Abstract
Neuropathic pain is triggered by the lesions to peripheral nerves which alter their structure and function. Neuroprotective approaches that limit the pathological changes and improve the behavioral outcome have been well explained in different experimental models of neuropathy but translation of such strategies to clinics has been disappointing. Experimental evidences revealed the role of free radicals, especially peroxynitrite after the nerve injury. They provoke oxidative DNA damage and consequent over-activation of the poly(ADP-ribose) polymerase (PARP) upregulates pro-inflammatory pathways, causing bioenergetic crisis and neuronal death. Along with these changes, it causes mitochondrial dysfunction leading to neuronal apoptosis. In related preclinical studies agents that neutralize the free radicals and pharmacological inhibitors of PARP have shown benefits in treating experimental neuropathy. This article reviews the involvement of PARP over-activation in trauma induced neuropathy and therapeutic significance of PARP inhibitors in the experimental neuropathy and neuropathic pain.
Keywords: neuropathic pain, poly(ADP-ribose) polymerase, neuroinflammation, oxidative stress, bioenergetic crisis
Introduction
Peripheral nerves which connect the brain and spinal cord to the body, if injured can lead to neuropathic pain, a chronic debilitating condition manifested as allodynia and hyperalgesia (Brookoff, 2000). It is a common clinical problem associated with 87% of traumatic conditions and 12% owing to surgeries. Peripheral nerve lesions occur approximately in 2.8% of multiple trauma patients, or 5% root and plexus injured patients (Hulsebosch et al., 2009). Clinical symptoms are characterized by abnormalities in the pain sensation which may be sensations like shooting pain, burning, tingling, numbness, allodynia and hyperalgesia (Baron et al., 2010). Peripheral nerve injury results in orchestrated changes similar to the Wallerian degeneration leading to structural and functional alterations which affect the whole peripheral nervous system including peripheral nerve endings, afferent fibers, dorsal root ganglion (DRG) and also central afferent terminals in the spinal cord (Austin et al., 2012). The changes include cell body swelling, loss of Nissl bodies, and displacement of the nucleus from the center of the neuron to a position near the cell membrane. After peripheral nerve injury, the nerve derangement, axon degeneration, endoneurial edema and massive demyelination were observed in peripheral nerves whereas there was axon degeneration, swelling and immune cell infiltration in DRG and dorsal horns of lumbar spinal cord (Zochodne, 2012). Aftermath of nerve injury includes functional and behavioural deficits which pose challenges for the identification of novel therapeutic strategies for the treatment of neuropathic pain. Unfortunately, despite several years of research experience in repair of peripheral nerve, functional recovery after the injury is disappointing. The available drugs provide symptomatic relief from neuropathic pain and suffer from several limitations like resistance (opioids), dose-limiting side effects (antidepressants and anticonvulsants) and no uniform success (Nickel et al., 2012). Therefore, understanding the exact pathomechanism is necessary for finding better treatment options as well as the development of novel pharmacological interventions.
The next question was to select a suitable experimental model which can be as close as possible to human pathophysiology of trauma/injury induced neuropathy and neuropathic pain. The animal model of chronic constriction injury (CCI) of the sciatic nerve was preferred, as it is one of the most commonly used peripheral neuropathic pain models which is a reliable and easily reproducible model (Bennett and Xie, 1988; Austin et al., 2012). The constriction of the sciatic nerve by placing 4 loose ligatures around the sciatic nerve proximal to the trifurcation is associated with an epineural inflammatory lesion, intraneural edema, focal ischemia and Wallerian degeneration. The behavioral signs of spontaneous pain, excessive guarding and licking, limping off the ipsilateral hind paw and avoidance of placing weight on the injured paw have been reported (Wang and Wang, 2003). Various other behavioral changes like mechanical and thermal hyperalgesia, chemical cold allodynia have been noted to develop within a week with maximal pain-related behaviors and postural asymmetries during the second week of post-surgery. Electrophysiological studies have also revealed a decrease in nerve conduction velocity. The pathological alterations in both myelinated and non-myelinated neurons may be responsible for this decrease in nerve conduction velocities (Gabay and Tal, 2004). Partial damage to the nerve leads to sensitization of both A and C fibers and thus plays a major role in initiating and maintenance of pain behavior. Hence, it produces unilateral peripheral mononeuropathy and it has been observed that symptoms in this rat model match to causalgia or complex regional pain syndrome is seen in patients suffering from trauma-induced neuropathic pain (Campbell and Meyer, 2006). Therefore, evaluating the therapeutic potential of pharmacological interventions in the animal model of CCI can be correlated to the clinical trauma-induced neuropathy and neuropathic pain.
Then we took an overview of all the known mechanisms reported for contributing to the genesis of neuropathy associated changes. Pathogenesis of nerve injury involves peripheral and central sensitization in which oxidative/nitrosative stress play a major role. Prominently the role of various transcription pathways like nuclear factor-κB (NF-κB), p38 mitogen-activated protein (MAP) kinase (MAPK), Jun amino-terminal kinases (JNK), Wnt/β-Catenin has been well studied and reported (Hulsebosch et al., 2009). Under such extreme stress conditions, hydroxyl radicals, superoxide and nitric oxide are produced in the cytoplasm by various enzymatic reactions, which are activated by increased intracellular calcium (Areti et al., 2014). Oxidative modification of neuronal biomolecules leads to mitochondrial dysfunction and drives the cell towards apoptosis (Ott et al., 2007). Superoxide and nitric oxide can readily convert to a highly reactive and toxic peroxynitrite that attacks nucleic acids. Peroxynitrite, a strong oxidizing and nitrating agent attacks various biomolecules like proteins, enzymes and nucleic acids etc. and results in their malfunctioning (Sandireddy et al., 2014). Nitrite species generally attack the proteins and render them nonfunctional due to various modifications including nitrosylation. Especially, peroxynitrite radicals attack mitochondrial superoxide dismutase and cause its inactivation (MacMillan-Crow et al., 1998). It also alters the glutamatergic transmission and inactivates glutamine transferase which helps in the synthesis of endogenous antioxidant, glutathione (Little et al., 2012). This results in loss of antioxidant defenses and alters the neurotransmission which aggravates the vicious cycle of oxidative/nitrosative stress and loss of nerve functionality. In addition to protein nitrosylation, peroxynitrite also attacks DNA and can possibly amplify poly(ADP-ribose) polymerase (PARP) overactivation (Moylan et al., 2014). PARP is a ubiquitous enzyme linked to DNA repair and associated with cellular functions such as preservation of genomic stability and cell death (Jagtap and Szabo, 2005). But oxidative DNA damage overactivated PARP which rapidly depletes the cellular NAD+ and ATP leading to the bioenergetic crisis. PARP acts on mitochondria and initiates cell death process through mitochondrial depolarization/membrane permeability transition (MPT) and release of cytochrome c (Cyt c), apoptosis-inducing factor (AIF)/endonuclease G into the cytosol. PARP activation also leads to transport and binding of poly (ADP) ribosyl (PAR) units to mitochondrial membranes thereby catalyzes mitochondrial MPT and initiates apoptotic cell death (Galluzzi et al., 2011). The cellular dysfunction is further enhanced by the activation of pro-inflammatory gene expression by PARP, through the promotion of MAP kinase, NF-κB and activator protein-1 (AP-1) activation. The PARP overactivation especially in neuronal tissues results in an increased expression of AP-1 and NF-κB dependent genes such as inducible nitric oxide synthase (iNOS), intracellular adhesion molecule-1 (ICAM-1), monocyte chemotactic protein-1 (MCP-1α), complement proteins (C3), cyclooxygenase -2 (COX-2) and proinflammatory cytokines (such as IL-1α, TNF-α) (de La Lastra et al., 2007). Activation of transcription factor NF-κB induces inflammatory process through pro-inflammatory cytokines plays a critical role in the maintenance of persistent pain. COX-2 enhances the synthesis of prostaglandins and sensitize the nociceptors whereas TNF-α and IL-6 modulate excitatory and inhibitory synaptic transmission, respectively in spinal dorsal horn causing hyperalgesia and allodynia (Komirishetty et al., 2016b). Hence, PARP overactivation causes energy depletion, mitochondrial dysfunction through excess ribosylation of proteins and neuroinflammation, thus aggravates the cycle of nerve damage (Tentori et al., 2002). This consequently forces the cell to undergo death because of bioenergetic crisis & neuronal dysfunction. PARP overactivation is associated with several nervous system disorders like neurodegenerative diseases, ischemia-reperfusion and traumatic injury (Jagtap and Szabo, 2005). There has been substantial research on the role of peroxynitrite, overactivation of PARP and inflammatory mediators in the progression of different types of neuropathies and neuropathic pain (Ilnytska et al., 2006). Mounting evidence indicates that targeting PARP overactivation could counteract the pathogenic effects against diabetic and chemotherapy induced neuropathies and interestingly PARP inhibitors also provide neuroprotection from the unanticipated pathomechanisms in neuropathy conditions (Obrosova et al., 2005). But the role of PARP overactivation and related neuroinflammation has not been clearly explored for their involvement in the peripheral nerve injury-induced neuropathy. Hence, we have explored the role of oxidative/nitrosative stress and PARP overactivation in the animal model of CCI-induced neuropathic pain. CCI produced significant rise in the levels of oxidative stress markers like nitrites and malondialdehyde (MDA), an indicative of nitro-oxidative stress in the tissues of the sciatic nerve and spinal cord (Komirishetty et al., 2016b). The extreme nitrosative stress can be explained by the expression of nitrotyrosine positive cells in both the tissues. PARP overactivation showed the mitochondrial dysfunction through the loss of membrane potential and electron transport chain inactivation in the sciatic nerve (Komirishetty et al., 2016c). Oxidant-induced DNA damage and PARP-induced neuronal apoptosis were prominently observed in three types of neuronal tissues i.e., ipsilateral sciatic nerve, L4–6 DRG and lumbar spinal cord through TUNEL positive cells (Komirishetty et al., 2016a). Increased PAR immunoreactivity in sciatic nerve and lumbar spinal cord sections indicated the oxidative/nitrosative stress induced PARP overactivation. It also led to NAD and ATP depletion driving the cell towards bioenergetic crisis induced cell death. Hence, PARP overactivation after peripheral nerve injury has been demonstrated to switch cell death pathways from the well-controlled and highly regulated apoptosis to the more inflammatory necrosis in the event of high levels of reactive oxygen species. The role of PARP-induced inflammation is also multifaceted. It also serves as a co-factor for NF-κB thus its activation elevates the expression levels of proinflammatory cytokines TNF-α, IL-6, iNOS, COX-2 in the peripheral nerve tissues (Kumar et al., 2011, 2012). Therefore, PARP-induced neuroinflammation aggravates the neurodegeneration process and challenges for the cell survival after the peripheral injury. CCI-induced alterations in the expression levels of oxidative/nitrosative stress markers and inflammatory markers can modulate excitatory and inhibitory synaptic transmission in spinal dorsal horn which can be correlated to the observed hyperalgesia and allodynia (Komirishetty et al., 2016a, b). Functional and behavioural deficits after the nerve injury can be explained through the PARP-induced bioenergetic failure and neuronal dysfunction. PARP inhibition ameliorates PAR accumulation in neuronal vasculature and axons, thus restores normal neuronal functions like conduction velocities and nerve blood flow (Obrosova et al., 2004). Sciatic functional evaluation is important to know the extent of myelin degradation and nerve injury (Jessen and Mirsky, 2008). A significant sciatic functional loss and foot deformity has been observed in the CCI animals. CCI of sciatic nerve induced morphological & structural changes like axon degeneration, ganglia nucleolar, nuclear and somatic size reduction with nucleolar segregation induced by damage to cell bodies in dorsal root ganglia, peripheral sensory and motor neurons makes them more susceptible to oxidant-induced nervous damage (Komirishetty et al., 2016b). The recent findings also suggest the nitro-oxidative stress and PARP overactivation is bidirectional rather than unidirectional (Obrosova et al., 2005). Hence, PARP overactivation after the nerve injury feed forwards the neurodegenerative mechanisms leading to the functional, behavioural and biochemical deficits (Figure 1). Identifying the pathological role of PARP overactivation in trauma-induced neuropathy, our research group evaluated the neuroprotective potential of PARP inhibitors like morin hydrate, 3-aminobenzamide (3-AB), 1,5-isoquinolinediol (ISO) and 4-amino 1,8-napthalimide (4-ANI) in the CCI model of neuropathic pain. Interestingly, administration of PARP inhibitors for 14 days in rats recovered the sciatic functional index, foot posture. They also attenuated the CCI-induced behavioral changes including thermal hyperalgesia, cold allodynia, dynamic mechanical allodynia, mechanical hyperalgesia, mechanical allodynia and spontaneous pain. Improved functional and behavioural deficits with the treatment indicate the therapeutic potential of PARP inhibitors against sciatic nerve constriction induced axon degeneration, demyelination and disturbed sensory motor inputs. Our studies also demonstrated that treatment with PARP inhibitors attenuated the CCI-induced bioenergetic crisis, neuronal apoptosis, cellular dysfunction and neuroinflammation through improved NAD & ATP levels, reduced TUNEL positive cells, PAR immunoreactivity and NF-κB activation respectively in the sciatic nerve and lumbar spinal cord after the nerve injury (Komirishetty et al., 2016a, b).
Figure 1.
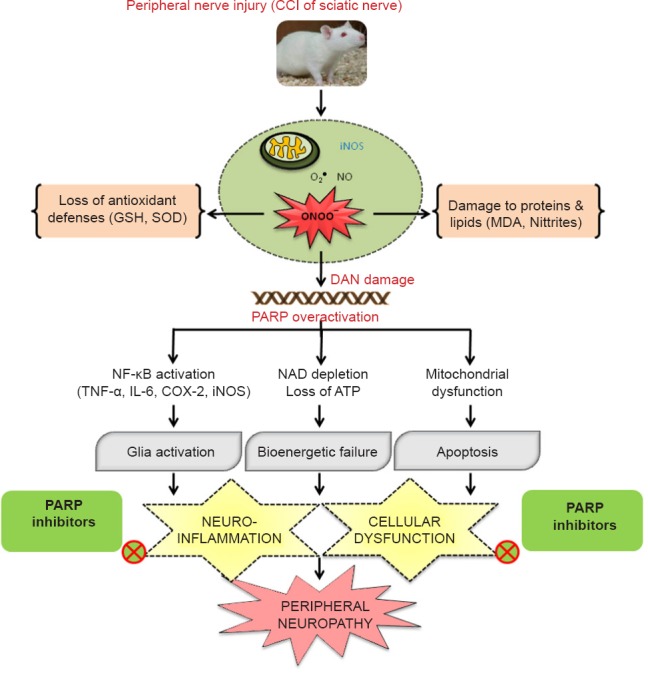
Schematic representation of the role of oxidative/nitrosative stress & PARP overactivation and therapeutic potential of PARP inhibitors in CCI-induced peripheral neuropathy.
ATP: Adenosine triphosphate; CCI: chronic constriction injury; GSH: glutathione; IL-6: interleukin 6; iNOS: inducible nitric oxide synthase; MDA: malondialdehyde; NAD: nicotinamide adenine dinucleotide; NF-κB: nuclear factor-κB; NO: nitric oxide; PARP: poly (ADP-ribose) polymerase; SOD: superoxidase dismutase; TNF-α: tumor necrosis factor α.
In summary, our studies demonstrated the role of nitro-oxidative stress and PARP overactivation in an experimental model of trauma-induced neuropathic pain. PARP inhibitors mitigated the neuroinflammation and nitro-oxidative stress induced functional, behavioural and biochemical deficits, thus showed protection against PARP-overactivation induced neuronal damage in an experimental model of trauma-induced neuropathy. Hence, it can be speculated that PARP inhibitors may facilitate promising therapeutic benefits in the treatment of peripheral nerve injury-induced neuropathic pain.
Footnotes
Funding: Authors would like to thank Department of Biotechnology Govt of India, for their financial support to Dr. Ashutosh Kumar via grant BT/527/NE/TBP/2013, to carry out the current study. Authors would also like to acknowledge the financial support from Department of Pharmaceuticals, Ministry of Chemical and Fertilizers and NIPER Hyderabad for their support.
References
- Areti A, Yerra VG, Naidu VGM, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PJ, Wu A, Moalem-Taylor G. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp. 2012:e3393–3393. doi: 10.3791/3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brookoff D. Chronic pain: 1. A new disease? Hosp Pract. 1995;35:45–59. doi: 10.1080/21548331.2000.11444031. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Lastra CA, Villegas I, Sanchez-Fidalgo S. Poly (ADP-ribose) polymerase inhibitors: new pharmacological functions and potential clinical implications. Curr Pharm Des. 2007;13:933–962. doi: 10.2174/138161207780414241. [DOI] [PubMed] [Google Scholar]
- Gabay E, Tal M. Pain behavior and nerve electrophysiology in the CCI model of neuropathic pain. Pain. 2004;110:354–360. doi: 10.1016/j.pain.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vanden Berghe T, Vanlangenakker N, Buettner S, Eisenberg T, Vandenabeele P, Madeo F, Kroemer G. Programmed necrosis from molecules to health and disease. Int Rev Cell Mol Biol. 2011;289:1–35. doi: 10.1016/B978-0-12-386039-2.00001-8. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Poly (ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly (ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Jessen KnR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Komirishetty P, Areti A, Sistla R, Kumar A. Morin mitigates chronic constriction injury (CCI)-induced peripheral neuropathy by inhibiting oxidative stress induced PARP over-activation and neuroinflammation. Neurochem Res. 2016a;41:2029–2042. doi: 10.1007/s11064-016-1914-0. [DOI] [PubMed] [Google Scholar]
- Komirishetty P, Areti A, Yerra VG, Ruby PK, Sharma SS, Gogoi R, Sistla R, Kumar A. PARP inhibition attenuates neuroinflammation and oxidative stress in chronic constriction injury induced peripheral neuropathy. Life Sci. 2016b;150:50–60. doi: 10.1016/j.lfs.2016.02.085. [DOI] [PubMed] [Google Scholar]
- Komirishetty P, Areti A, Gogoi R, Sistla R, Kumar A. Combination strategy of PARP inhibitor with antioxidant prevent bioenergetic deficits and inflammatory changes in CCI-induced neuropathy. Neuropharmacology. 2016c;113:137–147. doi: 10.1016/j.neuropharm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Duraikannu A, Zochodne DW. Releasing “brakes” to nerve regeneration: intrinsic molecular targets. Eur J Neurosci. 2015;43:297–308. doi: 10.1111/ejn.13018. [DOI] [PubMed] [Google Scholar]
- Kumar A, Negi G, Sharma SS. JSH-23 targets nuclear factor-kappa B and reverses various deficits in experimental diabetic neuropathy: effect on neuroinflammation and antioxidant defence. Diabetes Obes Metab. 2011;13:750–758. doi: 10.1111/j.1463-1326.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Negi G, Sharma SS. Suppression of NF-kB and NF-kB regulated oxidative stress and neuroinflammation by BAY 11-7082 (IkB phosphorylation inhibitor) in experimental diabetic neuropathy. Biochimie. 2012;94:1158–1165. doi: 10.1016/j.biochi.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Little JW, Doyle T, Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino acids. 2012;42:75–94. doi: 10.1007/s00726-010-0633-0. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, Hayley AC, Pasco JA, Anderson G, Jacka FN. Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev. 2014;45:46–62. doi: 10.1016/j.neubiorev.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Nickel FT, Seifert F, Lanz S, Maihofner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22:81–91. doi: 10.1016/j.euroneuro.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly (ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjáti K, Pacher P, Szabo C, Stevens MJ. Role of poly (ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol 2014. 2014 doi: 10.1155/2014/674987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentori L, Portarena I, Graziani G. Potential clinical applications of poly (ADP-ribose) polymerase (PARP) inhibitors. Pharmacol Res. 2002;45:73–85. doi: 10.1006/phrs.2001.0935. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Reversing neuropathic deficits. J Peripher Nerv Syst. 2012;17:4–9. doi: 10.1111/j.1529-8027.2012.00388.x. [DOI] [PubMed] [Google Scholar]


