Abstract
Brucella species are facultative intracellular bacteria that cause brucellosis, a chronic debilitating disease significantly impacting global health and prosperity. Much remains to be learned about how Brucella spp. succeed in sabotaging immune host cells and how Brucella spp. respond to environmental challenges. Multiple types of bacteria employ the prokaryotic second messenger cyclic di-GMP (c-di-GMP) to coordinate responses to shifting environments. To determine the role of c-di-GMP in Brucella physiology and in shaping host-Brucella interactions, we utilized c-di-GMP regulatory enzyme deletion mutants. Our results show that a ΔbpdA phosphodiesterase mutant producing excess c-di-GMP displays marked attenuation in vitro and in vivo during later infections. Although c-di-GMP is known to stimulate the innate sensor STING, surprisingly, the ΔbpdA mutant induced a weaker host immune response than did wild-type Brucella or the low-c-di-GMP guanylate cyclase ΔcgsB mutant. Proteomics analysis revealed that c-di-GMP regulates several processes critical for virulence, including cell wall and biofilm formation, nutrient acquisition, and the type IV secretion system. Finally, ΔbpdA mutants exhibited altered morphology and were hypersensitive to nutrient-limiting conditions. In summary, our results indicate a vital role for c-di-GMP in allowing Brucella to successfully navigate stressful and shifting environments to establish intracellular infection.
INTRODUCTION
Brucella species are Gram-negative, facultative intracellular bacterial pathogens that cause brucellosis, the most prevalent zoonosis worldwide (1, 2). With more than 500,000 infections per year, the high incidence of brucellosis in southeastern Europe, the Mediterranean, South America, and Africa causes a major economic burden (2, 3). In animals, brucellosis is characterized by increased abortion, weak offspring, and decreased milk production. Brucella melitensis is the predominant cause of human brucellosis; however, B. abortus, B. suis, and B. canis can also infect humans (4). Human brucellosis is typically acquired by consuming contaminated milk products or via inhalation of aerosolized bacteria from occupational hazards (5). Human brucellosis is a debilitating disease in which most people initially experience a period of undulating fever which can progress to a chronic infection if untreated or if antibiotic treatment fails. Complications of chronic infections include liver damage, orchitis, endocarditis, and arthritis (1, 4).
Brucella spp. have the ability to infect both professional and nonprofessional phagocytes (6). Because of this, Brucella spp. encounter varied environments both throughout the body and within a cell and must adapt accordingly. To date, few virulence factors have been identified in Brucella, and even less is known about how these virulence factors are regulated. Subsequently, little is known how Brucella adapts to its rapidly changing environments and how it alternates between acute and chronic virulence.
The second messenger cyclic di-GMP (c-di-GMP) is an important bacterial regulator that alters metabolism and virulence in response to environmental cues such as phosphate and nutrient availability, oxygen and nitric oxide levels, and light (7, 8). Additionally, bacteria such as Vibrio cholerae and Pseudomonas aeruginosa regulate levels of c-di-GMP to switch between an acute virulent and a chronic persistent lifestyle (i.e., phase switching) (9, 10). c-di-GMP is synthesized from two GTP monomers by diguanylate cyclases containing a GGDEF domain and degraded by c-di-GMP-specific phosphodiesterases containing an EAL or HD-GYP domain. Elevated levels of c-di-GMP in bacteria are associated with vegetative growth, sessility, and biofilm formation, whereas decreased levels of c-di-GMP are associated with a potentially more virulent state involving biofilm dispersal and increased motility (8). To date, very little is known about how this ubiquitous second messenger regulates Brucella physiology and virulence.
Previously, we showed that B. melitensis contains 11 c-di-GMP metabolism genes: 6 contain a diguanylate cyclase domain, 2 contain a phosphodiesterase domain, and 3 contain both domains. A ΔbpdA phosphodiesterase (increased c-di-GMP) mutant showed significant attenuation in immunocompromised IRF1−/− mice, whereas a ΔcgsB diguanylate cyclase (decreased c-di-GMP) mutant had increased virulence compared to that of wild-type 16M B. melitensis; however, the mechanisms by which c-di-GMP modulates virulence in vivo were unclear (11).
In this study, initial proliferation of the ΔbpdA mutant in macrophages in vitro and in vivo was intact; however, later infections displayed marked attenuation. To understand how c-di-GMP regulates Brucella virulence, we investigated how alterations in c-di-GMP affect Brucella physiology and host responses. Although predicted to induce a greater host immune response via the c-di-GMP sensing stimulator of interferon genes (STING), the ΔbpdA mutant surprisingly induced less of an immune response than wild-type 16M and the ΔcgsB mutant. Analysis of ΔbpdA morphology via scanning electron microscopy revealed larger, atypically shaped bacteria. Additionally, proteomics analysis revealed the effect of c-di-GMP levels on multiple processes impacting virulence, including nutrient acquisition, cell wall formation, and the type IV secretion system (T4SS). Together, these data suggest that bacterial fitness rather than an increased host immune response is responsible for the attenuation of the ΔbpdA mutant.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Brucella melitensis 16M (ATC 23456), B. melitensis ΔbpdA (ΔBMEI 1453), and B. melitensis ΔcgsB (ΔBMEI 1520) were grown in brucella broth or on brucella agar plates (Difco); mutants were supplemented with 50 μg/ml of kanamycin. Brucella abortus ΔbpdA was generated as previously described (11). For minimal-medium experiments, strains were grown in 2XYT (tryptone, yeast extract, and NaCl), TSAYE (tryptic soy agar plus 0.1% [wt/vol] yeast extract), or Gerhardt's minimal medium with the addition of 2% glycerol and 1% lactic acid (12). To measure relative levels of intracellular c-di-GMP, a ribolux plasmid was created. Briefly, the c-di-GMP-responsive riboswitch was cloned from 348 bp upstream of the Vibrio cholerae VC1722 gene (13) through the VC1722 start codon and inserted into pEP3, upstream of a promoterless luxCDABE operon (14). Bioluminescence was assayed on a Veritas microplate luminometer (Promega) 24 h posttransfection. Bacterial expression vector for td-Tomato was constructed by inserting the CDS for td-Tomato (Clontech) into pNSTrcD (15).
Mice, BMDMs, and infections.
BALB/c, C57BL/6, or STING knockout (STING−/−) mice were maintained in AAALAC-accredited facilities and used at 6 to 8 weeks of age. BALB/c mice were obtained from Harlan (Indianapolis, IN). Female BALB/c mice (three mice per group) were not infected or were infected intraperitoneally with 107 B. melitensis 16M or the ΔbpdA, ΔbpdA-pbpdA, or ΔcgsB mutant for 24 h or 7 days. The infectious dose was based on previous experience generating chronic undulating systemic infection in mice (16). Spleens were removed and assessed for CFU by standard dilution assays or pooled within groups, and splenic macrophages were isolated using CD11b+ magnetic cell separation according to the manufacturer's instructions (Miltenyi). For RNA analysis, macrophages were immediately resuspended in TRIzol reagent. To generate bone marrow-derived macrophages (BMDMs), tibial and femur bone marrow cells from 6- to 8-week-old BALB/c, C57BL/6, or STING−/− female mice were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 1% HEPES, and 10% LADMAC cell-conditioned medium (LCCM) as the source of macrophage colony-stimulating factor (M-CSF). On day 4, 100 μl/well of LCCM was added; by day 7 of culture, cells had phenotypically differentiated into macrophages. BMDMs were infected with late-log-phase bacteria at a multiplicity of infection (MOI) of 100:1. Culture supernatants were collected 17 h postinfection and assayed for IP-10/CXCL10 by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Immunofluorescence microscopy.
BALB/c BMDMs were seeded into two-well chambered coverslips (Ibidi), allowed to adhere for 24 h, and then infected with td-Tomato-expressing B. melitensis 16M or the ΔbpdA or ΔcgsB mutant at an MOI of 1,000 for 24 h. Cells were washed three times with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min, followed by permeabilization with 0.1% Triton X-100 for 10 min. Cells were then treated with blocking buffer containing 5% serum and 50 mM NH4Cl in 1× PBS for 30 min and then washed and incubated with a 1:100 dilution of anti-calreticulin antibody (Thermo Scientific) in PBS containing 0.1% normal serum for 1 h. Cells were washed three times with PBS, incubated with a 1:10,000 dilution of Dylight 488 (Thermo Scientific), washed three times in PBS, and mounted in ProLong Gold antifade reagent (Thermo Scientific). Images were acquired on a Nikon A1R confocal laser microscope.
Crystal violet biofilm staining.
Crystal violet staining was performed as previously described (17). Briefly, stationary-phase cultures of B. melitensis 16M or mutants were added to BD PureCoat amine plates for 5 days at 37°C. After 2 water washes, a 0.1% aqueous solution of crystal violet was added for 15 min at room temperature (RT), and then plates were washed in water again three times and dried overnight. Acetic acid (30%) was added for 15 min at RT, and then samples were transferred to a 96-well plate for optical density reading at 570 nm (BioTek).
Total carbohydrate content determination.
Stationary-phase cultures of B. melitensis 16M or the ΔbpdA mutant were heat killed at 56°C for 1 h. Each sample was transferred to a 96-well plate and phenol was added. Then, sulfuric acid was added to each sample and the plate was incubated at RT for 30 min before optical density reading at 490 nm (BioTek).
Scanning electron microscopy and ImageJ quantification.
Stationary-phase B. melitensis 16M or mutants were transferred to 0.2-μm polycarbonate filters placed on sheep blood agar plates for 72 h. Samples were fixed overnight in Karnovsky's fixative (3% glutaraldehyde and 2% formaldehyde in 0.1 M phosphate buffer), washed for 10 min in PBS, and then placed in osmium tetroxide (1% in PBS) for 1 h, followed by another PBS wash. Samples were dehydrated in a series of ethanol washes (30%/50%/70%/95%/100% ethanol washes), dried via critical-point drying, and then coated in gold using sputter coating. Images were taken on a Hitachi S-3200N scanning electron microscope at the Biological & Biomaterials Preparation, Imaging, and Characterization Laboratory (BBPIC) of the University of Wisconsin-Madison. ImageJ (NIH) was used to quantify cell area in singular distinct bacteria.
Proteomics.
A total of 3 × 109 stationary-phase cells of B. melitensis 16M or mutants were pelleted and then resuspended in 6 M guanidine hydrochloride with 50 mM Tris (pH 8.0), boiled for 5 min, and precipitated by adding methanol to a final concentration of 90%. The precipitate was centrifuged at a relative centrifugal force of 10,000 for 5 min, decanted, and air dried. The protein pellet was resuspended in 8 M urea with 100 mM Tris (pH 8.0), 10 mM Tris (2-carboxyethyl) phosphine, and 40 mM chloroacetamide and then diluted to 1.5 M urea with 50 mM Tris (pH 8.0). Trypsin was added to a final ratio of 1:50 (enzyme to protein), and the samples were incubated at RT overnight. Peptides were desalted over Strata-X cartridges, dried in a speed vacuum, resuspended in 0.2% formic acid, and quantified (Pierce peptide assay kit). Proteomics was performed at the Center for Quantitative Biology of Complex Systems at the University of Wisconsin-Madison. For each analysis, 2 μg of peptides was separated across a 30-cm column (inner diameter, 75 μm) packed with 1.7 μm of ethylene-bridged hybrid C18 particles. Mobile phase A was 0.2% formic acid, and mobile phase B was 0.2% formic acid, 70% acetonitrile, and 5% dimethyl sulfoxide (DMSO). The gradient was 5 to 50% mobile phase B over 100 min, followed by a 100% mobile phase B wash and reequilibration with 0% mobile phase B. Eluted peptides were analyzed on a Thermo Fusion Orbitrap. Orbitrap survey scans were performed at a resolution of 60,000, followed by ion-trap tandem mass spectrometry (MS/MS) analyses of the most intense precursors (with z = 2 to 6) for less than 3 s and using a dynamic exclusion of 15 s. The maximum injection time for each MS/MS was 25 ms, and the ion trap resolution was set to turbo. Peptides were identified and quantified from the mass spectrometry data using the MaxQuant software suite with the Andromeda and MaxLFQ search and quantitation algorithms, respectively (18). Spectra were searched against a Uniprot Brucella proteome and common contaminant database concatenated with the reverse sequences. “Match between runs” was toggled on with the default settings. Peptide and protein identifications were filtered to a 1% false-discovery rate (FDR), and proteins were quantified by the MaxLFQ algorithm using the default settings. Significance was determined using the FDR as previously described (19).
RNA-seq.
BALB/c BMDMs were either left uninfected or infected with B. melitensis 16M or mutants; they were then harvested in TRI reagent (Sigma-Aldrich), RNA extracted, DNase I (Invitrogen) treated, and cleaned using the RNeasy minikit according to the manufacturer's instructions (Qiagen). Transcriptome sequencing (RNA-seq) analysis was performed at the University of Chicago's Genomics Facility. Briefly, RNA quality was verified on an Agilent 2100 bioanalyzer. Eukaryotic rRNA was depleted using a Ribo-Zero rRNA removal kit according to the manufacturer's instructions. RNA was reverse transcribed and adaptors were added. Single-end 50-bp reads were performed on a HiSeq2500. Expression analysis was performed using TopHat and Cufflinks. Briefly, TopHat was used to align sequences to the BALB/c mouse reference genome. The Cufflinks package was then used to assemble transcripts and compare assemblies to annotation. Finally, Cuffdiff was used to find differentially expressed transcripts (20). Statistical analysis was performed using Student's t test on averaged transcript expression.
Real-time RT-PCR.
Purified CD11b+ cells isolated from 6-week-old female BALB/c mice either left uninfected or infected with B. melitensis 16M or mutants were homogenized in TRIzol reagent (Invitrogen) to isolate total RNA. RNA was reversed transcribed using random primers (Bio-Rad), and cDNA was quantified on a CFX96 real-time PCR machine (Bio-Rad). The primers used in this study were designed using Beacon Designer software (Premier Biosoft) and are as follows: for 18S, 5′-GGACACGGACAGGATTGACAG-3′ (forward) and 5′-ATCGCTCCACCAACTAAGAACG-3′ (reverse); for Nos2, 5′-ATTGTACTATTGTGGACTACTAA-3′ (forward) and 5′-AAGTGCTTCAGTCAGGAG-3′ (reverse); for interleukin 12b (IL-12b), 5′-GTGACAACCAATAAGAACAT-3′ (forward) and 5′-GTCTCCAAATACCACCTAT-3′ (reverse); for STING, 5′-TTAAGTAGATGGCGAGAG-3′ (forward) and 5′-TTATTAAGAAGGCAGTTGAC-3′ (reverse); for Itg8a, 5′-TCACCTCATCTAAGTCAT-3′ (forward) and 5′-TGGATTGTTTCTGTAGTG-3′ (reverse); and for Fgd2, 5′-CCTGATGTCTATACCGAGA-3′ (forward) and 5′-ACTTGGTGGTATCTTCCT-3′ (reverse). All data are presented as relative expression units after normalization to 18S rRNA.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 6.0. All data are shown as means ± standard errors of the means (SEMs) unless otherwise noted. Differences between data were evaluated using Student's t test, one-way analysis of variance (ANOVA) with Tukey's posttest, or two-way ANOVA with Sidak's posttest. In all tests, P values of <0.05 were considered statistically significant. P values are indicated in figures as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
RESULTS
The ΔbpdA mutant fails to establish long-term infection in vitro and in vivo.
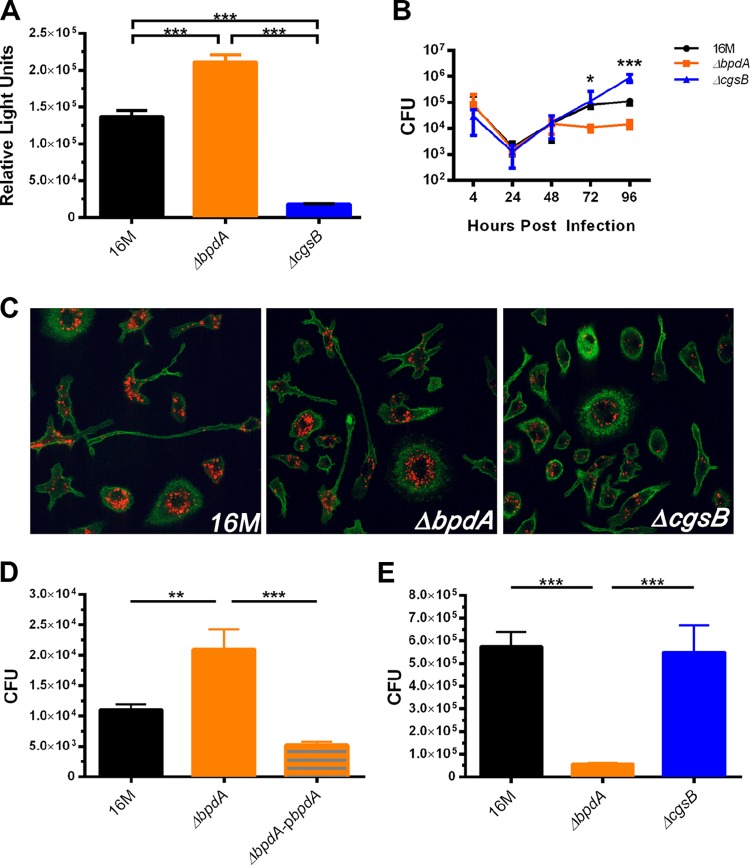
In our previous study, we used a Vibrio reporter system to indirectly determine the effects of individual c-di-GMP regulatory proteins on global c-di-GMP levels (11). To confirm the phenotype of each mutant in Brucella, the parental wild-type strain 16M and ΔbpdA and ΔcgsB mutants were all transformed with a plasmid containing a c-di-GMP-responsive riboswitch that drives lux expression (Fig. 1A). Both mutants displayed the predicted phenotype, with the ΔbpdA mutant having higher levels of c-di-GMP and the ΔcgsB mutant having lower levels of c-di-GMP than wild-type 16M. Our previous study had suggested altered survival in immunocompromised mice over time (11). To better delineate this chronology, bone marrow-derived macrophages (BMDMs) were infected with 16M or the ΔbpdA or ΔcgsB mutant for 4 to 96 h to determine if altered levels of c-di-GMP lead to altered intracellular growth (Fig. 1B). Initial replication (24 to 48 h) was preserved in the mutants. By 72 h, ΔbpdA mutant CFU diverged from those of 16M and the ΔcgsB mutant. Interestingly, the ΔcgsB mutant continued to increase replication at 96 h, whereas 16M and the ΔbpdA mutant plateaued, with the ΔbpdA mutant reaching lower levels of CFU. To confirm that the ΔbpdA mutant was able to establish its intracellular niche at the endoplasmic reticulum (ER), we performed confocal microscopy on infected BMDMs. BMDMs were infected with tD-Tomato-expressing 16M or the ΔbpdA or ΔcgsB mutant for 24 h and stained with anti-calreticulin to view the ER (Fig. 1C). As shown in the micrographs, the ΔbpdA mutant is able to infect macrophages in vitro and transit to an intracellular location similar to that of 16M and the ΔcgsB mutant, suggesting that its attenuation is not due to a trafficking defect. To determine if the ΔbpdA mutant exhibited differences in survival at an early versus later time point in vivo, BALB/c mice were infected with 16M or the ΔbpdA, ΔbpdA-pbpdA, or ΔcgsB mutant for either 24 h or 7 days. After 24 h, splenocyte CFU showed that the ΔbpdA mutant initially had higher intracellular growth than did 16M, and complementation (ΔbpdA-pbpdA) restored CFU to wild-type levels (Fig. 1D). After 7 days of infection, the ΔbpdA mutant had significantly lower CFU than did both 16M and the ΔcgsB mutant, indicating that the ΔbpdA mutant fails to establish a long-term infection both in vitro and in vivo (Fig. 1E).
FIG 1.
Intracellular c-di-GMP levels and survival differences of c-di-GMP mutants in vitro and in vivo. (A) 16M and the ΔbpdA and ΔcgsB mutants were transformed with a c-di-GMP-responsive riboswitch that drives lux expression. Bacteria were grown until stationary phase, and relative light units were measured (n = 4 replicates). (B) Bone marrow-derived macrophages (BMDMs) were infected with a multiplicity of infection (MOI) of 100 for 4 to 96 h and CFU were determined (n = 4 to 8 independent experiments). (C) Confocal micrographs of BMDMs infected with an MOI of 100 with td-Tomato-expressing 16M or the ΔbpdA or ΔcgsB mutant for 24 h. Endoplasmic reticulum was stained with anti-calreticulin antibody (green). Images are broad field (40×) and are representative of images from 3 independent experiments. (D) BALB/c mice were infected with 16M or the ΔbpdA or ΔbpdA-pbpdA mutant for 24 h and splenocyte CFU were plated (n = 3 independent experiments). (E) BALB/c mice were infected with 16M or the ΔbpdA or ΔcgsB mutant for 7 days and splenocyte CFU were determined (n = 2 independent experiments). Error bars represent SEMs; significance was determined using one-way ANOVA with Tukey's posttest.
ΔbpdA mutant attenuation is not a result of increased STING-dependent host IFN responses.
Bacterial cyclic dinucleotides are known stimuli of the innate immune receptor activating stimulator of interferon (IFN) genes (STING), leading to production of type I IFNs and specific chemokines (21–23). STING is an important mediator of IFN-β induced by Brucella (24). Because the growth defect of the ΔbpdA mutant was not related to failure to infect macrophages or traffic to the ER, we hypothesized that the ΔbpdA mutant, which leads to increased levels of c-di-GMP, induces a stronger immune response than the wild type, via activation of STING.
To test this hypothesis, BMDMs from STING−/− mice and age-matched wild-type littermates were infected with B. abortus 2308 and B. abortus ΔbpdA and production of CXCL10/IP-10 was assessed (Fig. 2A). Surprisingly, B. abortus ΔbpdA-infected BMDMs produced significantly less CXCL10/IP-10 than did B. abortus 2308. CXCL10/IP-10 production was suppressed in STING−/− BMDMs infected with each strain, confirming the dependence of this IFN-regulated response on STING stimulation. To determine the role of STING in regulating intracellular survival, BMDMs from STING−/− mice and wild-type littermates were infected for 72 h with B. abortus 2308 and B. abortus ΔbpdA (Fig. 2B). A significant increase in B. abortus 2308 CFU was detected when comparing STING−/− to wild-type BMDMs. Though not statistically significant, there is a trend showing increased B. abortus ΔbpdA in STING−/− BMDMs compared to wild-type BMDMs. Together, these data indicate that B. abortus ΔbpdA induces a weaker type I interferon response and that STING is critical for the control of B. abortus intracellular CFU in vitro.
FIG 2.
The ΔbpdA mutant induces a lesser CXCL10 response in mice via STING. BMDMs from wild-type C57BL/6 and STING−/− mice were infected with B. abortus S2308 or B. abortus ΔbpdA for 17 h (A) or 72 h (B). Culture supernatants were assayed for concentration of CXCL10/IP-10. Error bars represent SEMs; significance was determined using two-way ANOVA with Sidak's posttest (n = 3 independent experiments). (B) CFU were measured at 72 h postinfection. Parental Brucella strains are depicted with circles and squares and ΔbpdA mutants with triangles. Wild-type macrophage infections are depicted by circles and upward triangles, and STING−/− macrophages are depicted by squares and downward triangles. Error bars represent SEMs; significance was determined using one-way ANOVA with Tukey's posttest (n = 3 independent experiments).
ΔbpdA mutant attenuation is not a result of increased host immune response.
Although the ΔbpdA mutant induced a weaker type I interferon response than did wild-type B. abortus, it was possible that an otherwise increased host immune response is responsible for the attenuation of the ΔbpdA mutant. To test this hypothesis, and confirm the decreased interferon findings for B. melitensis 16M, BALB/c BMDMs were either left uninfected or infected with 16M or the ΔbpdA or ΔcgsB mutant for 24 h and RNA was submitted for RNA-seq analysis; this approach allows for an unbiased detection of changes in host gene expression. Genes with an absolute fold difference of 1.6 or greater (compared to both 16M and the other mutant) and that had a P value of <0.05 were considered significant. The host gene expression profiles of 16M and the ΔcgsB mutant were incredibly similar, with only 53 genes differentially regulated between the two strains, whereas there were over 2,000 differentially regulated host genes in the ΔbpdA mutant versus 16M and the ΔcgsB mutant.
As expected, each strain induced a significant immune response compared to uninfected BMDMs; however, the ΔbpdA mutant induced a drastically weaker immune response than did both 16M and the ΔcgsB mutant (Table 1), which is not attributable to a lower level of intracellular growth (Fig. 1B to D). The genes significantly downregulated fell into several categories, including complement activation, major histocompatibility complex (MHC)/antigen presentation, interferon-induced genes, immune signaling, and inflammation. Interferon-induced genes that were downregulated include those for guanylate binding proteins 1 to 11 (Gbp1 to -11), interferon-induced protein with tetratricopeptide repeats (Ifit) 1 to 3, 2′-5′ oligoadenylate synthetase 3 (Oas3), and chemokines (Ccl12, Cxcl10, and Cxcl11). The downregulation of Cxcl10 in ΔbpdA mutant-infected BMDMs confirms the result in Fig. 2, indicating that ΔbpdA elicits similar immune responses in both the B. melitensis and B. abortus backgrounds. Genes involved in inflammation that were significantly downregulated included CCL2, CCL3, CCL5, CCL7, CCL9, CCL22, IL-5, IL-10, IL12a, IL12b, IL-18, IL-27, and TNF-α.
TABLE 1.
Significantly downregulated immune genes in BMDMs infected with the ΔbpdA mutanta
| Functional category | Gene symbol | Downregulation factor |
|
|---|---|---|---|
| vs 16M | vs ΔcgsB mutant | ||
| Complement activation | |||
| Complement component 2 (within H-2S) | C2 | 0.40 | 0.44 |
| Complement factor B | Cfb | 0.31 | 0.29 |
| MHC/antigen presentation | |||
| Histocompatibility 2, class II antigen A, beta 1 | H2-Ab1 | 0.23 | 0.26 |
| Histocompatibility 2, M region locus 2 | H2-M2 | 0.24 | 0.18 |
| Histocompatibility 2, O region alpha locus | H2-Oa | 0.15 | 0.18 |
| Histocompatibility 2, O region beta locus | H2-Ob | 0.10 | 0.11 |
| Proteasome (prosome, macropain) subunit, beta type 6 | Psmb6 | 0.48 | 0.46 |
| Proteasome (prosome, macropain) 26S subunit, non-ATPase, 8 | Psmd8 | 0.50 | 0.50 |
| Transporter 1, ATP-binding cassette, subfamily B (MDR/TAP) | Tap1 | 0.48 | 0.49 |
| Interferon-induced genes | |||
| 2′-5′ Oligoadenylate synthetase 3 | Oas3 | 0.48 | 0.49 |
| 2′-5′ Oligoadenylate synthetase-like 1 | Oasl1 | 0.14 | 0.17 |
| 2′-5′ Oligoadenylate synthetase-like 2 | Oasl2 | 0.45 | 0.45 |
| Chemokine (C-C motif) ligand 12 | Ccl12 | 0.32 | 0.30 |
| Chemokine (C-X-C motif) ligand 10 | CXCL10 | 0.08 | 0.08 |
| Chemokine (C-X-C motif) ligand 11 | CXCL11 | 0.05 | 0.04 |
| Guanylate binding protein 1 | Gbp1 | 0.18 | 0.17 |
| Guanylate binding protein 10 | Gbp10 | 0.32 | 0.29 |
| Guanylate binding protein 11 | Gbp11 | 0.28 | 0.32 |
| Guanylate binding protein 2 | Gbp2 | 0.19 | 0.18 |
| Guanylate binding protein 3 | Gbp3 | 0.11 | 0.11 |
| Guanylate binding protein 4 | Gbp4 | 0.21 | 0.22 |
| Guanylate binding protein 5 | Gbp5 | 0.09 | 0.09 |
| Guanylate binding protein 6 | Gbp6 | 0.29 | 0.29 |
| Guanylate binding protein 7 | Gbp7 | 0.19 | 0.17 |
| Guanylate binding protein 8 | Gbp8 | 0.32 | 0.30 |
| Guanylate binding protein 9 | Gbp9 | 0.35 | 0.34 |
| Interferon gamma-induced GTPase | Igtp | 0.48 | 0.44 |
| Interferon-induced protein with tetratricopeptide repeats 1B like 2 | Ifit1bl2 | 0.35 | 0.31 |
| Interferon-induced protein with tetratricpeptide repeats 1B like 1 | Ifit1bl1 | 0.17 | 0.15 |
| Interferon-inducible GTPase 1 | Iigp1 | 0.40 | 0.33 |
| Interferon-induced protein with tetratricopeptide repeats 1 | Ifit1 | 0.27 | 0.25 |
| Interferon-induced protein with tetratricopeptide repeats 2 | Ifit2 | 0.21 | 0.21 |
| Interferon-induced protein with tetratricopeptide repeats 3 | Ifit3 | 0.39 | 0.36 |
| MX dynamin-like GTPase 1 | Mx1 | 0.27 | 0.29 |
| MX dynamin-like GTPase 2 | Mx2 | 0.19 | 0.20 |
| TAP binding protein-like | Tapbpl | 0.48 | 0.47 |
| Inflammation | |||
| Chemokine (C-C motif) ligand 2 | Ccl2 | 0.19 | 0.17 |
| Chemokine (C-C motif) ligand 22 | Ccl22 | 0.20 | 0.29 |
| Chemokine (C-C motif) ligand 3 | Ccl3 | 0.29 | 0.25 |
| Chemokine (C-C motif) ligand 5 | Ccl5 | 0.10 | 0.12 |
| Chemokine (C-C motif) ligand 7 | Ccl7 | 0.17 | 0.16 |
| Chemokine (C-C motif) ligand 9 | Ccl9 | 0.33 | 0.30 |
| Chemokine (C-C motif) receptor 7 | Ccr7 | 0.08 | 0.10 |
| Chemokine (C-C motif) receptor-like 2 | Ccrl2 | 0.12 | 0.11 |
| Chemokine (C-X-C motif) ligand 1 | CXCL1 | 0.23 | 0.21 |
| Chemokine (C-X-C motif) ligand 2 | CXCL2 | 0.27 | 0.25 |
| Chemokine (C-X-C motif) ligand 3 | CXCL3 | 0.27 | 0.32 |
| Chemokine (C-X-C motif) receptor 4 | Cxcr4 | 0.36 | 0.39 |
| Colony-stimulating factor 3 (granulocyte) | Csf3 | 0.05 | 0.03 |
| Colony-stimulating factor 3 receptor (granulocyte) | Csf3r | 0.48 | 0.47 |
| Interleukin 1 alpha | Il1a | 0.06 | 0.06 |
| Interleukin 1 receptor antagonist | Il1rn | 0.04 | 0.03 |
| Interleukin 10 | IL-10 | 0.19 | 0.13 |
| Interleukin 11 receptor, alpha chain 1 | IL-11ra1 | 0.36 | 0.36 |
| Interleukin 12a | IL-12a | 0.09 | 0.09 |
| Interleukin 12b | IL-12b | 0.02 | 0.02 |
| Interleukin 13 receptor, alpha 1 | IL-13ra1 | 0.28 | 0.27 |
| Interleukin 15 receptor, alpha chain | IL-15ra | 0.12 | 0.11 |
| Interleukin 17 receptor A | IL-17ra | 0.30 | 0.24 |
| Interleukin 18 | IL-18 | 0.38 | 0.42 |
| Interleukin 27 | IL-27 | 0.12 | 0.11 |
| Interleukin 6 | IL-6 | 0.04 | 0.04 |
| Interleukin 7 receptor | IL-7r | 0.12 | 0.10 |
| Killer cell lectin-like receptor subfamily K, member 1 | Klrk1 | 0.40 | 0.36 |
| Nitric oxide synthase 2, inducible | Nos2 | 0.01 | 0.01 |
| Tumor necrosis factor | TNF | 0.28 | 0.28 |
| Tumor necrosis factor (ligand) superfamily, member 10 | Tnfsf10 | 0.43 | 0.41 |
| Tumor necrosis factor (ligand) superfamily, member 15 | Tnfsf15 | 0.17 | 0.17 |
| Tumor necrosis factor (ligand) superfamily, member 9 | Tnfsf9 | 0.22 | 0.28 |
| Tumor necrosis factor receptor superfamily, member 12a | Tnfrsf12a | 0.22 | 0.27 |
| Tumor necrosis factor receptor superfamily, member 1b | Tnfrsf1b | 0.40 | 0.43 |
| Tumor necrosis factor receptor superfamily, member 8 | Tnfrsf8 | 0.24 | 0.25 |
| Tumor necrosis factor receptor superfamily, member 9 | Tnfrsf9 | 0.03 | 0.03 |
| Immune signaling | |||
| CCAAT/enhancer binding protein beta | Cebpb | 0.40 | 0.49 |
| CD164 antigen | Cd164 | 0.45 | 0.43 |
| CD200 antigen | Cd200 | 0.08 | 0.06 |
| CD24a antigen | Cd24a | 0.01 | 0.00 |
| CD274 antigen | Cd274 | 0.17 | 0.16 |
| CD300 antigen-like family member F | Cd300lf | 0.08 | 0.09 |
| CD33 antigen | Cd33 | 0.47 | 0.39 |
| CD40 antigen | Cd40 | 0.10 | 0.11 |
| CD69 antigen | Cd69 | 0.41 | 0.39 |
| CD83 antigen | Cd83 | 0.23 | 0.25 |
| CD86 antigen | Cd86 | 0.19 | 0.19 |
| Fas death domain-associated protein | Daxx | 0.44 | 0.46 |
| Fc receptor, IgG, low affinity IIb | Fcgr2b | 0.38 | 0.37 |
| Interferon regulatory factor 7 | Irf7 | 0.46 | 0.47 |
| Janus kinase 2 | Jak2 | 0.47 | 0.43 |
| Nucleotide-binding oligomerization domain containing 1 | Nod1 | 0.43 | 0.43 |
| Secretory leukocyte peptidase inhibitor | Slpi | 0.35 | 0.23 |
| Signal transducer and activator of transcription 3 | Stat3 | 0.49 | 0.50 |
| Signaling lymphocytic activation molecule family member 1 | Slamf1 | 0.01 | 0.01 |
| SLAM family member 7 | Slamf7 | 0.20 | 0.19 |
| SLAM family member 8 | Slamf8 | 0.21 | 0.18 |
| Suppressor of cytokine signaling 1 | Socs1 | 0.28 | 0.31 |
| Suppressor of cytokine signaling 3 | Socs3 | 0.32 | 0.32 |
| TNF receptor-associated factor 1 | Traf1 | 0.29 | 0.29 |
| TNF receptor-associated factor 5 | Traf5 | 0.32 | 0.32 |
| TNF receptor-associated factor 6 | Traf6 | 0.34 | 0.35 |
| Toll-like receptor 3 | TLR3 | 0.43 | 0.37 |
| Tumor necrosis factor, alpha-induced protein 3 | Tnfaip3 | 0.22 | 0.21 |
All genes presented are at least 2-fold different in the ΔbpdA mutant versus 16M and the ΔbpdA mutant versus the ΔcgsB mutant and are statistically significant (P < 0.001).
Nearly as many genes were upregulated by the ΔbpdA mutant by 16M and the ΔcgsB mutant (469 up versus 605 down); thus, there was not a failure of infection. Upregulated genes included those involved in cytoskeleton regulation, cell adhesion (lectins and integrins), and intracellular trafficking. Though most of the differentially expressed host immune genes were downregulated in BMDMs infected with the ΔbpdA mutant, there were a few immune genes significantly upregulated. For example, nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, zeta (Nfkbiz), Toll-like receptors (TLR4 and TLR13), TRAF3-interacting protein 3 (traf3ip3), and IL-16 were significantly upregulated. Interestingly, the upregulation of TLR4, lysozyme 1, lysozyme 2, and traf3ip3 (all lipopolysaccharide [LPS]-responsive genes) was suggestive of a change in bacterial LPS or outer membrane composition in the ΔbpdA mutant. Inflammasome components (Nlrp1 and Pycard) were also upregulated in the ΔbpdA mutant compared to 16M. Most surprising was the significant downregulation of STING in all three strains compared to uninfected BMDMs; however, STING gene expression was significantly upregulated in BMDMs infected with the ΔbpdA mutant compared to 16M and the ΔcgsB mutant. The relative downregulation of IL-6, Nos2, and IL12b and the relative upregulation of Fgd2 and STING in the ΔbpdA mutant versus 16M were confirmed in vivo in splenic macrophages (see Fig. S1 in the supplemental material).
Loss of bpdA results in microcolonies, biofilm, and pleomorphic bacteria.
The unexpected decrease in host immune response at 24 h prompted a closer evaluation of the effects of c-di-GMP dysregulation in the bacteria. Previous studies with Vibrio cholerae and Pseudomonas aeruginosa have shown that elevated c-di-GMP levels regulate bacterial colony size. For example, higher intracellular levels of c-di-GMP lead to small-colony variants in P. aeruginosa (25). To investigate whether the increased levels of c-di-GMP in the ΔbpdA mutant produce a microcolony phenotype in Brucella, the ΔbpdA mutant and 16M were plated on brucella broth agar plates (rich medium). After 3 days, no significant difference in colony size was observed between 16M and the ΔbpdA mutant (data not shown). However, it was possible that elevated c-di-GMP might lead to a microcolony phenotype after passage through macrophages, a notoriously hostile environment for Brucella. Further, the colonies that were recovered after growth in BMDMs appeared small (data not shown). To determine if these in vitro BMDM findings occurred in vivo, 6-week-old BALB/c mice were infected with 16M or the ΔbpdA or ΔbpdA-pbpdA mutant for 24 h. Splenocytes were lysed and CFU were plated to view colony size (Fig. 3A). As shown, the ΔbpdA mutant displays a microcolony phenotype after passage through mouse spleens, consistent with previous findings of elevated levels of c-di-GMP leading to microcolonies in other bacteria.
FIG 3.
Altered morphology in the ΔbpdA mutant. (A) BALB/c mice were infected with 16M or the ΔbpdA or ΔbpdA-pbpdA mutant for 24 h and splenocyte CFU were plated (pictures representative of those from 3 independent experiments). (B) Scanning electron micrographs of 16M and the ΔbpdA, ΔcgsB, and ΔbpdA-pbpdA mutants. White scale bars represent a distance of 5 μm. C. Quantification of cell area from scanning electron micrographs using ImageJ. Only single, distinct bacteria were used for quantification. (D) 16M and the ΔcgsB, ΔbpdA, and ΔbpdA-pbpdA mutants were stained with crystal violet to assess biofilm formation, and fold change absorbance was calculated with the value for parental 16M set equal to 1 (n = 6 independent experiments). Error bars represent SEMs; significance was determined using one-way ANOVA with Tukey's posttest.
These data suggest that the intracellular lifestyle imposes significant constraints on the ΔbpdA mutant. To determine if altered c-di-GMP impacted the morphology of individual bacteria, even in the absence of nutritional or other host pressure, we performed scanning electron microscopy on cultures of 16M and the ΔbpdA, ΔbpdA-pbpdA, and ΔcgsB mutants (Fig. 3B). 16M and the ΔcgsB mutant display the coccobacillus shape prototypical of Brucella, whereas ΔbpdA bacteria displayed a more round and amorphous coccus shape. The ΔbpdA-pbpdA genotype completely restores morphology to the typical coccobacillus shape, indicating that the mutant morphological changes related specifically to bpdA. To quantify these differences, ImageJ (NIH) was used to measure cell area (Fig. 3C). The coccus morphology of the ΔbpdA mutant leads to a significantly larger cell area than for both 16M and the ΔcgsB mutant. Taken together, these data suggest that elevated levels of c-di-GMP lead to a significantly disrupted bacterial shape, even prior to infection, and the formation of microcolonies when the organism is passaged through a hostile environment.
In other bacterial systems, besides generating microcolonies, c-di-GMP is also well known to increase biofilm production (8, 26). Previous literature has shown that under certain conditions, Brucella is able to produce a biofilm (27). To test whether the ΔbpdA mutation increases biofilm production, we used crystal violet staining. As shown in Fig. 3D, the ΔbpdA mutant has significantly higher crystal violet staining than both 16M and the ΔcgsB mutant. Microscopic examination also revealed greater numbers of adherent ΔbpdA bacteria (data not shown). Additionally, complementation (ΔbpdA-pbpdA) restores crystal violet staining to levels similar to those of 16M and significantly lower than for the ΔbpdA mutant.
Increased c-di-GMP leads to global metabolic changes affecting cell wall, type IV secretion, and nutrient acquisition.
Previous studies with other bacterial pathogens have revealed widespread effects of c-di-GMP on cell wall structure, biofilm, mobility, and replication (7, 8). As shown in Fig. 1E and our previous work, the ΔbpdA mutant exhibited a major disadvantage in survival over time in spite of an initial growth sufficiency (Fig. 1) and decreased host response (Fig. 2 and Table 1). The phosphodiesterase mutant also displayed altered morphology for both colonies and individual ΔbpdA bacteria (Fig. 3). To investigate the protein expression changes underlying this phenotype and further define the global effects of c-di-GMP on Brucella physiology, 16M and the ΔbpdA, and ΔcgsB mutants were compared using a proteomics approach. As shown in Fig. 4A, the numbers of proteins detected were 1,822, 1,866, and 1,898 for 16M and the ΔcgsB and ΔbpdA mutants, respectively, representing 57 to 59% of the Brucella orfeome. Of these, 1,734 proteins were shared among the three strains. There were 56, 23, and 45 unique proteins found in 16M and the ΔcgsB and ΔbpdA mutants, respectively. Proteins with an absolute fold difference of 1.6 or greater (compared to both counterparts) and that had a P value of <0.05 were considered significant. To classify these proteins, we used the COG (Clusters of Orthologous Groups) database for B. melitensis.
FIG 4.
Proteomics analysis of c-di-GMP mutants. (A) The proteomes of 16M and the ΔbpdA and ΔcgsB mutants at stationary phase were analyzed using mass spectroscopy for shared and unique proteins (n = 3 replicates). (B) Distribution of significantly up- or downregulated proteins found in the ΔbpdA or ΔcgsB mutant. Proteins were classified using the Clusters of Orthologous Groups (COGs) designations. Bars represent numbers of proteins in each COG uniquely present or absent and proteins with at least 1.6-fold difference compared to 16M (wild type) and the other c-di-GMP mutant.
The functional groups with the largest number of characterized proteins upregulated in the ΔbpdA mutant were cell wall/membrane/envelope biogenesis (10%) and intracellular trafficking, secretion, and vesicular transport (7.5%) (Fig. 4B; see also Table S5 in the supplemental material). This last group included type IV secretion proteins. Interestingly, type IV secretion proteins VirB4, VirB8, and VirB11 were all upregulated in the ΔbpdA mutant. Also upregulated were VirB9 and VirB10, although they did not meet all criteria for the tables in the supplemental material. Proteins involved in LPS and cell wall synthesis, in particular peptidoglycan synthesis, were also upregulated. For example, peptidoglycan cross-linking proteins d-alanyl-alanine synthetase A and penicillin-binding protein 1A were upregulated, as well as peptidoglycan synthesis proteins ybis and phospho-N-acetylmuramoyl-pentapeptide-transferase.
The proteins downregulated in the ΔbpdA mutant were classified as proteins involved in amino acid transport and metabolism (28%), carbohydrate transport and metabolism (13.4%), energy production and conversion (11%), inorganic ion transport and metabolism (7.3%), and defense mechanisms (7.3%). More specifically, branched-chain amino acid transferases and dehydrogenases were downregulated in the ΔbpdA mutant, in addition to a multitude of proteins involved in amino acid or peptide transport. Also, proteins involved in sugar transport (d-ribose-binding periplasmic proteins and sugar-binding proteins) and glyoxylate metabolism [hydroxypyruvate reductase and (S)-2-hydroxy-acid oxidase subunits D and E] were also downregulated. These decreases in sugar transport correlated with a significantly decreased overall carbohydrate content in the ΔbpdA mutant, suggesting an impact on nutrient acquisition (see Fig. S2 in the supplemental material). Vitamin B12 and thiamine biosynthesis proteins were downregulated in the ΔbpdA mutant. Finally, as confirmation of genetic deletion, bpdA itself was abundantly present only in 16M and the ΔcgsB mutant.
In the ΔcgsB mutant, many fewer significant changes versus both 16M and the ΔbpdA mutant were observed, consistent with the small departure from the wild type in virulence, host response (Table 1), in vivo growth (Fig. 1), and morphology (Fig. 3). Primarily, we observed a few increases in proteins regulating amino acid transport, carbohydrate metabolism, and inorganic ion transport. Most notably, iron transport protein Fe3+ ion import ATP-binding protein FbpC (compared to 16M) and bacterial extracellular solute-binding protein (compared to 16M and the ΔbpdA mutant) were significantly increased in the ΔcgsB mutant. Acquiring iron is essential for the survival of many different bacteria, and specifically in Brucella, iron transport is required for virulence (28). Interestingly, cold shock protein A (CspA) and type IV secretion system regulator VjbR were significantly downregulated in the ΔcgsB mutant, seemingly in contrast to its more virulent in vivo phenotype in immunocompromised mice but consistent with the VirB increases in the ΔbpdA mutant (11). Finally, in contrast to the case with the ΔbpdA mutant, there were slight decreases in proteins involved in cell wall processing, such as cell wall degradation protein, bactoprenol glucosyl transferase, and UDP-N-acetylmuramoyl-l-alanyl–d-glutamate synthetase.
The ΔbpdA mutant fails to adapt to nutrient-poor environments.
Proteomics analysis of the c-di-GMP mutants (Fig. 4; see also tables in the supplemental material) revealed many changes in metabolic proteins, including decreased transporters for amino acids, carbohydrates, and iron. These data, along with the differences in colony size between bacteria grown in rich medium versus macrophages, led to the hypothesis that the ΔbpdA mutant does not establish a chronic state of infection because it is unfit to adapt to the challenges of an intracellular lifestyle compared to 16M and the ΔcgsB mutant. The Brucella-containing vacuoles within macrophages are thought to be nutritionally hostile environments (29). To simulate and exaggerate a nutritional environment switch, bacteria were grown in 2XYT rich medium (Fig. 5A) or modified Gerhardt's minimal medium (Fig. 5B) and then plated on either rich (2XYT) or minimal (TSAYE) medium agar plates. Note that the 2XYT medium is less rich than the brain heart infusion (BHI) medium typically used to maintain the c-di-GMP mutant strains. As shown in Fig. 5A, the ΔbpdA mutant had significantly lower CFU than 16M and the ΔcgsB mutant in both instances, but the effect is more profound in the transfer from rich to minimal medium. Interestingly, in Fig. 5B, both the ΔbpdA and ΔcgsB mutants had significantly lower CFU than 16M even when transferred back to rich medium plates, indicating that both c-di-GMP mutants cope less well with nutritionally harsh environments than the parental 16M strain. In particular, the ΔbpdA mutant displays an extreme growth disadvantage upon exposure to low nutrients, and this growth curtailment is maintained, even after transfer back to a condition of increased nutrient availability. Further, on visual inspection the recovered ΔbpdA colonies displayed a microcolony phenotype similar to that observed post-macrophage infection (data not shown). In comparison, the parental 16M wild type began to show a decrease in CFU only when cultured and plated in minimal medium (Fig. 5B). Together, these data support the hypothesis that the high-c-di-GMP mutant exhibits decreased virulence in vivo, marked by decreased CFU and microcolony phenotype, because of an inability to cope with nutritionally hostile environments.
FIG 5.
Decreased growth of c-di-GMP mutants in minimal media. (A) 16M and the ΔbpdA and ΔcgsB mutants were cultured until stationary phase in rich medium (2XYT) and then plated on either rich (2XYT) or minimal (TSAYE) agar plates. (B) 16M and the ΔbpdA and ΔcgsB mutants were cultured until stationary phase in a modified Gerhardt's minimal medium and then plated on either rich (2XYT) or minimal (TSAYE) agar plates. Error bars represent SEMs; significance was determined using two-way ANOVA with Sidak's posttest (n = 2 independent experiments).
DISCUSSION
In its natural host, Brucella engages in a highly successful two-stage lifestyle. First, Brucella establishes its intracellular niche in macrophages, subverting immune surveillance while ensuring its long-term chronic survival (30). Second, Brucella develops into a virulent state by trafficking to nonprofessional phagocytic cells (e.g., trophoblasts) in the gravid uterus and replicating to high numbers, causing trophoblast death, placentitis, and abortion (6, 31, 32). Currently, very little is known about how Brucella spp. alternate between their acute virulent and chronic persistent lifestyles. In other bacterial genera, the ubiquitous second messenger c-di-GMP controls phase switching in response to varied environments (8). To gain a better understanding of how Brucella c-di-GMP levels modulate virulence, we used a systems-based approach to determine the effect of c-di-GMP dysregulation on host immune response and Brucella physiology. Elevated levels of c-di-GMP led to an increase in type IV secretion system proteins, altered morphology, biofilm production, and in vivo attenuation, whereas decreased levels of c-di-GMP led to a decrease in type IV secretion system proteins and increased nutrient acquisition. As previously described, the guanylate cyclase mutant (with low c-di-GMP) exhibits increased virulence in vivo in immunocompromised mice (11).
Many of the characteristics displayed by the ΔbpdA mutant are in accordance with the characteristics of other bacteria with high levels of c-di-GMP. For example, similar to Pseudomonas aeruginosa small-colony variants expressing high levels of c-di-GMP, the ΔbpdA mutant produces a microcolony phenotype (25). Specifically, this phenotype is observed after passaging through macrophages, a notoriously hostile environment for Brucella, or when grown in nutritionally minimal media. When grown in rich media (e.g., BHI medium), the ΔbpdA mutant produces a colony size similar to that of wild-type 16M. At the individual bacterium scale, however, ΔbpdA bacteria are pleomorphic and atypically coccus-shaped, in contrast to the coccobacillus shape of 16M bacteria. Furthermore, ΔbpdA bacteria have a larger average cell area than does the wild type.
Increased c-di-GMP is also associated with decreased motility and biofilm formation in other bacteria. Previously, we have shown that the ΔbpdA mutant has decreased flagellar transcription (11). Flagellar proteins were not observed in the proteomics analysis, potentially due to lack of expression in stationary-phase Brucella or lack of detection (assay sensitivity). In this study, we showed that the ΔbpdA mutant has increased crystal violet staining, consistent with production of a biofilm. Biofilms generally confer a survival advantage for pathogens that cause chronic infections by protecting the bacteria against host defenses (33). Additionally, biofilms allow for increased adhesion to host cells and various surfaces. Brucella persists long term in nutritionally scarce niches both in the environment and in infected hosts, potentially utilizing a biofilm to evade host immune detection or to resist environmental stress (34, 35). Although biofilms typically play an important role in chronic infections, and Brucella has previously been shown to produce components of a biofilm, the role of a biofilm in Brucella virulence is unclear (27, 36). Importantly, crystal violet staining, colony size, and individual bacterium size phenotypes displayed by the ΔbpdA mutant were restored to wild-type levels or appearance via complementation with a plasmid containing bpdA, indicating that these phenotypes are the result of loss of bpdA. The concordance with other systems supports our interpretation of the effects of bpdA as being related to altered levels of c-di-GMP.
Beyond the aforementioned general effects, c-di-GMP also controls organism-specific characteristics, such as spore formation in Streptomyces venezuelae (37). In this study, we utilized a proteomics approach to identify Brucella-specific proteins and pathways affected by changes in global c-di-GMP levels. This approach detected over half the orfeome (∼1,900 proteins) of Brucella, significantly more than previously reported (typically <500 proteins) (38–40). In the attenuated, high-c-di-GMP-expressing ΔbpdA mutant, proteins involved in cell wall biogenesis were upregulated compared to both 16M and the ΔcgsB mutant. Although not statistically significant, proteins involved in cell division and chromosome segregation (FtsK, minD, and minE) were also upregulated in the ΔbpdA mutant. Taken together, upregulation of cell wall biogenesis, cell division, and chromosome segregation proteins possibly explain the atypical morphology of the ΔbpdA mutant.
Although highly attenuated, the ΔbpdA mutant upregulates many proteins in the type IV secretion system (T4SS), a known Brucella virulence factor. Brucella activates the T4SS early in infection (3 to 4 h postinfection) to avoid phagolysosome degradation and eventually establish its intracellular niche at the endoplasmic reticulum (ER) (31). The increase in T4SS proteins in the ΔbpdA mutant may in part explain the initial higher CFU observed at 24 h. However, once established at the ER, Brucella shuts down T4SS proteins and begins to transition to stationary-phase physiology, approximately 48 h postinfection (41). The inability of the ΔbpdA mutant to regulate c-di-GMP levels and thus turn off the T4SS could explain our previous report of the failure of the ΔbpdA mutant to disseminate in BALB/c mice, as well as the attenuation observed in this study 72 to 96 h postinfection in BMDMs. Conversely, the more virulent ΔcgsB mutant significantly downregulates the T4SS regulator VjbR as well as cold shock protein A (CspA), in accordance with the recent finding that B. melitensis NI ΔcspA had significant downregulation of VjbR and genes involved in the T4SS (42). However, the ΔcgsB mutant significantly increases proteins involved in nutrient acquisition and iron transport, setting itself up for better survival in its nutrient-limiting replicative site at the ER. These ion and nutrient acquisition advantages may contribute to its increased virulence in immunocompromised mice.
We had initially hypothesized that the attenuation of the ΔbpdA mutant both in vitro and in vivo was a result of an increased host immune response via STING, a sensor of bacterial cyclic dinucleotides which leads to type I interferon production (21, 23). CFU analysis suggests that STING is critical for controlling Brucella, as STING−/− BMDMs had higher CFU than wild-type BMDMs 72 h postinfection. Further, STING was required for CXCL10 production, consistent with previous reports. However, the ΔbpdA mutant induced significantly smaller amounts of CXLC10 protein than did wild-type 2308, indicating that STING does not mediate an increased host immune response during infection with the ΔbpdA mutant. Differential gene expression analysis via RNA-seq of infected BMDMs revealed that surprisingly, the ΔbpdA mutant induced a drastically weaker overall host immune response than both 16M and the ΔcgsB mutant; specifically, over 100 genes involved in immune signaling, inflammation, MHC/antigen presentation, complement activation, and interferon-induced genes were significantly downregulated in BMDMs infected with the ΔbpdA mutant, indicating that the ΔbpdA mutant elicits a weaker host immune response in both the B. abortus and B. melitensis backgrounds. RNA-seq also revealed downregulation of STING by wild-type Brucella and the c-di-GMP mutants, suggesting a potentially novel mechanism by which Brucella evades host immune responses.
The reasons behind the unexpected decrease in host immune response to the ΔbpdA mutant are not currently clear. One possibility is that the ΔbpdA mutant does not survive long enough to elicit an immune response. However, the increases in host gene expression at 24 h, immunofluorescence microscopy findings suggesting successful trafficking, increased CFU at 24 h in vivo, and comparable CFU at 48 h in vitro would all suggest that this is not the case. Another potential explanation is the increase in T4SS components. Based on increased T4SS activity, one would predict enhanced trafficking to the ER and evasion of lysosomal destruction, thus perhaps limiting the triggering of cytosolic innate immune sensors. These mechanisms of immune evasion as well as STING downregulation warrant further investigation.
In summary, this is the first study to use a broad unbiased systems approach to analyze the effects of c-di-GMP regulatory Brucella proteins on host response and bacterial physiology. Beyond confirming expected effects of c-di-GMP levels on cell wall processing enzymes, microcolony formation, and biofilm production, we revealed unanticipated Brucella-specific effects on type IV secretion and modulation of host immune responses. The impact of c-di-GMP levels on nutrient acquisition, and thus bacterial fitness, could in large part explain the observed differences in virulence between the c-di-GMP regulatory mutants. Together, our results suggest a chronologic model whereby Brucella elevates c-di-GMP during early infection to enhance type IV secretion system activity but then must decrease c-di-GMP levels to ramp up nutrient acquisition (iron in particular) during its residence in ER-derived compartments. Evaluation of this model will be pursued in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Erik Petersen for providing us with the ribolux plasmid, Adam Ericsen for generating the RNA-seq data, and Glen Barber for providing the STING−/− mice.
The authors contributed to this article as follows: conceptualization, M.K., J.S.H., M.B., S.C.O., G.A.S., and J.A.S.; methodology, M.K., J.S.H., F.M.M., L.A., C.L.H., and Y.-P.L.; investigation, M.K., J.S.H., and J.A.S.; writing of the original draft, M.K.; review and editing, M.K., S.C.O., G.A.S., and J.A.S.; funding acquisition, M.B., S.C.O., G.A.S., and J.A.S.
This work was supported by BARD grant US-4378-11 and NIH grants R01 AI073558, R01 AI116453, and F31 AI115931.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00531-16.
REFERENCES
- 1.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N Engl J Med 352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect Dis 6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 3.Donev DM. 2010. Brucellosis as priority public health challenge in South Eastern European countries. Croat Med J 51:283–284. doi: 10.3325/cmj.2010.51.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco MP, Mulder M, Gilman RH, Smits HL. 2007. Human brucellosis. Lancet Infect Dis 7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 5.Pappas G, Panagopoulou P, Christou L, Akritidis N. 2006. Brucella as a biological weapon. Cell Mol Life Sci 63:2229–2236. doi: 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Watarai M, Suzuki H, Makino S, Kodama T, Shirahata T. 2004. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb Pathog 37:11–19. doi: 10.1016/j.micpath.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Kalia D, Merey G, Nakayama S, Zheng Y, Zhou J, Luo Y, Guo M, Roembke BT, Sintim HO. 2013. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p) ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev 42:305–341. doi: 10.1039/C2CS35206K. [DOI] [PubMed] [Google Scholar]
- 8.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bomchil N, Watnick P, Kolter R. 2003. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J Bacteriol 185:1384–1390. doi: 10.1128/JB.185.4.1384-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gjermansen M, Ragas P, Tolker-Nielsen T. 2006. Proteins with GGDEF and EAL domains regulate Pseudomonas putida biofilm formation and dispersal. FEMS Microbiol Lett 265:215–224. doi: 10.1111/j.1574-6968.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 11.Petersen E, Chaudhuri P, Gourley C, Harms J, Splitter G. 2011. Brucella melitensis cyclic di-GMP phosphodiesterase BpdA controls expression of flagellar genes. J Bacteriol 193:5683–5691. doi: 10.1128/JB.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna N, Ouahrani-Bettache S, Drake KL, Adams LG, Kohler S, Occhialini A. 2013. Global Rsh-dependent transcription profile of Brucella suis during stringent response unravels adaptation to nutrient starvation and cross-talk with other stress responses. BMC Genomics 14:459. doi: 10.1186/1471-2164-14-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. 2008. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rambow-Larsen AA, Rajashekara G, Petersen E, Splitter G. 2008. Putative quorum-sensing regulator BlxR of Brucella melitensis regulates virulence factors, including the type IV secretion system and flagella. J Bacteriol 190:3274–3282. doi: 10.1128/JB.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seleem MN, Jain N, Alqublan H, Vemulapalli R, Boyle SM, Sriranganathan N. 2008. Activity of native vs. synthetic promoters in Brucella. FEMS Microbiol Lett 288:211–215. doi: 10.1111/j.1574-6968.2008.01358.x. [DOI] [PubMed] [Google Scholar]
- 16.Durward-Diioia M, Harms J, Khan M, Hall C, Smith JA, Splitter GA. 2015. CD8+ T cell exhaustion, suppressed gamma interferon production, and delayed memory response induced by chronic Brucella melitensis infection. Infect Immun 83:4759–4771. doi: 10.1128/IAI.01184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Toole GA. 2011. Microtiter dish biofilm formation assay. J Vis Exp 2011(47):pii=2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. 2014. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics 13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diz AP, Carvajal-Rodriguez A, Skibinski DO. 2011. Multiple hypothesis testing in proteomics: a strategy for experimental work. Mol Cell Proteomics 10:M110.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konno H, Barber GN. 2014. The STING controlled cytosolic-DNA activated innate immune pathway and microbial disease. Microbes Infect 16:998–1001. doi: 10.1016/j.micinf.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TL, Vasconcelos AC, Nogueira L, Bafica A, Silva AM, Oliveira SC. 2011. MyD88 and STING signaling pathways are required for IRF3-mediated IFN-beta induction in response to Brucella abortus infection. PLoS One 6:e23135. doi: 10.1371/journal.pone.0023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starkey M, Hickman JH, Ma L, Zhang N, De Long S, Hinz A, Palacios S, Manoil C, Kirisits MJ, Starner TD, Wozniak DJ, Harwood CS, Parsek MR. 2009. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol 191:3492–3503. doi: 10.1128/JB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan RP. 2013. Cyclic di-GMP signalling and the regulation of bacterial virulence. Microbiology 159:1286–1297. doi: 10.1099/mic.0.068189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godefroid M, Svensson MV, Cambier P, Uzureau S, Mirabella A, De Bolle X, Van Cutsem P, Widmalm G, Letesson JJ. 2010. Brucella melitensis 16M produces a mannan and other extracellular matrix components typical of a biofilm. FEMS Immunol Med Microbiol 59:364–377. doi: 10.1111/j.1574-695X.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- 28.Eskra L, Covert J, Glasner J, Splitter G. 2012. Differential expression of iron acquisition genes by Brucella melitensis and Brucella canis during macrophage infection. PLoS One 7:e31747. doi: 10.1371/journal.pone.0031747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rambow-Larsen AA, Petersen EM, Gourley CR, Splitter GA. 2009. Brucella regulators: self-control in a hostile environment. Trends Microbiol 17:371–377. doi: 10.1016/j.tim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Köhler S, Michaux-Charachon S, Porte F, Ramuz M, Liautard JP. 2003. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol 11:215–219. doi: 10.1016/S0966-842X(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 31.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med 198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samartino LE, Enright FM. 1993. Pathogenesis of abortion of bovine brucellosis. Comp Immunol Microbiol Infect Dis 16:95–101. doi: 10.1016/0147-9571(93)90001-L. [DOI] [PubMed] [Google Scholar]
- 33.Reckseidler-Zenteno SL, DeVinney R, Woods DE. 2005. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect Immun 73:1106–1115. doi: 10.1128/IAI.73.2.1106-1115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwida M, Al Dahouk S, Melzer F, Rosler U, Neubauer H, Tomaso H. 2010. Brucellosis—regionally emerging zoonotic disease? Croat Med J 51:289–295. doi: 10.3325/cmj.2010.51.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almirón MA, Roset MS, Sanjuan N. 2013. The Aggregation of Brucella abortus occurs under microaerobic conditions and promotes desiccation tolerance and biofilm formation. Open Microbiol J 7:87–91. doi: 10.2174/1874285801307010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uzureau S, Godefroid M, Deschamps C, Lemaire J, De Bolle X, Letesson JJ. 2007. Mutations of the quorum sensing-dependent regulator VjbR lead to drastic surface modifications in Brucella melitensis. J Bacteriol 189:6035–6047. doi: 10.1128/JB.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tschowri N, Schumacher MA, Schlimpert S, Chinnam NB, Findlay KC, Brennan RG, Buttner MJ. 2014. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell 158:1136–1147. doi: 10.1016/j.cell.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Dahouk S, Jubier-Maurin V, Neubauer H, Kohler S. 2013. Quantitative analysis of the Brucella suis proteome reveals metabolic adaptation to long-term nutrient starvation. BMC Microbiol 13:199. doi: 10.1186/1471-2180-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandalakis V, Psaroulaki A, De Bock PJ, Christidou A, Gevaert K, Tsiotis G, Tselentis Y. 2012. Investigation of rifampicin resistance mechanisms in Brucella abortus using MS-driven comparative proteomics. J Proteome Res 11:2374–2385. doi: 10.1021/pr201122w. [DOI] [PubMed] [Google Scholar]
- 40.Paredes-Cervantes V, Flores-Mejia R, Moreno-Lafont MC, Lanz-Mendoza H, Tello-Lopez AT, Castillo-Vera J, Pando-Robles V, Hurtado-Sil G, Gonzalez-Gonzalez E, Rodriguez-Cortes O, Gutierrez-Hoya A, Vega-Ramirez MT, Lopez-Santiago R. 2011. Comparative proteome analysis of Brucella abortus 2308 and its virB type IV secretion system mutant reveals new T4SS-related candidate proteins. J Proteomics 74:2959–2971. doi: 10.1016/j.jprot.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 41.López-Goñi I, O'Callaghan D. 2012. Brucella: molecular microbiology and genomics. Caister Academic Press, Norfolk, UK. [Google Scholar]
- 42.Wang Z, Liu W, Wu T, Bie P, Wu Q. 2016. RNA-seq reveals the critical role of CspA in regulating Brucella melitensis metabolism and virulence. Sci China Life Sci 59:417–424. doi: 10.1007/s11427-015-4981-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.