Abstract
Aim:
To investigate childhood abuse victimization in relation to adult DNA methylation levels in a novel region of NR3C1, with emotional support as a possible modifier.
Materials & methods:
295 participants from the Black Women's Health Study. Multivariable linear regression models were used to compute differences in mean percent methylation levels.
Results:
Women reporting childhood abuse victimization exhibited higher mean NR3C1 methylation levels than nonabused women, with a clear dose–response relationship. Childhood emotional support appeared to attenuate associations only among women with the highest levels of physical and sexual abuse.
Conclusion:
NR3C1 mean methylation was higher among women who reported childhood abuse. Further research is warranted to clarify whether or the extent to which childhood emotional support buffers the association.
Keywords: : abuse, African–Americans, child maltreatment, CpG island shore, DNA methylation, epigenetic epidemiology, glucocorticoid receptor, health disparities, NR3C1, violence
Over the past decade, several epigenetics studies have emerged linking various forms of childhood adversity with an altered hypothalamic–pituitary–adrenal axis stress response in humans [1,2]. Among the genes in the stress pathway, the glucocorticoid receptor gene, NR3C1, has been shown to be hypermethylated in response to various forms of early life psychosocial stress, such as physical or emotional abuse [3] or parental loss [4]. Increased methylation of NR3C1 is associated with a range of deleterious outcomes, including depression [5], borderline personality disorder [6,7] and cancer [8–10].
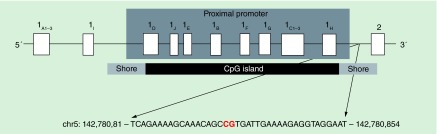
Nearly all methylation studies conducted on NR3C1 have focused on the CpG island located within the proximal promoter region, particularly in exon 1F and the 1F promoter. Epigenetics research to date has tended to focus on methylation in CpG islands, which are small stretches of unmethylated DNA found in the promoters of a handful of human genes [11], because methylation in these regions is assumed to have the greatest functional significance [12]. However, more recent research has shown that the majority of functionally important DNA methylation occurs not in CpG islands, but in CpG island shores, which are genomic regions located within 2 kb of a CpG island [13]. Our prior research suggests that DNA methylation at the CpG island shore located within the proximal promoter of NR3C1 is sensitive to environmental stressors [14–16], and thus may be a suitable candidate for methylation studies of external and internal stressors. None of the published studies demonstrating a link between early life psychosocial stressors and epigenetic modification within the proximal promoter region of NR3C1 have explored CpG island shores as a more sensitive or functional methylation site for psychosocial stress.
Furthermore, given the strong connection between NR3C1 methylation and psychosocial stress documented in previous research, NR3C1 regulation may be an important link in explaining the disparate burden of inflammatory and stress-related diseases experienced by African–Americans (AAs) relative to white populations. AA women also bear a disproportionate burden of child abuse and neglect [17].
In the present study, we assess the impact of child abuse victimization on methylation of a novel CpG site located in a CpG island shore within the NR3C1 promoter among 295 AA women drawn from the Black Women's Health Study. We further assess the potential buffering effect of having received emotional support (e.g., feeling encouraged by family members, feeling cared for and protected) in childhood. We hypothesized that women reporting childhood abuse victimization would demonstrate increased methylation at the downstream CpG island shore located in the NR3C1 proximal promoter relative to women who were not abused in childhood, and that this increased methylation would be attenuated among women who were abused but also reported receiving emotional support in childhood. To our knowledge, this is the first epigenetics study of NR3C1 to investigate childhood abuse victimization in relation to DNA methylation of a CpG island shore as a more sensitive methylation site for assessing the biological impact of psychosocial stress. It is also the first to investigate the epigenetic effects of psychosocial stress on NR3C1 methylation in a cohort of AA women, and the first to investigate positive psychosocial factors (e.g., emotional support in childhood) that may buffer against the deleterious epigenetic effects of psychosocial stress.
Materials & methods
Ethics statement
This study was approved by the Harvard School of Public Health Institutional Review Board and the Boston University Institutional Review Board.
Selection of study participants
The Black Women's Health Study (BWHS) is a prospective cohort study established in 1995, when 59,000 AA women aged 21–69 years from across the USA completed health questionnaires. The baseline questionnaire elicited data on demographic and lifestyle factors, reproductive history, dietary intake and medical conditions. The cohort has been followed biennially through mailed questionnaires, and follow-up has been successful for 88% of potential person years through the last completed follow-up in 2013.
Blood specimen collection
During July 2006 through July 2007, 1500 BWHS participants aged 40 years and older without a history of cancer and residing in New York, NY; Chicago, IL; or Atlanta (GA, USA) were randomly selected and invited to provide blood samples [18]. Each potential participant was sent a packet containing an introductory letter and brochure, consent forms, instructions for locating a blood collection site and a preprinted laboratory requisition form. Blood specimens were collected and processed by Quest Diagnostics (NJ, USA [19]), an accredited national clinical laboratory [20,21]. Women who were willing to participate went to a conveniently located Quest Patient Service Center, where the blood was drawn. Blood samples were provided by 532 women. The women who provided blood samples were similar to nonparticipants with regard to many health-related characteristics, including age, BMI, education, income, alcohol consumption, vigorous exercise, menopausal hormone use, and prevalence of diabetes, hypertension and high cholesterol.
Due to cost issues, we were able to conduct methylation assays on only 300 of the approximately 500 samples. The 300 participants were selected such that there would be representation of both extremes of the exposure of interest (childhood violence victimization). The final analytic sample consisted of 295 women.
Description of variables
Abuse victimization
On the 2005 BWHS follow-up questionnaire, participants were asked questions about physical and sexual abuse (‘abuse victimization’) across the lifespan, including exposure as a ‘child’ (up to age 11 years), ‘teenager’ (age 12–18 years) and ‘adult’ (ages 19 years and older). We created a nine-item abuse instrument adapted from the Conflict Tactics Scale [22] and the Pregnancy Abuse Assessment Screen [23], which has been used previously to successfully study the association between childhood abuse and early menarche [24], eating disorders [25] and risk of smoking onset [26]. Response categories were ‘never’, ‘1–3 times’ or ‘≥4 times’. We defined physical abuse as any report of a perpetrator having ‘pushed, grabbed or shoved me’, ‘threw something at me that could hurt me’, ‘kicked, bit or punched me’, ‘hit me with something including hand or fist’, or ‘physically attacked me in some other way’ at a frequency of greater than or equal to four-times; or either ‘choked or burned me’ or ‘seriously harmed someone I loved’ at any frequency. We defined sexual abuse as any report of a perpetrator having ‘exposed genitals against my will’ greater than or equal to four-times; or ‘been sexual with me against my will’ at any frequency.
As shown in Supplementary Table 1, we created a physical abuse summary score variable by assigning one point for each report of a physical abuse item occurring more than or equal to four-times, with the exception of ‘choked or burned’ or ‘seriously harmed someone I loved’, where one point was assigned for reports that these occurred one to three-times and two points for reports that these occurred more than or equal to four-times, because these events were considered more severe. The resulting physical abuse severity score, which ranged from zero to nine, was further categorized as low (score = 1), intermediate (score = 2) and high (score ≥3). We also created a summary variable for sexual abuse that classified more than or equal to four incidents separately from one to three incidents of sexual assault. Finally, we created a ‘severity’ variable that defined ‘mild’ abuse as one type of physical abuse that occurred more than or equal to four-times or a more severe form of physical abuse (burn or choke or seriously harm someone I love) occurring one to three-times; ‘moderate’ abuse as sexual abuse one to three-times and/or two forms of physical abuse occurring more than or equal to four-times or a more severe form of physical abuse (burn or choke or seriously harm someone I love) occurring at any frequency; ‘severe’ abuse as three or more types of physical abuse occurring more than or equal to four-times and/or sexual abuse more than or equal to four-times; and ‘very severe’ abuse includes three or more types of physical abuse occurring more than or equal to four-times and sexual abuse more than or equal to four-times. These abuse definitions have been used in previous publications from the BWHS [24,27–30] and other studies [31,32].
Financial hardship
Financial hardship in childhood has been associated with increased DNA methylation of other stress-related genes, and was therefore included as a potential covariate in our analyses [33]. On the 2011 follow-up questionnaire, financial hardship as a child was assessed using the following questions: (in childhood [up until age 11]), ‘Was there at least one time when your household did not have enough money for food or housing?’ and ‘Was there at least one time when your household received public assistance or welfare?’ Women who responded positively to either question were considered to have experienced financial hardship as a child.
Emotional support in childhood
The 2011 follow-up questionnaire also queried women about receipt of nurturing and emotional support in childhood using the following two questions: ‘When you were growing up, did people in your family show confidence in you and encourage you to achieve?’ and ‘When you were growing up, did you feel that there was someone to take care of you and protect you?’ Response categories were ‘never’, ‘almost never’, ‘sometimes’, ‘fairly often’, and ‘very often’. Women who answered ‘fairly often’ or ‘very often’ to both questions were considered to have had emotional support in childhood.
NR3C1 promoter methylation sequencing
Buffy coat from whole blood was collected from each participant and immediately stored at -80°C until genomic DNA was isolated with QIAamp DNA Blood Kit (QIAGEN, CA, USA). The isolated genomic DNA was stored at -80°C for future use. Details on sample collection and DNA isolation for each study have been described in previous publications [34–36]. Four hundred nanogram of genomic DNA was treated using the EZ DNA Methylation-Gold Kit (Zymo Research, CA, USA) according to the manufacturer's protocol. Final elution was performed with 30 μl M-Elution Buffer. Bisulfite-treated DNA was aliquoted and stored at -80°C until ready for use. A 30 μl of PCR was carried out in 15 μl of Promega GoTaq Hot Start Green Master Mix (Promega), 10 pmol forward and 10 pmol reverse primers, 2 μl of bisulfite-treated DNA and water to reach 30 μl final volume. PCR products were sequenced by pyrosequencing using 0.3 μm sequencing primer. Briefly, pyrosequencing reaction was performed with PSQ Q96 MD pyrosequencing System (QIAGEN), as previously described [37]. Pyrogram peak pattern from every sample was visually inspected to confirm the quality of the reaction. PCR primer sequences are NR3C1-F (Biotin): TTATATGTATTGGTTTTTAGAAAA, NR3C1-R: TACTCCCATTCAACATACCACATT, NR3C1-Seq: ATTCCTACCTCTTTTCAA and sequence to analyze was CACA/GACTATTT [15,16]. Forward primer is located in chr5:143,401,282–143,401,305, reverse primer is in chr5:143,401,030–143,401,053, sequencing primer is in chr5:143,401,250–143,401,267 and target CpG is in chr5: 143,401,272 (Genome Reference Consortium Human Reference 38Genome: GRCh38).
Rationale for CpG site chosen
The specific CpG site analyzed is located in a CpG island shore downstream of the CpG island located in the NR3C1 proximal promoter (Figure 1), and was identified via bioinformatic analysis using the Genomatix software (Genomatix Software, Inc, MI, USA). Specifically, the site is 2803 bp downstream from the first CpG site analyzed by Oberlander et al. [2] in the 1F promoter, the region of NR3C1 that has been most frequently studied in methylation analyses [5,10,38–47]. We selected this region in light of the growing evidence that non-CpG island regions, and in particular, CpG island shores, are enriched with functional methylation sites that control gene expression [13,48–50]. Previous investigations suggest that DNA methylation at this CpG site is sensitive to environmental stressors. For instance, Bollati et al. showed that higher methylation at this specific CpG site is associated with number of years in stressful work conditions, such as shift work [14]. Individuals with higher methylation at this site have also been shown to exhibit poorer lung function as a result of air pollution [15]. These studies have demonstrated that this genomic region is sensitive to various stress exposures. Taken together with the above-mentioned functional relevance of this CpG island shore, this specific CpG site seems an ideal candidate for methylation studies of external and internal stressors.
Figure 1. . CpG island shore site analyzed.
Statistical analysis
We used multivariable linear regression models to compute differences in mean percent NR3C1 DNA methylation levels (β) and 95% CI comparing women who reported abuse victimization with those who reported no abuse. In models where sexual abuse was the independent variable of interest, we adjusted for physical abuse, and vice versa. Age is known to affect cortisol response [51], and was thus controlled for in all models. We further adjusted for parental education as a covariate to control for other potential confounders that track with low educational attainment. Mean NR3C1 methylation values for women reporting financial hardship as a child were 0.57 units higher than women reporting no financial hardship in childhood (95% CI: -0.71, 1.86). However, the inclusion of ‘financial hardship as a child’ as a variable in our multivariable model did not appreciably change the magnitude of the association between abuse and methylation, indicating little evidence of confounding by this variable. Therefore, we did not adjust for child financial hardship in our final multivariable models.
We assessed the association of various forms of childhood abuse victimization (ever abused, physical abuse frequency, sexual abuse frequency, abuse severity) with methylation levels of one CpG site in the NR3C1 proximal promoter located at a CpG shore. The reference group for all multivariate analyses was having no report of any prior child abuse. Tests for trend were computed by inserting the ordinal categorical variable for each abuse variable into the regression model and evaluating the associated Wald test statistic.
Based on our a priori hypothesis that emotional support in childhood would mitigate the impact of child abuse on NR3C1 methylation, we stratified the data by emotional support in childhood. As a formal test of interaction, we created cross-product terms between childhood emotional support (yes vs no) and each abuse variable – modeled as a binary variable for child abuse (ever vs never) or as ordinal variables for physical abuse, sexual abuse and severity of abuse – and evaluated their respective Wald test statistics. A two-sided p < 0.05 was considered statistically significant. SAS software version 9.4 [52] was used to conduct all analyses (SAS Institute Inc., Cary, NC, USA).
Results
The 295 study participants were aged 43–78 years (median = 53, interquartile range: 48–59) in 2005. Approximately 52% (n = 153) reported a history of childhood abuse victimization (Table 1). Those reporting a history of abuse were, on average, slightly younger than those reporting no abuse, more likely to report financial hardship in childhood, and less likely to report emotional support in childhood.
Table 1. . Baseline characteristics of 295 women by child abuse, Black Women's Health Study (2005).
| Characteristic† |
Abuse in childhood |
||
|---|---|---|---|
| No | Yes | p-value | |
| Number of women |
142 |
153 |
– |
| Age in 2005, years (mean) |
54.9 |
52.8 |
0.01 |
| BMI in 2005, kg/m2 (mean) |
29.2 |
30.9 |
0.03 |
| Personal education, years (%): | 0.39 | ||
| – <12 | 0.7 | 1.0 | |
| – 12 | 11.3 | 11.8 | |
| – 13–15 | 36.0 | 38.0 | |
| – ≥16 |
52.1 |
48.6 |
|
| Parental education, years (%)†: | 0.28 | ||
| – <12 | 27.5 | 24.2 | |
| – 12 | 30.3 | 31.4 | |
| – 13–15 | 22.5 | 13.1 | |
| – ≥16 |
14.1 |
19.6 |
|
| Financial hardship as a child, yes (%) |
16.9 |
35.3 |
<0.001 |
| Emotional support as a child, yes (%) | 78.0 | 55.4 | <0.001 |
†Highest level of education reached by either parent. Percentages do not sum to 100% because of missing data.
49% of women reported physical abuse and 32% reported sexual abuse in childhood (Table 2). Unadjusted mean percent methylation levels were 51.9, 52.0 and 53.6 for those reporting low, intermediate and high frequencies of child physical abuse, respectively, and 54.1 and 53.6 for women reporting one to three incidents and more than or equal to four incidents of child sexual abuse, respectively. Unadjusted mean percent methylation levels increased monotonically with increasing categories of abuse severity, ranging from 52.0 to 53.4 for women reporting mild to very severe child physical and sexual abuse, respectively.
Table 2. . Differences in mean NR3C1 levels by child abuse among 295 Black Women's Health Study participants (2005).
| Severity and type of abuse | Number of women (%) |
NR3C1 % |
Unadjusted β (95% CI)† | Age-adjusted β (95% CI) | Multivariable-adjusted β (95% CI)† | |
|---|---|---|---|---|---|---|
| Mean | Std Err | |||||
| No child abuse |
142 (48.1) |
51.8 |
0.37 |
Referent (0.00) |
Referent (0.00) |
Referent (0.00) |
| Any child abuse |
153 (51.9) |
52.7 |
0.36 |
0.88 (-0.14, 1.89) |
0.93 (-0.10, 1.96) |
1.02 (-0.02, 2.06) |
|
Physical abuse frequency
| ||||||
| Low |
51 (18.4) |
51.9 |
0.63 |
0.22 (-1.29, 1.73) |
0.30 (-1.21, 1.81) |
0.36 (-1.16, 1.88) |
| Intermediate |
32 (11.6) |
52.0 |
0.79 |
0.27 (-1.51, 2.06) |
0.31 (-1.47, 2.09) |
0.60 (-1.22, 2.43) |
| High |
52 (18.8) |
53.6 |
0.63 |
1.89 (0.39, 3.40) |
2.06 (0.54, 3.59) |
2.20 (0.65, 3.75) |
| p-trend |
|
|
|
0.022 |
0.014 |
0.008 |
|
Sexual abuse frequency
| ||||||
| One to three incidents |
41 (19.6) |
54.1 |
0.92 |
2.85 (0.48, 5.22) |
2.79 (0.38, 5.21) |
2.70 (0.30, 5.10) |
| Four or more incidents |
26 (12.4) |
53.6 |
1.12 |
2.33 (-0.37, 5.04) |
2.29 (-0.44, 5.02) |
2.13 (-0.53, 4.89) |
| p-trend |
|
|
|
0.110 |
0.126 |
0.159 |
|
Severity of abuse
| ||||||
| Mild |
33 (10.9) |
52.0 |
0.77 |
0.21 (-1.48, 1.89) |
0.25 (-1.44, 1.94) |
0.34 (-1.37, 2.05) |
| Moderate |
52 (17.1) |
52.7 |
0.62 |
0.87 (-0.54, 2.28) |
0.92 (-0.50, 2.34) |
1.02 (-0.42, 2.45) |
| Severe |
58 (19.1) |
52.9 |
0.58 |
1.13 (-0.23, 2.49) |
1.20 (-0.17, 2.57) |
1.34 (-0.04, 2.73) |
| Very severe |
10 (3.3) |
53.4 |
1.40 |
1.63 (-1.22, 4.49) |
1.69 (-1.17, 4.56) |
1.45 (-1.45, 4.34) |
| p-trend | 0.045 | 0.037 | 0.029 | |||
Tests for trend include zero level of exposure (i.e., no child abuse).
No child abuse is reference group for all comparisons. All models of sexual abuse control for physical abuse, and vice versa.
†Adjusted for age at baseline (centered at median age: 43 years) and parental education (<12, 12, 13–15, ≥16 years, missing/unknown).
β: Mean difference; Std Err: Standard error.
In multivariable-adjusted models (Table 2), women who reported any child abuse exhibited higher mean percent methylation levels than women who did not report child abuse (β = 1.02, 95% CI: -0.02, 2.06), although the difference was not statistically significant. Relative to women who did not report child physical abuse, mean percent methylation levels were significantly higher (β = 2.20, 95% CI: 0.65, 3.75) in women with the highest physical abuse frequency, and there was evidence of a dose–response relation (p-trend: 0.008). Mean percent methylation levels were also higher among women who reported one to three incidents (β = 2.70, 95% CI: 0.30, 5.10) of child sexual abuse relative to no abuse, but there was no evidence of a dose–response association (p-trend: 0.159). There was a statistically significant trend of increasing methylation level with increased severity of abuse (p-trend = 0.029).
When we stratified the data by emotional support in childhood, patterns of association were not consistent. Associations between child abuse and methylation levels in the most severe categories of abuse were attenuated among those with emotional support in childhood relative to those without, but numbers (given our sample size) were extremely small (Table 3). In addition, while associations were attenuated for physical and sexual abuse among those reporting child emotional support, the overall abuse association showed no evidence of attenuation and was actually stronger among those reporting emotional support in childhood.
Table 3. . Differences in mean NR3C1 levels by child abuse in the Black Women's Health Study (2005), stratified by emotional support in childhood.
| Severity and type of abuse |
Emotional support in childhood |
p-value, test for interaction | |||||
|---|---|---|---|---|---|---|---|
|
Yes (n = 164)
|
No (n = 84)
|
||||||
| Number of women (%) | NR3C1 % mean (SE) | Adjusted β (95% CI)† | Number of women (%) | NR3C1 % mean (SE) | Adjusted β (95% CI)† | ||
| No child abuse |
92 (54.4) |
51.9 (0.50) |
Referent (0.00) |
26 (30.2) |
52.4 (0.72) |
Referent (0.00) |
0.6924 |
| Any child abuse |
72 (45.6) |
52.7 (0.56) |
0.94 (-0.60, 2.48) |
58 (69.8) |
52.5 (0.48) |
0.37 (-1.45, 2.19) |
|
|
Physical abuse frequency
| |||||||
| Low |
23 (13.6) |
51.7 (1.06) |
-0.01 (-2.55, 2.42) |
19 (22.1) |
51.2 (0.81) |
-0.60 (-2.92, 1.72) |
0.9085 |
| Intermediate |
18 (10.6) |
51.9 (1.15) |
0.37 (-2.25, 2.98) |
12 (14.0) |
51.8 (1.04) |
-0.24 (-3.19, 2.71) |
|
| High |
21 (12.4) |
53.0 (1.06) |
1.39 (-1.06, 3.84) |
22 (25.6) |
53.9 (0.76) |
2.31 (0.01, 4.62) |
|
| p-trend |
|
|
0.276 |
|
|
0.038 |
|
|
Sexual abuse frequency
| |||||||
| One to three incidents |
23 (13.6) |
54.6 (1.45) |
2.81 (-0.68, 6.30) |
16 (18.6) |
53.3 (1.12) |
3.09 (-0.96, 7.13) |
0.6544 |
| Four or more incidents |
9 (5.3) |
53.8 (1.92) |
1.38 (-2.98, 5.74) |
10 (11.6) |
54.8 (1.48) |
4.44 (-0.27, 9.15) |
|
| p-trend |
|
|
0.443 |
|
|
0.076 |
|
|
Severity of abuse
| |||||||
| Mild |
13 (7.7) |
50.7 (1.33) |
-1.22 (-4.06, 1.62) |
12 (14.0) |
51.9 (1.06) |
-0.24 (-2.92, 2.44) |
0.8178 |
| Moderate |
31 (18.3) |
53.1 (0.86) |
1.54 (-0.46, 3.54) |
18 (20.9) |
51.8 (0.87) |
-0.38 (-2.76, 2.00) |
|
| Severe |
26 (15.4) |
53.1 (0.94) |
1.28 (-0.83, 3.39) |
24 (27.9) |
53.1 (0.75) |
0.92 (-1.29, 3.14) |
|
| Very severe |
2 (1.2) |
52.1 (3.39) |
0.44 (-6.32, 7.20) |
4 (4.7) |
54.4 (1.84) |
1.86 (-2.23, 5.96) |
|
| p-trend | 0.115 | 0.288 | |||||
Tests for trend include zero level of exposure (i.e., no child abuse). No child abuse is reference group for all comparisons. All models of sexual abuse control for physical abuse, and vice versa.
†Adjusted for age and parental education.
β: Mean difference; SE: Standard error.
Discussion
In this paper, we present results of the first study assessing methylation of a novel CpG site located in a CpG island shore within the NR3C1 proximal promoter in relation to childhood abuse victimization within AA women. We found that women with a history of childhood abuse had increased methylation levels at this CpG site compared with women who reported no childhood abuse. Positive associations were observed for both physical and sexual abuse. In addition, we found a significant dose–response pattern for severity of abuse. In the group of women exposed to the highest level of physical abuse, methylation was >2% points higher, and 1.5% points higher for the highest severity of overall abuse. Differences of this magnitude in NR3C1 have been previously demonstrated to be clinically significant. For example, as little as half a percent increase in methylation of exon 1F and the 1F promoter in NR3C1 has been associated with an attenuated cortisol response into adulthood using leukocyte DNA [53]. Increases in exon 1B methylation of 1.8% in blood DNA have been associated with borderline personality disorder [6], and increases in exon 1F and 1F promoter methylation of 2% have been associated with depression using saliva, which is comprised mainly of leukocyte DNA [5]. Moreover, increased methylation at the same exact CpG site we examined has been associated with a significant modifying effect on decreased lung function in humans, which the authors believe is caused by attenuating the body's glucocorticoid response [15]. Also, mouse models investigating the CpG island shore of NR3C1 have shown that early life stress is associated with increased methylation, which was shown to affect transcription factor binding and gene expression [54]. Therefore, it is quite likely that methylation in this CpG site we chose possesses a functional significant effect, which needs to be confirmed in future analysis.
This study is the first of its kind to investigate NR3C1 methylation at a CpG island shore, as opposed to the CpG island itself, in association with childhood victimization and abuse. Our findings of increased CpG methylation at this CpG site corroborate numerous other human studies exploring childhood abuse and NR3C1 exon 1F promoter methylation, which is the predominant genomic region that has been investigated in previous studies [1,3].
Human studies similar to our study design analyzing peripheral blood have documented associations between physical abuse, childhood maltreatment and parental loss or low levels of parental care, and hypermethyaltion in the NR3C1 exon 1F promoter [3–4,55]. Two studies conducted by Perroud et al. also demonstrated the same dose–response relationship of increased NR3C1 methylation with increased frequency and severity of abuse that was found in our study [3,56]. The mean methylation levels reported for their Swiss samples are similar to those observed in our study of AA women. Our results also connect with the wider body of literature that has demonstrated an association between NR3C1 exon 1F promoter methylation and various other forms of early life psychosocial stress, ranging from parental loss [4] to neglect [3,56] to perceived stress [4]. Increased NR3C1 methylation has even been documented in the cord blood of fetuses whose mother's had a history of abuse or depression during pregnancy [2,57].
Our findings regarding the potential attenuating effect of childhood emotional support on methylation levels among women who were abused were largely inconclusive, owing to our small sample size and the subsequent small numbers of exposed women. Specifically, while emotional support did not attenuate increased methylation among those who experienced any abuse victimization overall (52.5% mean methylation among those without emotional support vs 52.7% among those with support), it did attenuate methylation levels among women experiencing the highest levels of physical and sexual abuse. The results for severity of abuse were equivocal. A larger sample would have allowed us to capture more individuals in the severe abuse categories, and provided greater precision to evaluate our hypothesis. Further investigation of the potential protective effect of emotional support on the association between early life adversity and methylation is warranted.
This study has limitations that should be noted. Our study was limited to 295 women, and thus we may not have been adequately powered to detect some important relationships. We are currently undertaking a larger study to address this. Our measure of sexual abuse had response categories of ‘never’, ‘1–3 times’ or ‘4 or more times’, and did not allow for distinguishing between those who had been abused a single time from those who had been abused multiple times. Furthermore, participants’ recall about childhood events may have been inaccurate, but at least one prospective study has shown that recall bias is relatively minimized when in reference to traumatic life events, as opposed to nontraumatic events [58]. We were unable to determine whether childhood abuse took place before, after or during the time respondents were referring to when answering questions about emotional support in childhood. Future studies could ask more detailed questions to ascertain the time sequence between receipt of emotional support and experiences of abuse, and the relationships of respondents to those perpetrating abuse and providing support. Last, although our CpG site overlaps with transcription factor binding sites, including binding sites for FOXA1 and SPI1 (Supplementary Figure 1), our study lacks functional analyses, and therefore we cannot confirm the downstream biological effects this methylation may have.
Despite these limitations, this study provides the first data among AA women regarding the relationship between childhood physical and sexual abuse and methylation of NR3C1. This study also provides the first evidence of an association between childhood abuse victimization and a novel CpG site located in a CpG island shore within the proximal promoter of NR3C1, with a clear dose–response relationship. Further studies are needed to examine in great detail whether hypermethylation associated with childhood abuse victimization can be attenuated by sources of emotional support and encouragement in childhood. Should future research confirm this possibility, this would only further emphasize the importance of policies aimed at providing early supportive interventions to children in the early, formative years, particularly for children and communities known to expereince a disproportionate burden of soocial or environmental stressors. As increasing evidence demonstrates the link between psychosocial stress in early childhood and risk of chronic illness in adulthood, public efforts to provide supportive environments for children and their families may come to be seen as an essential part of any comprehensive public health agenda.
Conclusion
Gaining a better understanding of the mechanistic pathways through which early disadvantage translates into increased vulnerability to disease later in life, and is even transferred intergenerationally, may be an important component to a comprehensive strategy for eliminating health disparities in the USA. Not only are targeted psychosocial interventions needed to mitigate the powerful effects of early life stressors, as well as ongoing life stressors, on individuals’ health, but public policies that support struggling families and small children in their early developmental years may be especially critical to ensuring a healthy adult population [59].
Executive summary.
Early life childhood adversity has been associated with hypothalamic–pituitary–adrenal axis dysregulation in adulthood via epigenetic modification of the glucocorticoid receptor gene, NR3C1.
Recent research has shown the functional importance of DNA methylation within CpG island shores, which are genomic regions located within 2 kb of a CpG island.
No previous study has assessed the impact of child abuse on NR3C1 methylation within an African–American (AA) population, although AAs are more likely to experience childhood abuse and conditions associated with increased NR3C1 methylation.
This is the first epigenetics study of NR3C1 to: investigate childhood abuse victimization in relation to DNA methylation of a CpG island shore as a more sensitive methylation site for assessing the biological impact of psychosocial stress; study the epigenetic effects of psychosocial stress on NR3C1 methylation in a cohort of AA women; and explore positive psychosocial factors that may buffer against the deleterious epigenetic effects of psychosocial stress.
AA women who reported child abuse exhibited higher mean NR3C1 methylation levels than women reporting no child abuse, after adjusting for a number of covariates.
Both physical and sexual abuses were positively associated with methylation levels.
Comparing mild, moderate, severe and very severe abuse with no abuse, severity of abuse demonstrated a significant dose-response trend in relation to mean differences in NR3C1 methylation (β). Greater severity of abuse showed greater mean NR3C1 methylation.
Emotional support in childhood appeared to attenuate associations with physical and sexual abuse, but further research with larger samples sizes is warranted to clarify whether the extent to which childhood emotional support buffers the association.
Gaining a better understanding of the mechanistic pathways through which early disadvantage translates into increased vulnerability to disease later in life, and is even transferred intergenerationally, may be an important component to a comprehensive strategy for eliminating health disparities in the USA.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of all Black Women's Health Study participants. The authors thank M Farvid for comments on a previous version of this manuscript, and A Schachter and MA Argentieri for their excellent research assistance.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers or the NIH.
Financial & competing interests disclosure
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers (AA Baccarelli). This work was also supported by The John Templeton Foundation Grant #48424 (AE Shields) and the Black Women's Health Study (R01 CA 058420 and UM1CA 164974). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the Harvard School of Public Health Institutional Review Board and the Boston University Institutional Review Board. The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.McGowan PO, Sasaki A, D'Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 3.Perroud N, Paoloni-Giacobino A, Prada P, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl. Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Adults with a history of childhood sexual abuse demonstrated a positive dose–response relationship with severity of abuse in exon1F of NR3C1 using peripheral blood.
- 4.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Lower levels of parental care, parental loss, maltreatment and perceived stress were associated with increased NR3C1 promoter methylation using peripheral blood.
- 5.Melas PA, Wei Y, Wong CC, et al. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int. J. Neuropsychopharmacol. 2013;16(7):1513–1528. doi: 10.1017/S1461145713000102. [DOI] [PubMed] [Google Scholar]
- 6.Dammann G, Teschler S, Haag T, Altmuller F, Tuczek F, Dammann RH. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics. 2011;6(12):1454–1462. doi: 10.4161/epi.6.12.18363. [DOI] [PubMed] [Google Scholar]
- 7.Steiger H, Labonte B, Groleau P, Turecki G, Israel M. Methylation of the glucocorticoid receptor gene promoter in bulimic women: associations with borderline personality disorder, suicidality, and exposure to childhood abuse. Int. J. Eat. Disorder. 2013;46(3):246–255. doi: 10.1002/eat.22113. [DOI] [PubMed] [Google Scholar]
- 8.Lind GE, Kleivi K, Meling GI, et al. ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell. Oncol. 2006;28(5–6):259–272. doi: 10.1155/2006/949506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kay P, Schlossmacher G, Matthews L, et al. Loss of glucocorticoid receptor expression by DNA methylation prevents glucocorticoid induced apoptosis in human small cell lung cancer cells. PLoS ONE. 2011;6(10):e24839. doi: 10.1371/journal.pone.0024839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesset KA, Perri AM, Mueller CR. Frequent promoter hypermethylation and expression reduction of the glucocorticoid receptor gene in breast tumors. Epigenetics. 2014;9(6):851–859. doi: 10.4161/epi.28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad. Sci. USA. 2002;99(6):3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes. Dev. 2011;25(10):1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irizarry RA, Ladd-Acosta C, Wen B, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009;41(2):178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The methylome study reveals that a majority of functionally important DNA methylation occurs not in CpG islands, but in CpG island shores, which are genomic regions located within 2 kb of a CpG island.
- 14.Bollati V, Baccarelli A, Sartori S, et al. Epigenetic effects of shiftwork on blood DNA methylation. Chronobiol. Int. 2010;27(5):1093–1104. doi: 10.3109/07420528.2010.490065. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Years of shiftwork significantly increased DNA methylation at the same exact CpG island shore we used in this study.
- 15.Lepeule J, Bind MA, Baccarelli AA, et al. Epigenetic influences on associations between air pollutants and lung function in elderly men: the normative aging study. Environ. Health Perspect. 2014;122(6):566–572. doi: 10.1289/ehp.1206458. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Individuals with higher methylation at the CpG island shore we used in this study exhibited worse lung function as a result of air pollution.
- 16.Madrigano J, Baccarelli A, Mittleman MA, et al. Aging and epigenetics: longitudinal changes in gene-specific DNA methylation. Epigenetics. 2012;7(1):63–70. doi: 10.4161/epi.7.1.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedlak A, McPherson K, Das B. Washington, DC, USA: US Department of HHS; Supplementary analyses of race differences in child maltreatment rates in the NIS-4.www.acf.hhs.gov/sites/default/files/opre/nis4_supp_analysis_race_diff_mar2010.pdf [Google Scholar]
- 18.Cozier YC, Albert MA, Castro-Webb N, et al. Neighborhood socioeconomic status in relation to serum biomarkers in the Black Women's Health Study. J. Urban Health. 2006;93(2):279–291. doi: 10.1007/s11524-016-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quest Diagnostics. www.QuestDiagnostics.com
- 20.Department of Health and Human Services. Regulation and Guidance. Center for Medicare and Medicaid Services.www.cms.hhs.gov/CLIA/ [Google Scholar]
- 21.IL, USA: College of American Pathologists.www.cap.org/ [Google Scholar]
- 22.Straus MA. Measuring intrafamily conflict and violence: the Conflict Tactics (CT) scales. J. Marriage Fam. 1979;41:75–88. [Google Scholar]
- 23.McFarlane J, Parker B, Soeken K, Bullock L. Assessing for abuse during pregnancy. Severity and frequency of injuries and associated entry into prenatal care. JAMA. 1992;267(23):3176–3178. doi: 10.1001/jama.267.23.3176. [DOI] [PubMed] [Google Scholar]
- 24.Wise LA, Palmer JR, Rothman EF, Rosenberg L. Childhood abuse and early menarche: findings from the black women's health study. Am. J. Public Health. 2009;99(Suppl. 2):S460–S466. doi: 10.2105/AJPH.2008.149005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayworth BB, Wise LA, Harlow BL. Childhood abuse and risk of eating disorders in women. Epidemiology. 2004;15(3):271–278. doi: 10.1097/01.ede.0000120047.07140.9d. [DOI] [PubMed] [Google Scholar]
- 26.Nichols HB, Harlow BL. Childhood abuse and risk of smoking onset. J. Epidemiol. Community Health. 2004;58(5):402–406. doi: 10.1136/jech.2003.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise LA, Palmer JR, Boggs DA, Adams-Campbell LL, Rosenberg L. Abuse victimization and risk of breast cancer in the Black Women's Health Study [corrected] Cancer Causes Control. 2011;22(4):659–669. doi: 10.1007/s10552-011-9738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boynton-Jarrett R, Rosenberg L, Palmer JR, Boggs DA, Wise LA. Child and adolescent abuse in relation to obesity in adulthood: the Black Women's Health Study. Pediatrics. 2012;130(2):245–253. doi: 10.1542/peds.2011-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coogan PF, Wise LA, O'connor GT, Brown TA, Palmer JR, Rosenberg L. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J. Allergy Clin. Immunol. 2013;131(4):1058–1063. doi: 10.1016/j.jaci.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wise LA, Palmer JR, Rosenberg L. Lifetime abuse victimization and risk of uterine leiomyomata in black women. Am. J. Obstet. Gynecol. 2013;208(4):272.e1–272.e13. doi: 10.1016/j.ajog.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wise LA, Zierler S, Krieger N, Harlow BL. Adult onset of major depressive disorder in relation to early life violent victimisation: a case–control study. Lancet. 2001;358(9285):881–887. doi: 10.1016/S0140-6736(01)06072-X. [DOI] [PubMed] [Google Scholar]
- 32.Rayworth BB, Wise LA, Harlow BL. Childhood abuse and risk of eating disorders in women. Epidemiology. 2004;15(3):271–278. doi: 10.1097/01.ede.0000120047.07140.9d. [DOI] [PubMed] [Google Scholar]
- 33.Swartz JR, Hariri AR, Williamson DE. An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Mol. Psychiatry. 2016 doi: 10.1038/mp.2016.82. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am. J. Respir. Crit. Care Med. 2009;179(7):572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollati V, Baccarelli A, Hou L, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67(3):876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 36.Baccarelli A, Barretta F, Dou C, et al. Effects of particulate air pollution on blood pressure in a highly exposed population in Beijing, China: a repeated-measure study. Environ. Health. 2011;10(1):108. doi: 10.1186/1476-069X-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byun HM, Barrow TM. Analysis of pollutant-induced changes in mitochondrial DNA methylation. Methods Mol. Biol. 2015;1265:271–283. doi: 10.1007/978-1-4939-2288-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao J, An Q, Goldberg J, Quyyumi AA, Vaccarino V. Promoter methylation of glucocorticoid receptor gene is associated with subclinical atherosclerosis: a monozygotic twin study. Atherosclerosis. 2015;242(1):71–76. doi: 10.1016/j.atherosclerosis.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vangeel E, Van Den Eede F, Hompes T, et al. Chronic fatigue syndrome and DNA hypomethylation of the glucocorticoid receptor gene promoter 1F region: associations with HPA axis hypofunction and childhood trauma. Psychosom. Med. 2015;77(8):853–862. doi: 10.1097/PSY.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Fan J, Shou Q, Zhang L, Ma H, Fan Y. Hypermethylation of glucocorticoid receptor gene promoter results in glucocorticoid receptor gene low expression in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Rheumatol. Int. 2015;35(8):1335–1342. doi: 10.1007/s00296-015-3266-5. [DOI] [PubMed] [Google Scholar]
- 41.Na KS, Chang HS, Won E, et al. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS ONE. 2014;9(1):e85425. doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alt SR, Turner JD, Klok MD, et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35(4):544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Yehuda R, Daskalakis NP, Desarnaud F, et al. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front. Psychiatry. 2013;4:118. doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yehuda R, Flory JD, Bierer LM, et al. Lower methylation of glucocorticoid receptor gene promoter 1 in peripheral blood of veterans with posttraumatic stress disorder. Biol. Psych. 2015;77(4):356–364. doi: 10.1016/j.biopsych.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Vukojevic V, Kolassa IT, Fastenrath M, et al. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. J. Neurosci. 2014;34(31):10274–10284. doi: 10.1523/JNEUROSCI.1526-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Blanco A, Ferrer M, Soler J, et al. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J. Psychiatr. Res. 2014;57:34–40. doi: 10.1016/j.jpsychires.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Moser D, Molitor A, Kumsta R, Tatschner T, Riederer P, Meyer J. The glucocorticoid receptor gene exon 1-F promoter is not methylated at the NGFI-A binding site in human hippocampus. World J. Biol. Psychiatry. 2007;8(4):262–268. doi: 10.1080/15622970701429862. [DOI] [PubMed] [Google Scholar]
- 48.Doi A, Park IH, Wen B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat. Genet. 2009;41(12):1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu X, Davison J, Du L, et al. Identification of differentially methylated markers among cytogenetic risk groups of acute myeloid leukemia. Epigenetics. 2015;10(6):526–535. doi: 10.1080/15592294.2015.1048060. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The genome-wide DNA methylation analysis shows that a majority of the differentially methylated regions in humans are located within CpG island shores as opposed to the CpG islands.
- 50.Wilson GA, Butcher LM, Foster HR, et al. Human-specific epigenetic variation in the immunological leukotriene B4 receptor (LTB4R/BLT1) implicated in common inflammatory diseases. Genome. Med. 2014;6(3):19. doi: 10.1186/gm536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veldhuis JD. Changes in pituitary function with ageing and implications for patient care. Nat. Rev. Endocrinol. 2013;9(4):205–215. doi: 10.1038/nrendo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sas Institute, Inc. The SAS system for Windows. 2013. www.sas.com/en_us/home.html
- 53.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bockmuhl Y, Patchev AV, Madejska A, et al. Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics. 2015;10(3):247–257. doi: 10.1080/15592294.2015.1017199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romens SE, McDonald J, Svaren J, Pollak SD. Associations between early life stress and gene methylation in children. Child Dev. 2014;86(1):303–309. doi: 10.1111/cdev.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perroud N, Dayer A, Piguet C, et al. Childhood maltreatment and methylation of the glucocorticoid receptor gene NR3C1 in bipolar disorder. Br. J. Psychiatry. 2014;204:30–35. doi: 10.1192/bjp.bp.112.120055. [DOI] [PubMed] [Google Scholar]
- 57.Radtke KM, Ruf M, Gunter HM, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lalande K, Bonanno G. Retrospective memory bias for the frequency of potentially traumatic events: a prospective study. Psychol. Trauma. 2011;3(2):165–170. [Google Scholar]
- 59.Phillips DA, Shonkoff JP. From Neurons to Neighborhoods: The Science of Early Childhood Development. National Academies Press; Washington, DC, USA: 2000. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.