Abstract
Apoptosis is a widespread phenomenon that occurs in the brain in both physiological and pathological conditions. Dead cells must be quickly removed to avoid the further toxic effects they exert in the parenchyma, a process executed by microglia, the brain professional phagocytes. Although phagocytosis is critical to maintain tissue homeostasis, it has long been either overlooked or indirectly assessed based on microglial morphology, expression of classical activation markers, or engulfment of artificial phagocytic targets in vitro. Nevertheless, these indirect methods present several limitations and, thus, direct observation and quantification of microglial phagocytosis is still necessary to fully grasp its relevance in the diseased brain. To overcome these caveats and obtain a comprehensive, quantitative picture of microglial phagocytosis we have developed a novel set of parameters. These parameters have allowed us to identify the different strategies utilized by microglia to cope with apoptotic challenges induced by excitotoxicity or inflammation. In contrast, we discovered that in mouse and human epilepsy microglia failed to find and engulf apoptotic cells, resulting in accumulation of debris and inflammation. Herein, we advocate that the efficiency of microglial phagocytosis should be routinely tested in neurodegenerative and neurological disorders, in order to determine the extent to which it contributes to apoptosis and inflammation found in these conditions. Finally, our findings point towards enhancing microglial phagocytosis as a novel therapeutic strategy to control tissue damage and inflammation, and accelerate recovery in brain diseases.
Keywords: microglia, phagocytosis, apoptosis, impairment, epilepsy, brain diseases, neurodegeneration, inflammation, neuroinflammation
Microglial Phagocytosis is a Multistep Process
Apoptosis, or programmed cell death, is a widespread phenomenon that occurs in the brain during development (embryonic and adult neurogenesis) and in pathological conditions (Madden and Cotter, 2008). Apoptotic dead cells are rapidly and efficiently removed through phagocytosis executed by microglia, the brain professional phagocyte (Sierra et al., 2013). Microglia are the resident brain macrophages and they have been traditionally studied as orchestrators of the brain inflammatory response during infection and diseases (Ransohoff, 2016). In addition, microglia have a more benign, less explored role as the brain professional phagocytes (Sierra et al., 2013), as they engulf and degrade dead cells, microbes, axonal and myelin debris, amyloid beta (Aβ) protein deposits, and supernumerary synapses under different conditions. Phagocytosis is a complex mechanism that comprises three different phases: “find-me”, “eat-me” and “digest-me”.
First, in the “find-me” stage, apoptotic cells release signals to attract phagocytes, such as the extracellular nucleotides adenosine triphosphate (ATP) and uridine 5’-triphosphate (UTP) (Elliott et al., 2009). ATP signals to microglia on a plethora of promiscuous P2X (ionotropic) and P2Y (metabotropic) receptors and is degraded by extracellular ectonucleotidases to adenosine 5’-diphosphate (ADP), adenosine monophosphate (AMP), and adenosine. Many of these receptors are expressed by microglia (Domercq et al., 2013) and regulate phagocytosis, such as P2X7 (Fang et al., 2009; Orr et al., 2009). Similarly, uridine diphosphate (UDP), the product of degradation of UTP by extracellular ectonucleotidases, acts on microglial P2Y6 receptors to facilitate phagocytosis (Koizumi et al., 2007). More recently, the existence of “help-me” signals released from damaged or diseased neurons has been proposed (Xing and Lo, 2016). These signals include several chemokines, cytokines, and growth factors such as interleukin 34 (IL-34), tumor necrosis factor alpha (TNFα), fractalkine (CX3CL1), and fibroblast growth factor 2 (FGF2), and may shift glial and vascular cells into potentially beneficial phenotypes by providing neuroprotection, promoting neurogenesis and angiogenesis, and enhancing clearance of cellular debris (Xing and Lo, 2016). Second, in the “eat-me” stage of phagocytosis, microglia recognize their targets through specific ligands present in apoptotic cells such as phosphatidylserine, integrins, immunoglobulins (IgG superfamily), and complement proteins (Sierra et al., 2013). Finally, in the “digest-me” stage, phagocytes engulf and completely degrade apoptotic cells in the lysosomal compartment (Arandjelovic and Ravichandran, 2015).
Microglial phagocytosis is therefore an essential process to remove cellular debris in the developing brain, in adult neurogenic niches, and in neurodegenerative and neurological disorders. However, its efficiency in the living brain remains poorly explored, largely because of intrinsic limitations in the classical methods used for its assessment. Here we first describe the different technical approaches to quantify microglial phagocytosis in vitro and in vivo, and their disadvantages. Next, we describe how novel quantitative approaches have allowed us to test microglial phagocytosis efficiency in the diseased mouse and human brain for the first time. Finally, we propose enhancing microglial phagocytosis as a novel therapeutical tool to accelerate recovery in the diseased brain.
The Importance of Choosing the Correct Methods for Assessing Microglial Phagocytosis of Apoptotic Cells
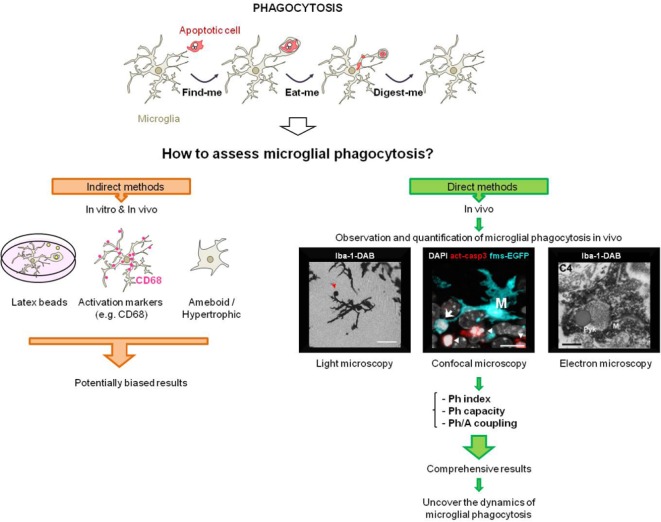
Traditionally, microglial phagocytosis has been studied in vitro using microglial cultures derived from the neonatal brain (Sierra et al., 2013) (Figure 1). One of the main problems presented by this approach is that cultured microglia have not been exposed to the postnatal development of the brain and, therefore, have not maturated as they would have done so in physiological conditions (Hellwig et al., 2013). Furthermore, in vivo microglia are kept under the restraint of various inhibitory inputs, such as fractalkine, a chemokine and microglial chemoattractant which has been proved to dramatically affect the transcription profile of microglia when genetically removed in vivo (Hellwig et al., 2013). These inhibitory inputs are absent in cultures and can cause microglia to respond differently in vitro compared to in vivo. Furthermore, cultured microglia are often used with synthetic phagocytic targets such as latex beads. Performing either in vitro or in vivo phagocytic assays with beads is artificial, as these particles do not release any chemoattractants and therefore do not activate conventional finding mechanisms in phagocytes (Park et al., 2011). Moreover, although these beads are frequently coated with serum to facilitate their recognition, it is unclear whether in vivo microglia come in contact with serum components (Hellwig et al., 2013). In addition, when these synthetic materials are engulfed the phagocytic process cannot be completed, as the beads cannot be degraded. Although the use of beads and cultured microglia has been proved useful to dissect out some molecular pathways involved in phagocytosis, they still impose inevitable limitations compared to the living brain (Sierra et al., 2013).
Figure 1.
Quantification of microglial phagocytosis.
Microglial phagocytosis is an essential mechanism to remove apoptotic cells from the tissue, and consists in three stages: finding, engulfing, and degrading the apoptotic cells. Although it is a critical process to maintain homeostasis, it has long been either overlooked or assessed using indirect methods based on engulfment of artificial phagocytic targets, expression of classical phagocytic markers, or morphology, which are inconclusive as they do not reliably represent the efficiency of phagocytosis. Importantly, there are some studies that have developed different strategies to directly quantify microglial phagocytosis in vivo, such as light microscopy, confocal microscopy, or electron microscopy. The light microscopy image was modified from (Pérez-Pouchoulen et al., 2015) and shows a microglia (Iba-1) with a phagocytic cup (red arrow) in the cerebellum during postnatal development (scale bar = 25 μm). The confocal microscopy image was obtained from (Abiega et al., 2016). The photomicrograph shows a 3D-rendered confocal z-stack of the epileptic mouse hippocampus. Microglia (M), visualized with transgenic expression of fms-EGFP (cyan) engulf an apoptotic cell with abnormal nuclear morphology (pyknosis, with the DNA dye DAPI, white) and expressing activated caspase-3 (a marker of apoptosis, red) (arrow). However, other nearby apoptotic cells are not engulfed (arrowheads), showing the phagocytosis impairment (scale bar = 8.4 μm, z = 14.1 μm). The electron microscopy image is reprinted from (Sierra et al., 2010) with permission from Elsevier. The image shows electron micrograph of an apoptotic cell engulfed by an Iba-1+ microglia in the young adult subgranular zone (SGZ). M: Microglia; Pyk: pyknotic body (scale bar = 500 nm). To obtain a comprehensive understanding of the dynamics of microglial phagocytosis we have developed a novel set of parameters based on confocal imaging of microglia: Ph index, Ph capacity, and Ph/A coupling (Abiega et al., 2016) that have allowed us to uncover the modus operandi of microglia in the healthy and diseased brain.
Some of these limitations can be overcome using organotypic cultures, in which apoptosis occurs naturally and the connectivity of the brain parenchyma is largely preserved. Nevertheless, the exposure to culture medium also modifies microglial responses, which sometimes greatly differ from the responses evoked by the same treatment in vivo (Abiega et al., 2016). Altogether, the issues discussed above suggest that the results obtained when analyzing phagocytosis in vitro should be cautiously interpreted and validated in vivo with physiological targets.
A major issue when studying microglial phagocytosis in vivo is the use of indirect methods based on microglial morphology or their expression of different molecular markers. In contrast to the long-standing assumption that phagocytosis is executed only by ameboid-shaped microglia (Sierra et al., 2013), we have observed that phagocytosis can effectively be performed by either ramified microglia (in physiological conditions) or a more hypertrophic microglia (in acute inflammation), which renders the function-morphology association misleading (Sierra et al., 2010). Moreover, classical microglial “activation markers”, such as macrosialin (CD68) (da Silva and Gordon, 1999; Ekdahl et al., 2003) have also been used in some studies to indirectly determine microglial phagocytosis (Perego et al., 2011; Schafer et al., 2012). CD68 is a lysosomal and membrane protein which is overexpressed during inflammatory challenge. While the location of CD68 previously suggested its involvement in phagocytosis, its loss of function does not result in phagocytosis deficits and, thus, its function still remains unknown (Sierra et al., 2013). In addition, there is no correlation between the expression of CD68 and phagocytosis, since we have observed that microglia with impaired phagocytosis during seizures overexpress CD68 (Abiega et al., 2016). Therefore, it is important to note that using “markers” as a proxy for phagocytosis can be misleading and that phagocytosis efficiency should be directly quantified.
Importantly, there are some studies that have developed different methodological approaches to directly quantify microglial phagocytosis in vivo (Figure 1). A potential method is 3D electron microscopy (EM) reconstruction, a technique that has been already implemented in the measurement of the phagocytosis of synaptic elements (Tremblay et al., 2010). EM is considered the gold standard to confirm apoptosis (Savill et al., 2002) and has been used to visualize microglial phagocytosis of apoptotic bodies (Sierra et al., 2010), although this strategy presents many disadvantages such as the large time expenditure, and the limitation to analyze only small regions.
Another strategy is to use light microscopy and immunostaining. Perez-Pouchoulen et al. (2015) defined microglial cups as round cup-shaped invaginations of the plasma membrane located at the tip of microglia processes in the developing cerebellum. These cups could potentially contain cellular debris, infectious agents, or dead cells. However, this study separately assessed microglial cups and pyknotic bodies and could not ensure that each cup contained an apoptotic cell.
Confocal microscopy and immunofluorescence provide a more powerful approach to study phagocytosis. We defined phagocytosis as the formation of a three dimensional pouch, usually located in terminal or en passant branches of microglia, completely surrounding an apoptotic cell (Sierra et al., 2010). We suggest that the term “pouch” is more appropriate than “cup” to describe the completely closed structure engulfing the dead cells. Furthermore, it is paramount to test the presence of cargo within every pouch, as some are apparently empty, i.e., do not contain an apoptotic cell. While these pouches may engulf other structures such as dendritic spines, axon terminals, or myelin debris (Sierra et al., 2013), their quantification alone without assessing whether they contain any kind of cargo may lead to overestimating phagocytosis.
Further information will come from live imaging experiments using 2-photon microscopy, which have already been implemented to determine phagocytosis of apoptotic cells in the developing zebra fish brain (Sieger et al., 2012). Altogether, confocal and 2-photon imaging are the most reliable methods to study phagocytosis in vivo, since they enable us to directly obtain quantitative data of the process of phagocytosis at the population level in fixed tissue (confocal microscopy) or individual cells in real time (2-photon microscopy).
Quantifying Microglial Phagocytosis is Critical for Understanding its Contribution to Brain Pathologies
Traditionally, quantitative analysis of microglial phagocytosis has focused on the study of single parameters, such as the number of pouches (with or without confirming that they actually engulf an apoptotic cell), or the number of apoptotic cells. However, in order to understand the dynamics of phagocytosis, we need to combine the analysis of the total number of live and apoptotic cells, the number of phagocytosed apoptotic cells, and the proportion of phagocytic microglia. To integrate this information and obtain a comprehensive, quantitative picture of microglial phagocytosis efficiency in vivo we have developed a novel set of parameters.
First, we established the Phagocytic index (Ph index), the proportion of apoptotic cells engulfed and undergoing degradation, which in physiological conditions in the adult hippocampus is around 90% (Sierra et al., 2010). We also determined the Phagocytic capacity (Ph capacity), i.e., the proportion of microglia with one or more phagocytic pouches, each containing one apoptotic cell (Sierra et al., 2010). In young mice the phagocytic capacity value is 0.35, that is, on average one third of microglia phagocytose a single apoptotic cell in the adult hippocampal neurogenic niche, where newborn neurons naturally undergo apoptosis (Sierra et al., 2010). These parameters allow us to fully comprehend the changes that microglia could undergo in different conditions.
Using this approach we have now discovered that in certain pathological models (excitotoxicity, and acute and chronic inflammation), microglia boost their phagocytic efficiency to counterbalance the increase in the number of apoptotic cells by combining different strategies: recruiting more phagocytic microglia, increasing the phagocytic capacity of each microglia, and/or increasing the number of microglial cells (Abiega et al., 2016). These results suggest that microglia have a substantial reservoir for phagocytosis, as they could reach their maximum Ph capacity by recruiting up to 100% microglia for phagocytosis, each engulfing several apoptotic cells. In addition we have observed that in excitotoxicity and inflammation, the increase in apoptosis is matched by a parallel increase in the Ph capacity. To analyze this response we introduced the Phagocytosis/Apoptosis coupling ratio (Ph/A coupling), i.e., the net phagocytosis (number of microglia multiplied by their phagocytic capacity) divided by the number of apoptotic cells. In excitotoxicity and inflammatory conditions, the Ph index and the Ph/A coupling ratio are maintained, suggesting a tight coupling between phagocytosis and apoptosis (Abiega et al., 2016) (Figure 2).
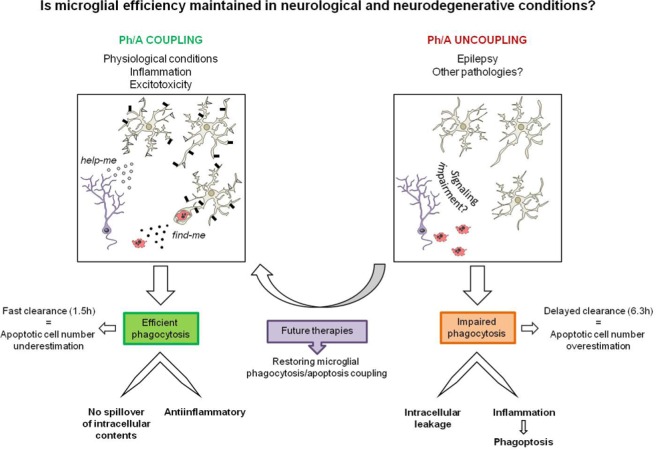
Figure 2.
Microglial phagocytosis/apoptosis coupling in health and disease.
Using our novel set of parameters, we have discovered a widespread microglial response to apoptotic challenge that occurs in physiological and some pathological conditions. Here, apoptosis and microglial phagocytosis are tightly coupled thanks to a complex signaling machinery such as “find-me” and “help-me” signals between neurons and microglia. An efficient phagocytosis prevents the spillover of toxic intracellular content of apoptotic cells and is also antiinflammatory, thus maintaining tissue homeostasis. In these conditions, a fast clearance of apoptotic cells leads to a low probability of detection and therefore an underestimation of their total numbers. In contrast, microglial phagocytosis is impaired in mouse and human epilepsy and possibly in other neurodegenerative and neurological disorders. The impairment in phagocytosis results in delayed clearance of apoptotic cells and intracellular leakage, and contributes to the development of an inflammatory response, which may initiate further engulfment of healthy cells (phagoptosis). In uncoupling conditions, a slow clearance of apoptotic cells may be interpreted as de novo apoptosis rather than accumulation of non phagocytosed cells, and ultimately lead to an overestimation of their total numbers. Therefore, our findings point towards enhancing microglial phagocytosis as a novel therapeutic strategy to control tissue damage and inflammation, and accelerate recovery in neurodegenerative and neurological diseases.
Surprisingly, when we assessed microglial phagocytosis efficiency for the first time in a brain disease we found the opposite. In a mouse model of medial temporal lobe epilepsy, seizures result in an increased number of apoptotic cells that is not counteracted by a proportional increase in phagocytosis. Rather, the Ph index decreases to 10% and the microglial phagocytic capacity is rapidly diminished. As a result, the Ph/A ratio is dramatically reduced, indicating that apoptosis and phagocytosis become uncoupled during epilepsy (Abiega et al., 2016). While non-professional phagocytes (astrocytes or neuroblasts) become engaged in phagocytosis in epilepsy, they only engulf a small proportion of the apoptotic cells compared to microglia (Abiega et al., 2016). Therefore, although there is an attempt to compensate for the microglial uncoupling by recruiting non-professional phagocytes, the cell type that contributes the most to phagocytosis in the epileptic tissue is still the impaired microglia.
We have observed that this uncoupling is a complex phenomenon triggered by the overlap of different mechanisms (Abiega et al., 2016). First, there is a massive release of ATP, a well known neurotransmitter and gliotransmitter, during epileptic seizures. This bath of ATP blinds microglia to the local microgradients of ATP released as a “find me” signal and prevents their targeting. Moreover, microglial motility is also impaired. Finally, seizures decrease the expression of phagocytic receptors in microglia, which implies an additional impairment in the cargo recognition. In addition, there may be further unexplored mechanisms underlying the phagocytosis impairment, such as alteration of different “find-me” and “help-me” signals released by apoptotic neurons, or alterations in the microglial machinery that regulates the different stages of phagocytosis. This data highlights the importance of analyzing all the stages of the process to understand the efficiency of phagocytosis.
Microglial Phagocytosis Conceals the Apoptotic Readout in the Healthy and Diseased Brains
Phagocytosis is a time-related process. In physiological conditions phagocytosis is highly efficient, as apoptotic cells are removed by microglia in 1.5 hours (Sierra et al., 2010). This short clearance time implies that only 5% of the apoptotic cells that undergo apoptosis in a 24 hours period are visualized, as the rest of them are already degraded by microglia and are therefore undetectable. This issue is already evident in adult neurogenic niches, where newborn cells undergo apoptosis but nonetheless very few apoptotic newborn cells are found (Dayer et al., 2003; Sierra et al., 2010). Thus, a speedy phagocytosis results in an underestimation of the total number of dead cells.
In contrast, the impairment of phagocytosis leads to overestimation of the number of apoptotic cells. During the phagocytosis-apoptosis uncoupling induced by seizures, the clearance time increases to 6.3 hours and as a result the non phagocytosed apoptotic cells accumulate in the parenchyma (Abiega et al., 2016) (Figure 2). This increase in the number of apoptotic cells may be ascribed to induction of apoptosis by a researcher oblivious of phagocytosis. To avoid false readouts of the apoptotic cell dynamics it is therefore crucial to assess microglial phagocytosis efficiency and determine whether increases in the number of apoptotic cells are due to de novo apoptosis or impairment of phagocytosis. Because up until now microglial phagocytosis in vivo has not been directly quantified, it is difficult to speculate the extent to which this impairment contributes to the increased apoptosis found in neurodegenerative and neurological disorders, such as stroke, Alzheimer's disease, Parkinson's disease, or multiple sclerosis.
Microglial Phagocytosis is Critical for the Maintenance of Brain Homeostasis
Phagocytosis is a vital process for the tissue (Figure 2). First, it prevents the apoptotic cells from losing membrane integrity and the subsequent leakage of potentially toxic intracellular contents into the surrounding parenchyma (Arandjelovic and Ravichandran, 2015). These intracellular contents engage receptors for damage-associated molecular patterns and contribute to immune responses to self antigens (Arandjelovic and Ravichandran, 2015). Accordingly, defects in the clearance of apoptotic cells by macrophages have been attributed to the onset of persistent inflammatory disorders and autoimmunity (Lauber et al., 2004).
In addition, clearance of apoptotic cells by phagocytes actively suppresses the initiation of inflammatory and immune responses, in part through the release of anti-inflammatory cytokines (Byrne and Reen, 2002; Huynh et al., 2002), thus preventing an immune response against the processed proteins of the apoptotic debris. The anti-inflammatory role of phagocytosis in microglia has also been explored in vitro and involves the release of anti-inflamamtory cytokines such as transforming growth factor β1 (TGFβ1) and trophic factors such as NGF (nerve growth factor) (De Simone et al., 2003); (Fraser et al., 2010) that may potentially facilitate the functional recovery of the surrounding compromised neurons. Therefore, an impairment in phagocytosis would release the brake imposed on the inflammatory response. As we expected, we have found that the phagocytic impairment correlates with the development of an inflammatory response during epilepsy (Abiega et al., 2016), although the signal/s which initiate this response in the epileptic brain remain to be determined. Prolonged inflammatory responses are detrimental for brain functioning, as they lead to neuronal degeneration (Glass et al., 2010).
Additionally, inflammation resulting from the phagocytosis/apoptosis uncoupling may have further detrimental consequences. Inflammation causes neurons to transiently expose phosphatidylserine in the outer cell membrane, leading to their recognition and engulfment by microglia (Brown and Neher, 2014). Indeed, we have observed this dysfunctional phagocytosis, termed “phagoptosis”, in mouse and human epilepsy, albeit the number of seemingly viable neurons phagocytosed was lower than the number of apoptotic cells engulfed. The presence of phagoptosis in a mouse model of epilepsy has been recently confirmed (Luo et al., 2016). Thus, it is conceivable that phagoptotic loss of neurons may contribute to the pathology of neurodegenerative conditions. Therefore, the detrimental features associated with microglial phagocytosis/apoptosis uncoupling such as accumulation of apoptotic cells, inflammation, and phagoptosis could exacerbate the pathology in brain diseases.
Modulation of Microglial Phagocytosis as a Novel Therapy in Brain Injury and Neurodegeneration
To this day, most therapies aimed at treating neurodegenerative diseases have focused on preventing the neuronal death. For example, some strategies have aimed at inhibiting caspases, the enzymes responsible for executing apoptosis (Troy and Jean, 2015); eliminating Aβ plaques in Alzheimer's disease (AD); or repressing the oxidative injury caused by ischemia (Patel, 2016). While the results in animal models are promising, many of these candidate strategies have failed in human clinical trials (Ginsberg, 2007; Snow et al., 2010).
Here, we suggest that in addition to reducing neuronal damage and death, a complementary therapeutical strategy could involve enhancing the “self-cleaning” mechanisms of the brain, such as microglial phagocytosis. Similar to what we have reported in epilepsy (Abiega et al., 2016), it is possible that microglial phagocytosis is impaired in other brain diseases. Inflammation and/or neuronal death are hallmarks of many other neurodegenerative and neurological disorders, such as Alzheimer's and Parkinson's disease, multiple sclerosis, ischemia/stroke, and mood disorders (Cappellano et al., 2013; Ransohoff, 2016). We speculate that the increase in apoptosis and the pro-inflammatory profile classically described in neurological and neurodegenerative diseases could be the consequence of an undetected phagocytosis impairment. Therefore, we urge to assess microglial phagocytosis efficiency in brain pathologies using comprehensive, quantitative approaches.
Moreover, we want to stress that the comprehension of the mechanisms that regulate phagocytosis, such as “help-me” and “find-me” signals coming from damaged neurons or apoptotic cells, or phagocytic receptors in microglia, becomes crucial for the development of new therapies based on the modulation of microglial phagocytosis. Novel pharmacological approaches aimed at enhancing or restoring microglial phagocytosis efficiency would accelerate the clearance of apoptotic cells and promote an anti-inflammatory response. At the same time they should avoid uncontrolled engulfment of healthy neurons (phagoptosis). We propose that the modulation of microglial phagocytosis is a novel and yet unexplored therapy to accelerate functional brain recovery from neurodegenerative and neurological diseases.
Conclusion
Microglial phagocytosis is an essential mechanism to maintain tissue homeostasis. However, technical limitations have so far prevented understanding the modus operandi of microglia under phagocytic challenge and its ultimate relevance. The aim of this review is to encourage the scientific community to implement a set of parameters based on direct methods of phagocytosis efficiency quantification in order to obtain a global overview of the phagocytic process. Moreover, we intend to increase awareness about the relevance of assessing microglial phagocytosis in neurodegenerative and neurological diseases. Microglial phagocytosis is at the core of the brain regenerative response and harnessing their phagocytic potential is a novel therapeutic alternative to promote clearance of apoptotic cells and anti-inflammatory response, in order to accelerate brain recovery in diseases such as epilepsy, Alzheimer's disease, Parkinson's disease, ischemia/stroke, or multiple sclerosis, among others.
Acknowledgments
We are grateful to Jorge Valero for critically reading the manuscript.
Footnotes
Funding: This work was supported by grants from the Spanish Ministry of Economy and Competitiveness with FEDER funds to AS (BFU2015-66689, RYC-2013-12817). OA is recipient of a predoctoral fellowship from the Basque Government, and IDA is recipient of a predoctoral fellowship from the University of the Basque Country EHU/UPV.
Conflicts of interest: None declared.
References
- Abiega O, Beccari S, Diaz-Aparicio I, Nadjar A, Layé S, Leyrolle Q, Gómez-Nicola D, Domercq M, Pérez-Samartín A, Sánchez-Zafra V, Paris I, Valero J, Savage JC, Hui CW, Tremblay MÈ, Deudero JJ, Brewster AL, Anderson AE, Zaldumbide L, Galbarriatu L, et al. Neuronal hyperactivity disturbs ATP microgradients, impairs microglial motility, and reduces phagocytic receptor expression triggering apoptosis/microglial phagocytosis uncoupling. PLoS Biol. 2016;14:e1002466. doi: 10.1371/journal.pbio.1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, Comi C. Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:89–107. [PMC free article] [PubMed] [Google Scholar]
- da Silva RP, Gordon S. Phagocytosis stimulates alternative glycosylation of macrosialin (mouse CD68), a macrophage-specific endosomal protein. Biochem J. 1999;338:687–694. [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Tirassa P, Minghetti L. Apoptotic PC12 cells exposing phosphatidylserine promote the production of anti-inflammatory and neuroprotective molecules by microglial cells. J Neuropathol Exp Neurol. 2003;62:208–216. doi: 10.1093/jnen/62.2.208. [DOI] [PubMed] [Google Scholar]
- Domercq M, Vazquez-Villoldo N, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci. 2013;7:49. doi: 10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang KM, Yang CS, Sun SH, Tzeng SF. Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J Neurochem. 2009;111:1225–1237. doi: 10.1111/j.1471-4159.2009.06409.x. [DOI] [PubMed] [Google Scholar]
- Fraser DA, Pisalyaput K, Tenner AJ. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem. 2010;112:733–743. doi: 10.1111/j.1471-4159.2009.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Life after cerovive: a personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke. 2007;38:1967–1972. doi: 10.1161/STROKEAHA.106.479170. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig S, Heinrich A, Biber K. The brain's best friend: microglial neurotoxicity revisited. Front Cell Neurosci. 2013;7:71. doi: 10.3389/fncel.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- Luo C, Koyama R, Ikegaya Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia. 2016;64:1508–1517. doi: 10.1002/glia.23018. [DOI] [PubMed] [Google Scholar]
- Madden SD, Cotter TG. Cell death in brain development and degeneration: control of caspase expression may be key! Mol Neurobiol. 2008;37:1–6. doi: 10.1007/s12035-008-8021-4. [DOI] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. 2009;12:872–878. doi: 10.1038/nn.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, Collins S, Lysiak JJ, Hoehn KL, Ravichandran KS. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–224. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci. 2016;37:768–778. doi: 10.1016/j.tips.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pouchoulen M, VanRyzin JW, McCarthy MM. Morphological and phagocytic profile of microglia in the developing rat cerebellum(1,2,3) eNeuro 2. 2015 doi: 10.1523/ENEURO.0036-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieger D, Moritz C, Ziegenhals T, Prykhozhij S, Peri F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev Cell. 2012;22:1138–1148. doi: 10.1016/j.devcel.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM. Protect Study G (2010) A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Dis. 25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Jean YY. Caspases: therapeutic targets in neurologic disease. Neurotherapeutics. 2015;12:42–48. doi: 10.1007/s13311-014-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Lo EH. Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery. 2016 doi: 10.1016/j.pneurobio.2016.04.004. doi:10.1016/j.pneurobio.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]