Abstract
Background
To evaluate the prevalence rates of non-amnestic neurological symptoms of autosomal dominant Alzheimer’s disease (ADAD) in the DIAN Observational Study (DIAN–OBS) and the published literature. Analyses were conducted to clarify the prevalence of neurological manifestations of ADAD mutation carriers as a group.
Methods
Using the DIAN-OBS study database and 189 peer-reviewed publications on ADAD families, we extracted individual-level data on age of symptom onset, disease course from onset to death, and the presence of fourteen neurological findings that have been reported in association with ADAD and included symptomatic subjects only. The primary outcomes were the rates of various neurological symptoms and the contribution of age and specific mutations on the prevalence of the neurological symptoms. Analyses were done using descriptive statistics, comparisons of means and frequencies and multivariable linear regression.
Findings
Our meta-analysis dataset includes 1228 affected individuals, with detailed clinical descriptions of 753. The DIAN–OBS dataset included 107 individuals with detailed clinical data. The most prevalent non-amnestic cognitive manifestations in DIAN were those typical of mild-moderate Alzheimer’s disease, including visual agnosia (95% CI 45·7%–64·6%), aphasia (43·8%–62·7%), and behavioral changes (51·5%–70·0%). The prevalence of non-amnestic cognitive manifestations from the published literature were (95% CI 3·9%–7·2%) for visual agnosia, (20%–26%) for aphasia, and (28·4%–35·1%) for behavioral changes. Prevalence of non-cognitive neurological manifestations in DIAN was low, including myoclonus and spasticity (3·8%–15·0%), seizures (0·5%–9·1%) and moderate for parkinsonism (5·3%–17·1%). Whereas, in the published literature the prevalence was (95% CI 16·6%–22·2% and 12·5%–17·6%) for myoclonus and spasticity, (10·1%–15·0%) for parkinsonism, and (17·4%–23·2%) for seizures. Age of onset appears to influence the prevalence of several non-cognitive manifestations in both groups, stroke being more prevalent at older ages of onset with motor symptoms being more prevalent at younger age of onset and at an older age of onset. Further, symptoms were overall more common in later clinical stages of disease.
Interpretation
Comparing the prevalence of non-amnestic and non-cognitive clinical features in DIAN with the published literature indicates that previous reports of non-cognitive features are likely overestimated whereas DIAN identifies higher non-amnestic cognitive symptoms in addition to memory impairment. The non-cognitive clinical manifestations of AD appear to be in a minor fraction of mild-moderate ADAD and is likely influenced by disease severity, environmental and genetic factors in addition to genetic status. The results of this work clarify the clinical presentations of ADAD including the effects of age and disease stage. Attention to these neurologic symptoms and screening for ADAD mutations are warranted if present. Future work is needed to determine the factors which cause these neurologic symptoms.
Introduction
Autosomal dominant Alzheimer’s disease (ADAD) is a rare, completely penetrant form of Alzheimer’s disease that typically presents at a much earlier age than sporadic forms of Alzheimer’s disease. Despite its rarity, ADAD has been used as a model for understanding pathological processes and developing potential therapies for sporadic Alzheimer’s disease due to similarities in both clinical course and pathophysiology (for a comprehensive review, see Bateman et al, 20111). Although the majority of carriers of symptomatic mutations in the amyloid precursor protein (APP), presenilin-1 (PSEN1), or presenilin-2 (PSEN2) present with early amnestic symptoms2 similar to those with sporadic Alzheimer’s disease, a significant portion of individuals with ADAD have been reported to exhibit additional behavioral and neurologic deficits, such as seizures, myoclonus, spastic paraparesis, or visual disturbances, with remarkable diversity in age of onset, clinical presentation, and rate of progression1,3-5. The location of mutations within genes has also been shown to affect pathophysiology and age of onset, as is the case for presenilin-1 mutations before and after codon 2006. As a consequence of the rarity of ADAD and the reported variability in presentation, it has been difficult to estimate the prevalence of neurological manifestations of ADAD mutation carriers as a group.
To this end, we aimed to better clarify the incidence and prevalence rates of non-amnestic manifestations of ADAD from a prospective global observational ADAD study– the Dominantly Inherited Alzheimer’s Network Observational Study (DIAN–OBS) – and also individual level data of symptomatic cases extracted from 189 published reports. Additionally, we aimed to assess relationships of these clinical manifestations with the age of symptom onset and the location of ADAD mutations within affected genes as this could provide important information on the pathophysiology of ADAD mutations. The DIAN–OBS findings complement the existing published literature by contributing uniform and extensive assessments in a prospective cohort with mild to moderate AD to the literature reports of pedigrees clinically followed to more advanced stages of dementia. The results of this work may help to clarify the clinical presentations of ADAD and hold implications for the structure and function of the presenilin proteins and APP.
Methods
The DIAN study is reviewed and approved by all participating sites Institutional/Ethical Review Boards (IRB). All participants (and as appropriate their legally authorized representatives) sign IRB-approved DIAN consent forms that include a statement informing participants that deidentified data will be shared with authorized investigators for future research following guidelines for preserving confidentiality through coded identifiers.
Literature database
In an expansion of our previously reported ADAD meta-analysis dataset7, clinical data on 1335 carriers of 183 known pathogenic mutations in APP, PSEN1, and PSEN2 was collected from publications cited in the Alzheimer’s Disease/Frontotemporal Dementia Mutation Database, the Alzheimer Research Forum database, and PubMed search results using the terms “dominant Alzheimer”, “dominant AD”, “ADAD”, “presenilin”, “PSEN1”, “PSEN2”, and “APP”. Genotype information, pedigree information, ages of onset and death, clinical descriptions of the disease course and symptomatology, and pathological findings for each affected individual were recorded, when available. Demographic characteristics of this population are provided in table 1.
Table 1. Study population.
| Literature | DIAN | |||
|---|---|---|---|---|
| N | Total | 1228 | 107 | |
| Clinical descriptions |
753 | 107 | ||
| Sex | M | 34·7% | 43·9% | |
| F | 38·0% | 56·1% | ||
| Unknown | 27·3% | - | ||
| Gene | PSEN1 | 74·2% | 80·4% | |
| PSEN2 | 5·0% | 1·9% | ||
| APP | 20·8% | 17·7% | ||
|
Age of symptom
onset |
p = 0·0004 | |||
| Mean | 46·0 | 42·9 | ||
| SD | 10·5 | 8·17 | ||
| Follow up (years) | Mean | 8·33 | 3·93 | p < 0·0001 |
| SD | 4·59 | 3·18 | ||
| CDR | Mean | - | 1·05 | |
| SD | - | 0·79 | ||
| CDR-SB | Mean | - | 5·39 | |
| SD | - | 5·06 | ||
| MMSE | Mean | - | 20.98 | |
| SD | - | 10.92 |
DIAN database
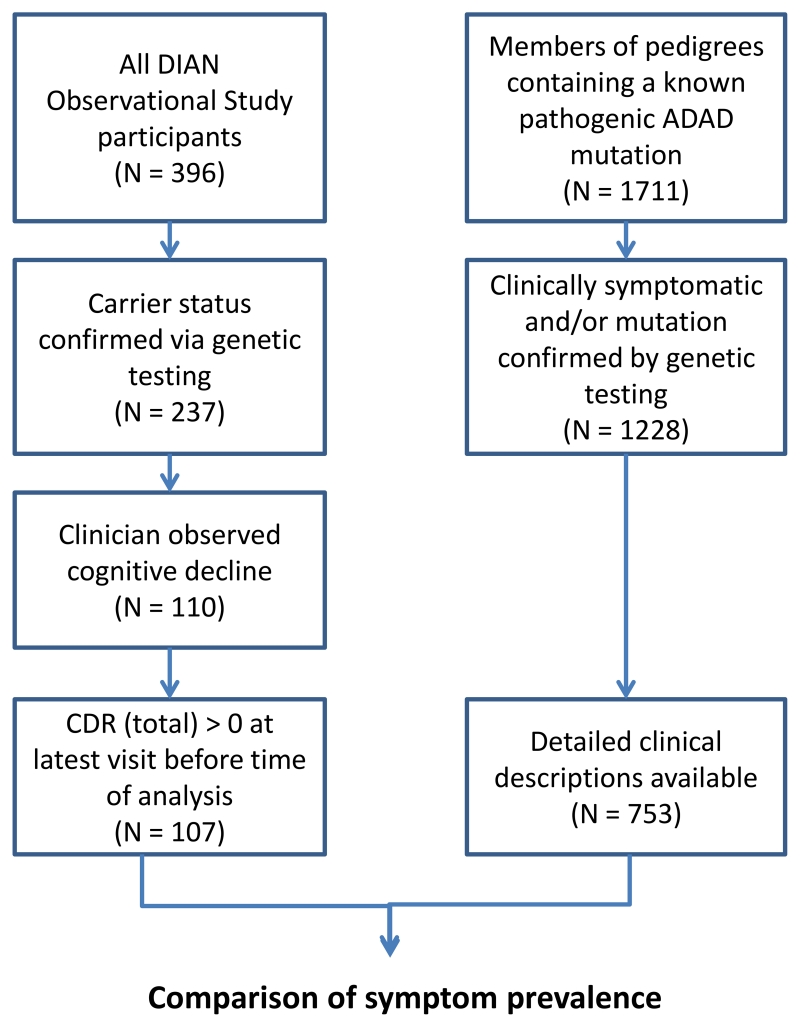
Analyses were performed on DIAN datafreeze 8. Participants in the DIAN observational study include families of carriers of mutations causing ADAD in APP, PSEN1 or PSEN28. Per standard DIAN protocols, each study participant and a collateral source underwent semi-structured interviews that included detailed demographics, medical history, and family history. All study staff underwent audiotape recordings of the clinical assessments at the beginning of the study and then every 10th participant to ensure compliance with the protocol and increase inter-rater reliability. In addition, each participant completed a physical and neurological examination conducted by a clinical evaluator who was blinded to the participant’s mutation status. A total of 107 individuals were considered to be symptomatic at time of analysis, based upon having both a Clinical Dementia Rating sum of boxes (CDRsb) score9 greater than 0 and a known pathogenic ADAD mutation as confirmed by genetic testing using methods previously described10,11. Using data from these individuals, we constructed a database including age, gender, mutated gene, mutation type (including specific amino acid change of the mutation, eg, PSEN1 E280A), APOE genotype, family history, medical history, list of medications, age of onset evaluation, physical exam, neurological exam, CDR (including supplemental boxes for behavior and language), Functional Activities Questionnaire (FAQ), Mini-Mental Status Exam (MMSE), Geriatric Depression Scale (GDS), Unified Parkinson’s disease rating scale (UPDRS), vascular contributions to dementia/or history of stroke (Hachinski Ischemic Score, (HIS), clinical judgment of symptoms, clinician diagnosis, and psychometric battery summary.
Individuals were assessed for the presence of non-amnestic cognitive or non-cognitive symptoms using neurological exams conducted during their initial visit and each visit thereafter and sections from the National Alzheimer’s Coordinating Center’s Uniform Data Set (UDS)12, paying specific attention to the health history (UDS A5, B2), UPDRS (UDS B3), and clinician judgment of symptoms (UDS B9). UPDRS scores were calculated based on review of performance in each of 27 motor domains (eg, body bradykinesia, facial expressiveness, gait, etc), with a maximum possible score of 108. If an individual exhibited a specific symptom during any visit, that symptom was marked as “present”. Demographic characteristics of this population are provided in table 1. A list of descriptions of the exact process used to extract this data is provided in supplemental table 1.
Subject selection
Only symptomatic individuals were studied. We included a total of 753 individuals from literature reports and 107 from the DIAN Observational Study in our analysis (table 1). In the literature group, individuals were designated as symptomatic by the authors of the publication in which they are found, and their age of symptom onset was recorded when available. Length of follow up time in this group is defined as the time from age of onset until the individual either died or was lost to follow up. Age of onset was determined by clinician judgment as the age at which the individual began to exhibit cognitive decline, and years of follow up is calculated by subtracting the individual’s age of onset from their age at the latest visit. Those APP mutations with predominant cerebral amyloid angiopathy (CAA), ie, the Dutch mutation, were not included in this analysis as they may be associated with less uniform pathology.
Statistical analysis
For the comparison between autosomal dominant and DIAN, we calculated the prevalence of a group of cognitive and non-cognitive symptoms in the literature database and the DIAN cohort, respectively. To compare symptom prevalence between mutations found in APP, PSEN1, and PSEN2, we constructed a generalized linear mixed model treating the mutated gene as a fixed effect, and including a unique identifier for family pedigree as a random effect, in order to take into account the impact of familial genetics. Age of onset was also included as a fixed effect. We did not specifically analyze the effect of APOE ε4 carrier status on disease course due to limitations in sample size. Additionally, we directly compared the symptom prevalence in carriers of PSEN1 mutations before and after codon 2006. We also explored the relationship between clinical severity as measured by CDR-SB and the frequency of clinical features in the DIAN–OBS group but were unable to perform a similar exploration in the literature group due to clinical ratings at time of non-amnestic symptoms not being reported in most.
Role of the funding source
Data collection and sharing for this project was supported by The Dominantly Inherited Alzheimer’s Network (DIAN, UF1 AG032438) funded by the National Institute on Aging (NIA), the German Center for Neurodegenerative Diseases (DZNE), The MRC Dementias Platform UK (MR/L023784/1 and MR/009076/1) and NIHR Queen Square Dementia Biomedical Research Unit. This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. The corresponding author had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
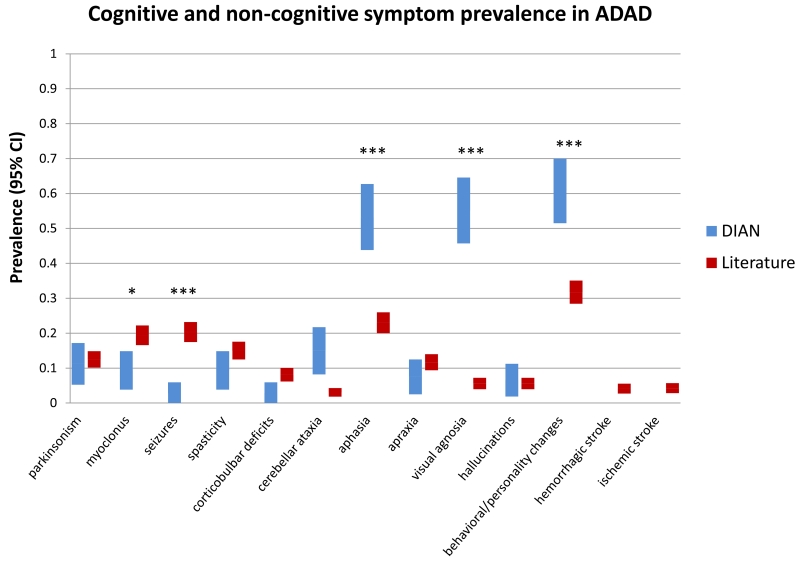
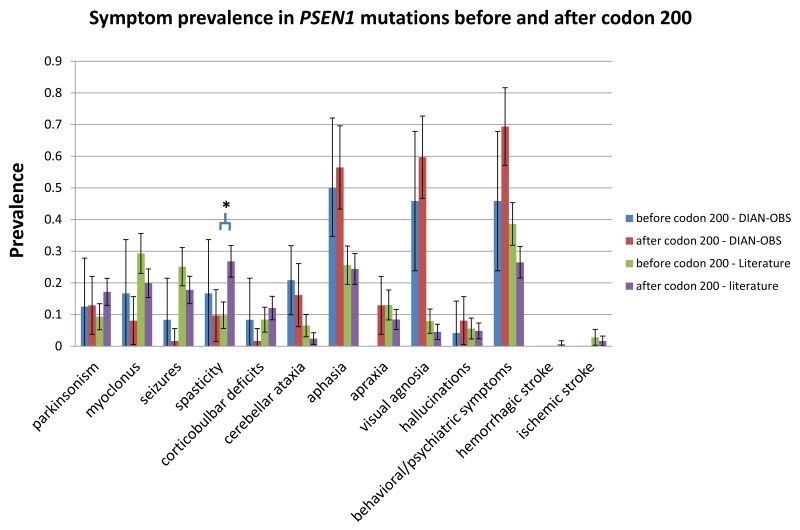
Compared to the literature group, the DIAN Observation Study cohort has a significantly earlier average age of onset and shorter average follow up time (Table 1). Overall, 36 of the 107 individuals in the DIAN–OBS displayed one or more abnormality on the neurological exam at any point during the time they were followed (figure 1). Significantly higher rates of cognitive symptoms were noted in the DIAN–OBS group than the literature group, including aphasia (57/107 (53%) vs. 173/753 (23%), p < 0·0001), visual agnosia (59/107 (55%) vs. 42/753 (5·6%), p < 0·0001), and behavioral/personality changes (65/107 (61%) vs. 239/753 (32%), p < 0·0001) (table 2). In contrast, motor symptoms such as myoclonus (10/107 (9·3%) vs. 146/753 (19%), p = 0·0117) and recent/active seizures (3/107 (2·8%) vs. 153/753 (20·3%), p < 0·0001) were less common in the DIAN–OBS group compared to the literature group; corticobulbar deficits were marginally less common in DIAN–OBS (3/107 (2·8%) vs. 61/753 (8·1%), p= 0·051). The rate of cerebellar ataxia was higher in the DIAN–OBS group than the literature group (16/107 (15%) vs. 23/753 (3·1%), p < 0·0001). The rates of parkinsonism were similar between DIAN–OBS and the literature group, (12/107 (11%) vs. 94/753 (12%), p = 0·71). Of the twelve individuals in DIAN who displayed parkinsonian symptoms, eleven were mildly symptomatic (UPDRS total score < 36), and one was moderately symptomatic with a score of 58. In DIAN–OBS compared to the literature group the rate of spasticity was not significantly different (10/107, (9·3%) vs. 113/753 (15%), p= 0·12). The rate of behavioral and personality changes was greater in the DIAN–OBS group compared to the literature group (65/107 (61%) vs. 239/753 (32%), p < 0·0001), but hallucinations were similar and low (7/107 (7%) vs. 42/753 (6%), p= 0·69) in both groups. No individuals in the DIAN–OBS cohort have reported recent or active hemorrhagic stroke or ischemic stroke whereas the rate from the reported literature was low (55/753, 7·3%).
Figure 1. Combined symptom prevalence in reported and prospectively observed ADAD.
Included are all individuals with detailed clinical descriptions from the DIAN prospective observational study and the ADAD literature (N = 107 and 753, respectively). Error bars are 95% confidence intervals.
Table 2. Comparisons of symptom prevalence between DIAN and Literature.
| Frequency (DIAN) |
95% CI | Frequency (Literature) |
95% CI | p-value | |
|---|---|---|---|---|---|
| Parkinsonism | 0·11 | [0·053, 0·17] | 0·12 | [0·10, 0·15] | 0·71 |
| Myoclonus | 0·094 | [0·038, 0·15] | 0·19 | [0·17, 0·22] | 0·012 |
| Seizures | 0·028 | [0, 0·059] | 0·20 | [0·17, 0·23] | <0·0001 |
| Spasticity | 0·094 | [0·038, 0·15] | 0·15 | [0·12, 0·18] | 0·12 |
|
Corticobulbar
deficits |
0·028 | [0, 0·059] | 0·081 | [0·06, 0·10] | 0·051 |
| Cerebellar ataxia | 0·149 | [0·082, 0·22] | 0·031 | [0·018, 0·043] |
<0·0001 |
| Aphasia | 0·53 | [0·44, 0·63] | 0·23 | [0·20, 0·26] | <0·0001 |
| Apraxia | 0·075 | [0·025, 0·12] | 0·12 | [0·094, 0·14] | 0·19 |
| Visual agnosia | 0·55 | [0·46, 0·65] | 0·056 | [0·039, 0·072] |
<0·0001 |
| Hallucinations | 0·065 | [0·019, 0·11] | 0·056 | [0·039, 0·072] |
0·69 |
|
Behavior/Personality
changes |
0·61 | [0·51, 0·70] | 0·32 | [0·28, 0·35] | <0·0001 |
|
Hemorrhagic
stroke |
0 | - | 0·041 | [0·027, 0·055] |
- |
| Ischemic stroke | 0 | - | 0·042 | [0·028, 0·057] |
- |
| N | 107 | 753 |
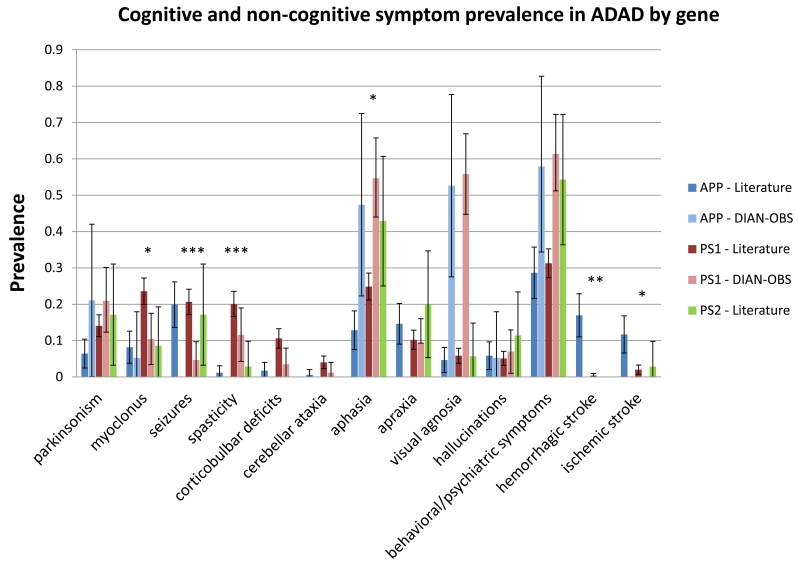
We also examined the prevalence of behavioral and neurological symptoms in the reported literature, by mutated gene. In order to account for other genetic factors specific to the family and physiological changes as an individual ages, pedigree ID and age of onset were included as covariates (figure 2). The number of PSEN2 mutation carriers was too small to make meaningful comparisons when these covariates are taken into consideration. Compared to APP mutation carriers, PSEN1 mutation carriers as reported by published literature are significantly more likely to exhibit myoclonus (OR = 4·25, 95% CI [1·37, 13·2], p = 0·0125), corticobulbar deficits (OR = 9·78, 95% CI [1·32, 72·4], p = 0·0257), and aphasia (OR = 3·76, 95% CI [1·33, 10·7], p = 0·0129); spasticity was also more common in PSEN1 mutation carriers (n=110 of 547) compared to APP mutation carriers (n=2 of 171). On the other hand, APP mutation carriers were significantly more likely to present with ischemic stroke (OR = 3·92, 95% CI [1·33, 11·6], p = 0·0135); a hemorrhagic stroke was also more common APP mutation carriers (n=29 of 171) compared to PSEN1 mutation carriers (n=2 of 547). There were no significant differences in the prevalence of parkinsonism, apraxia, visual agnosia, behavioral/personality changes, or hallucinations between the three groups in the literature. In contrast, there were no significant differences in the DIAN cohort in myoclonus, aphasia, or stroke.
Figure 2. Comparison of reported symptom prevalence in APP, PSEN1, and PSEN2 mutation carriers.
(Literature - N = 171 for APP, 547 for PSEN1, 35 for PSEN2; DIAN-OBS - N = 19 (APP), 86 (PSEN1), 2 (PSEN2)). Rates for PSEN2 carriers in DIAN-OBS were not calculated as there were only two symptomatic individuals in that group. Although significant variability in symptom prevalence is observed between mutations in the three genes in the reported literature, there were few differences between APP and PSEN1 in the DIAN-OBS cohort. Error bars shown are 95% confidence intervals.
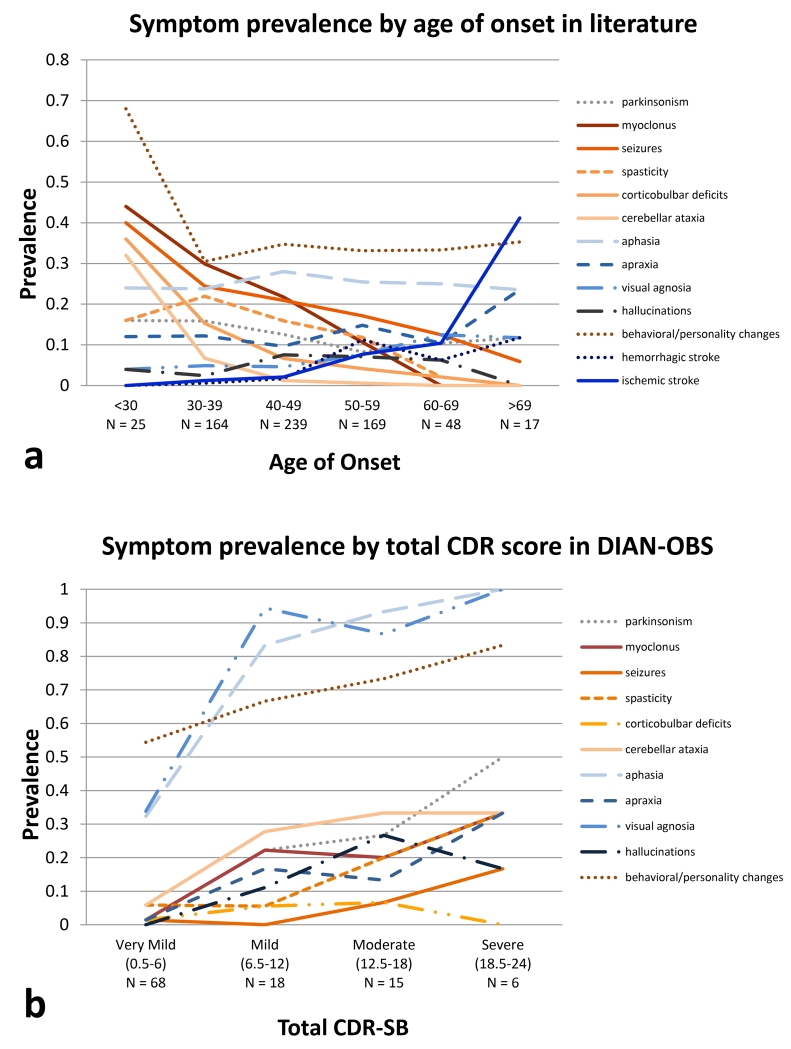
Clinical stage of disease was also associated with an increased frequency of all clinical features, with the exception of corticobulbar deficits, with increasing disease severity as measured by CDR–SB) in the DIAN–OBS (figure 3b).
Figure 3.
a. Comparison of reported prevalence of cognitive and non-cognitive neurological symptoms in ADAD by age of disease onset. Individuals were considered symptomatic if they developed the symptom at any point in their disease course. Solid lines represent symptoms for which a one-year increase in age of onset is associated with a statistically significant change in risk.
b. Symptom prevalence by CDR Sum of Box score, in DIAN-OBS. All cognitive symptoms and most non-cognitive symptoms (except corticobulbar deficits) increase in prevalence as the clinical stage worsens. Total CDR-SB = CDR sum of boxes + supplemental sum of boxes, possible scores 0-24. As all individuals included in the DIAN-OBS analysis are symptomatic, the lowest total CDR-SB in this group is 0.5.
Age at symptom onset was significantly associated with an individual’s likelihood of presenting with several symptoms in the literature cohort. Older age at onset is associated with elevated rates of ischemic stroke (p = 0·0003, OR for developing symptom = 1·09 per 1-year increase in age of onset, 95% CI [1·04, 1·14]) and decreased rates of myoclonus (p = 0·0007, OR = 0·93, 95% CI [0·90, 0·97]), seizures (p = 0·0018, OR = 0·95, 95% CI [0·92, 0 ·98]), corticobulbar deficits (p = 0·0012, OR = 0·91, 95% CI [0·86, 0·96]), and cerebellar ataxia (p = 0·0002, OR = 0·82, 95% CI [0·74, 0·91]) (figure 3).
For the DIAN–OBS cohort, prevalence rates were only calculated for PSEN1 and APP, as there were too few symptomatic individuals with PSEN2 mutations. After excluding Dutch mutation carriers, several symptoms were notably absent from APP mutation carriers in the DIAN–OBS population: new-onset seizures, stroke, and corticobulbar deficits.
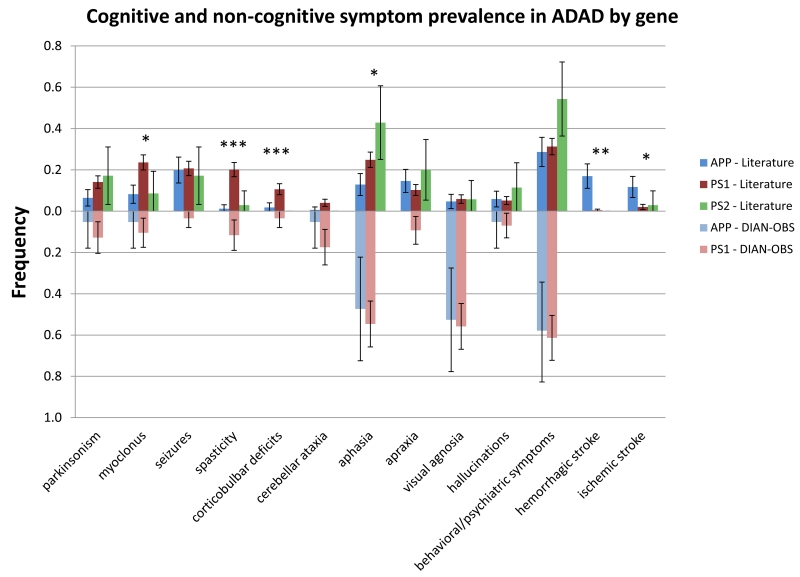
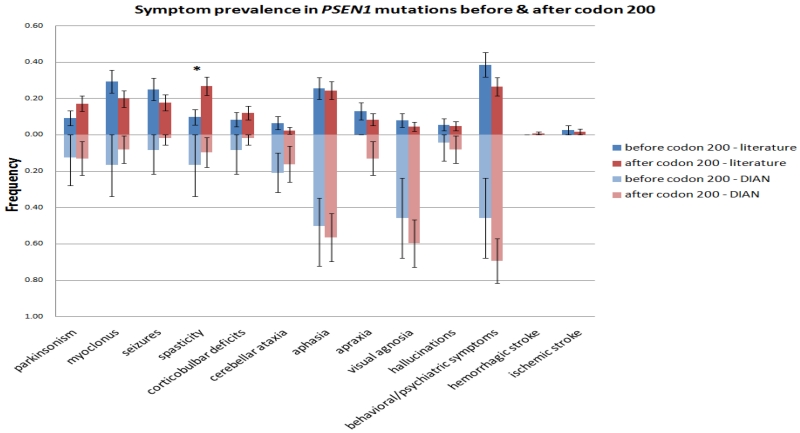
Finally, we compared PSEN1 mutation carriers before and after codon 200 in the DIAN–OBS and literature groups, and compared the rates at which they demonstrated behavioral and neurological deficits (figures 4 and 5). In the literature group, PSEN1 mutations after codon 200 were more likely to be associated with spasticity (21/215 (9·8%) vs. 89/332 (26·8%), p < 0·0001). However, in the DIAN–OBS cohort, there was no significant difference in the prevalence of any symptom for mutations before or after codon 200. Interestingly, mirroring recent findings by Ryan et al,5 the pre-codon 200 population in the DIAN–OBS cohort has a significantly earlier age of onset than the post-codon 200 population (37·3(6.9) vs. 45·0(8·1), p < 0·0001), a difference that was not seen in the literature population (42·8(10·4) vs. 43·7(8·3), p = 0·319).
Figure 4. Comparison of symptom prevalence for PSEN1 mutations before and after codon 200 in literature and DIAN-OBS cohort.
(Literature - N = 215, 332; DIAN-OBS - N = 24, 62).
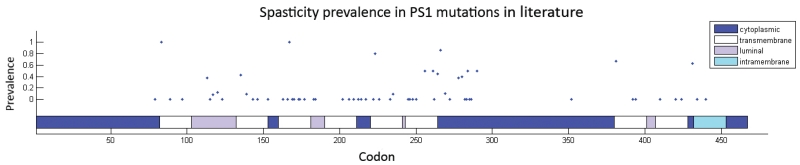
Figure 5. Distribution of known pathogenic PSEN1 mutations in literature and the rates at which carriers demonstrated spasticity in their disease course.
Discussion
In the DIAN–OBS, we found that the most frequently reported non-amnestic manifestations were cognitive, including visual agnosia, aphasia, and behavioral changes. However, in our meta-analysis of the literature, we found moderate rates of motor symptoms and seizures and lower rates in the DIAN–OBS. Interestingly, younger age of onset and more advanced stages of disease were related to a higher frequency of non-cognitive clinical features. A larger prospective cohort study now reports that a significant minority, 16% of the individuals with ADAD had non-amnestic cognitive phenotypes and about 25% had atypical neurologic symptoms in addition to an amnestic phenotype [Ryan et. Al 2016 Lancet Neurology], suggesting that in cases with unusual neurologic manifestations, genetic counseling and testing may be warranted.
One potential interpretation of these findings is that compared to clinical data collected prospectively in DIAN–OBS, case reports may overestimate the prevalence of non-cognitive neurologic manifestations (eg, myoclonus and seizures), while underestimating cognitive neurologic manifestations (eg, visual agnosia, aphasia, and behavioral/personality changes). Two sources of bias that could contribute include measurement bias and ascertainment bias. The DIAN–OBS prospective cohort study complements the literature reports to help account for these biases. Likewise, the literature reports provide a broader understanding with longer duration follow-up and more advanced disease.
With regards to measurement bias, our study demonstrates the impact of having systematic protocols in observational cohort studies (supplemental table 1). By employing uniform study procedures, symptoms are consistently identified, such as non-amnestic cognitive symptoms. The DIAN–OBS prospective and uniform assessments of earliest symptom onset may account for the earlier age of onset reported in the DIAN–OBS cohort. However, the limited follow up period in DIAN–OBS compared to literature likely resulted in a lower prevalence of certain symptoms such as seizures and myoclonus that were found to be higher in the published literature cohort, due to higher symptom prevalence at later stages of the disease (figure 3b). With further follow-up, the DIAN–OBS will be positioned to accurately prospectively measure symptoms with more advanced disease.
Non-amnestic cognitive phenotypes are more commonly reported in SAD and include language variants, executive-frontal variants and a visuoperceptual variant- posterior cortical atrophy (PCA)13. In general, these focal variants have been reported less, in ADAD14,15. Importantly, in SAD these variants appear to occur more frequently at younger ages of onset. A recent study found an odds ratio of greater than 5–12 for non-amnestic cognitive impairment in those with AD in the 6th decade versus those in the 9th decade.16 Similar to the common SAD presentation in DIAN-OBS the majority of subjects had amnestic impairments as the first presenting symptom2.
The current literature indicates that when non-amnestic variants are present, the symptoms are related to NFT pathology and not Aβ plaques17. Thus, in both SAD and DIAD, clinical cognitive symptoms appear to be more related to tau pathology18.
We sought to determine the age, disease stage, mutation, and other genetic effects on the manifestation of symptoms. Interestingly, age of onset appears to significantly impact the risk of neurologic manifestations. For example, in the literature cases, individuals who begin to decline at a younger age are more likely to develop myoclonus and seizures than their older age at onset counterparts. In contrast, stroke and hemorrhage were associated with older ages of onset.
However, the DIAN–OBS cohort showed lower overall incidences of myoclonus and seizures than the literature group, possibly due to milder stages of disease (figure 3b). In the DIAN-OBS study, we found a trend of increasing prevalence of all symptoms including cognitive symptoms such as apraxia, visual agnosia, and non-cognitive symptoms including such as seizures, myoclonus, spasticity, cerebellar ataxia, and parkinsonism, at later stages of disease. Several previous studies suggest that for individuals with ADAD, seizures are correlated with earlier age of onset and more severe disease19-23. Our work focusing on the published literature supports the importance of the age of onset as it relates to myoclonus and seizures, and now adds the association of disease duration and symptom frequency from the DIAN–OBS. In the sporadic Alzheimer population, there is also evidence to support that an earlier age of onset is associated with an increased risk of seizures24,25.
In order to account for other genetic or environmental factors that may influence disease presentation within a pedigree, we included family membership as a covariate in our analysis of symptom prevalence in PSEN1, PSEN2, and APP mutation carriers as reported in the literature. We demonstrated some differences between APP, PSEN1, and PSEN2 mutations in the prevalence of certain symptoms (eg, in myoclonus and spasticity for PSEN1). Further, we found a propensity for APP mutation carriers to present with stroke or hemorrhage. It has been previously reported that PSEN1 mutations before codon 200 are pathologically different from those after codon 200, likely due to differences in the severity of amyloid angiopathy and rates of amyloid deposition26. However, aside from spasticity, there are no apparent differences in symptom prevalence between PSEN1 pre-codon 200 and post-codon 200 mutations (figure 4). Significant heterogeneity exists within the pre- and post-codon 200 PSEN1 mutation groups. Additionally, within PSEN1, there is a notable paucity of pathogenic mutations between codon 290-350 (figure 5), which gives rise to three possibilities – that mutations in this region are asymptomatic, that they are lethal, or that these regions have intrinsically lower rates of mutation.
Although APOE ε4 is a major risk factor for SAD27, the evidence for APOE’s effect on ADAD presentation is less clear7,28-30. Our current analysis of symptomatic mutation carriers is too small for constructing a model that includes APOE status as a co–variate in addition to age of onset, pedigree membership, and mutated ADAD gene.
The strength of the DIAN Observational Study is that it is a prospective cohort study of many mutations and families implemented with uniform standard assessments. However, limitations of the DIAN–OBS include the relatively small number of symptomatic participants, with 107 individuals in various stages of dementia as determined by our inclusion criteria. Consequently, we could not construct a model that simultaneously takes into account factors that may influence disease course such as mutated gene, duration of follow up, and APOE genotype. Further, the DIAN–OBS dataset includes few severe stages of disease with the average stage at moderate dementia (mean MMSE 21.0 (10.9)).
Accurately determining the prevalence of specific clinical and neurological signs and symptoms is important for defining a clinical disease, understanding its prognosis and impact on patients, and for informing the conduct of clinical research. A more complete understanding of cognitive and other neurological manifestations of ADAD will allow for improvements in diagnosis, prognosis, and management, as well as the design of research studies in this unique and important population. Future studies will be able to compare the clinical presentation of ADAD patients with sporadic Alzheimer’s disease in greater detail, leading the field toward a deeper understanding of their shared clinical manifestations which will be critical to accurately interpret the findings of ongoing treatment trials in each disorder.
Supplementary Material
Research in context.
Evidence before this study
We reviewed publications up through January 27, 2015 cited in the AD/FTD Mutation Database and the Alzheimer Research Forum database, and searched PubMed identifying 189 peer-reviewed journal articles which reported individual-level data on age of symptom onset, disease course from onset to death, and the presence of fourteen neurological findings previously reported to be associated with ADAD. There is a large body of literature providing phenotypic information on specific autosomal dominant Alzheimer disease (ADAD) mutations. Over 170 of these reports are on a small number of subjects or families across a wide spectrum of clinical severity. These reports suggested a relatively high prevalence of non-cognitive neurologic manifestations including behavioral, motor symptoms, and seizures which may be further influenced by specific gene mutation. However, there are less than 7 reports of large cohorts from single centers and no compiled individual level data review.
Added value of this study
Our literature based dataset includes 1228 affected individuals from literature reports, with detailed clinical descriptions of disease course available for 753 of this group with an average of 8 years of follow-up. The DIAN-OBS dataset included 107 symptomatic individuals with detailed clinical data from an ongoing, observational study with an average of over 3 years of follow-up. From these two datasets we were able to report descriptive statistics, comparisons of prevalence between the DIAN study and the published data base, as well as determine correlations between clinical features and gene mutation type and position in both data sets. This study provides one of the largest and most diverse collections of prospectively followed, symptomatic, ADAD populations to provide more accurate estimates of non-amnestic clinical features.
With the large number of PSEN1 mutations we were also able to explore whether atypical clinical features were more commonly associated with specific codon position, as has been suggested previously. However, in the DIAN population we found no clear associations of clinical features with PSEN1 codon position.
Implications of available evidence
This study indicates that the prevalence of atypical clinical features in ADAD is low and may have been overestimated in the published literature. Non-cognitive neurologic symptoms of AD appear to affect the minority of ADAD mutation carriers, suggesting that the mutations are not the major factor for presentation of non-cognitive neurologic manifestations of AD. The factors that influence the presence of neurological symptoms include unidentified genetic and environmental factors with some impact from the age of onset, stage of disease and type of mutation. Further, non-amnestic cognitive impairment is common in ADAD, similar to sporadic AD. As ADAD has provided a wealth of understanding of AD pathophysiologic processes, future work comparing ADAD with sporadic AD will lead to a better understanding of both sporadic and dominantly inherited AD.
In our analysis, we recorded each individual as exhibiting a symptom if the manuscript in which they are found mentioned the symptom in connection with dementia in a detailed clinical description of the course of disease. For DIAN, some of these symptoms are covered by single variables in the UDS (supplemental table 1), such as parkinsonism, seizures, visual agnosia, aphasia, and stroke, while others were derived from a combination of several variables, such as hallucinations (combined visual and auditory), cerebellar ataxia (abnormal finger-nose-finger and/or heel-shin exam, or clinical report), and behavioral changes (disinhibition, agitation, irritation, and other personality changes). A third group (myoclonus, spasticity, corticobulbar deficits, and apraxia) did not have specific variables associated but were tested and searched for in the neurological exam conducted during each visit and the UPDRS (UDS B3). Differences in how variables are obtained present a challenge to interpretation, as symptoms specifically enumerated in the UDS may be more consistently detected and documented by clinicians. This appears to be the case for visual agnosia and behavioral/psychiatric changes, which are common in both sporadic and dominantly inherited Alzheimer’s disease (figure 1). The language used in the UDS may also influence the rate of detection. For instance, aphasia in the DIAN group is determined using UDS B9 4c, which is a clinician judgment of symptoms including “hesitant speech, trouble finding words, and using inappropriate words without self correction”, all of which may reflect word-finding difficulty which can be a consequence of memory impairment. Alternatively, the more stringent category of primary progressive aphasia is included as a possible clinician diagnosis (UDS D10), but so far there has not been a case documented as such in the DIAN cohort as of December 2014. Consequently, the higher prevalence of aphasia in the DIAN population may be an artifact of how the UDS question is constructed. Likewise, in the published literature it is likely that the predominant symptom at the time of clinical presentation is most likely to be reported. This would result in a tendency to under report symptoms such as language disorders, which are very common with disease progression in all forms of Alzheimer’s disease.
Acknowledgments
This manuscript has been reviewed by the DIAN study investigators for scientific content and consistency of data interpretation with previous DIAN study publications. We gratefully acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study (http://www.dian-info.org/institutions_map.htm). We also thank the many dedicated investigators involved in the prior studies included in this meta-analysis. We particularly thank M. Scot Fague for his assistance with the DIAN dataset. The DIAN Expanded Registry (http://dianxr.org) welcomes contact from any ADAD families or treating clinicians interested in research.
Funding: National Institutes of Health (UF1AG032438), German Center for Neurodegenerative Diseases (DZNE), MRC Dementias Platform UK (MR/L023784/1 and MR/009076/1) and NIHR Queen Square Dementia Biomedical Research Unit.
Abbreviations
- ADAD
autosomal dominant Alzheimer’s disease
- APOE
Apolipoprotein E
- APP
amyloid precursor protein
- CAA
Cerebral amyloid angiopathy
- CDR
Clinical Dementia Rating
- DIAN
Dominantly Inherited Alzheimer’s Network
- DIAN-OBS
DIAN Observational Study
- FAQ
Functional Activities Questionnaire
- GDS
Geriatric Depression Scale
- HIS
Hachinski Ischemic Score
- MMSE
Mini-Mental Status Exam
- PSEN1
presenilin-1
- PSEN2
presenilin-2
- UPDRS
Unified Parkinson’s disease rating scale
- UDS
Uniform Data Set
Author Information
Mengxuan Tang, AB, Washington University School of Medicine, Department of Neurology, 660 S. Euclid Ave, Campus Box 8111, Saint Louis, MO 63110, USA, Mengxuan.tang@wustl.edu
Davis C Ryman, MD, AbbVie, Inc., 1 North Waukegan Road, North Chicago, IL 60064, USA, davis.ryman@abbvie.com
Mateusz Jasielec, PhD, 616 N 7th St. Apt 913, Saint Louis, MO 63101, USA, msj3535@gmail.com
Virginia Buckles, PhD, Washington University School of Medicine, 660 South Euclid, Campus Box 8111, Saint Louis, MO 63110, USA, bucklesv@abraxas.wustl.edu
Nigel Cairns, PhD, Washington University School of Medicine, 660 South Euclid, Campus Box 8118, Saint Louis, MO 63110, USA, cairnsn@neuro.wustl.edu
Anne Fagan, PhD, Washington University School of Medicine, 660 South Euclid, Campus Box 8111, Saint Louis, MO 63110, USA, fagana@neuro.wustl.edu
Alison Goate, DPhil, Icahn School of Medicine at Mount Sinai, 1425 Madison Ave, Department of Neuroscience, B1065, New York, NY 10029,USA, alison.goate@mssm.edu
Daniel Marcus, PhD, Washington University School of Medicine, 660 South Euclid, Campus Box 8067, Saint Louis, MO 63110,USA, dmarcus@WUSTL.EDU
Chengjie Xiong, PhD, Washington University School of Medicine, 660 South Euclid, Campus Box 8067, Saint Louis, MO 63110, USA, chengjie@wubios.wustl.edu
Ricardo Allegri, MD, Neurological Research Institute Raul Carrea (FLENI), Montaneses 2325, Buenos Aires City, Buenos Aires, C1428AQK, Argentina, rallegri@fleni.org.ar
Jasmeer Chhatwal, MD, Massachusetts General Hospital, 149 13th Street, Gerontology Research Room 2669, Charlestown, MA 02129, USA, Chhatwal.Jasmeer@mgh.harvard.edu
Adrian Danek, MD, University Hospital Ludwig-Maximilians-Universitat, Marchioninistr. 15, Munich, D-81377 Germany, Adrian.Danek@med.uni-muenchen.de
Bernardino Ghetti, MD, Indiana University School of Medicine, Department of Pathology and Laboratory Medicine, 635 Barnhill Drive, MS A138, Indianapolis, IN 46202, USA, bghetti@iupui.edu
Martin Farlow, MD, Indiana University School of Medicine, Department of Neurology, 635 Barnhill Drive, MS A142, Indianapolis, IN 46202, USA, mfarlow@iupui.edu
Nick Fox, MD, Professor of Clinical Neurology, Dementia Research Centre, UCL Institute of Neurology, Queen Square, London WC1N 3NG, n.fox@ucl.ac.uk
Neill Graff-Radford, MD, Mayo Clinic Jacksonville, 4500 San Pablo Road, Jacksonville, FL 32224,USA, graffradford.neill@mayo.edu
Christoph Laske, MD, University Hospital of Tuebingen, Section for Dementia Research, Department of Psychiatry and Psychotherapy, Calwer-Street 14, Tuebingen D-72076 Germany, Christoph.Laske@med.uni-tuebingen.de
Ralph Martins, PhD, Edith Cowan University, Sir James McCusker Alzheimer’s Disease Research Unit, 184 Hampden Road, Nedlands, Western Australia 06009 AUS, r.martins@ecu.edu.au
Colin Masters, MD, Mental Health Research Institute of Victoria, Level 3, Alan Gilbert Building, University of Melbourne, Victoria 03130 AUS, c.masters@unimelb.edu.au
Richard Mayeux, MD, Columbia University, College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032, USA, rpm2@columbia.edu
Eric McDade, DO, Washington University School of Medicine, 660 South Euclid, Campus Box 8111, Saint Louis, MO 63110, USA, mcdadee@neuro.wustl.edu
John Ringman, MD, Keck School of Medicine of University of Southern California, Center for the Health Professionals, 1540 Alcazar Street, Suite 209F, Los Angeles, CA 90089, USA, john.ringman@med.usc.edu
Martin Rossor, MD, Dementia Research Center, Institute of Neurology, University College London, Queen Square, London WC1 3BG United Kingdom, m.rossor@drc.ion.ucl.ac.uk
Stephen Salloway, MD, Butler Hospital, 245 Blackstone Boulevard, Providence, RI 02906, USA, SSalloway@Butler.org
Peter Schofield, PhD, Neuroscience Research Australia, Barker Street, Randwick, Sydney 02031 Australia, p.schofield@neura.edu.au
John Morris, MD, Washington University School of Medicine, 660 South Euclid, Campus Box 8111, Saint Louis, MO 63110, USA, morrisj@abraxas.wustl.edu
Randall Bateman, MD, Washington University School of Medicine, 660 South Euclid, Campus Box 8111, Saint Louis, MO 63110, USA, batemanr@neuro.wustl.edu
DIAN, Washington University School of Medicine, 660 South Euclid, Campus Box 8111, Saint Louis, MO 63110, USA, http://www.dian-info.org/personnel.htm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MT and DR did the literature search and prepared the figures. MT, RJB, JM, DR, MJ, EM, and VB designed the study. NC, AG, AF, DM, RA, AD, BG, MF, NGR, CL, RM, CM, RM, EM, JR, SS, PS, JM and RB, with the Dominantly Inherited Alzheimer’s Network (DIAN), collected the data. CX, RB, DR, VB, MJ, NC, AF, AG, DM and MT did the data analysis. CX, MJ, MT and DR did the statistical analysis. MT, DR, RB, and EM interpreted the data. MT, DR, RB and EM wrote the report. VB, NC, AF, AG, DM, CX, RA, AD, MF, NF, BG, CL, CM, RM, JR, MR, SS, PS and investigators from the DIAN, critically reviewed the report. Part of the data used in preparation of this Article were obtained from the DIAN database.
References
- 1.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimer Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storandt M, Balota DA, Aschenbrenner AJ, Morris JC. Clinical and psychological characteristics of the initial cohort of the Dominantly Inherited Alzheimer Network (DIAN) Neuropsychol. 2014;28:19–29. doi: 10.1037/neu0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J Neurol. 2006;253:139–58. doi: 10.1007/s00415-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 4.Larner AJ, Doran M. Genotype-phenotype relationships of presenilin-1 mutations in Alzheimer’s disease: an update. J Alzheimers Dis. 2009;17:259–65. doi: 10.3233/JAD-2009-1042. [DOI] [PubMed] [Google Scholar]
- 5.Ryan NS, Rossor MN. Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomark Med. 2010;4:99–112. doi: 10.2217/bmm.09.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan NS, Biessels GJ, Kim L, et al. Genetic determinants of white matter hyperintensities and amyloid angiopathy in familial Alzheimer’s disease. Neurobiol Aging. 2015;36:3140–51. doi: 10.1016/j.neurobiolaging.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Ryman DC, Acosta-Baena N, Aisen PS, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–60. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JC, Aisen PS, Bateman RJ, et al. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clin Investig(Lond) 2012;2:975–84. doi: 10.4155/cli.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–14. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 10.Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with apoE epsilon 2. Lancet. 1994;343:1432–3. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- 11.Cruchaga C, Haller G, Chakraverty S, et al. Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PloS One. 2012;7:e31039. doi: 10.1371/journal.pone.0031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 13.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–78. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindquist SG, Hasholt L, Bahl JM, et al. A novel presenilin 2 mutation (V393M) in early-onset dementia with profound language impairment. Euro J Neurol Official J Euro Fed Neurological Soc. 2008;15:1135–39. doi: 10.1111/j.1468-1331.2008.02256.x. [DOI] [PubMed] [Google Scholar]
- 15.Sitek EJ, Narozanska E, Peplonska B, et al. A patient with posterior cortical atrophy possesses a novel mutation in the presenilin 1 gene. PloS One. 2013;8:e61074. doi: 10.1371/journal.pone.0061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimer Dementia. 2015;11:1349–57. doi: 10.1016/j.jalz.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Isla T, Growdon WB, McNamara MJ, et al. The impact of different presenilin 1 andpresenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuronal loss in the familial Alzheimer’s disease brain: evidence for other phenotype-modifying factors. Brain. 1999;122:1709–19. doi: 10.1093/brain/122.9.1709. [DOI] [PubMed] [Google Scholar]
- 19.Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18:285–94. doi: 10.1111/j.1755-5949.2011.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology. 1986;36:1226–30. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry MC, Jin S, He F, et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurolr. 2012;69:368–72. doi: 10.1001/archneurol.2011.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez MF, Catanzaro P, Doss RC, R AR, Frey WH., 2nd Seizures in Alzheimer’s disease: clinicopathologic study. J Geriatr Psychiatry Neurol. 1994;7:230–3. doi: 10.1177/089198879400700407. [DOI] [PubMed] [Google Scholar]
- 23.Pandis D, Scarmeas N. Seizures in Alzheimer disease: clinical and epidemiological data. Epilepsy Curr. 2012;12:184–7. doi: 10.5698/1535-7511-12.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vossel KA, Beagle AJ, Rabinovici GD, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–66. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–40. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann DM, Pickering-Brown SM, Takeuchi A, Iwatsubo T, Members of the Familial Alzheimer’s Disease Pathology Study G Amyloid angiopathy and variability in amyloid beta deposition is determined by mutation position in presenilin-1-linked Alzheimer’s disease. Am J Pathol. 2001;158:2165–75. doi: 10.1016/s0002-9440(10)64688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 28.Lendon CL, Martinez A, Behrens IM, et al. E280A PS-1 mutation causes Alzheimer’s disease but age of onset is not modified by ApoE alleles. Hum Mut. 1997;10:186–95. doi: 10.1002/(SICI)1098-1004(1997)10:3<186::AID-HUMU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein Eepsilon4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Annals Neurol. 2003;54:163–9. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 30.Van Broeckhoven C, Backhovens H, Cruts M, et al. APOE genotype does not modulate age of onset in families with chromosome 14 encoded Alzheimer’s disease. Neurosci Letters. 1994;169:179–80. doi: 10.1016/0304-3940(94)90385-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.