice and was therefore not a correlate of increased susceptibility. Thus, although TLR2 is an important mediator of the early inflammatory response, it is dispensable for protective immunity against O. tsutsugamushi. Increased susceptibility to O. tsutsugamushi infection in TLR2-competent mice rather suggests a TLR2-related immunopathologic effect.
INTRODUCTION
Scrub typhus is a neglected chigger-borne zoonosis caused by the obligate intracellular bacterium Orientia tsutsugamushi. Although scrub typhus has lost much of its former threat thanks to the advent of antibiotic therapy, fatal infections continue to be reported, yet it remains largely unknown how bacterial virulence factors and host immunity mutually contribute to the pathogenesis of scrub typhus (1).
In mammals, the Toll-like receptor (TLR) system has evolved as a germ line-encoded, ancient repertoire of pattern recognition receptors. Conserved structures on the surface of microorganisms consisting of lipoproteins and lipopeptides (2, 3) and of lipopolysaccharide (LPS) in Gram-negative bacteria (4) are recognized by TLR2 and TLR4, respectively, and trigger immediate antimicrobial responses. As part of the first line of immune defense, these responses help to limit infection success and shape the development of protective adaptive immunity. TLR ligation, mediated by MyD88- or TRIF-dependent pathways, results in activation of transcription factors, such as nuclear factor (NF)-κB, activated protein-1 (AP-1), or interferon regulatory transcription factors (IRFs) (5).
Many studies have supported a protective effect for TLR2 and TLR4 during bacterial and fungal infections in mouse models (6–10), including rickettsial infections (11, 12). However, the protective role of TLRs was recently challenged by studies demonstrating an increased susceptibility to infection or infection-induced pathology in the presence of TLR2 (13–15).
In the study described here, we investigated the involvement of TLR2 and TLR4 in the recognition of O. tsutsugamushi. Our study provides evidence for the requirement of TLR2 in the induction of early inflammation. We also studied the in vivo consequences of TLR2 deficiency in experimental mouse infections (16) and show that TLR2 is largely dispensable for protection. Instead, we demonstrate that TLR2-competent animals were more susceptible to O. tsutsugamushi infections. Our study therefore suggests a role of TLR2 as a host factor that increases the severity of experimental scrub typhus.
MATERIALS AND METHODS
Cell culture.
Bone marrow-derived dendritic cells (BMDCs) were obtained by generation from bone marrow progenitors according to the protocol described by Lutz et al. (17). Briefly, murine bone marrow cells were differentiated into myeloid dendritic cells (DCs) by culturing 2 × 106 cells in RPMI medium containing 10% heat-inactivated fetal calf serum (FCS), 1 mM l-glutamine, 50 μg/ml gentamicin, 10% culture supernatant from granulocyte-macrophage colony-stimulating factor (GM-CSF)-transfected Ag8653 myeloma cells (18, 19) as a source of GM-CSF, and 50 μM 2-mercaptoethanol. Cultures were fed with fresh medium on days 3 and 6, and DCs were collected on day 7 of culture.
TLR activation assays and infection of cells.
HEK293 cells were plated at a density of 5 × 104 cells/ml in 96-well plates in Dulbecco modified Eagle medium (PAA Laboratories) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS. On the following day, cells were transiently transfected using the Lipofectamine reagent according to the manufacturer's instructions (Invitrogen, Karlsruhe, Germany). The expression plasmid expressing human CD14 was a kind gift of D. T. Golenbock (Worcester, MA, USA), and the Flag-tagged version of human TLR2 was a kind gift from P. Nelson (Seattle, WA, USA). Flag-tagged human TLR4 (P. Nelson) was further subcloned into pREP9 (Invitrogen). The human MD-2 expression plasmid was a kind gift from K. Miyake (Tokyo, Japan). Plasmids were used at 100 ng (10 ng for CD14 and MD-2) per transfection. After 24 h, cells were stimulated for another 18 h.
Infection of TLR-overexpressing HEK293 cells in 96-well plates or macrophages or BMDCs in 24-well plates was achieved by 30 min of centrifugation of medium containing O. tsutsugamushi at 133 × g. Controls were treated with 1 μg/ml Salmonella enterica serovar Minnesota Re595 lipopolysaccharide (LPS; Sigma, St. Louis, MO, USA), 100 nM Pam3CysSK4 lipopeptide (Pam3C; EMC Microcollections, Tübingen, Germany), or 1 ng/ml human recombinant tumor necrosis factor alpha (TNF-α; a kind gift of Daniela Männel, Regensburg, Germany).
ELISA and cytokine bead array.
A CytoSet enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, Frederick, MD, USA) was used for measuring the concentrations of human interleukin-8 (IL-8). Murine cytokines TNF-α and IL-6 from cell culture supernatants were measured using DuoSet ELISA kits from R&D Systems (Wiesbaden, Germany). The concentrations of IL-6 and TNF-α in mouse sera were measured using FlowCytomix kits from eBioscience (Frankfurt, Germany).
Mice and infections.
Female C57BL/6 mice (age, 6 to 10 weeks) were purchased from Charles River Laboratories and kept in individually ventilated cages. Tlr2−/− mice were kindly provided by the animal facility of LMU Munich, Munich, Germany, and bred at the Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany. To achieve equal microbial colonization, litter containing pellets of feces were exchanged between cages of wild-type and knockout mice 2 weeks before the onset of the experiments.
Cryostocks of O. tsutsugamushi Karp inocula were generated as previously described (16). Infections were initiated by inoculation of 5 × 103 spot-forming units (SFU) in phosphate-buffered saline (PBS) in a volume of 50 μl for dermal inoculation in the right hind footpad or in 200 μl as an intraperitoneal (i.p.) injection. During the infection, the mice were scored by assessment of their fur and general condition (Table 1).
TABLE 1.
Scoring system for i.p. infected micea
| Score | Condition producing the indicated score |
|
|---|---|---|
| Condition of fur | General condition | |
| 0 | No signs of ruffled fur | No signs |
| 1 | Ruffled fur between ears | Tiredness |
| 2 | Ruffled fur on back | Distended abdomen, tiredness |
| 3 | Ruffled fur all over | Bloated body, hunched posture or physical wound, trembling because of pain, lethargy |
| 4 | Death | Death |
Mice were assessed every 2 days for the presence of changes in fur and general condition. Scores for the condition of the fur and the general condition were added up to obtain a combined score value.
Blood biochemistry.
For quantification of serum alkaline phosphatase (ALP) and aspartate aminotransferase (AST) activities, commercially available colorimetric assays were used (Reflotron; Roche Diagnostics, Mannheim, Germany).
Preparation of bacteria for in vitro infections.
O. tsutsugamushi Karp was cultured in L929 cells. At 14 days postinfection (p.i.), infected cells were collected from two 75-cm2 flasks (Greiner, Frickenhausen, Germany) and disrupted by rocking for >2 min with sterilized glass beads or silicon carbide granules. Cellular debris was removed by low-speed centrifugation (for 3 min at 3,000 rpm in a Micro 20 tabletop centrifuge [Hettich, Tuttlingen, Germany]). Cell-free bacteria were collected by high-speed centrifugation at 3,200 × g for 30 min and resuspended in RPMI medium.
Chemical and enzymatic treatment.
Purified bacterial preparations of O. tsutsugamushi Karp were resuspended in PBS and heat inactivated for 10 min at 95°C. Treatment protocols were established as described by Zähringer et al. (20). Proteinase K treatment was performed by incubation with 0.1 mg/ml proteinase K (Qiagen, Hilden, Germany) for 30 min at 37°C, followed by inactivation for 15 min at 70°C. LPS was neutralized by incubation with 1 mg/ml polymyxin B (Sigma, Deisenhofen, Germany) for 1 h at 37°C. Alkaline hydrolysis was performed by incubation with 10 mM NaOH (Merck, Darmstadt, Germany) for 1 h at room temperature, followed by neutralization with 37% (vol/vol) HCl. H2O2 treatment was performed with 1% H2O2 (Merck, Darmstadt, Germany) for 6 h at 37°C.
Nucleic acid extraction and qPCR.
Quantification of the bacterial loads in tissue samples was performed using the highly sensitive multicopy traD quantitative PCR (qPCR) as described by Keller et al. (16). Briefly, small tissue samples were transferred to 0.5 ml PBS and were mechanically homogenized using 1.4- and 2.8-mm-diameter ceramic beads (Peqlab, Erlangen, Germany) in a Precellys 24 homogenizer (PeqLab). Total DNA was extracted using a QIAamp DNA minikit (Qiagen, Hilden, Germany), and the total DNA concentration was adjusted to 10 ng/ml. Quantitative PCRs were run on a LightCycler 480 II instrument (Roche, Mannheim, Germany). Results were transformed to logarithmic scale and expressed as the number of traD copies per microgram of DNA.
mRNA transcription analysis.
Tissue samples (50 to 100 mg) were taken up in 1 ml TRIzol reagent (Life Technologies, Darmstadt, Germany) and homogenized using ceramic beads. RNA was extracted according to the recommended TRIzol procedure, followed by DNase (Qiagen, Hilden, Germany) treatment and RNA purification (RNeasy; Qiagen, Hilden, Germany). Two micrograms of RNA was used for reverse transcription to cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Quantification of mRNA transcription was performed using a HotStarTaq kit (Qiagen, Hilden, Germany). In a total volume of 10 μl, the reaction mix contained 1 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (Applied Biosystems, Foster City, CA, USA), 300 nM each sense and antisense primers (Tibmolbiol, Berlin, Germany), 1 μg bovine serum albumin (Roche Diagnostics, Risch, Switzerland), 0.1 μl of a 1:1,000 dilution of SYBR green I (Invitrogen, Darmstadt, Germany) in dimethyl sulfoxide, and 0.25 U Taq DNA polymerase. Oligonucleotide sequences specific for the murine genes rps9, tnf-a, and il-6 were chosen on the basis of the work of Helk et al. (21) and Song et al. (22) and were validated in silico (some with slight modifications; Table 2). Reactions were run in duplicate in 384-well plates on a LightCycler 480 II instrument (Roche, Mannheim, Germany). Cycling conditions were 95°C for 15 min and 35 cycles of 30 s at 95°C, 40 s at 58°C, and 30 s at 72°C with touchdown from 64°C to 58°C in cycles 1 to 6. Gene expression was expressed with reference to the mean for all day 18 wild-type values and normalized to the levels of RPS9 expression, using the ΔΔCT (CT is threshold cycle) method [relative expression is 2(ΔCTtarget)/2(ΔCTRPS9)].
TABLE 2.
Primer sequences and references
Statistical analysis.
Data were analyzed using GraphPad Prism (version 5.0) software. Hypotheses were tested by one-way or two-way analysis of variance (ANOVA) with Bonferroni's postcorrection. A P value of ≤0.05 was considered significant.
Ethical approval.
The Animal Protection Commission and the Authority of Health of the State of Hamburg reviewed and approved the experimental protocol (approval number 74/09). The protocol adheres to the national guidelines and regulations of the German Animal Welfare Act.
RESULTS
O. tsutsugamushi has ligands for TLR2 but not TLR4.
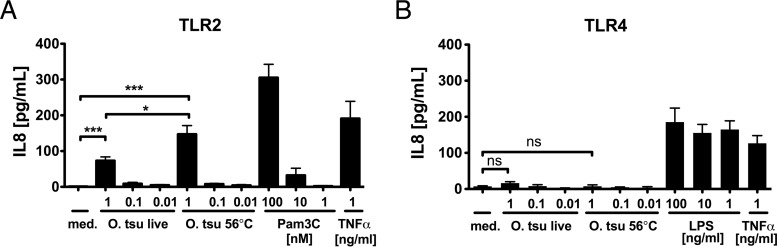
To investigate whether O. tsutsugamushi is recognized by human TLR2 or TLR4, we overexpressed TLR2 or TLR4 in HEK293 cells. This gain-of-function model has been widely used to characterize TLR ligands (20, 23–28). Only TLR-transfected HEK293 cells respond to TLR ligands. This response can be demonstrated by measuring the IL-8 concentrations in supernatants by ELISA (24). At 24 h after transfection, cells were infected with purified O. tsutsugamushi Karp or stimulated with heat-inactivated O. tsutsugamushi Karp or controls. After 18 h of incubation, the concentration of IL-8 in the supernatants was measured by ELISA. As positive controls, the synthetic triacylated lipopeptide Pam3C (TLR2 assays) and LPS from Salmonella enterica serovar Minnesota Re595 (TLR4 assays) were used. As shown in Fig. 1A, only TLR2-transfected HEK293 cells produced IL-8 following challenge with either live or heat (56°C)-inactivated O. tsutsugamushi in a dose-dependent fashion. The level of IL-8 production induced by heat-inactivated O. tsutsugamushi was approximately 2-fold higher than the level induced by live O. tsutsugamushi. Conversely, TLR4-transfected HEK293 cells produced IL-8 upon stimulation with LPS but not upon stimulation with either live or inactivated O. tsutsugamushi (Fig. 1B). Thus, O. tsutsugamushi provides ligands that are stable at 56°C and that are recognized by TLR2 but that are not recognized by TLR4.
FIG 1.
O. tsutsugamushi is recognized by TLR2 but not TLR4. HEK293 cells were transiently transfected with plasmids expressing human TLR2 or TLR4 and were infected with live O. tsutsugamushi (O. tsu live) or stimulated with heat (56°C)-inactivated O. tsutsugamushi (O. tsu 56°C). S. Minnesota LPS, Pam3C, and TNF-α were used as controls. After 18 h, IL-8 concentrations from cell culture supernatants were measured by a sandwich cytokine ELISA. (A) TLR2-transfected cells responded to live O. tsutsugamushi, heat (56°C)-inactivated O. tsutsugamushi, and Pam3C in a dose-dependent fashion. (B) TLR4-transfected cells responded to S. Minnesota LPS only. Shown are combined results from two independent experiments (2 to 3 replicates, mean ± SEM). med., medium. ***, P < 0.001 by one-way ANOVA; *, P < 0.05 by one-way ANOVA; ns, no significant difference.
Selective enzymatic and chemical degradation reveals the composition of TLR2 ligands.
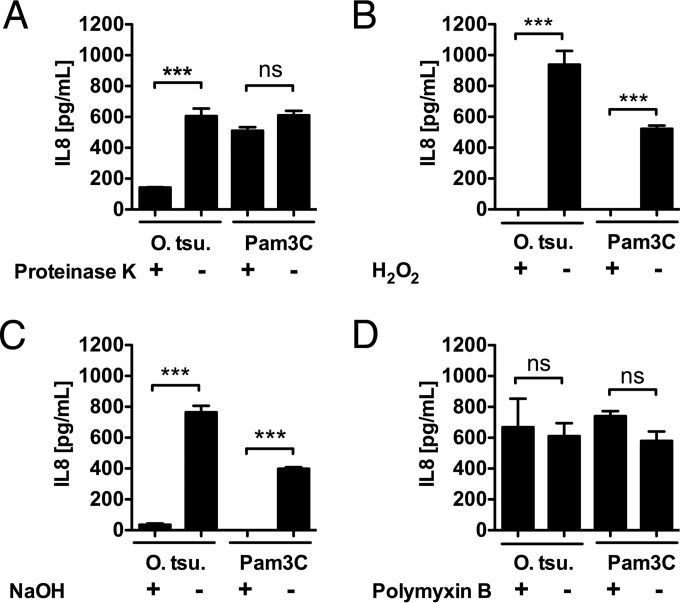
In order to further characterize the molecular structure sensed by TLR2, simple chemical and enzymatic degradation procedures were performed on heat (95°C)-inactivated whole O. tsutsugamushi extract (for degradation of the proteins, proteinase K; for hydrolysis of lipid esters, NaOH; for thioether oxidation in lipopeptides, H2O2 [20]). Polymyxin B, which neutralizes LPS, was used as a control. TLR2-transfected HEK293 cells were incubated with the chemically modified stimuli, and the IL-8 concentrations in cell culture supernatants were measured after 18 h. As shown in Fig. 2A, proteinase K digestion of heat-inactivated O. tsutsugamushi reduced the IL-8 concentration by over 75%, while the digestion of Pam3C did not result in a significantly reduced level of IL-8 release. This suggests that the TLR2-ligating components of O. tsutsugamushi contain a protein or peptide structure. H2O2 treatment of both heat-inactivated O. tsutsugamushi and Pam3C almost completely abrogated the production of IL-8 by TLR2-transfected HEK293 cells (Fig. 2B), suggesting that a lipid thioether structure partakes in recognition of O. tsutsugamushi by TLR2. Similarly, NaOH treatment reduced the IL-8 concentrations of both heat-inactivated O. tsutsugamushi and Pam3C almost completely, implying that fatty acids are part of the TLR2-activating components of O. tsutsugamushi (Fig. 2C). As expected, polymyxin B, which inactivates LPS as a TLR4 ligand, did not reduce the capacity of O. tsutsugamushi to trigger TLR2 (Fig. 2D). Taken together, our findings suggest that the major TLR2 ligand of O. tsutsugamushi could be a lipopeptide. On the other hand, the pronounced sensitivity to proteinase K treatment, which synthetic lipopeptides did not show (Fig. 2A), could also be consistent with the presence of a peptidic or proteinaceous part on the ligands or an additional protein ligand for TLR2 in O. tsutsugamushi.
FIG 2.
The TLR2 ligand of O. tsutsugamushi is sensitive to proteinase K, H2O2, and NaOH. (A to D) HEK293 cells transfected with human TLR2 were stimulated with heat-inactivated and crude lysates of O. tsutsugamushi pretreated with the indicated compound. IL-8 concentrations were measured 18 h later by ELISA. Representative results from one of two independent experiments are shown (n = 4 replicates, mean ± SEM). ***, P < 0.001 by one way-ANOVA; ns, no significant difference.
IL-6 and TNF-α production by BMDCs after O. tsutsugamushi infection requires TLR2 signaling.
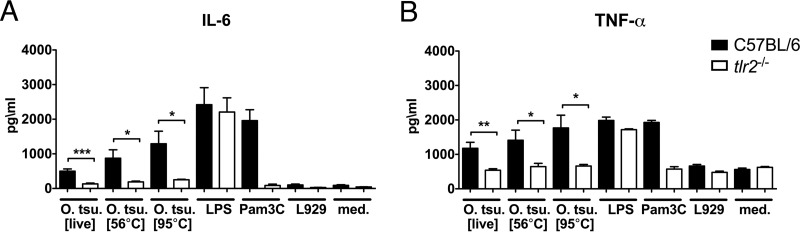
Dendritic cells show high levels of TLR expression and produce high levels of cytokines in response to pathogen-associated molecular pattern (PAMP) molecules. We therefore infected or stimulated BMDCs from C57BL/6 mice or TLR2-deficient mice with O. tsutsugamushi or treated them with LPS, Pam3C, or medium. As shown in Fig. 3, BMDCs produced both IL-6 (Fig. 3A) and TNF-α (Fig. 3B) in a TLR2-dependent manner in response to O. tsutsugamushi infection at 24 h posttreatment. Again, as in TLR2-transfected HEK293 cells, the concentration of cytokines increased slightly when BMDCs were stimulated with O. tsutsugamushi cells that had been inactivated at 56°C or 95°C, further substantiating that the previously described heat-stable component of O. tsutsugamushi is a ligand for TLR2.
FIG 3.
Production of IL-6 and TNF-α by BMDCs in response to O. tsutsugamushi requires TLR2. (A, B) BMDCs (2 × 105) were infected or stimulated with O. tsutsugamushi or treated with LPS (1 μg/ml), Pam3C (1 μM), or controls. At 24 h p.i., the levels of IL-6 (A) and TNF-α (B) in the supernatants were measured by ELISA (n = 4 replicates per experiment). Shown are pooled results from two independent experiments (mean ± SEM). *, P < 0.05 by one way-ANOVA; **, P < 0.01 by one way-ANOVA; ***, P < 0.001 by one way-ANOVA.
TLR2 is not required to limit the growth of O. tsutsugamushi in a resistant mouse model.
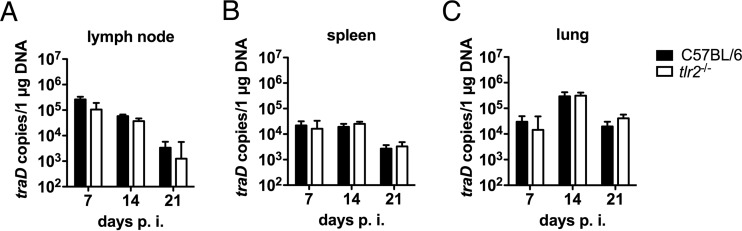
TLRs are crucial mediators of resistance to many infections and mediate resistance by limiting pathogen growth. We thus investigated whether TLR2 is required for survival and the control of O. tsutsugamushi Karp growth during the acute infection phase, using our recently developed mouse footpad infection model, which closely approximates natural inoculation via the dermis (16). All wild-type and tlr2−/− mice survived the acute infection phase until day 21 p.i. (data not shown). We next measured the bacterial load in draining lymph nodes, spleen, and lungs on days 7, 14, and 21 p.i. In the lymph nodes, the bacterial loads were the highest on day 7 p.i. and decreased thereafter, without showing significant differences between wild-type and tlr2−/− mice (Fig. 4A). This suggested that tlr2−/− mice are not defective in limiting the growth of O. tsutsugamushi. In the spleen and lungs, bacterial loads were efficiently reduced to similar levels in both groups between days 14 and 21 p.i. (Fig. 4B and C). Thus, TLR2 was not required to limit the growth of O. tsutsugamushi after dermal infection.
FIG 4.
tlr2−/− mice are able to restrict bacterial growth after dermal infection with O. tsutsugamushi. C57BL/6 and tlr2−/− mice were infected with 5,000 SFU of O. tsutsugamushi, which was injected into the right hind footpad. Bacterial loads in popliteal lymph node, spleen, and lung were measured by traD qPCR at the indicated time points. Pooled results from two independent experiments (n = 6, mean ± SEM) are shown.
TLR2 confers susceptibility to i.p. infection with O. tsutsugamushi.
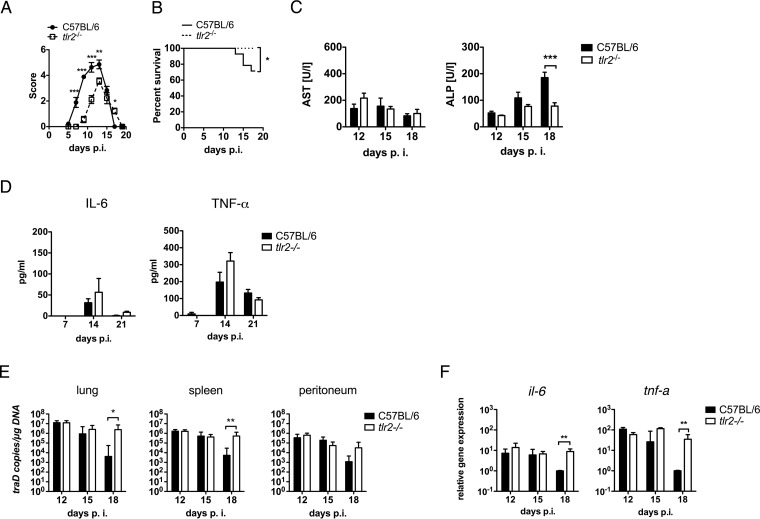
Compared with the intravenous, dermal, or subcutaneous inoculation routes, i.p. infection with O. tsutsugamushi Karp causes the most rapid and severe disease courses (29). The i.p. administration model thus allows investigation of both the protective and the pathological effects of TLR2 on the outcome of infection. C57BL/6 and tlr2−/− mice were infected with 5,000 SFU i.p., and survival was assessed daily. The development of symptoms was assessed every other day until the resolution of symptoms on day 19 p.i. Unexpectedly, wild-type mice became ill more rapidly than tlr2−/− mice and developed higher disease scores (Fig. 5A), suggesting that TLR2 aggravates the pathogenicity of severe i.p. O. tsutsugamushi infection. Moreover, about 30% of wild-type mice succumbed to infection between days 13 and 19 p.i., while tlr2−/− mice were protected against a lethal outcome (Fig. 5B). Thus, TLR2 had no protective role but, rather, increased mortality during the convalescent phase.
FIG 5.
TLR2-competent mice show higher levels of susceptibility to a lethal outcome, which is associated with a more efficient reduction of bacterial loads and increased serum ALP levels but not prolonged inflammation. C57BL/6 and tlr2−/− mice were infected i.p. with 5,000 SFU of O. tsutsugamushi. (A) Clinical scores were determined at the indicated time points p.i. (pooled results from two independent experiments, n = 9, mean ± SEM). (B) Kaplan-Meier curves show the survival of i.p. infected C57BL/6 and tlr2−/− mice. Data from three independent experiments were pooled (n = 15). (C) AST and ALP concentrations (n = 5 to 8) in serum samples obtained at the indicated times were measured using Reflotron colorimetric analysis (pooled results from two independent experiments, n = 5 to 8, mean ± SEM). (D) Serum concentrations of IL-6 (left) and TNF-α (right) were measured by use of a cytokine bead array on days 7, 14, and 21 p.i. (n = 3, mean ± SEM). (E) Bacterial loads in the lung, spleen, and peritoneum were measured by traD qPCR on days 12, 15, and 18 p.i. (n = 7 to 8, data pooled from two independent experiments, mean ± SEM). (F) RNA was extracted from peritoneum samples obtained on days 12, 15, and 18 p.i., and the relative expression of il-6 and tnf-a mRNA was measured by quantitative real-time PCR (n = 4, means ± SEMs). *, P < 0.05 by two way-ANOVA (A and C to E); **, P < 0.01 by two way-ANOVA (A and C to E); ***, P < 0.001 by two way-ANOVA (A and C to E); *, P < 0.05 by log-rank (Mantel-Cox) test (B).
Next, we questioned whether TLR2 caused an increase in the biochemical parameters of tissue injury during the convalescent phase. To that end, we measured the serum concentrations of AST and ALP, two parameters that were shown to be biochemical predictors of mortality in humans (30). As shown in Fig. 5C, the levels of AST did not differ significantly between wild-type and tlr2−/− mice. In contrast, the ALP level continued to increase from day 12 to day 18 p.i. in wild-type mice and was 4-fold higher on day 18 than day 12 p.i. No such rise in the ALP level was seen in tlr2−/− animals (Fig. 5C). Hence, TLR2-related mortality was associated with increased tissue injury, reflected by an increase in serum ALP levels.
TLR2 accelerates bacterial clearance from infected tissues but does not prolong inflammation.
Lethal infections may be linked to an excessive systemic inflammatory response and elicit a cytokine storm. We thus measured the concentrations of TNF-α and IL-6 in the sera of i.p. infected mice at the beginning, at the peak, and after the resolution of symptoms (days 7, 14, and 21 p.i., respectively; Fig. 5D). Increased serum concentrations of TNF-α and IL-6 were measured in both groups on day 14 p.i., but the concentrations decreased again by day 21 p.i. and were not significantly higher in wild-type mice (Fig. 5D). Thus, wild-type mice did not develop an excessive systemic inflammatory response during convalescence that could explain the increased mortality.
Since the cause of tissue injury may be a local inflammatory response in infected tissues rather than a consequence of systemic inflammation, we next investigated the influence of TLR2 on bacterial clearance and the transcription of inflammatory genes in infected tissue during the convalescent phase. To that end, we measured the bacterial load in infected lung, spleen, and peritoneum on days 12, 15, and 18 p.i. Bacterial loads did not differ at days 12 and 15 p.i. and sharply decreased in the lungs and spleens of the wild-type animals but not tlr2−/− animals on day 18 p.i. Also, in peritoneum samples, a tendency to higher loads in tlr2−/− animals was found on day 18 p.i. (Fig. 5E). Therefore, TLR2 accelerated the clearance of O. tsutsugamushi from infected organs between day 15 and day 18 p.i., in the late phase of acute i.p. infection.
In the i.p. infection model, the peritoneum is the primary tissue of infection and becomes severely inflamed. We thus measured the levels of transcription of the il-6 and tnf-a genes in peritoneum samples recovered on days 12, 15, and 18 p.i. The relative transcription of the il-6 and tnf-a genes was equal in the wild-type and tlr2−/− mice on days 12 and 15 p.i. but was significantly decreased in the wild-type mice compared to that in the TLR2-deficient mice on day 18 p.i. (Fig. 5F). Thus, TLR2 signaling did not exacerbate the transcription of the inflammatory cytokines IL-6 and TNF-α in infected tissue. Rather, decreasing bacterial loads were associated with the decreased transcription of these cytokines in wild-type animals. Our data suggest that death in mice infected i.p. with O. tsutsugamushi is an event that is not linked to the increased transcription of inflammatory cytokines or an inability to reduce the infection.
DISCUSSION
Scrub typhus is a neglected, emerging infectious disease caused by O. tsutsugamushi that is associated with severe complications during acute infections, including mortality. Little is understood about how host immunity contributes to protection or disease. Although the recognition of conserved bacterial surface structures by TLR2 and TLR4 has been well studied for many Gram-negative bacteria, their role in the recognition of O. tsutsugamushi has remained elusive. Here we show that O. tsutsugamushi provides heat-stable ligands for TLR2, while TLR4 has no role in the recognition of either live or heat-inactivated organisms. This finding completes previous studies that demonstrated an absence of lipopolysaccharide from the cell wall of O. tsutsugamushi (31) and the lack of genes for the biosynthesis and transport of lipid A, which is the moiety recognized by TLR4/MD-2 (32). A component of O. tsutsugamushi with heat stability up to 100°C had been shown before to be polymyxin B resistant (33), and we demonstrate here that this heat-stable component is an agonist for TLR2.
Furthermore, we characterized the chemical nature of the TLR2 ligand by specific chemical or enzymatic treatments. The treatment of cell wall extracts with H2O2 completely abolished TLR2 reactivity. H2O2 destroys the thioether present in the characteristic N-terminal motifs of all lipoproteins or lipopeptides by oxidation, converting the N-terminal cysteine-thioether substructure into TLR2-inactive sulfoxide derivatives (20, 34). Abrogation of the TLR2-activating ability was also obtained after alkaline hydrolysis with NaOH. These data indicate that the TLR2 agonist of O. tsutsugamushi is a lipid-carrying molecule with a functional group that is susceptible to oxidative degradation. Moreover, digestion of O. tsutsugamushi extracts with proteinase K reduced the IL-8 concentrations from TLR2-transfected HEK293 cell culture supernatants by over 75%. Thus, the TLR2 ligand of O. tsutsugamushi contains a protein or peptide structure. In fact, since most lipopeptides are resistant to proteases (35, 36), O. tsutsugamushi might provide more than one TLR2 agonist, e.g., a lipopeptide and a protein, as it was shown for Francisella tularensis (36).
We also demonstrated in the present study that heat-inactivated O. tsutsugamushi elicited concentrations of IL-8 in the supernatants of TLR2-transfected HEK293 cells higher than those elicited by live bacteria. Either dead or live bacteria are more potent activators of proinflammatory responses, but this is dependent on the species: Listeria, e.g., is more efficient in stimulating TNF-α release as live organisms (37), whereas only live Brucella bacteria, e.g., inhibit TNF-α release by macrophages via a secretory protein (38). It has been suggested that live O. tsutsugamushi suppresses the proinflammatory response against its own heat-stable compound (33). One possible scenario is active inhibition of TLR2-mediated signaling by live bacteria, e.g., by bacterial components secreted via the large available arsenal of type 1 and 4 secretion systems (39).
TLR2 ligation is linked to the activation of intracellular MyD88-dependent signaling pathways. The best understood consequences of TLR2 ligation are (i) activation of mitogen-activated protein (MAP) kinases, leading to activated protein-1 (AP-1)-mediated gene transcription; (ii) activation of the IκB kinase complex, causing the nuclear translocation of NF-κB and induction of NF-κB-dependent genes; and (ii) interferon-related factor (IRF)-dependent induction of type I interferons (5, 40). However, noncanonical pathways, such as the calcium/calmodulin-dependent protein kinase II (CaMKII) pathway, may be involved in TLR2-related transcriptional responses (41). Several previous studies have demonstrated that O. tsutsugamushi activates the NF-κB and AP-1 pathways (42–44). The inflammatory response induced by O. tsutsugamushi appears to be tightly regulated, depending on cell and cytokine type: monocyte chemoattractant protein 1 transcription in macrophages is NF-κB dependent (43), while TNF-α depends on the activation of MAP kinases (45) and AP-1 is the dominant pathway in endothelial cells (44, 46). More recent work has suggested that the induction of pathways in human macrophages may depend on infectious doses (47). It will therefore be of interest to study in more depth the signaling pathway involved in TLR2-dependent recognition of O. tsutsugamushi.
TLR2 deficiency increases the susceptibility to many experimental infections, including Gram-positive bacterial infections (8–10, 48) and Gram-negative bacterial infections (49–52). In order to study the influence of TLR2 on host resistance to O. tsutsugamushi, we first used an experimental mouse model based on the dermal inoculation route via the hind footpad (16). In this model, all tlr2−/− mice survived and were able to control bacterial growth as well as wild-type mice did, as shown by the kinetics of the bacterial loads from draining lymph nodes, spleen, and lung. Thus, TLR2 had no influence on survival or the pathogen burden in this model.
While dermal inoculation is an appropriate model for self-limiting infections, rapid and severe infections with O. tsutsugamushi Karp, whose pathogenesis must be different, can be more appropriately modeled by i.p. inoculation (29). For the i.p. model, we chose an infection dose of 5,000 SFU, which kills about 30% of wild-type animals between days 13 and 19 p.i. While we expected that the tlr2−/− mice would show increased susceptibility to O. tsutsugamushi, they actually developed fewer symptoms and were protected from lethal infection. We investigated serum ALP and AST concentrations as biochemical markers of tissue injury from samples taken between day 12 and day 18 p.i. and found continuously increased ALP concentrations in wild-type mice, while AST concentrations did not differ significantly between the two groups. This difference may be related to the fact that a cell type which expresses ALP but not AST is predominantly susceptible to cellular injury in TLR2-competent wild-type mice but not tlr2−/− mice. Importantly, our data show that inflammation and tissue injury are independent events. Although our study did not specify the cellular or tissue origin of increased ALP concentrations, which is a limitation, our finding parallels observations in human infections in which increased ALP concentrations were suggested to be a predictor of mortality (30). Since all animals were still alive at the times indicated above and euthanized before serum was collected, a correlation with mortality was not possible. Future experimental studies should address the question whether an elevation of ALP levels and mortality also correlate in mice.
We also investigated whether increased susceptibility in wild-type mice was associated with an increased systemic inflammatory response by measuring the concentrations of TNF-α and IL-6 in serum, but the wild-type and tlr2−/− mice showed no significant differences. Since the systemic cytokine response might not reflect the inflammatory processes in inflamed tissue, we related the pathogen burden and the transcription of proinflammatory genes in the infected peritoneum during the phase between days 13 and 19 p.i., i.e., when lethal outcomes were observed. Our data showed that a sharp decrease of O. tsutsugamushi loads in wild-type mice on day 18 p.i. was paralleled by a decrease in the level of transcription of tnf-a and il-6 mRNA, which was not observed in tlr2−/− mice. Thus, TLR2 accelerated bacterial clearance but did not trigger an increased or prolonged transcription of inflammatory genes.
Our in vitro data showed lower levels of cytokine production by tlr2−/− mouse BMDCs at 24 h p.i. In vivo, tlr2−/− mice had no defect in cytokine transcription, as measured between days 12 and 18 p.i. Rather than suggesting incongruence, these results reflect two fundamentally different phases of infection: while TLR2 appears to have a role in early recognition, as shown in vitro, TLR2-independent pathways are able to maintain inflammation once the infection is established in vivo, e.g., via cytosolic receptors such as NOD1 (57) or via the inflammasome (53). tlr2−/− mouse BMDCs may equally be able to respond to O. tsutsugamushi infection with cytokine secretion at time points later than 24 h p.i. via TLR2-independent pathways, but this possibility was not experimentally addressed. We conclude from our data that TLR2 is important for the reduction of infection but does not do so via cytokines. Moreover, sudden death in wild-type mice is not associated with a cytokine storm.
Surprisingly, the differences in survival and signs of tissue injury were not found in the early phase of infection but were found during convalescence, between days 13 and 19 p.i. This timing makes an early innate effect of TLR2 unlikely and might instead indicate a difference in adaptive immunity, as it has been known, e.g., that the cytotoxic function of CD8+ T cells may also be enhanced by TLR2 signaling (54, 55). However, this possibility was not experimentally addressed as it was beyond the scope of the present study.
Our data show that O. tsutsugamushi belongs to the group of pathogens against which TLR2 induces pathological rather than protective effects. Other examples of TLR2 mediating pathological effects encompass Chlamydia trachomatis, which triggers a TLR2-dependent pathology in oviduct and mesosalpinx (13); Burkholderia pseudomallei infection, in which mortality and organ injury are ameliorated in the absence of TLR2 (14); or secondary pneumococcal pneumonia after influenza virus infection (15). In our mouse i.p. O. tsutsugamushi infection model, TLR2 not only increased resistance to O. tsutsugamushi by helping to reduce the pathogen burden in the convalescent phase but also appeared to reduce tolerance to the disease caused by it (56).
Taken together, the results of our study show that TLR2 mediates proinflammatory responses to O. tsutsugamushi infection in vitro. Although TLR2 helps in vivo to reduce the infection during the convalescent phase, the TLR2-dependent response is not required for protection from infection. Rather, TLR2 increases the susceptibility to immunopathology during severe O. tsutsugamushi infection in the i.p. model, and this increase in susceptibility is not paralleled by an excessive production of cytokines. The present study suggests that TLR2 could be a host susceptibility factor in scrub typhus. TLR2 thus deserves further attention in human scrub typhus studies.
ACKNOWLEDGMENTS
We gratefully acknowledge the outstanding technical expertise of Ute Mehlhoop in establishing the mRNA expression analysis platform and maintaining O. tsutsugamushi cell culture, as well as Ina Goroncy for excellent technical assistance.
Funding Statement
This work, including the efforts of Mohammad Gharaibeh, was supported by the German Academic Exchange Service (DAAD). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Paris DH, Shelite TR, Day NP, Walker DH. 2013. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO. 2009. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31:873–884. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. 2006. TLR signaling. Cell Death Differ 13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 6.Bernheiden M, Heinrich JM, Minigo G, Schutt C, Stelter F, Freeman M, Golenbock D, Jack RS. 2001. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J Endotoxin Res 7:447–450. [PubMed] [Google Scholar]
- 7.Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. 2002. The role of Toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis 185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 8.Torres D, Barrier M, Bihl F, Quesniaux VJF, Maillet I, Akira S, Ryffel B, Erard F. 2004. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect Immun 72:2131–2139. doi: 10.1128/IAI.72.4.2131-2139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drennan MB, Nicolle D, Quesniaux VJF, Jacobs M, Allie N, Mpagi J, Frémond C, Wagner H, Kirschning C, Ryffel B. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol 164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi O, Hoshino K, Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 11.Quevedo-Diaz MA, Song C, Xiong Y, Chen H, Wahl LM, Radulovic S, Medvedev AE. 2010. Involvement of TLR2 and TLR4 in cell responses to Rickettsia akari. J Leukoc Biol 88:675–685. doi: 10.1189/jlb.1009674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan JM, Woods ME, Olano J, Walker DH. 2008. The absence of Toll-like receptor 4 signaling in C3H/HeJ mice predisposes them to overwhelming rickettsial infection and decreased protective Th1 responses. Infect Immun 76:3717–3724. doi: 10.1128/IAI.00311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darville T, O'Neill JM, Andrews CW, Nagarajan UM, Stahl L, Ojcius DM. 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol 171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 14.Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M, Florquin S, de Vos AF, White N, Dondorp AM, Day NP, Peacock SJ, van der Poll T. 2007. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med 4:e248. doi: 10.1371/journal.pmed.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlstrom A, Heston SM, Boyd KL, Tuomanen EI, McCullers JA. 2011. Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis 204:1358–1366. doi: 10.1093/infdis/jir522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller CA, Hauptmann M, Kolbaum J, Gharaibeh M, Neumann M, Glatzel M, Fleischer B. 2014. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl Trop Dis 8:e3064. doi: 10.1371/journal.pntd.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 18.Volkmann A, Neefjes J, Stockinger B. 1996. A conditionally immortalized dendritic cell line which differentiates in contact with T cells or T cell-derived cytokines. Eur J Immunol 26:2565–2572. doi: 10.1002/eji.1830261105. [DOI] [PubMed] [Google Scholar]
- 19.Zal T, Volkmann A, Stockinger B. 1994. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med 180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zähringer U, Lindner B, Inamura S, Heine H, Alexander C. 2008. TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Helk E, Bernin H, Ernst T, Ittrich H, Jacobs T, Heeren J, Tacke F, Tannich E, Lotter H. 2013. TNFalpha-mediated liver destruction by Kupffer cells and Ly6Chi monocytes during Entamoeba histolytica infection. PLoS Pathog 9:e1003096. doi: 10.1371/journal.ppat.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song M, Jin J, Lim JE, Kou J, Pattanayak A, Rehman JA, Kim HD, Tahara K, Lalonde R, Fukuchi K. 2011. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. J Neuroinflammation 8:92. doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem 274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 24.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. 2005. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J 272:6354–6364. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 25.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 26.Heine H, Kirschning CJ, Lien E, Monks BG, Rothe M, Golenbock DT. 1999. Cutting edge: cells that carry a null allele for Toll-like receptor 2 are capable of responding to endotoxin. J Immunol 162:6971–6975. [PubMed] [Google Scholar]
- 27.Underhill DM, Ozinsky A, Smith KD, Aderem A. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A 96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manukyan M, Triantafilou K, Triantafilou M, Mackie A, Nilsen N, Espevik T, Wiesmuller KH, Ulmer AJ, Heine H. 2005. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur J Immunol 35:911–921. doi: 10.1002/eji.200425336. [DOI] [PubMed] [Google Scholar]
- 29.Shelite TR, Saito TB, Mendell NL, Gong B, Xu G, Soong L, Valbuena G, Bouyer DH, Walker DH. 2014. Hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus [corrected]. PLoS Negl Trop Dis 8:e2966. doi: 10.1371/journal.pntd.0002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P, Abraham OC, Kavitha ML. 2014. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis 23:39–43. doi: 10.1016/j.ijid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, Fukushi K. 1987. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun 55:2290–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min CK, Yang JS, Kim S, Choi MS, Kim IS, Cho NH. 2008. Genome-based construction of the metabolic pathways of Orientia tsutsugamushi and comparative analysis within the Rickettsiales order. Comp Funct Genomics 2008:623145. doi: 10.1155/2008/623145:623145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MK, Kang JS. 2001. Orientia tsutsugamushi suppresses the production of inflammatory cytokines induced by its own heat-stable component in murine macrophages. Microb Pathog 31:145–150. doi: 10.1006/mpat.2001.0457. [DOI] [PubMed] [Google Scholar]
- 34.Morr M, Takeuchi O, Akira S, Simon MM, Muhlradt PF. 2002. Differential recognition of structural details of bacterial lipopeptides by Toll-like receptors. Eur J Immunol 32:3337–3347. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. 2006. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol 18:355–362. [DOI] [PubMed] [Google Scholar]
- 36.Thakran S, Li H, Lavine CL, Miller MA, Bina JE, Bina XR, Re F. 2008. Identification of Francisella tularensis lipoproteins that stimulate the Toll-like receptor (TLR) 2/TLR1 heterodimer. J Biol Chem 283:3751–3760. doi: 10.1074/jbc.M706854200. [DOI] [PubMed] [Google Scholar]
- 37.Zhan Y, Cheers C. 1995. Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro. Infect Immun 63:720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caron E, Gross A, Liautard JP, Dornand J. 1996. Brucella species release a specific, protease-sensitive, inhibitor of TNF-alpha expression, active on human macrophage-like cells. J Immunol 156:2885–2893. [PubMed] [Google Scholar]
- 39.Cho NH, Kim HR, Lee JH, Kim SY, Kim J, Cha S, Kim SY, Darby AC, Fuxelius HH, Yin J, Kim JH, Kim J, Lee SJ, Koh YS, Jang WJ, Park KH, Andersson SG, Choi MS, Kim IS. 2007. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci U S A 104:7981–7986. doi: 10.1073/pnas.0611553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira-Nascimento L, Massari P, Wetzler LM. 2012. The role of TLR2 in infection and immunity. Front Immunol 3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. 2009. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol Cell 35:48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MJ, Kim MK, Kang JS. 2006. Orientia tsutsugamushi inhibits tumor necrosis factor alpha production by inducing interleukin 10 secretion in murine macrophages. Microb Pathog 40:1–7. doi: 10.1016/j.micpath.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Cho NH, Seong SY, Huh MS, Han TH, Koh YS, Choi MS, Kim IS. 2000. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun 68:594–602. doi: 10.1128/IAI.68.2.594-602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho NH, Seong SY, Huh MS, Kim NH, Choi MS, Kim IS. 2002. Induction of the gene encoding macrophage chemoattractant protein 1 by Orientia tsutsugamushi in human endothelial cells involves activation of transcription factor activator protein 1. Infect Immun 70:4841–4850. doi: 10.1128/IAI.70.9.4841-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun JH, Koo JE, Koh YS. 2009. Mitogen-activated protein kinases are involved in tumor necrosis factor alpha production in macrophages infected with Orientia tsutsugamushi. Microbiol Immunol 53:349–355. doi: 10.1111/j.1348-0421.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- 46.Cho NH, Seong SY, Choi MS, Kim IS. 2001. Expression of chemokine genes in human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect Immun 69:1265–1272. doi: 10.1128/IAI.69.3.1265-1272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai MH, Chang CH, Tsai RK, Hong YR, Chuang TH, Fan KT, Peng CW, Wu CY, Hsu WL, Wang LS, Chen LK, Yu HS. 2016. Cross-regulation of proinflammatory cytokines by interleukin-10 and miR-155 in Orientia tsutsugamushi-infected human macrophages prevents cytokine storm. J Investig Dermatol 136:1398–1407. doi: 10.1016/j.jid.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol 169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 49.Abplanalp AL, Morris IR, Parida BK, Teale JM, Berton MT. 2009. TLR-dependent control of Francisella tularensis infection and host inflammatory responses. PLoS One 4:e7920. doi: 10.1371/journal.pone.0007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K. 2007. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol 56:305–312. doi: 10.1099/jmm.0.46913-0. [DOI] [PubMed] [Google Scholar]
- 51.Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. 2008. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol 10:388–403. [DOI] [PubMed] [Google Scholar]
- 52.Dickinson GS, Piccone H, Sun G, Lien E, Gatto L, Alugupalli KR. 2010. Toll-like receptor 2 deficiency results in impaired antibody responses and septic shock during Borrelia hermsii infection. Infect Immun 78:4579–4588. doi: 10.1128/IAI.00438-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koo JE, Hong HJ, Dearth A, Kobayashi KS, Koh YS. 2012. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS One 7:e39042. doi: 10.1371/journal.pone.0039042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, Davila E. 2010. When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood 116:3494–3504. doi: 10.1182/blood-2010-02-268169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mercier BC, Cottalorda A, Coupet CA, Marvel J, Bonnefoy-Berard N. 2009. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol 182:1860–1867. doi: 10.4049/jimmunol.0801167. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho KA, Jun YH, Suh JW, Kang JS, Choi HJ, Woo SY. 2010. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog 49:95–104. doi: 10.1016/j.micpath.2010.05.001. [DOI] [PubMed] [Google Scholar]