Abstract
Fragile X syndrome (FXS), due to transcriptional silencing of fragile X mental retardation protein (FMRP), is characterized by excess synaptic connections and impaired dendrite maturation. Programmed cell death (PCD) is critical for synaptogenesis and elimination of aberrant neuronal connections in the developing brain; however, the role of FMRP in PCD is unknown. The aim of this work was to assess the intrinsic apoptosis pathway in the developing brain of Fmr1 mutants. To accomplish this, we evaluated two different Fmr1 mutant strains of 10-day-old male mice compared with appropriate controls. We performed immunohistochemistry for activated caspase-3 and TUNEL assays, quantified the number of neurons in neocortex and hippocampus, determined cytochrome c peroxidase activity, measured the amount of cytochrome c release from forebrain mitochondria, and assessed levels of key pro- and antiapoptotic mediators with immunoblot analysis. Both Fmr1 mutant strains demonstrated decreased apoptosis in neocortex, hippocampus, and basolateral amygdala, impaired cytochrome c and procaspase-9 release from mitochondria despite intact Bax translocation, increased expression of the antiapoptotic protein, BCL-xL, and increased number of neurons. Taken together, the data suggest that PCD is impaired due to increased BCL-xL expression and is associated with excess neurons in the developing brain of FMRP-deficient mice. It is possible that deficient PCD prevents neuron elimination and results in abnormal retention of developmentally transient neurons. Thus, defective PCD may contribute to the excess synaptic connections known to exist in Fmr1 mutants and could play a role in the behavioral phenotype of children with FXS.
Keywords: Programmed cell death, Apoptosis, Brain, Development, Fragile X syndrome, Autism
Introduction
Fragile X syndrome (FXS) is the leading known genetic cause of autism [1, 2]. Due to CGG triplet repeat expansion in the Fmr1 gene and hypermethylation of the Fmr1 promoter region, the full mutation results in reduced gene transcription, and loss or significant reduction in fragile X mental retardation protein (FMRP) expression [3, 4]. FMRP is an RNA-binding protein thought to regulate several mRNAs important for synapse development and function and deficiency results in abnormal synapse maturation, failure of synapse elimination, excess and aberrant synaptic connections, and impaired dendrite maturation and pruning [4].
Several regions of the autistic brain have been shown to be relatively large, containing significantly more neurons and higher neuronal density compared to the brain of unaffected children [5–7]. Loss of FMRP expression results in synaptic overgrowth and excess number of neurons in the developing Drosophila brain [8, 9]. Since cortical postmitotic neurons are not generated postnatally, pathologically increased numbers must be due to increased cell proliferation, impaired programmed cell death (PCD), or both [7]. PCD is a widespread phenomenon that occurs within the central nervous system coincident with proliferation, migration, and differentiation and is a natural process that is necessary for normal brain development and patterning [10]. The postnatal wave of PCD is critical for synaptogenesis and elimination of aberrant neuronal connections [10].
Defective apoptosis has been demonstrated in the developing Drosophila brain in dFmr1 null mutants leading to abnormal retention of developmentally transient neurons [8]. However, developmental PCD has never been assessed in the established murine models of FXS. Furthermore, the role of FMRP in developmental PCD is unknown and the specific defect(s) in mitochondrial pathway of apoptosis caused by FMRP deficiency have never been elucidated. Here we demonstrate that PCD is impaired in the developing postnatal brain of FMRP-deficient mice. Importantly, we identify distinct defects and aberrancies in the intrinsic apoptosis pathway of two different Fmr1 mutant strains. The results suggest that impaired PCD during development could play a role in FXS with regard to deficient neuron elimination leading to excess and aberrant synapse connections.
Materials and Methods
Animals
The care of the animals in this study was in accordance with NIH and Institutional Animal Care and Use Committee guidelines. Study approval was granted by the Children’s National Medical Center IACUC. All experimental studies were performed on 10-day-old male Fmr1−/y and Fmr1 I304N mouse pups with appropriate controls. Postnatal day 10 (P10) was chosen because synaptogenesis peaks at day 7 in rodents and is completed by the 2nd or 3rd week of life [11, 12]. Thus, P10 probably equates to a timepoint in postnatal human infancy [13–15]. For Fmr1 null mice (FVB.129P2-Pde6b + Tyr c-ch Fmr1 tm1Cgr /J), 6- to 8-week-old paired hemizygous male (Fmr1−/y) and homozygous female (Fmr1−/−) mice were acquired (Jackson Laboratory, Bar Harbor, Me., USA) and bred to yield fully affected newborn male pups. Appropriate control (FVB.129P2-Pde6b + Tyr c-ch /AntJ) hemizygous male and homozygous female breeding pairs for the Fmr1 knockout strain were also acquired and bred (Jackson Laboratory). For the Fmr1 I304N strain (B6.129-Fmr1 tm1Rbd /J), 6- to 8-week-old paired hemizygous male (Fmr1−/y) and heterozygous female (Fmr1−/+) breeders were acquired and bred (Jackson Laboratory). Genotyping on tail clippings offspring using standard PCR was performed to identify hemizygous males. Appropriate wild-type C57Bl/6J paired breeders were also acquired (Jackson Laboratory) to control for the Fmr1I 304N strain.
Activated Caspase-3 Immunohistochemistry
At the time of euthanasia, following pentobarbital injection (150 mg/kg, i.p.), the brain was perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) via left ventricle injection for 30 min and then postfixed in additional fixative solution for 24 h at 4°C. Serial frozen sections were cut at a thickness of 6 μm in the coronal plane through the cerebral hemispheres beginning at −1.7 mm from bregma, 2.1 mm from interaural and individual sections were slide-mounted. Immunohistochemistry was performed on three to four nonserial nonadjacent sections using polyclonal anti-rabbit activated caspase-3 (Cell Signaling Technology, Beverly, Mass., USA, 9661), biotinylated secondary antibody (goat anti-rabbit, Cell Signaling Technology), and developed with diaminobenzidine. The number of activated caspase-3-positive cells per square millimeter was quantified at ×10 magnification in neocortex, hippocampus, caudate/putamen, and basolateral amygdala of both hemispheres in 3–4 nonserial sections in 3–4 animals per group.
Terminal Deoxynucleotidyl Transferase-Mediated UTP Nick End-Labeling Staining
At the time of euthanasia, the brain was perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) via left ventricle injection for 30 min and then postfixed in additional fixative solution for 24 h at 4°C. Paraffin-embedded brain sections were cut into 6-μm sections in the coronal plane through the cerebral hemispheres beginning at −1.7 mm from bregma, 2.1 mm from interaural, slide-mounted, and stained for terminal deoxynucleotidyl transferase-mediated UTP nick end-labeling (TUNEL). Sections were incubated in 0.5% Triton at room temperature, followed by proteinase K at 37°C, then immersed in terminal deoxynucleotidyl transferase buffer (30 mM Tris-HCl buffer, pH 7.2, 140 mM sodium cacodylate, and 1 mM cobalt chloride) at room temperature. This was followed by incubation with terminal deoxynucleotidyl transferase and biotin-16-dUTP for 60 min at 37°C. The reaction was terminated with TB buffer (300 mM sodium chloride with 30 mM sodium citrate) at room temperature, followed by immersion in 3% hydrogen peroxide and 2% fetal bovine serum at room temperature. The sections were then covered with an Avidin Biotin Complex (1: 200 dilution) for 30 min at room temperature, incubated with FITC-Avidin D for detection and counterstained with DAPI. The numbers of TUNEL-positive nuclei in neocortex, hippocampus, caudate/putamen, and basolateral amygdala were quantified in 3–4 nonserial sections per mouse and 3–4 mice per cohort were evaluated.
Quantification of Number of Neurons
At the time of euthanasia, following pentobarbital injection (150 mg/kg, i.p.), the brain was perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) via left ventricle injection for 30 min and then postfixed in additional fixative solution for 24 h at 4°C. Brains were stored in 30% sucrose for 24 h at 4°C and sectioned coronally (45 μm) on a freezing microtome. Sections were rinsed in phosphate-buffered saline, slide-mounted and stained with cresyl violet for 30 min [16]. Cresyl violet-positive neurons with a clear nucleus and nucleoli in the primary somatosensory cortex and in the pyramidal layer of the CA3 region of the hippocampus were counted by two blinded observers using an image analyzing system equipped with a computer-based CCD camera (Nikon Eclipse e800). Starting from the first section (interaural 2.10 mm, bregma −1.70 mm, 4.7 mm from the most rostral section), counts were taken from 5 coronal sections at 0.135-mm increments in 100,000 μm2 fields in both hemispheres per mouse [17]. Brain regions were defined in accordance with the Mouse Brain Atlas [18, 19]. Three mice per cohort were evaluated.
Cytochrome c Peroxidase Activity
Cytochrome c was extracted from fresh mitochondria as previously described [20]. Isolated forebrain mitochondria (20 mg/ml) were suspended in a hypotonic 0.015 M KCl solution for 10 min on ice and then centrifuged at 105,000 g for 15 min at 4°C. The pellet was resuspended in 0.15 M KCl solution for 10 min on ice and then centrifuged again at 105,000 g for 15 min at 4°C. The supernatant was collected and cytochrome c content quantified with spectrophotometry. The peroxidase activity of 0.5–1 μM cytochrome c was determined by measuring the rate of oxidation of 50 μM 2,2′-azinobis-(2-ethylbenzthiazoline-6-sulfonate) in 10 mM potassium phosphate buffer (pH 7.4) at 415 nm (ε415 = 3.6. 104 M−1 cm−1) following the addition of hydrogen peroxide [21]. Five animals per cohort were evaluated.
Heme c Determination
Forebrain mitochondria and cytosol were isolated by differential centrifugation [22]. As previously described, forebrain was harvested and homogenized in ice-cold H medium (70 mM sucrose, 220 mM mannitol, 2.5 mM Hepes, pH 7.4 and 2 mM EDTA) [22]. The homogenate was spun at 1,500 g for 10 min at 4°C. Supernatant was removed and centrifuged at 10,000 g for 10 min at 4°C. Cytosolic supernatant was collected and pellet was resuspended in H medium and centrifuged again at 10,000 g for 10 min at 4°C. Pellet was again resuspended in H medium and mitochondrial and cytosolic protein concentrations subsequently determined using the method of Lowry [22].
Mitochondrial and cytosolic heme c content were calculated from the difference in spectra (dithionate/ascorbate reduced minus air-oxidized) of mitochondria or cytosolic protein (0.5–1 mg) solubilized in 10% lauryl maltoside using an absorption coefficient of 20.5 mM−1 cm−1 at 550–535 nm [23, 24]. Five animals per cohort were evaluated.
Immunoblot Analysis
Ten-microgram samples of homogenized forebrain mitochondrial or cytosolic protein were subjected to SDS-acrylamide gel electrophoresis and immunoblotting. Blots were labeled with a primary rabbit monoclonal antibody to procaspase-9 (GeneTex Inc., Irvine, Calif., USA) or Bax (Santa Cruz Biotechnology Inc., Santa Cruz, Calif., USA), mouse monoclonal antibody to APAF-1 (Santa Cruz Biotechnology Inc.), rabbit polyclonal antibody to BCL-xL or BCL-2 (GeneTex Inc.), or rabbit monoclonal antibody to Akt, p-Akt (Thr308), p-Akt (Ser473), Bad, or p-Bad (Ser112) (Cell Signaling Technology). Blots were secondarily exposed to the appropriate secondary antibody: either donkey anti-rabbit IgG or rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc.). Mitochondrial protein loading was assessed with a primary monoclonal antibody to mouse VDAC (Molecular Probes, Eugene, Oreg., USA) and secondarily exposed to rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc.). Cytosolic protein loading was assessed with a primary monoclonal antibody to mouse actin (Thermo Fisher Scientific Inc., Rockford, Ill., USA) and secondarily exposed to rabbit anti-mouse IgG (Santa Cruz Biotechnology Inc.). For p-Akt and p-Bad, samples were processed with phosphatase inhibitors (Sigma, St. Louis, Mo., USA). Signal was detected with enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, N.J., USA), and density was measured using scanning densitometry. Five animals per cohort were evaluated.
Statistics
Data are presented as mean ± standard deviation (SD). Statistical significance was assessed using ANOVA and post hoc Tukey’s test with p < 0.05.
Results
PCD Is Impaired in the Developing Brain of Fmr1 Mutants
Cell death ascends from subcortical to upper cortical regions during rodent development with the majority of apoptosis occurring in the somatosensory cortex, medial cortical regions, the cortical subplate, and hippocampus in the first 10 days of life [25–27]. Thus, we assessed PCD by measuring levels of activated caspase-3 with immunohistochemistry and TUNEL staining on slide-mounted brain sections from P10 male pups. We focused on cerebral cortex, hippocampus, and amygdala because synapse development in these regions has been previously shown to be pathologic in Fmr1 knockout mice [28].
Consistent with the natural process of developmental PCD, the majority of activated caspase-3-positive cells and TUNEL-positive nuclei localized to layers II, IV, and V of the neocortex and CA1, CA3, and the dentate gyrus of the hippocampus (fig. 1, 2). Activated caspase-3-positive cells and the number of TUNEL-positive nuclei were significantly decreased in number in neocortex, hippocampus, and basolateral amygdala of both Fmr1 mutant strains compared to their respective controls (fig. 1, 2). Fmr1 knockouts demonstrated a 20–25% decrease in the number of activated caspase-3 cells and TUNEL-positive nuclei in neocortex and hippocampus compared to FVB controls and a 60% decline in basolateral amygdala (fig. 1, 2). Similar decreases were seen in identical brain regions of Fmr1 I304N mutants compared to C57Bl6J controls (fig. 1, 2). However, there was no measurable difference in the number of activated caspase-3-positive cells or TU-NEL-positive nuclei in the caudate and putamen region of either mutant strain versus control mice (fig. 1, 2).
Fig. 1.
Activated caspase-3 is decreased in the developing brain of Fmr1 mutant mice. Immunohistochemistry for activated caspase-3 was performed on coronal sections. a Representative sections imaged at ×10 from somatosensory neocortex (NC), hippocampus (HC), caudate/putamen (CPu), and basolateral amygdala (BLA) are depicted. Activated caspase-3-positive cells undergoing degeneration or lack thereof within the box in each section are magnified in the inset. Black arrowheads indicate activated caspase-3-stained cells. CA1, CA3, dentate gyrus (DG) regions of HC, lateral ventricle (LV), internal capsule (ic), globus pallidus (GP), dorsolateral (LaDL), ventrolateral (LaVL), and central nucleus (CeC) of the amygdala are labeled. Scale bar, 100 μm. b Quantification of activated caspase-3-stained cells in NC, HC, CPu, and BLA is demonstrated. Values are expressed as means + SD. * p < 0.05 vs. control; † p < 0.01 vs. control; n = 3–4 animals per cohort.
Fig. 2.
Developmental PCD is decreased in both Fmr1 mutant strains. TUNEL assays were performed on coronal sections. a Representative sections imaged at ×10 from somatosensory neocortex (NC), hippocampus (HC), caudate/putamen (CPu), and basolateral amygdala (BLA) are depicted. Green TUNEL-positive nuclei are visible and are indicated by white arrowheads. Nuclei counterstained with DAPI appear blue. CA1, CA3, dentate gyrus (DG) regions of HC, lateral ventricle (LV), dorsolateral (LaDL), and ventrolateral (LaVL) nucleus of the amygdala are labeled. Scale bar, 100 μm. b Quantification of total TUNEL-positive nuclei from NC, HC, CPu, and BLA in 3–4 nonserial coronal sections is demonstrated. Values are expressed as means + SD. * p < 0.05 vs. control; † p < 0.01 vs. control; n = 3–4 animals per cohort.
Impaired Developmental PCD Is Associated with Excess Neurons in the Developing Brain of Fmr1 Mutants
PCD is a natural process that is necessary for normal brain development and patterning [29]. Cell death eliminates the entire neuron and leads to loss of all neurites associated with the dying cell [29]. Impaired neuronal apoptosis results in persistence of the progenitor pool resulting in excess number of neurons [29]. Thus, we quantified the number of neurons in the primary somatosensory cortex and in the pyramidal layer of the CA3 region of the hippocampus using cresyl violet staining of slide-mounted brain sections from P10 male pups. Consistent with impaired PCD, both Fmr1 mutant strains demonstrated significantly increased number of neurons in the neocortex and hippocampus compared to their respective controls indicating a defect in neuron elimination (fig. 3).
Fig. 3.
The number of neurons in neocortex and hippocampus is increased in the developing brain of Fmr1 mutant mice. a Representative cresyl violet-stained coronal sections of 10-day-old mice are depicted. S1 = Primary somatosensory cortex; CA3 = CA3 region of hippocampus. Scale bar, 200 μm. b Quantification of the number of neurons in the primary somatosensory cortex and CA3 region of hippocampus is demonstrated. * p < 0.05 vs. control; † p < 0.01 vs. control; n = 3 animals per cohort.
Release of Proapoptotic Mediators from Mitochondria Is Impaired in the Developing Brain of Fmr1 Mutants
The postnatal wave of PCD is mediated by the mitochondrial pathway of apoptosis [12]. Cytochrome c, bound to cardiolipin on the inner mitochondrial membrane via both electrostatic and hydrophobic interactions, has peroxidase activity and, in the presence of hydrogen peroxide, oxidizes cardiolipin to hydroperoxicardiolipin [22]. This important upstream event mobilizes cytochrome c from the inner membrane and facilitates its release. Following translocation from cytosol to mitochondria, Bax then permeabilizes the outer mitochondrial membrane, permitting release of both cytochrome c and procaspase-9 from mitochondria to cytosol [30, 31]. Procaspase-9 undergoes autoactivation, and along with APAF-1 and cytochrome c forms the apoptosome which, in turn, activates the effector caspase of the intrinsic pathway, caspase-3 [31].
We isolated forebrain mitochondria from 10-day-old mice, extracted cytochrome c, and measured cytochrome c peroxidase activity with spectrophotometry. To assess for cytochrome c release in the developing brain, we measured the amount of heme c (the heme moiety of cytochrome c) in forebrain mitochondrial and cytosolic fractions and performed immunoblot analyses for the various proapoptotic mediators.
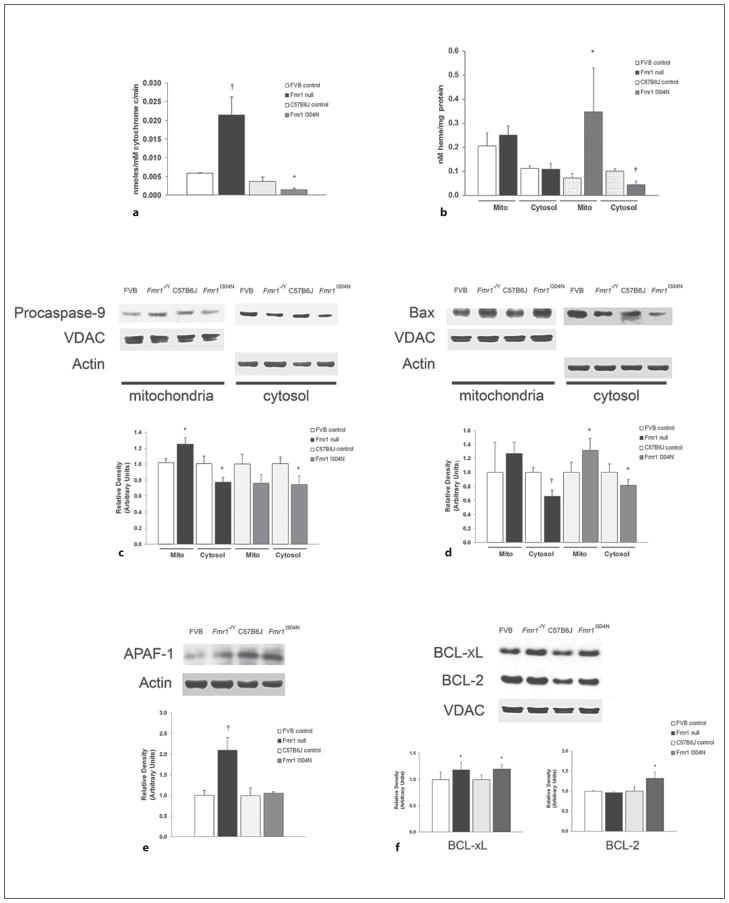
Compared with their respective controls, Fmr1 nulls demonstrated a significant and marked increase in cytochrome c peroxidase activity while F mr1I 304N mutants exhibited significantly decreased peroxidase activity (fig. 4 a). While there was no difference in the relative amount of heme c in mitochondria or cytosol of Fmr1 knockout forebrain, steady-state levels of procaspase-9 were significantly increased in mitochondria and significantly decreased in cytosol versus controls (fig. 4 b, c). The procaspase-9 findings coupled with increased cytochrome c peroxidase activity and a lack of increased cytosolic heme c indicate impaired release of both procaspase-9 and cytochrome c in the forebrain of Fmr1 knockouts.
Fig. 4.
Pro- and antiapoptotic changes in the forebrain of Fmr1 mutant mice. a Cytochrome c peroxidase activity. Steady-state cytochrome c peroxidase activity was measured in cytochrome c extracted from isolated forebrain mitochondria in 10-day-old mice. Values are expressed as means + SD. b Cytochrome c release from mitochondria. Heme c was measured in isolated forebrain mitochondria and cytosol. Heme c content within mitochondria (Mito) and cytosol is demonstrated. Values are expressed as means + SD. c Procaspase-9 release from mitochondria. Representative immunoblots of procaspase-9 in mitochondria (Mito) and cytosol are depicted. VDAC served as a loading control for mitochondrial protein and actin was used to control for cytosolic protein loading. Graphical representations of procaspase-9 relative densities are shown. Controls were arbitrarily set to 1. Values represent means + SD. d Bax translocation to mitochondria. Representative immunoblots of Bax in mitochondria (Mito) and cytosol are depicted. VDAC served as a loading control for mitochondrial protein and actin was used to control for cytosolic protein loading. Graphical representations of Bax relative densities are shown. Controls were arbitrarily set to 1. Values represent means + SD. e APAF-1 expression. Representative immunoblot of APAF-1 is depicted. Actin served as a loading control for cytosolic protein. Graphical representation of APAF-1 relative densities is shown. Controls were arbitrarily set to 1. Values represent means + SD. f BCL-xL and BCL-2 expression. Representative immunoblots of BCL-xL and BCL-2 are depicted. VDAC served as a loading control for mitochondrial protein. Graphical representations of BCL-xL and BCL-2 relative densities are shown. Controls were arbitrarily set to 1. Values represent means + SD. n = 5 animals per cohort; * p < 0.05 vs. control; † p < 0.01 vs. control.
On the other hand, levels of heme c were significantly higher in mitochondria and significantly lower in cytosol in Fmr1 I304N brain compared to forebrain of C57Bl6J controls, also indicating decreased cytochrome c release (fig. 4 b). Steady-state cytosolic levels of procaspase-9 were significantly decreased in Fmr1 I304N mice versus controls and, although not statistically significant, there was a trend toward decreased procaspase-9 in mitochondria of Fmr1I 304N mutants (fig. 4 c). Steady-state levels of cytosolic APAF-1 were significantly increased in Fmr1 null mice compared to FVB controls while there was no difference in APAF-1 between Fmr1I 304N and C57Bl6J controls (fig. 4 e).
Bax Translocation Is Intact in the Developing Forebrain of Fmr1 Mutants
In Fmr1I 304N mutants, steady-state Bax levels were significantly decreased in cytosol compared to C57Bl6J controls and significantly increased in the mitochondrial fraction indicating increased translocation (fig. 4 d). Similarly, steady-state cytosolic Bax levels were significantly decreased in the forebrain of Fmr1 knockouts compared to controls with a trend toward an increase in mitochondria (fig. 4 d). The presence of Bax within the mitochondrial fraction at or above wild-type levels in the context of reduced cytosolic levels suggests that Bax translocation is intact in the forebrain of Fmr1 null mice.
Antiapoptotic Protein Expression Is Increased in the Developing Brain of Both Fmr1 Mutants
The antiapoptotic members of the BCL-2 family of proteins, BCL-2 and BCL-xL, can inhibit permeabilization of the mitochondrial outer membrane by binding to and interacting with the proapoptotic effectors, Bax and Bak [32, 33]. Thus, we isolated forebrain mitochondria and performed immunoblot analysis for both BCL-2 and BCL-xL.
Steady-state levels of forebrain BCL-2 were significantly increased in Fmr1I 304N mutants compared to C57Bl6J controls (fig. 4 f). However, there was no difference in steady-state levels of BCL-2 between Fmr1 knockout mice and FVB controls (fig. 4 f). On the other hand, steady-state levels of BCL-xL were significantly increased in both Fmr1 mutant strains compared to their respective controls (fig. 4 f). These findings suggest increased expression of antiapoptotic proteins in Fmr1 mutant forebrain.
Phosphorylation of Both Akt and Bad Is Increased in the Developing Forebrain of Fmr1 Mutants
Akt/PKB, a serine/threonine kinase, is the most widely known kinase to promote cell survival [34]. Phosphorylation of Akt/PKB activates the kinase and results in phosphorylation and inactivation of the proapoptotic mediator, Bad [34, 35]. Nonphosphorylated Bad inactivates BCL-xL while phosphorylation inactivates Bad and preserves BCL-xL levels [34]. Thus, we isolated forebrain cytosol and performed immunoblot analysis for Akt, phosphorylated Akt, Bad and phosphorylated Bad.
Phosphorylation of Akt at threonine 308 was significantly increased in the forebrain of Fmr1 I304N mutants compared to C57Bl6J controls (fig. 5 a). However, there was no difference in the degree of Akt phosphorylation at threonine 308 between Fmr1 knockout mice and FVB controls (fig. 5 a). On the other hand, both Fmr1 mutant strains demonstrated significantly increased phosphorylation of Akt at serine 473 as well as phosphorylation of Bad at serine 112 compared to controls (fig. 5 a). These findings indicate pro-survival activation of the Akt pathway in Fmr1 mutant forebrain.
Fig. 5.
Phosphorylation of Akt and Bad in the forebrain of Fmr1 mutant mice. a Representative immunoblots of phosphorylated Akt (p-Akt) at threonine 308 and serine 473, total Akt, phosphorylated Bad (p-Bad) at serine 12, and total Bad are depicted. b Graphical representations of p-Akt/Akt and p-Bad/Bad ratios are shown. Control ratios were arbitrarily set to 1. Values represent means + SD. n = 5 animals per cohort; * p < 0.05 vs. control; † p < 0.01 vs. control.
Discussion
Our findings indicate that PCD is impaired in the developing postnatal brain of FMRP-deficient mice. Importantly, defects were identified in neocortex, hippocampus, and basolateral amygdala of both Fmr1 mutant strains and the magnitude of impairment appeared to be similar in each mutant strain compared to healthy control PCD levels. Interestingly, reduced levels of apoptosis were identified in the specific brain regions that are known to demonstrate pathologic synapse development in Fmr1 knockouts, sparing the caudate and putamen.
Fmr1 null mice, produced by a targeted knockout of the Fmr1 gene, and Fmr1 I304N mutants, generated by an I304N missense mutation, have been developed as models of FXS [36, 37]. Like the human condition, Fmr1 knockouts demonstrate increased synapse number and both mutant strains have been shown to display abnormal social behaviors [36, 37]. Fmr1−/y mice do not express any FMRP while F mr1I 304N mice express between 13 and 30% of wild-type FMRP levels [37]. However, the mutant FMRP synthesized by Fmr1 I304N mice lacks association with polyribosomes and is not capable of RNA binding [37]. Thus, both mutant strains share a defect in FMRP-dependent regulation of mRNAs via RNA binding.
In order to conclude that FMRP is important for developmental PCD, similar abnormalities in the mitochondrial apoptosis pathway must be evident in both Fmr1 mutant strains. As such, identical aberrancies in both strains were identified: increased steady-state levels of the antiapoptotic protein, BCL-xL and increased phosphorylation of Akt ser473 and Bad ser112. BCL-xL is known to inhibit permeabilization of the mitochondrial outer membrane by binding to and interacting with Bax and Bak and overexpression of BCL-xL has been shown to prevent cytochrome c release and activation of caspase-3 in the setting of proapoptotic stimuli [32, 33, 38]. Thus, it is possible that FMRP deficiency results in increased expression of BCL-xL in the developing brain, preventing the release of proapoptotic mediators from mitochondria and subsequent caspase activation. Since PCD is a natural process that is necessary for normal brain development and patterning, defects in developmental apoptosis could result in abnormal neuron elimination and retention of developmentally transient neurons. Consistent with this, we found that the number of neurons in the somatosensory neocortex and hippocampus was significantly increased in both Fmr1 mutant strains compared to their respective controls.
Increased expression of BCL-xL in FMRP deficiency is likely related to the dysregulation of the mammalian target of rapamycin (mTOR) pathway [35]. The mTOR pathway is initiated following activation of group 1 metabotropic glutamate receptors [35, 39]. Receptor activation promotes formation of a complex between metabotropic glutamate receptors, the signaling molecule, Homer, and the phosphatidylinositol-3-kinase (PI3K) enhancer, PIKE [35, 39]. PIKE stimulates PI3K which, in turn, phosphorylates and activates Akt/PKB, resulting in stimulation of mTOR signaling [34, 35, 39]. Wild-type FMRP represses PIKE and, as such, negatively regulates the mTOR pathway [35]. In Fmr1 knockout mice, FMRP deficiency has been shown to increase PI3K/Akt activity and mTOR signaling [35]. Our finding of increased Akt phosphorylation in both mutant strains supports this concept. Importantly, activated Akt/PKB also promotes cell survival by phosphorylating Bad, thereby inactivating it [34, 35]. Here we demonstrate that Akt phosphorylation is associated with increased Bad phosphorylation in the forebrain of both Fmr1 mutants. Because Bad inactivation is known to result in preservation of the antiapoptotic mediator, BCL-xL [34], it is quite possible that overactivation of PI3K/Akt signaling in FMRP deficiency provides an explanation for increased BCL-xL expression in the Fmr1 mutant brain.
Fmr1I 304N mutant mice demonstrated a propensity toward antiapoptosis in the developing brain as evidenced by decreased cytochrome c peroxidase activity, impaired cytochrome c release, increased BCL-xL and BCL-2 expression, decreased activated caspase-3, and decreased number of TUNEL-positive nuclei despite increased mitochondrial translocation of Bax. Although activated caspase-3 and the number of TUNEL-positive nuclei were also decreased in Fmr1 knockout brain, there were components of the mitochondrial apoptosis pathway that were activated and divergent from abnormalities seen in Fmr1I 304N mutant mice. For example, cytochrome c peroxidase activity and steady-state APAF-1 expression were both significantly increased. These changes likely result from the proapoptotic stimulus of oxidative stress known to exist in the developing brain of Fmr1 null mice [40]. However, an antiapoptotic state prevails due to increased BCL-xL expression and inhibition of mitochondrial outer membrane permeabilization. Evidence of such inhibition is found in the lack of enhanced cytochrome c and procaspase-9 release from mitochondria despite increased cytochrome c peroxidase activity and intact Bax translocation. Thus, although there were differences seen in various components of the intrinsic apoptosis pathway between mutant strains, the common shared features were increased steady-state BCL-xL levels with decreased PCD and increased neuron numbers as an end result.
Taken together, the data suggest that increased BCL-xL expression due to FMRP deficiency inhibits PCD in the developing brain. Deficient PCD, in turn, likely prevents adequate neuron elimination and may lead to excess number of neurons. It is possible that abnormal retention of developmentally transient neurons contributes to excess and aberrant synaptic connections known to exist in Fmr1 mutants. Thus, defective PCD and impaired neuron elimination during development may contribute to the behavioral phenotype of children with FXS. Although assessing developmentally regulated PCD at a single postnatal time point and restricting our evaluation to the forebrain represent limitations of this descriptive study, our findings lay the ground for assessing how impairments in PCD change over time in the Fmr1 mutant developing brain and exploring potential targets for novel therapeutic interventions designed to restore the process of neuron elimination. Future work will, therefore, focus on targeting the PI3K/Akt survival pathway and defective PCD in an effort to eliminate excess neurons in the FMRP-deficient brain.
References
- 1.Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, et al. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budimirovic DB, Kaufmann WE. What Can We Learn about Autism from Studying Fragile X Syndrome? Dev Neurosci. 2011;33:379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olmos-Serrano JL, Corbin JG, Burns MP. The GABAA Receptor Agonist THIP Ameliorates Specific Behavioral Deficits in the Mouse Model of Fragile X Syndrome. Dev Neurosci. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodkin ES. Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. Behav Neurosci. 2008;122:483–489. doi: 10.1037/0735-7044.122.2.483. [DOI] [PubMed] [Google Scholar]
- 5.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 8.Gatto CL, Broadie K. Fragile X mental retardation protein is required for programmed cell death and clearance of developmentally-transient peptidergic neurons. Dev Biol. 2011;356:291–307. doi: 10.1016/j.ydbio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callan MA, Cabernard C, Heck J, Luois S, Doe CQ, Zarnescu DC. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet. 2010;19:3068–3079. doi: 10.1093/hmg/ddq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan WY, Lorke DE, Tiu SC, Yew DT. Proliferation and apoptosis in the developing human neocortex. Anat Rec. 2002;267:261–276. doi: 10.1002/ar.10100. [DOI] [PubMed] [Google Scholar]
- 11.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HA, Komljenovic D, Tarabykin V, Erzurumlu RS, Tucker KL. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J Neurosci. 2010;30:4221–4231. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornig M, Chian D, Lipkin WI. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol Psychiatry. 2004;9:833–845. doi: 10.1038/sj.mp.4001529. [DOI] [PubMed] [Google Scholar]
- 15.Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 16.Stenqvist A, Agerman K, Marmigere F, Minichiello L, Ernfors P. Genetic evidence for selective neurotrophin 3 signalling through TrkC but not TrkB in vivo. EMBO Rep. 2005;6:973–978. doi: 10.1038/sj.embor.7400512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon MS, et al. Neuroprotective Effect of Visnagin on Kainic Acid-induced Neuronal Cell Death in the Mice Hippocampus. Korean J Physiol Pharmacol. 2010;14:257–263. doi: 10.4196/kjpp.2010.14.5.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 19.Paxinos G, Watson C. Atlas of the developing mouse brain: at E17.5, PO, and P6. San Diego: Academic Press; 2007. [Google Scholar]
- 20.Jacobs EE, Sanadi DR. The reversible removal of cytochrome c from mitochondria. J Biol Che. 1960;235:531–534. [PubMed] [Google Scholar]
- 21.Kim NH, Jeong MS, Choi SY, Kang JH. Peroxidase Activity of Cytochrome c. Bull Korean Chem Soc. 2004;25:1889–1892. [Google Scholar]
- 22.Cheng Y, Thomas A, Mardini F, Bianchi SL, Tang JX, Peng J, Wei H, Eckenhoff MF, Eckenhoff RG, Levy RJ. Neurodevelopmental consequences of sub-clinical carbon monoxide exposure in newborn mice. PLoS One. 2012;7:e32029. doi: 10.1371/journal.pone.0032029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozawa T, Tanaka M, Shimomura Y. Crystallization of the middle part of the mitochondrial electron transfer chain: Cytochrome bc1-cytochrome c complex. Proc Natl Acad Sci USA. 1980;77:5084–5086. doi: 10.1073/pnas.77.9.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka M, Ogawa N, Ihara K, Sugiyama Y. Mukohata, Y: Cytochrome aa(3) in Haloferax volcanii. J Bacteriol. 2002;184:840–845. doi: 10.1128/JB.184.3.840-845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrer I, Bernet E, Soriano E, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer I, Serrano T, Soriano E. Naturally occurring cell death in the subicular complex and hippocampus in the rat during development. Neurosci Res. 1990;8:60–66. doi: 10.1016/0168-0102(90)90058-m. [DOI] [PubMed] [Google Scholar]
- 27.Kim WR, Sun W. Programmed cell death during postnatal development of the rodent nervous system. Dev Growth Differ. 2011;53:225–235. doi: 10.1111/j.1440-169X.2010.01226.x. [DOI] [PubMed] [Google Scholar]
- 28.Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Precht TA, Phelps RA, Linseman DA, Butts BD, Le SS, Laessig TA, Bouchard RJ, Heidenreich KA. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ. 2005;12:255–265. doi: 10.1038/sj.cdd.4401552. [DOI] [PubMed] [Google Scholar]
- 31.Singh BK, Tripathi M, Chaudhari BP, Pandey PK, Kakkar P. Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS One. 2012;7:e34200. doi: 10.1371/journal.pone.0034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renault TT, Teijido O, Antonsson B, Dejean LM, Manon S. Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): Keep your friends close but your enemies closer. Int J Biochem Cell Biol. 2013;45:64–67. doi: 10.1016/j.biocel.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Sen P, Mukherjee S, Ray D, Raha S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol Cell Biochem. 2003;253:241–246. doi: 10.1023/a:1026020101379. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comery TA, Harris JB, Willems PJ, Oostra BA, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zang JB, Nosyreva ED, Spencer CM, et al. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genet. 2009;5:e1000758. doi: 10.1371/journal.pgen.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He L, Perkins GA, Poblenz AT, Harris JB, Hung M, Ellisman MH, Fox DA. Bcl-xL overexpression blocks bax-mediated mitochondrial contact site formation and apoptosis in rod photoreceptors of lead-exposed mice. Proc Natl Acad Sci U S A. 2003;100:1022–1027. doi: 10.1073/pnas.0333594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.el Bekay R, Romero-Zerbo Y, Decara J, Sanchez-Salido L, Del Arco-Herrera I, Rodriguez-de Fonseca F, de Diego-Otero Y. Enhanced markers of oxidative stress, altered antioxidants and NADPH-oxidase activation in brains from Fragile X mental retardation 1-deficient mice, a pathological model for Fragile X syndrome. Eur J Neurosci. 2007;26:3169–3180. doi: 10.1111/j.1460-9568.2007.05939.x. [DOI] [PubMed] [Google Scholar]