Abstract
Staphylococcus aureus is a leading cause of community- and nosocomial-acquired infections, with a propensity for biofilm formation. S. aureus biofilms actively skew the host immune response toward an anti-inflammatory state; however, the biofilm effector molecules and the mechanism(s) of action responsible for this phenomenon remain to be fully defined. The essential bacterial second messenger cyclic diadenylate monophosphate (c-di-AMP) is an emerging pathogen-associated molecular pattern during intracellular bacterial infections, as c-di-AMP secretion into the infected host cytosol induces a robust type I interferon (IFN) response. Type I IFNs have the potential to exacerbate infectious outcomes by promoting anti-inflammatory effects; however, the type I IFN response to S. aureus biofilms is unknown. Additionally, while several intracellular proteins function as c-di-AMP receptors in S. aureus, it has yet to be determined if any extracellular role for c-di-AMP exists and its release during biofilm formation has not yet been demonstrated. This study examined the possibility that c-di-AMP released during S. aureus biofilm growth polarizes macrophages toward an anti-inflammatory phenotype via type I interferon signaling. DacA, the enzyme responsible for c-di-AMP synthesis in S. aureus, was highly expressed during biofilm growth, and 30 to 50% of total c-di-AMP produced from S. aureus biofilm was released extracellularly due to autolytic activity. S. aureus biofilm c-di-AMP release induced macrophage type I IFN expression via a STING-dependent pathway and promoted S. aureus intracellular survival in macrophages. These findings identify c-di-AMP as another mechanism for how S. aureus biofilms promote macrophage anti-inflammatory activity, which likely contributes to biofilm persistence.
INTRODUCTION
Staphylococcus aureus is a leading cause of prosthetic joint infections (1, 2), whereupon adherence to the implant surface facilitates biofilm formation. These infections are particularly challenging to treat and typically require a two-stage surgical process for insertion of a new device (1). Moreover, biofilms actively skew the host immune response toward an anti-inflammatory state, thereby contributing to the chronic nature of biofilm-mediated infections (3–5). This is evident by macrophage polarization toward an alternatively activated phenotype and the recruitment of myeloid-derived suppressor cells (MDSCs) (3, 6–8). While this immune deviation appears to be driven by S. aureus biofilm products, the effectors and their mechanism(s) of action remain to be fully identified.
To further understand mechanisms of immune modification by S. aureus biofilms, we investigated the role of the essential bacterial second messenger cyclic diadenylate monophosphate (c-di-AMP), since it has been identified as an emerging pathogen-associated molecular pattern (PAMP) that influences immune responsiveness (9, 10). While c-di-AMP has been found to regulate many important functions in bacteria, including cell wall stress and peptidoglycan homeostasis (11–15), antibiotic resistance (16–18), biofilm formation (19), growth and metabolism (20–23), and DNA damage response (24, 25), it can also modulate leukocyte activation. For example, c-di-AMP secretion into the macrophage cytosol induces a robust type I interferon (IFN [IFN-β]) response during intracellular bacterial infections, including those caused by Listeria monocytogenes (26, 27), Chlamydia trachomatis (28), and Mycobacterium spp. (29, 30). Cytosolic c-di-AMP is sensed by both the endoplasmic reticulum resident protein stimulator of interferon genes (STING) (31–33) and the helicase DDX41 (34); however, the individual contributions of these proteins in sensing c-di-AMP remain unclear.
While several intracellular proteins function as c-di-AMP receptors in S. aureus (11, 35–38), the spatial and temporal production as well as any extracellular role for c-di-AMP during biofilm development has yet to be determined. Furthermore, the ability of extracellular c-di-AMP to trigger type I IFN production in macrophages has not yet been demonstrated and may serve as a potential mechanism to polarize macrophages to an anti-inflammatory state characteristic of biofilm infections (3, 5–8). The data presented here demonstrate that the gene responsible for c-di-AMP synthesis (dacA) is expressed in a temporal manner during biofilm growth, and quantitative mass spectrometry revealed that c-di-AMP is released extracellularly from S. aureus biofilm during cell lysis. Additionally, macrophage exposure to extracellular c-di-AMP, coculture with S. aureus biofilms, and treatment with conditioned medium from mature S. aureus biofilm all induced a robust type I IFN response. In terms of functional implications, c-di-AMP promoted S. aureus intracellular survival in human monocyte-derived macrophages. Collectively, these data represent the first evidence for the recognition of exogenous c-di-AMP from an extracellular pathogen and provide support for bacterial cell lysis as a mechanism for polarizing macrophages toward a type I IFN response.
MATERIALS AND METHODS
Bacterial strains, plasmid construction, and growth conditions.
Escherichia coli DH5α was used for cloning and grown at 37°C in lysogeny broth (LB) with ampicillin (100 μg ml−1) for selection. DNA ligase and restriction enzymes were obtained from New England BioLabs (Beverly, MA). Plasmids were purified using the Wizard Plus SV Miniprep DNA purification system (Promega Corporation, Madison, WI) and analyzed using Vector NTI (Invitrogen, Carlsbad, CA). The S. aureus strain used (LAC) is a USA300 isolate from a skin and soft tissue infection, cured of plasmid p03, and designated LAC-13C (39). LAC-13C is referred to as LAC throughout the text. The strains and plasmid used in the study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Characteristic(s) | Source(s) |
|---|---|---|
| Bacterial strains | ||
| LAC-13C | Wild-type S. aureus CA-MRSAa (USA300) isolate cured of plasmid p03 | 40 |
| ΔgdpP | Transduction of gdpP::ΦNΣ in LAC-13C | This study and ref. 40 |
| CMG8 | Transduction of SAUSA300_2126, SAUSA_2298, and SAUSA2360::ΦNΣ in LAC-13C | This study and ref. 40 |
| RN4220 | Highly transformable restriction-deficient strain | 64 |
| Plasmid | ||
| pCMG2 | Divergent PdacA::DsRed and PgdpP::GFP cloned into pDM4 | This study and ref. 41 |
CA-MRSA, community-acquired methicillin-resistant S. aureus.
S. aureus growth, biofilm development, and analysis.
For static biofilm development, single isolated colonies grown on tryptic soy agar (TSA) were inoculated into 3 ml of RPMI 1640 plus 1% Casamino Acids (CAA; Becton, Dickinson, Franklin Lakes, NJ) and grown overnight at 37°C with orbital shaking at 250 rpm. Wells of a 12-, 24-, or 48-well plate were precoated with 20% human plasma in 0.1% carbonate-bicarbonate buffer (Sigma, St. Louis, MO) overnight at 4°C. Cultures were diluted to an optical density at 600 nm (OD600) of 0.05 in fresh RPMI 1640 plus 1% CAA and added to wells at a surface-to-volume ratio of 0.19. Chloramphenicol (10 μg ml−1) was supplemented when plasmid maintenance was required. Biofilms were grown statically in a 37°C, 5% CO2 incubator. After every 24 h of growth, 50% of the medium was removed and replaced with fresh medium.
Confocal analysis of fluorescence from transcription reporter plasmids during static biofilm growth was performed in sterile two-well glass chamber slides (Thermo Scientific Nunc) using a Zeiss 710 META laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) with ×40 magnification and analyzed using ZEN 2009 Black (Carl Zeiss) software. Green fluoresceint protein (GFP) and DsRed fluorescence were acquired with 488-nm and 560-nm excitation (Ex) wavelengths, respectively.
Analysis of dacA and gdpP transcription levels was performed by isolating RNA from static biofilms at 1 to 6 days of growth. Supernatants were removed and biofilms resuspended in 1 ml of TRIzol (Life Technologies). Cell suspensions were transferred to 2 ml lysing matrix B tubes containing 0.1-mm silica beads (MP Biomedicals, Santa Ana, CA), lysed in an OMNI Bead Ruptor 24 (Omni International, Kennesaw, GA), and bead beat twice for 23 s at speed 6. Subsequent RNA purification was performed according to the manufacturer's instructions. cDNA was generated using the high-capacity cDNA reverse transcription (RT) kit (Applied Biosystems, Foster City, CA), and quantitative RT-PCR (qRT-PCR) was performed using iTaq Universal SYBR green supermix (Bio-Rad, Hercules, CA) with primers specific to sigA, dacA, and gdpP (Table 2).
TABLE 2.
Primers used in the study
| Primer | Sequencea | Application | Source |
|---|---|---|---|
| dacA-F | TGCGGTTGGTATTTCAGAAG | dacA qRT-PCR | This study |
| dacA-R | TTTCTTTTGAAAGCGTGTGC | dacA qRT-PCR | This study |
| gdpP-F | TTAGTCGATGGGCAACTGAG | gdpP qRT-PCR | This study |
| gdpP-R | TTAATTGGGCACGATAACCA | gdpP qRT-PCR | This study |
| sigA-F | AACTGAATCCAAGTGATCTTAGTG | sigA qRT-PCR | 65 |
| sigA-R | TCATCACCTTGTTCAATACGTTTG | sigA qRT-PCR | 65 |
| CMG2 | CCCTGCAGATTGATAAATTGGGGATAAAGGAA | 500 bp 5′ dacA | This study |
| CMG4 | CCGTCGACTTATTTCACACCTTTCTTTTGAAAG | 3′ dacA | This study |
| CMG7 | CCCTGCAGGCCGATGCGAATATGAC | 500 bp 5′ gdpP | This study |
| CMG9 | CCGTCGACTCATGCATCTTCACTCCTAC | 3′ gdpP | This study |
| CMG10 | CCGAATTCGCGCGACTTTTCATTT | SAUSA300_2126 | This study |
| CMG11 | CCGTCGACTAAAATTTCCTTCTATTACTTTCTATTTCT | SAUSA300_2126 | This study |
| CMG12 | CCGAATTCAAATTAAATCACTTAATGTTAAACAAGGTGA | SAUSA300_2298 | This study |
| CMG13 | CCGTCGACTTATTCATGATTGATACTATTATCTGC | SAUSA300_2298 | This study |
| CMG14 | CCGAATTCATGTACACAAGGAGTGAGT | SAUSA300_2360 | This study |
| CMG15 | CCGTCGACTTATTGACGAGAATCAACTTC | SAUSA300_2360 | This study |
Primer oligonucleotide sequences are provided in the 5′-to-3′ orientation. Italics indicate nonhomologous sequences added for cloning.
To assess fluorescence from the transcription reporter plasmids during planktonic growth, S. aureus was grown in trypic soy broth (TSB) overnight (16 to 18 h) at 37°C with shaking at 250 rpm. Cultures were diluted to an OD600 of 0.1 in TSB and added to a 96-well black-wall, clear-bottom plate (Corning, Inc., Corning, NY), where growth (OD600), GFP fluorescence (Ex, 488 nm; emission [Em], 518 nm), and DsRed fluorescence (Ex, 560 nm; Em, 590 nm) were monitored in real-time by using a TECAN 200 Infinite Pro system (Tecan Group Ltd., Männedorf, Switzerland). For fluorescence analysis during biofilm formation under flow conditions, methods previously described were employed for use with the Bio-Flux microfluidics system (40, 41).
Cyclic di-AMP measurements.
Biofilms were propagated in 12-well plates containing 2 ml/well of medium as described above. At the desired time point, biofilm cells were resuspended in the existing 2 ml of biofilm-conditioned medium (no fresh medium change) and placed in a 2-ml tube on ice. A 10-μl aliquot was removed for bacterial numeration. Bacteria were centrifuged at 14,000 rpm for 5 min at 4°C. Bacterial cells were resuspended in ice-cold extraction buffer (methanol/acetonitrile/H2O, 40:40:20) and stored at −80°C until assessed for c-di-AMP content. The insoluble fraction was pelleted in a benchtop centrifuge (15,000 rpm for 5 min), and 200 μl of the supernatant was filtered with a 0.45-μm-pore-size filter (Titan 4 mm; SUN-Sri, Rockwood, TN). Next, 100 μl of the filtered sample was transferred and the extraction buffer was evaporated using a vacuum manifold. The pellet was resuspended in 100 μl ultrapure water. Ten microliters of each sample was then analyzed on a Quattro Premier XE mass spectrometer (Waters, Milford, MA) coupled with an Acquity Ultra Performance liquid chromatography (LC) system (Waters) as previously described for c-di-GMP (42). Ion settings for the mass spectrometer were electrospray negative, and the specific ion pair being analyzed for quantification of c-di-AMP was 657 → 134.
For quantitation of c-di-AMP release from S. aureus biofilms, biofilm-conditioned medium was removed and lyophilized using vacuum centrifugation. Prior to high-performance LC/mass spectrometry (MS) measurements, lyophilized supernatant samples were suspended in extraction buffer and processed for c-di-AMP quantification as described above. For reporting both intracellular and extracellular c-di-AMP levels, the measured mass of c-di-AMP was normalized to viable bacteria collected from biofilms.
Preparation of bone marrow-derived macrophages, RNA isolation, qRT-PCR, and enzyme-linked immunosorbent assay (ELISA).
To prepare bone marrow-derived macrophages (BMDMs), bone marrow was isolated from the long bones (femur and tibia) of C57BL/6 or STING knockout (KO) mice (Jackson Laboratories, Bar Harbor, ME) as previously described, with minor modifications (3). Briefly, bone marrow was cultured in a 12-well plate at 37°C in a 5% CO2 incubator in RPMI 1640 medium supplemented with 1% penicillin/streptomycin/amphotericin B (Corning, Corning, NY), 50 μM beta-mercaptoethanol, 10 mM HEPES, 10% fetal bovine serum, and 20% supernatant from L-929 fibroblasts as a source of macrophage colony-stimulating factor (M-CSF). Medium was replaced at 2 to 3 and 4 to 5 days in vitro, and macrophages were used for experiments at days 7 to 10. These studies were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (43). The protocol was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
BMDMs were either cocultured with mature (6-day) S. aureus biofilms for 3 h, or treated with purified c-di-AMP (InvivoGen, San Diego, CA) or biofilm-conditioned medium diluted to 22% for the indicated intervals, whereupon RNA was isolated using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. In some experiments, diluted biofilm-conditioned medium was treated with 10 U/ml of snake venom phosphodiesterase (SVP; Affymetrix, Santa Clara, CA), an enzyme capable of degrading c-di-AMP (26), for 45 min at 37°C and handled according to the manufacturer's recommendations. Macrophage cDNA was generated using iScript RT supermix (Bio-Rad), and qRT-PCR was performed using TaqMan gene expression master mix (Applied Biosystems) in a Bio-Rad CFX Connect real-time system with TaqMan primers specific to ifn-β and il-6 (interleukin-6 [IL-6]), and to gadph (glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) as a housekeeping gene. Data were analyzed using CFX Manager software (Bio-Rad) with gene expression levels normalized to those of GAPDH and are presented as the fold induction (2−ΔΔCT) value relative to the level for untreated BMDMs. IFN-β and IL-6 ELISA kits were purchased from BioLegend (San Diego, CA, USA) and R&D BioScience (Minneapolis, MN, USA), respectively.
Biofilm autolysis assay.
Triton X-100-induced autolysis assays were performed using a modification of previously published methods (44). Biofilms were grown in RPMI plus 1% Casamino Acids for 6 days as described above. In some experiments, polyanethole sulfonate (PAS) was added at day 4 of biofilm growth to a final concentration of 50 μg/ml to inhibit autolysis. Biofilms were disrupted, washed with ice-cold H2O, and resuspended in autolysis buffer (50 mM Tris-HCl [pH 7.2] with 0.05% Triton X-100) to an OD600 of less than 1.0. Subsequently, 1 ml of cell suspension was placed in a 12-well plate and incubated at 30°C with constant orbital shaking in a Tecan 200 Infinite Pro apparatus. The OD580 was measured at 30-min intervals for a period of 13 h, and results are reported as the percentage of the initial OD580 for each sample.
Human monocyte-derived macrophages and gentamicin protection assays.
Human monocytes were obtained from healthy human donors by the University of Nebraska Medical Center Elutriation Core Facility by countercurrent centrifugal elutriation, in full compliance and approval of the Institutional Review Board. Cells were cultured in suspension in Teflon flasks in Dulbecco's modified Eagle's medium supplemented with human M-CSF (100 ng ml−1), 10% human serum, gentamicin, and ciprofloxacin for 7 days prior to experimentation.
To assess the effects of c-di-AMP on macrophage microbicidal activity, human MDMs were subjected to gentamicin protection assays conducted similarly to previously described methods (3). Briefly, macrophages were pretreated with various concentrations of c-di-AMP (0.01 to 1 μM) for 1 h, followed by a 2-h incubation with live S. aureus LAC at an multiplicity of infection (MOI) of 5:1. MDMs were washed three times with 1× phosphate-buffered saline and treated with gentamicin (100 μg ml−1) for 1 h, whereupon fresh medium containing 1 μg ml−1 gentamicin was added and cells were incubated for 24 h. At this point, MDMs were washed and lysed using sterile double-distilled water and plated onto blood agar plates for enumeration of intracellular bacteria.
Statistics.
Significant differences between experimental groups were determined with either an unpaired two-tailed Student's t test or a one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test, within Prism 6 (GraphPad, La Jolla, CA). For all analyses, a P value of less than 0.05 was considered statistically significant.
RESULTS
S. aureus expresses dacA but minimal gdpP during post-exponential-phase and biofilm growth.
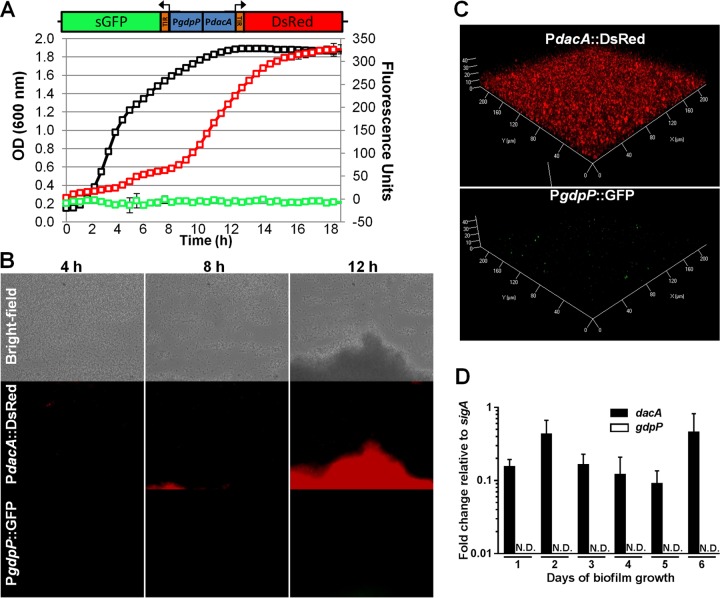
To gain insight into the spatial and temporal production of c-di-AMP, we analyzed the expression patterns of the S. aureus diadenylyl cyclase (dacA) and phosphodiesterase (gdpP) genes, which are responsible for c-di-AMP synthesis and degradation, respectively. Utilizing divergent promoter-fusion plasmids (PdacA::DsRed and PgdpP::GFP), dacA expression was observed primarily during the post-exponential and early stationary phases of planktonic growth in TSB (Fig. 1A). Surprisingly, gdpP expression was nearly undetectable throughout the 18-h experiment. To assess dacA and gdpP expression in a developing biofilm under flow conditions, BioFlux microfluidic microscope technology was utilized. As shown in Fig. 1B, strong dacA expression was localized to developing biofilm tower structures, whereas gdpP expression was below the limit of detection. Regulation was also examined under static biofilm growth conditions in RPMI 1640 supplemented with 1% Casamino Acids. Widespread dacA expression was observed throughout the static biofilm, and gdpP expression was only detected in a small number of cells (Fig. 1C). Differential dacA and gdpP regulation during static biofilm growth was confirmed by qRT-PCR (Fig. 1D), showing constitutive dacA expression throughout 1 to 6 days of static biofilm development, whereas gdpP was below the limit of detection.
FIG 1.
S. aureus expresses dacA but minimal gdpP during post-exponential and biofilm growth. (A to C) To assess dacA and gdpP promoter activity, fluorescence from a transcriptional reporter plasmid (divergent PdacA::DsRed and PgdpP::GFP) was monitored in S. aureus LAC during planktonic growth in TSB by using a TECAN fluorescent plate reader (black line indicates the OD600; green reflects GFP fluorescence; red line indicates DsRed fluorescence) (A); at 4, 8, and 12 h in a flow cell biofilm grown in 50% TSB in a Bio-Flux system (B); or in a 5-day static biofilm grown in RPMI 1640 plus 1% Casamino Acids and examined using confocal microscopy (C). (D) Expression levels of dacA and gdpP in static biofilms grown for 1 to 6 days were analyzed using qRT-PCR. No significant changes in dacA expression were observed across the time points based on a one-way ANOVA with Tukey post hoc analysis. Results are representative of at least two independent experiments. ND, not detected.
Cyclic di-AMP is released extracellularly from S. aureus biofilm.
To compare dacA/gdpP expression patterns with c-di-AMP production by S. aureus biofilms, quantitative analysis of intracellular and extracellular c-di-AMP levels from wild-type (WT) and isogenic gdpP mutant (ΔgdpP) static biofilms was performed using ultrahigh-performance LC–tandem MS (UPLC-MS/MS) and results were normalized for viable bacteria (see Fig. S1A in the supplemental material). A dacA mutant could not be examined, since this is an essential gene in S. aureus (45). As shown in Fig. 2A, 59.1% ± 8.9% (mean ± standard deviation) of total c-di-AMP detected was extracellular following 2 days of static biofilm growth. Despite the inability to detect significant gdpP expression during static biofilm growth using the reporter-fusion plasmid or qRT-PCR (Fig. 1C and D), the ΔgdpP strain resulted in significantly more intracellular and extracellular c-di-AMP during static biofilm growth (∼5-fold increase). These results are consistent with previous findings assessing planktonic S. aureus (12). Interestingly, a similar percentage of total c-di-AMP (48.8% ± 12.9%) was extracellular in ΔgdpP biofilm-conditioned medium after 2 days of growth. These phenotypes continued through days 4 and 6, with ∼30 to 40% of total c-di-AMP detected extracellularly in biofilm-conditioned medium (Fig. 2B and C).
FIG 2.
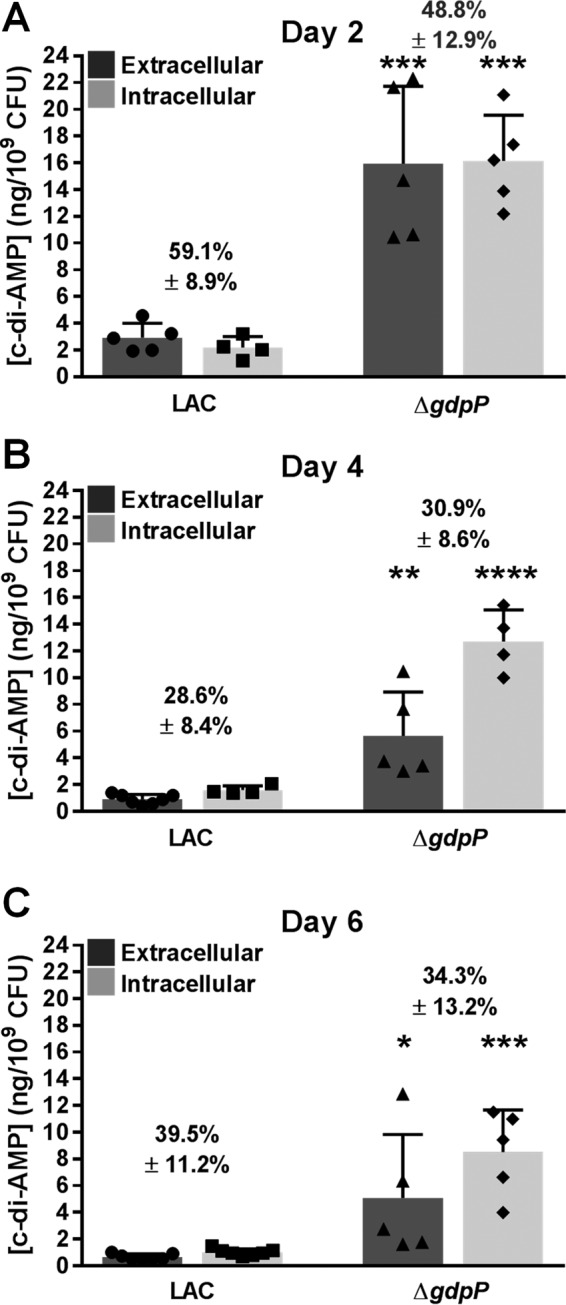
c-di-AMP is released from S. aureus biofilms. c-di-AMP levels were quantified in extracellular fractions (biofilm-conditioned medium) and intracellular fractions from wild-type S. aureus (LAC) and isogenic ΔgdpP static biofilms by using UPLC-MS/MS. Viable bacteria were quantified, and c-di-AMP values were plotted per 109 CFU for normalization. The percentage of total c-di-AMP detected in biofilm-conditioned medium after 2 (A), 4 (B), and 6 (C) days of growth are indicated. Error bars represent standard deviations of the average results from at least two independent experiments, with individual data points plotted. Results were analyzed using Student's t test to compare wild-type LAC and ΔgdpP fractions. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
In silico analysis identified three proteins in the S. aureus USA300 reference strain FPR3757 that share homology with the L. monocytogenes MdrM and MdrT c-di-AMP secretion proteins (Table 1 and data not shown). A triple mutant of all three homologues (CMG8) revealed minor overall changes in extracellular c-di-AMP levels (see Fig. S2 in the supplemental material), suggesting that other mechanisms exist for c-di-AMP release from S. aureus biofilms.
Extracellular c-di-AMP induces a STING-dependent type I IFN response in macrophages.
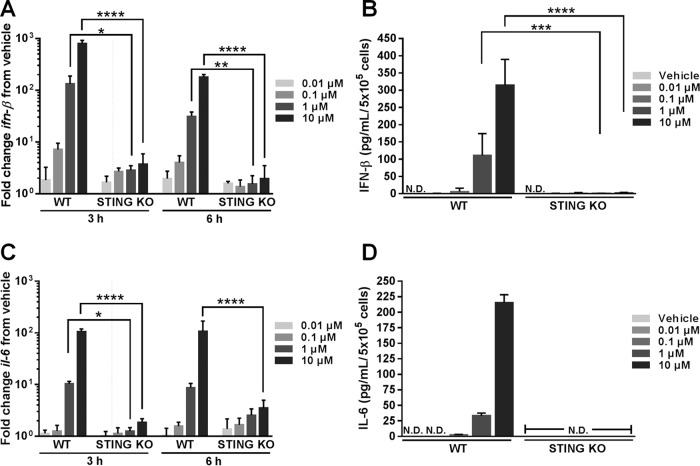
Cyclic di-AMP released from intracellular pathogens is sensed by STING within the host cytosol and induces a type I IFN response (26–34). A previous report described IFN-β production following c-di-AMP treatment in vivo and in human and murine dendritic cells in vitro (46); however, effects of extracellular c-di-AMP on macrophages have not yet been reported. To assess the impact of extracellular c-di-AMP on macrophage activation, murine primary BMDMs were treated with purified c-di-AMP (0.01 to 10 μM) for 3 or 6 h, whereupon RNA was isolated and analyzed for cytokine expression. Although all but one concentration of c-di-AMP examined was higher than those detected in biofilm-conditioned medium (see Fig. S1B in the supplemental material), the known heterogeneity in biofilms and the fact that dacA expression was localized to biofilm towers (Fig. 1B) suggest that macrophages may be exposed to higher local concentrations of c-di-AMP that are reminiscent of the levels examined here. The expression of 14 proinflammatory (i.e., IL-1β, IFN-β, and inducible nitric oxide synthase [iNOS]) and anti-inflammatory (i.e., IL-10, arginase) mediators was examined (data not shown), and the two most strongly induced genes were ifn-β (Fig. 3A) followed by il-6 (Fig. 3C), which were selected for further analysis. Increases in gene expression correlated with elevated IFN-β and IL-6 protein secretion (Fig. 3B and D). The increase in both cytokines by c-di-AMP was dose dependent and showed early, yet sustained induction. Interestingly, no concentration of c-di-AMP tested induced il-1β, ifn-γ, or inos expression (data not shown).
FIG 3.
Extracellular c-di-AMP induces macrophage IFN-β and IL-6 production in a STING-dependent manner. BMDMs were treated with c-di-AMP (0.01 to 10 μM) for 3 or 6 h, whereupon RNA and supernatants were collected to quantify IFN-β and IL-6 mRNA (A and C) and protein (B and D) expression by qRT-PCR and ELISA, respectively. (A and C) Gene expression levels were normalized to the housekeeping gene GAPDH and are presented as the fold induction (2−ΔΔCT) value relative to that for untreated BMDMs (vehicle). (B and D) Cytokine levels were normalized to cell number and are expressed per milliliter of supernatant per 5 × 105 cells. Results depict averages from three independent experiments with standard deviations shown. Statistical analysis was performed using a one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. ND, not detected.
To examine the mechanism of ifn-β and il-6 induction by exogenous c-di-AMP, BMDMs from STING KO mice were examined. As shown in Fig. 3, the response to c-di-AMP was predominantly STING dependent at all concentrations tested, indicating that extracellular c-di-AMP is detected in the macrophage cytosol and induces a type I IFN response via a STING-mediated signaling process.
Cyclic di-AMP is released from S. aureus biofilm during cell lysis to induce STING-dependent ifn-β expression.
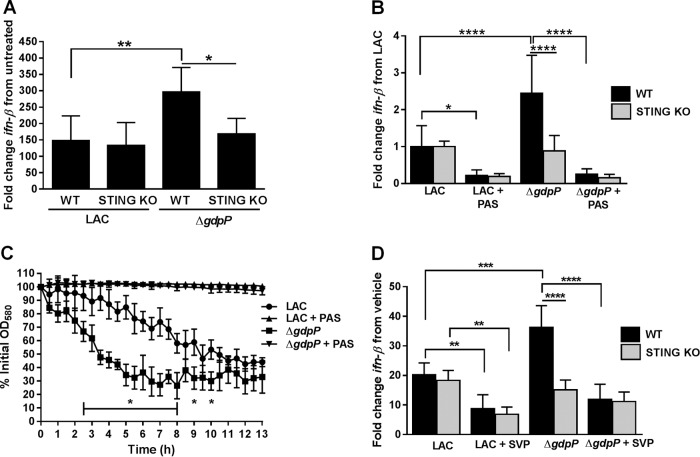
To directly assess whether c-di-AMP released from S. aureus biofilm induces a type I IFN response, WT and STING KO BMDMs were cocultured for 3 h with mature (day 6) WT LAC and isogenic ΔgdpP static biofilms. As shown in Fig. 4A, WT BMDMs expressed significantly higher levels of ifn-β following coculture with the ΔgdpP strain compared to WT LAC biofilms. This increase was absent in STING KO BMDMs. To further assess the impact of c-di-AMP released from the biofilm, WT and STING KO BMDMs were treated for 3 h with biofilm-conditioned medium from mature (day 6) WT LAC and isogenic ΔgdpP static biofilms (Fig. 4B). Due to the rapid killing of BMDMs with undiluted biofilm-conditioned medium (data not shown), supernatants were diluted to examine effects on macrophage ifn-β induction. Conditioned medium from ΔgdpP biofilms induced significantly greater ifn-β expression than WT biofilms, reflecting similar trends to the concentrations of extracellular c-di-AMP released from the respective biofilms (see Fig. S1B in the supplemental material). This increase in ifn-β expression was absent following treatment of STING KO BMDMs, indicating that the excess c-di-AMP released from ΔgdpP biofilms triggered a STING-dependent type I IFN response. Importantly, there were no observable increases in ifn-β expression following treatment of BMDMs with planktonic ΔgdpP supernatant compared to WT LAC, indicating a mechanism of c-di-AMP release specific for biofilm growth (see Fig. S3 in the supplemental material).
FIG 4.
S. aureus biofilms release c-di-AMP via cell lysis to induce STING-dependent macrophage type I IFN production. (A) WT and STING KO BMDMs were cocultured for 3 h with mature (6-day) WT LAC or ΔgdpP static biofilms, whereupon RNA was isolated to assess changes in ifn-β expression by qRT-PCR. (B) Expression of ifn-β in WT and STING KO BMDMs exposed for 3 h to biofilm-conditioned medium collected from WT LAC or ΔgdpP biofilms, where lysis was inhibited with PAS. (C) Autolytic rates of cells harvested from WT LAC and isogenic ΔgdpP static biofilms with and without PAS treatment. (D) WT and STING KO BMDMs were exposed for 3 h to LAC or ΔgdpP biofilm-conditioned medium treated with SVP to degrade c-di-AMP. For qRT-PCR (A, B, and D), ifn-β expression was normalized to expression of the housekeeping gene gapdh and is presented as the fold induction (2−ΔΔCT) value relative to untreated BMDMs (A and D) or wild-type LAC biofilm-conditioned medium (B). Results depict averages from three independent experiments with standard deviations shown. Statistical analysis was performed using a one-way ANOVA with Tukey's post hoc analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
As previously shown, an isogenic S. aureus mutant in which all three homologues of the L. monocytogenes c-di-AMP secretion proteins were interrupted had a minimal effect on extracellular c-di-AMP release (see Fig. S2 in the supplemental material), suggesting that alternative mechanisms were involved. To assess the possibility that c-di-AMP is passively released from S. aureus biofilms via cell lysis, mature biofilms were treated for 2 days with 50 μg/ml PAS. PAS has been shown to inhibit S. aureus autolysis without affecting cell viability (47, 48). PAS had no observable effect on biofilm structure or viability (see Fig. S4 in the supplemental material), likely because treatment was initiated with established biofilms. PAS treatment of both WT and ΔgdpP biofilms significantly reduced ifn-β expression in both WT and STING KO BMDMs (Fig. 4B). Importantly, supplementation of PAS-treated biofilm-conditioned medium with 1 μM c-di-AMP restored ifn-β expression only in WT but not STING KO BMDMs (see Fig. S5 in the supplemental material). An S. aureus gdpP mutant was previously reported to have an altered rate of autolysis under planktonic conditions, which resulted from increased peptidoglycan cross-linking (12). To examine the impact of the gdpP mutation on autolysis in a mature (6-day) biofilm, WT LAC and ΔgdpP biofilms were subjected to a Triton X-100-induced autolysis assay. As shown in Fig. 4C, the gdpP mutation significantly increased the rate of cellular autolysis from a biofilm, suggesting that elevated extracellular c-di-AMP likely results from both the lack of GdpP hydrolysis and release due to cell lysis.
To further assess the contribution of extracellular c-di-AMP released from S. aureus biofilm on macrophage type I IFN expression, biofilm-conditioned medium from WT LAC and ΔgdpP was treated with SVP, an enzyme known to degrade c-di-AMP (26). SVP treatment of WT LAC biofilm-conditioned medium significantly reduced ifn-β induction in both WT and STING KO BMDMs compared to results with untreated supernatant (Fig. 4D). However, SVP treatment of ΔgdpP biofilm-conditioned medium did not affect ifn-β expression in STING KO BMDMs, further supporting a STING-dependent type I IFN response elicited by extracellular c-di-AMP. Together, these data provide direct evidence that biofilm-derived c-di-AMP is responsible for macrophage ifn-β induction and signals primarily via a STING-dependent pathway.
c-di-AMP promotes S. aureus intracellular survival in macrophages.
To evaluate the functional and translational implications of c-di-AMP on macrophage-S. aureus interactions, the type I IFN response and intracellular survival of S. aureus were examined in human monocyte-derived macrophages (MDMs). Human MDMs were treated with exogenous c-di-AMP (0.01 to 10 μM), after which ifn-β expression was assessed. As shown in Fig. 5A, ifn-β was induced in a dose-dependent manner following c-di-AMP treatment. To determine whether extracellular c-di-AMP modulated macrophage functional activity, human MDMs were treated with purified c-di-AMP (0.01 to 1 μM) prior to live S. aureus exposure, whereupon intracellular survival was assessed in gentamicin protection assays. As shown in Fig. 5B, pretreatment with 0.1 μM c-di-AMP significantly promoted S. aureus intracellular survival, as revealed by elevated bacterial counts, which was less evident at a 10-fold higher concentration of c-di-AMP (1 μM). Currently, we cannot determine whether this results from increased S. aureus fitness in response to potential changes in the macrophage intracellular milieu following c-di-AMP exposure or from reduced microbicidal activity of c-di-AMP-treated macrophages. Collectively, these data indicate that c-di-AMP-induced signaling pathways bias macrophages toward a nonproductive phenotype, reflected by impaired S. aureus clearance.
FIG 5.
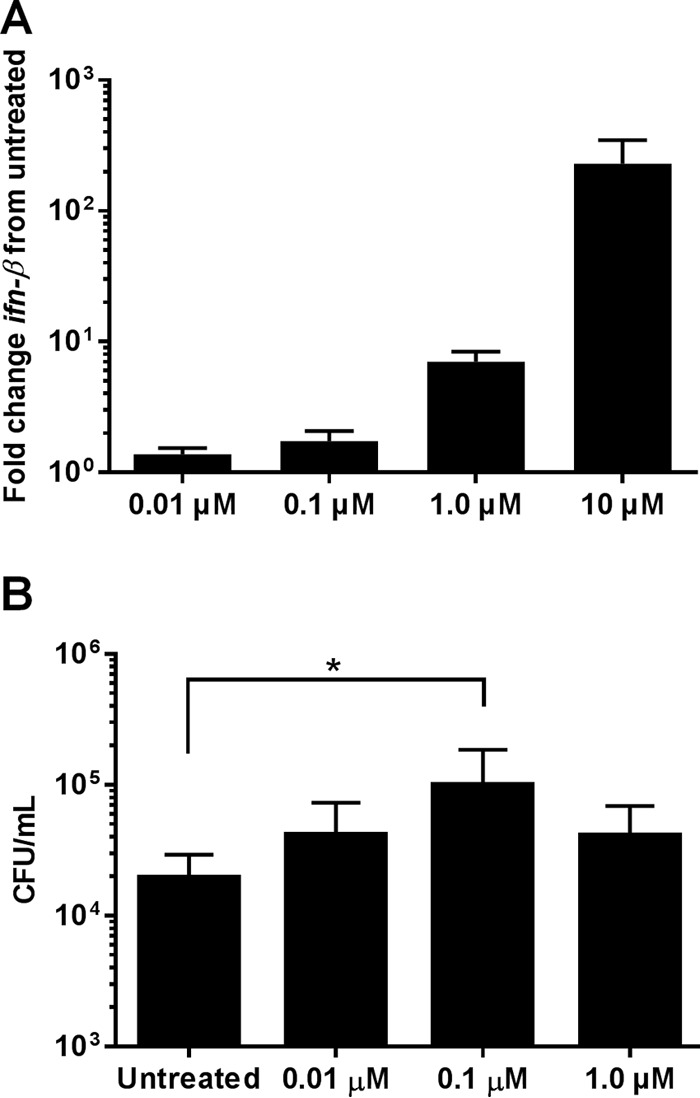
c-di-AMP induces ifn-β expression and promotes S. aureus intracellular survival in human MDMs. (A) Human MDMs were treated with c-di-AMP (0.01 to 10 μM) for 3 h, whereupon RNA was isolated and analyzed for changes in ifn-β expression by qRT-PCR. Gene expression levels were normalized to the housekeeping gene gapdh and are presented as the fold induction (2−ΔΔCT) value relative to untreated MDMs. (B) MDMs were pretreated with c-di-AMP (0.01 to 1.0 μM) for 1 h, whereupon cells were incubated with live S. aureus at an MOI of 5 for 2 h and subjected to a gentamicin protection assay, in which MDMs were lysed 24 h later to quantify intracellular bacteria. Statistical analysis was performed using a one-way ANOVA method with Tukey post hoc analysis. *, P < 0.05.
DISCUSSION
Since its discovery in 2008 (24), c-di-AMP has been reported to function in a variety of essential processes in various bacterial species (49, 50). However, the role of c-di-AMP in the major human pathogen S. aureus has been limited to mediating ion homeostasis by regulating channel activity (11, 36, 37). In this study, we investigated an extracellular role for c-di-AMP during S. aureus biofilm growth and regulation of macrophage activation and functional activity. Biofilms represent an interesting paradigm in the bacterial lifestyle, since as a community of cells organized within a self-produced matrix, their gene expression patterns, metabolism, and fate are dramatically altered from those for planktonic cells (51). In contrast to planktonic cells, the immune response to S. aureus biofilm is suppressive, as reflected by macrophage polarization toward an anti-inflammatory state and MDSC recruitment (3, 7). To better understand how S. aureus biofilms are able to skew host immune responses and promote their own survival, we sought to identify molecules that alter macrophage signaling.
c-di-AMP secretion during intracellular bacterial infections is known to induce a robust type I IFN response (26, 27). These reports have established the importance of c-di-AMP in bacterial signaling and innate immune sensing of intracellular pathogens; however, a role for exogenous c-di-AMP produced by extracellular bacteria has not yet been examined. It has been proposed that c-di-AMP production is triggered during metabolic switches involved in biofilm formation (52). Indeed, we found rapid induction of dacA but minimal gdpP expression during the post-exponential and early stationary phases of planktonic growth that extended to biofilm formation, where dacA was concentrated within biofilm tower structures formed within a flow cell and ubiquitously throughout a static biofilm. These data defining the spatial and temporal expression of dacA and gdpP suggest that c-di-AMP production is induced as S. aureus cultures reach high cell densities, such as those found within biofilms, and that c-di-AMP synthesis is lower in rapidly growing, planktonic cells and maximal in post-exponential-phase cells. While it is tempting to speculate that c-di-AMP may be acting as a quorum-sensing molecule, the addition of exogenous c-di-AMP to S. aureus planktonic or biofilm cultures had no effect on dacA and gdpP expression (data not shown).
To understand the relationship between dacA and gdpP expression on c-di-AMP production and release, intracellular and extracellular c-di-AMP levels from S. aureus static biofilms were quantified using UPLC-MS/MS. These data demonstrated that 30 to 50% of the total c-di-AMP produced by S. aureus biofilms was extracellular. Surprisingly, despite the small amount of gdpP promoter activity detected, ΔgdpP biofilms displayed a 5-fold increase in total c-di-AMP; however, no difference in the percentage of extracellular c-di-AMP was observed. This appears to be due to a combination of highly efficient c-di-AMP hydrolysis by GdpP and an increased rate of autolysis by the ΔgdpP strain. Mutations in all three S. aureus MdrM orthologues revealed minor overall changes in extracellular c-di-AMP, and while these data do not exclude the possibility for c-di-AMP secretion, they do suggest other mechanisms exist for c-di-AMP release. To this end, we identified a novel mode of c-di-AMP release via cell autolysis, revealed by the finding that macrophage IFN-β induction was attenuated when biofilm autolysis was inhibited by PAS and the phenotype could be complemented with the addition of exogenous c-di-AMP.
c-di-AMP secreted by intracellular bacterial pathogens is sensed by the endoplasmic reticulum-associated protein STING (28), a potent inducer of type I IFN signaling (26), via phosphorylation of the transcription factor interferon response factor 3 (IRF3) (53). Prior to this study, it was unknown whether extracellular c-di-AMP is sensed and what effect(s) it may have on macrophage function. Many of the transcription factor activation pathways that induce anti-inflammatory cytokine production are regulated by type I IFN signaling (54). Secreted type I IFNs (IFN-α and -β) bind to the same receptor (IFNAR) in an autocrine and paracrine manner, triggering Janus kinase (JAK)/tyrosine kinase (TYK)-mediated phosphorylation of signal transducer and activator of transcription (STAT) proteins. STATs then translocate into the nucleus and promote the expression of many cytokines, including IFN-α and IFN-β, forming a positive feedback loop. Anti-inflammatory pathways of type I IFN signaling result from STAT3 phosphorylation (55) and can drive an alternatively activated macrophage phenotype and MDSC development, both of which are immune hallmarks of S. aureus biofilm-mediated infections (8, 56). It is currently unknown what STAT pathways are elicited in response to S. aureus biofilms, although the anti-inflammatory profile of infiltrating macrophages and MDSCs suggests that STAT3 may play a role (3, 6–8). IFN-β protein expression was significantly elevated in our S. aureus orthopedic implant biofilm infection model at days 7 to 14 compared to animals receiving sterile implants (data not shown). Our preliminary studies suggest that IFN receptor (IFNAR)-deficient mice do not display drastic changes in biofilm burdens or leukocyte infiltrates during early infection; however, the impact of IFNAR signaling may be more evident during later disease, which remains to be determined. Alternatively, due to the complexity of host immune responses during biofilm formation, it is possible that multiple pathways involved in type I IFN production exert redundant effects that would necessitate the generation of double/triple mutant mice to discern overt phenotypes (i.e., IFNAR/STING or IFNAR/SOCS KO).
To assess the effects of exogenous c-di-AMP on macrophage activation, cytokine expression was first evaluated in BMDMs treated with purified c-di-AMP. The gene most strongly induced was ifn-β, followed by il-6. Importantly, this response was STING dependent, which represents the first evidence linking extracellular c-di-AMP to STING in macrophages. IL-6 can also regulate anti-inflammatory pathways (57) and MDSC expansion (58), as cells expressing the IL-6 receptor (macrophages, MDSCs) respond to IL-6 by STAT3 activation (57, 59). The ability of c-di-AMP to promote S. aureus intracellular survival further indicates that extracellular c-di-AMP biases macrophages toward an anti-inflammatory state. These data are the first to demonstrate that macrophages are able to internalize c-di-AMP, where it can be sensed by STING; however, the mechanism of c-di-AMP uptake remains unknown. Pretreatment of BMDMs with cytochalasin D, a drug which depolymerizes actin filaments and inhibits endocytosis (60), had no effect on c-di-AMP-mediated ifn-β expression (data not shown). Additionally, analysis of MyD88 KO BMDMs revealed that this major Toll-like/IL-1 receptor adaptor protein also plays no role in the type I IFN response to c-di-AMP (data not shown). Other possibilities are that c-di-AMP enters the cell either through fluid-phase pinocytosis or protein-mediated membrane transport, which remain to be investigated. Another interesting concept is whether other PRRs are being activated concurrently with STING to modulate macrophage type I IFN production in response to S. aureus biofilms. One possibility is NOD2, which recognizes muramyl dipeptides following PGN degradation (61, 62) and could account for the minor STING-independent induction of type I IFN that was observed in our study, although this remains speculative. The failure of STING KO macrophages to augment type I IFN production in response to gdpP mutant biofilms coupled with the finding that SVP treatment of biofilm-conditioned medium is capable of inhibiting macrophage ifn-β expression implies a dominant action of c-di-AMP; however, a contribution from the cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS) in detecting extracellular DNA present in biofilm-conditioned medium cannot be excluded (63).
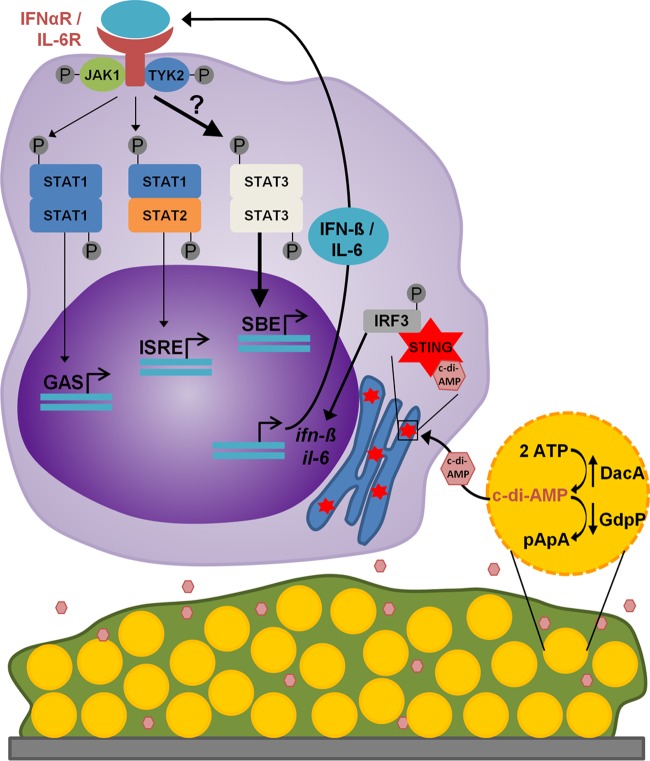
While the anti-inflammatory response to S. aureus biofilms appears to be driven by biofilm products, the effectors and their mechanism(s) of action remain ill-defined. These data provide evidence for the ability of S. aureus biofilms to induce macrophage type I IFN production via extracellular c-di-AMP (Fig. 6). Lending support to this hypothesis, increased c-di-AMP levels observed in ΔgdpP biofilms corresponded with increased ifn-β expression in BMDMs and was STING dependent. Furthermore, inhibiting biofilm autolysis dampened the ifn-β response to levels similar to those in untreated macrophages, revealing that c-di-AMP is released passively during cell lysis. Remarkably, despite the multitude of toxins and other bacterial antigens released from S. aureus biofilms that can cause macrophage dysfunction (5), the macrophage type I IFN response has high specificity to extracellular c-di-AMP. Taken together, these data identify a novel extracellular role for S. aureus c-di-AMP in polarizing macrophages toward an anti-inflammatory state.
FIG 6.
Model for the S. aureus biofilm c-di-AMP-induced macrophage type I IFN response. Increased expression of dacA, the enzyme responsible for c-di-AMP synthesis, relative to minimal expression of the phosphodiesterase gdpP, suggests that c-di-AMP production is favored during S. aureus biofilm growth. Furthermore, autolytic events occurring during biofilm growth allow for c-di-AMP release from S. aureus. STING-mediated sensing of c-di-AMP by surrounding macrophages induces a type I IFN response, including the expression of IL-6. Upon secretion, type I IFNs and IL-6 bind their cognate receptors in an autocrine and paracrine manner. It is proposed that type I IFN and IL-6 signaling in macrophages induces a STAT3-mediated anti-inflammatory response, ultimately contributing to biofilm persistence. GAS, gamma interferon activation site; ISRE, IFN-stimulated response element; SBE, STAT-binding element.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paul Fey, Keer Sun, and Jessica Snowden for critical review of the manuscript and Marat Sadykov for supplying the sigA qRT-PCR primers.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00447-16.
REFERENCES
- 1.Del Pozo JL, Patel R. 2009. Infection associated with prosthetic joints. N Engl J Med 361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. 2011. Protective role of IL-1beta against post-arthroplasty Staphylococcus aureus infection. J Orthop Res 29:1621–1626. doi: 10.1002/jor.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T. 2015. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. mBio 6:e01021-15. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanke ML, Angle A, Kielian T. 2012. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS One 7:e42476. doi: 10.1371/journal.pone.0042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. 2013. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol 190:2159–2168. doi: 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T. 2014. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol 192:3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho KH, Kang SO. 2013. Streptococcus pyogenes c-di-AMP phosphodiesterase, GdpP, influences SpeB processing and virulence. PLoS One 8:e69425. doi: 10.1371/journal.pone.0069425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ. 2013. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4:e00282-13. doi: 10.1128/mBio.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Grundling A. 2013. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci U S A 110:9084–9089. doi: 10.1073/pnas.1300595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan Zeevi M, Shafir NS, Shaham S, Friedman S, Sigal N, Nir Paz R, Boneca IG, Herskovits AA. 2013. Listeria monocytogenes multidrug resistance transporters and cyclic di-AMP, which contribute to type I interferon induction, play a role in cell wall stress. J Bacteriol 195:5250–5261. doi: 10.1128/JB.00794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Helmann JD. 2012. Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol 83:623–639. doi: 10.1111/j.1365-2958.2011.07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y, Yang J, Zarrella TM, Zhang Y, Metzger DW, Bai G. 2014. Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. J Bacteriol 196:614–623. doi: 10.1128/JB.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths JM, O'Neill AJ. 2012. Loss of function of the gdpP protein leads to joint beta-lactam/glycopeptide tolerance in Staphylococcus aureus. Antimicrob Agents Chemother 56:579–581. doi: 10.1128/AAC.05148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dengler V, McCallum N, Kiefer P, Christen P, Patrignani A, Vorholt JA, Berger-Bachi B, Senn MM. 2013. Mutation in the c-di-AMP cyclase dacA affects fitness and resistance of methicillin resistant Staphylococcus aureus. PLoS One 8:e73512. doi: 10.1371/journal.pone.0073512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng X, Zhang Y, Bai G, Zhou X, Wu H. 2015. Cyclic di-AMP mediates biofilm formation. Mol Microbiol 99:945–949. doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai Y, Yang J, Eisele LE, Underwood AJ, Koestler BJ, Waters CM, Metzger DW, Bai G. 2013. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J Bacteriol 195:5123–5132. doi: 10.1128/JB.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehne FM, Gunka K, Eilers H, Herzberg C, Kaever V, Stulke J. 2013. Cyclic di-AMP homeostasis in bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem 288:2004–2017. doi: 10.1074/jbc.M112.395491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sureka K, Choi PH, Precit M, Delince M, Pensinger DA, Huynh TN, Jurado AR, Goo YA, Sadilek M, Iavarone AT, Sauer JD, Tong L, Woodward JJ. 2014. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158:1389–1401. doi: 10.1016/j.cell.2014.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteley AT, Pollock AJ, Portnoy DA. 2015. The PAMP c-di-AMP is essential for Listeria monocytogenes growth in rich but not minimal media due to a toxic increase in (p)ppGpp. Cell Host Microbe 17:788–798. doi: 10.1016/j.chom.2015.05.006 (Erratum, 18:132, 2015.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte G, Hartung S, Buttner K, Hopfner KP. 2008. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell 30:167–178. doi: 10.1016/j.molcel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. 2011. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep 12:594–601. doi: 10.1038/embor.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer KA, Durack J, Portnoy DA. 2014. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog 10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker JR, Koestler BJ, Carpenter VK, Burdette DL, Waters CM, Vance RE, Valdivia RH. 2013. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. mBio 4:e00018-13. doi: 10.1128/mBio.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. 2015. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med 21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. 2015. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun 79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. 2012. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller M, Hopfner KP, Witte G. 2015. c-di-AMP recognition by Staphylococcus aureus PstA. FEBS Lett 589:45–51. doi: 10.1016/j.febslet.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Moscoso JA, Schramke H, Zhang Y, Tosi T, Dehbi A, Jung K, Grundling A. 2015. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J Bacteriol 198:98–110. doi: 10.1128/JB.00480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Youn SJ, Kim SO, Ko J, Lee JO, Choi BS. 2015. Structural studies of potassium transport protein KtrA regulator of conductance of K+ (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP). J Biol Chem 290:16393–16402. doi: 10.1074/jbc.M115.641340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campeotto I, Zhang Y, Mladenov MG, Freemont PS, Grundling A. 2015. Complex structure and biochemical characterization of the Staphylococcus aureus cyclic diadenylate monophosphate (c-di-AMP)-binding protein PstA, the founding member of a new signal transduction protein family. J Biol Chem 290:2888–2901. doi: 10.1074/jbc.M114.621789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moormeier DE, Endres JL, Mann EE, Sadykov MR, Horswill AR, Rice KC, Fey PD, Bayles KW. 2013. Use of microfluidic technology to analyze gene expression during Staphylococcus aureus biofilm formation reveals distinct physiological niches. Appl Environ Microbiol 79:3413–3424. doi: 10.1128/AEM.00395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moormeier DE, Bayles KW. 2014. Examination of Staphylococcus epidermidis biofilms using flow-cell technology. Methods Mol Biol 1106:143–155. doi: 10.1007/978-1-62703-736-5_13. [DOI] [PubMed] [Google Scholar]
- 42.Bobrov AG, Kirillina O, Ryjenkov DA, Waters CM, Price PA, Fetherston JD, Mack D, Goldman WE, Gomelsky M, Perry RD. 2011. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol Microbiol 79:533–551. doi: 10.1111/j.1365-2958.2010.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 44.Mani N, Tobin P, Jayaswal RK. 1993. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol 175:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corrigan RM, Bowman L, Willis AR, Kaever V, Grundling A. 2015. Cross-talk between two nucleotide-signaling pathways in Staphylococcus aureus. J Biol Chem 290:5826–5839. doi: 10.1074/jbc.M114.598300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skrnjug I, Rueckert C, Libanova R, Lienenklaus S, Weiss S, Guzman CA. 2014. The mucosal adjuvant cyclic di-AMP exerts immune stimulatory effects on dendritic cells and macrophages. PLoS One 9:e95728. doi: 10.1371/journal.pone.0095728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wecke J, Lahav M, Ginsburg I, Kwa E, Giesbrecht P. 1986. Inhibition of wall autolysis of staphylococci by sodium polyanethole sulfonate “liquoid”. Arch Microbiol 144:110–115. [DOI] [PubMed] [Google Scholar]
- 49.Corrigan RM, Grundling A. 2013. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol 11:513–524. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- 50.Commichau FM, Dickmanns A, Gundlach J, Ficner R, Stulke J. 2015. A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP. Mol Microbiol 97:189–204. doi: 10.1111/mmi.13026. [DOI] [PubMed] [Google Scholar]
- 51.Resch A, Rosenstein R, Nerz C, Gotz F. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71:2663–2676. doi: 10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valle J, Solano C, Garcia B, Toledo-Arana A, Lasa I. 2013. Biofilm switch and immune response determinants at early stages of infection. Trends Microbiol 21:364–371. doi: 10.1016/j.tim.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL. 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol 187:2595–2601. doi: 10.4049/jimmunol.1100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Navajas JM, Lee J, David M, Raz E. 2012. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. 2012. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22:275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 58.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. 2007. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodge DR, Hurt EM, Farrar WL. 2005. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Mooren OL, Galletta BJ, Cooper JA. 2012. Roles for actin assembly in endocytosis. Annu Rev Biochem 81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 61.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 62.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem 278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 63.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 65.Sadykov MR, Thomas VC, Marshall DD, Wenstrom CJ, Moormeier DE, Widhelm TJ, Nuxoll AS, Powers R, Bayles KW. 2013. Inactivation of the Pta-AckA pathway causes cell death in Staphylococcus aureus. J Bacteriol 195:3035–3044. doi: 10.1128/JB.00042-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.