Incidence and consequences of spinal cord injuries: Worldwide, every year 250,000–500,000 people suffer from spinal cord injury (SCI; www.who.int, 2013). Traumatic lesions of the spinal cord lead to primary and secondary injury mechanisms, which result in axon damage, loss of signal conduction, demyelination of axons and long-lasting deficits in motor and sensory function. The extent of the damage and the subsequent functional loss depend on the spinal level and the severity of the primary injury. Furthermore, pathophysiological and pathomorphological responses in acute and chronic SCI share similar but also different requirements for treatment.
To date, SCI is a generally incurable condition, and, although numerous therapeutic strategies to treat SCI have been developed and tested in the last decades (Bradbury and McMahon, 2006), no experimental treatment to achieve substantial axonal regeneration and myelination with accompanying significant behavioral recovery has been successfully translated into a clinical therapy. In this regard, it is also important to take into consideration that even sophisticated combinatorial and sometimes ethically problematic treatment approaches (i.e., embryonic stem cell grafting) face limitations and lack substantial additive benefit regarding functional outcome compared to single component treatments in complete SCI. In SCI patients, rehabilitative training is currently the treatment of choice (Awai and Curt, 2016) that can facilitate the process of recovery to certain but limited extents particularly in severely injured patients.
Making use of the basic concepts and safe methods of mechanical and biochemical bridging, it is, however, possible to achieve a remarkable improvement of locomotor function after complete thoracic transection of the spinal cord as shown in adult rats. Two novel treatment procedures, the low pressure mechanical adaptation of severed spinal stumps (Brazda et al., 2013, 2016) and a simple biochemical polymer approach (Estrada et al., 2014; Brazda et al., 2016), as introduced here, promoted beneficial effects on axon growth as well as invasion and activity of various other cell types, which are also affected by SCI. Examples are endothelial cells which were involved in angiogenetic events, and peripheral Schwann cells which myelinated regenerating axons. Very importantly, both treatments led to locomotor improvements after complete thoracic spinal transection, which has, to our knowledge, not been exceeded by any other single component or combinatorial treatment.
Applying basic treatment concepts for acute and chronic spinal cord injuries: Currently there are numerous treatment approaches for experimental SCI (Kabu et al., 2015), which have been developed in the past decades. However, it is widely accepted in the field that there is an urgent need for the development and optimization of combinatorial (multi-component) therapies in order to treat this multifactorial disease. Although to date, there is no clinical treatment for SCI, which has proven to result in significant locomotor recovery in human patients, occasional individual reports have previously described impressive outcomes (Tabakow et al., 2014). However, such anecdotic reports are received rather critically by SCI experts. Reasons are (1) the extremely low number of treated subjects and the resulting lack of reproducibility of described outcomes, and (2) the great variety of spontaneous improvements observed in SCI patients.
Two innovative and safe basic treatment approaches to bridge tissue gaps in severely injured spinal cord that yielded impressive results regarding, i.e., hindlimb locomotion in rat, could be modified and optimized for future preclinical and eventually clinical application. The first approach uses the implantable mechanical microconnector system (Brazda et al., 2013, 2016), a device that was specifically designed to re-adapt severed spinal tissue in a submillimeter range (Figure 1b). The microconnector system is equipped with two ports, one outlet and one inlet port, and both are attached to plastic tubes. The outlet can be used to connect the system to an external vacuum pump for the application of gentle negative pressure after its implantation. Via the inlet port therapeutics can be delivered through, e.g., minipumps and/or catheters directly into the lesion center. Either single, repeated or long-term continuous infusions are possible. Design and application of the mechanical device, which brings the two spinal cord stumps into close apposition merely by mechanical means via negative pressure, are unique. However, to bridge larger gaps after experimental SCI, polymer scaffolds and/or cell transplantation are frequently used (Madigan et al., 2009; Li and Lepski, 2013). And nerve guidance channels are common to provide directional axon growth (Straley et al., 2010). While the directional growth is a reasonable goal, guidance channels have the disadvantage that, although they support axonal regeneration through the channel, the fibers usually have difficulties to exit the implants due to the presence of inhibitory scar tissue in the border regions. The microconnector system was designed to bypass such problems: in addition to mechanical stabilization of the lesion, the system allows pharmacological modulation of unwanted processes such as scar formation at any time point via drug infusion into the internal microchannel system.
Figure 1.
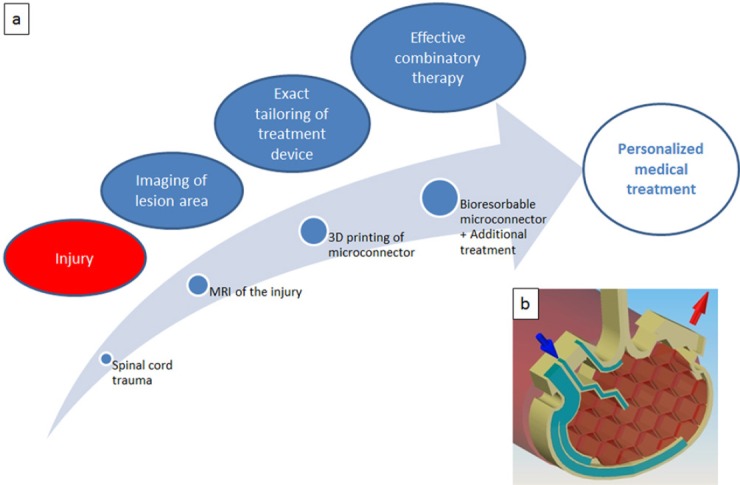
Mechanical adaptation as treatment for spinal cord injury (SCI) using a novel microconnector system.
(a) Hypothetical paradigm for a personalized SCI treatment using the implantable microconnector system for adaptation of spinal cord stumps. (b) Schematic representation of one half of the microconnector system; inlet port for infusion (blue) and outlet port for vacuum application (red) are marked with arrows. Note the honeycomb structures on the connector's inner surface, which are important for tissue anchorage.
The second approach, polyethylene glycol (PEG) biopolymer treatment (Figure 2), was developed in a rat model of chronic complete spinal cord transection injury to overcome larger tissue gaps (Estrada et al., 2014). Following the resection of the inhibitory scar tissue, PEG filling of the resection cavity resulted not only in the invasion of regeneration promoting cells such as endothelial and glial cells followed by the formation of a stable tissue bridge, but it also supported long-distance growth and (re-)myelination of axons accompanied by significant functional locomotor improvements of the hindlimbs. The use of PEG is often described for the “PEGylation” of agents and therapeutics since it facilitates the uptake of molecules, increases their solubility and decreases their immunogenicity and antigenicity. PEGs are often cross-linked into networks to form hydrogels due to their hydrophilic properties. The previously proposed mechanism of action of systemic PEG treatment after acute SCI in dogs and rodents is based on the fusogenic nature of PEG presumably leading to rapid fusion of axonal membranes thus reconstituting some morphological continuity of the transected spinal cord (Borgens and Shi, 2000). For the chronic PEG approach we propose other physicochemical properties of PEG 600 for its efficacy as a therapeutic. The fusion of membranes must definitely be ruled out as the mechanism of action in our chronic injury model, which comprises degeneration of the separated distal axon fragments, the removal of an entire segment of spinal cord tissue (containing fibrotic scar) and filling of the resulting gap with pure and undiluted PEG solution. Out of several PEGs with different molecular weights that were tested only PEG 600 led to the formation of a stable biomatrix in the resection cavity, which, in turn, resulted in the beneficial cellular invasion and functional outcome. Since the tested PEGs with different molecular weights led to different outcomes after their implantation into the injured spinal cord, it can be concluded that the biophysical properties of the material – most of all the viscosity – is crucial for the effectiveness. Moreover, in our paradigm PEG acts as the only active component of the treatment. Therefore, it presents a highly promising approach, which can easily be combined with other molecular and cellular treatments and may thus further enhance their efficacy.
Figure 2.
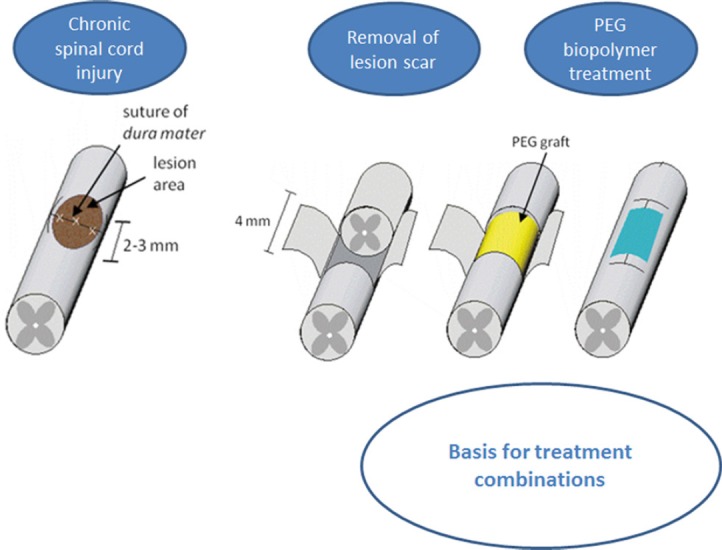
Biochemical treatment for spinal cord injury (SCI) with polyethylene glycol (PEG) implantation as a basis for future combinatory treatments.
Schematic representation of chronically injured spinal cord, tissue resection, and PEG grafting.
Personalized and combinatory treatments to improve the outcome of spinal cord injuries: The resection of tissue is a standard procedure for, e.g., the removal of tumors, and the resection of scar tissue after SCI has been tested preclinically and clinically. Both the microconnector as well as the PEG biopolymer are suitable to bridge the resulting tissue gap and they each have their specific advantages: While the microconnector brings separated spinal stumps into very close proximity again via low pressure mechanical bridging, PEG 600 biopolymer can – due to its biophysical properties - easily be applied also to larger and irregularly shaped tissue gaps, where it promotes, the formation of a tissue bridge via enhancing beneficial cellular invasion and regeneration processes.
The internal microchannel system that is integrated into the microconnector turns the device into an excellent tool for combinatory treatments. Via these internal channels therapeutics can be delivered directly into the lesion center (see schematic presentation Figure 1b). Thus, the connector holds great potential as a treatment device for personalized medical treatment for spinal cord trauma. While it has already proven a remarkable efficacy when composed of a rigid material (polymethylmethacrylate), on-going and future studies will make use of implantable microconnector systems composed of biodegradable material for a controlled degradation over time. Compared to rodent models, the SCI lesion gaps, which need to be bridged in large animal models and eventually in human patients, will be more extensive. The controlled degradation of the connector's material is necessary after the formation of a stable tissue bridge has developed since non-biodegradable long-term implants might cause late side effects and result in additional impairments. The microconnector can be adjusted in its size to fit between severed tissue stumps in both small and large animals or in patients, respectively. A further step into the direction of an optimal tool for personalized medical treatments, which will most likely be the future of the multi-symptom disease, is to customize the connector system according to the specific shape and size of the individual spinal lesion via bioprinting techniques. Ideally, the respective requirements for personalized design will be determined by imaging techniques, such as MRI scans, which will allow the precise representation of the position and shape of the severe injury. Finally, because of its unique properties the microconnector system is ideally suited for the application of combinatory therapies. The possibility of customizing the connector's shape and size makes it a useful medical tool not only for acute but also for chronic lesions after the resection of scar tissue. The different prospective steps of the so far hypothetical personalized medical treatment cascade are summarized in Figure 1a.
The efficacy of both treatments presented here needs to be further investigated in preclinical large animal models since the treatment success in rodent models of SCI does not necessarily imply the same outcome in larger species. Therefore, an extensive collaborative investigation to study the effects of the implantation of the microconnector system into the severely injured porcine spinal cord is currently in progress to analyze the cross-species transferability of the beneficial therapeutic effects from rodents into a large animal model.
Although the road to recovery is long and rocky, both the mechanical adaptation and the PEG biopolymer treatment – with the challenges and opportunities they entail - are promising new therapeutic concepts as both individually effective approaches offer the possibility and the basis for multiple innovative treatment combinations.
Work of the authors’ laboratory mentioned in this work has been funded by the DGUV (Deutsche Gesetzliche Unfallversicherung), BMBF (German Federal Ministry for Education and Research), DSQ (German Paraplegia Foundation), Manchot Foundation and Research Commission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf.
References
- Awai L, Curt A. Locomotor recovery in spinal cord injury: insights beyond walking speed and distance. J Neurotrauma. 2016;33:1428–1435. doi: 10.1089/neu.2015.4154. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000;14:27–35. doi: 10.1096/fasebj.14.1.27. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Brazda N, Estrada V, Voss C, Seide K, Trieu HK, Muller HW. Experimental strategies to bridge large tissue gaps in the injured spinal cord after acute and chronic lesion. J Vis Exp. 2016;110:e53331. doi: 10.3791/53331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazda N, Voss C, Estrada V, Lodin H, Weinrich N, Seide K, Muller J, Muller HW. A mechanical microconnector system for restoration of tissue continuity and long-term drug application into the injured spinal cord. Biomaterials. 2013;34:10056–10064. doi: 10.1016/j.biomaterials.2013.09.057. [DOI] [PubMed] [Google Scholar]
- Estrada V, Brazda N, Schmitz C, Heller S, Blazyca H, Martini R, Muller HW. Long-lasting significant functional improvement in chronic severe spinal cord injury following scar resection and polyethylene glycol implantation. Neurobiol Dis. 2014;67:165–179. doi: 10.1016/j.nbd.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Kabu S, Gao Y, Kwon BK, Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Control Release. 2015;219:141–154. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int 2013. 2013:786475. doi: 10.1155/2013/786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan NN, McMahon S, O’Brien T, Yaszemski MJ, Windebank AJ. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir Physiol Neurobiol. 2009;169:183–199. doi: 10.1016/j.resp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma. 2010;27:1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakow P, Raisman G, Fortuna W, Czyz M, Huber J, Li D, Szewczyk P, Okurowski S, Miedzybrodzki R, Czapiga B, Salomon B, Halon A, Li Y, Lipiec J, Kulczyk A, Jarmundowicz W. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23:1631–1655. doi: 10.3727/096368914X685131. [DOI] [PubMed] [Google Scholar]
- 2013. www.who.int . Fact sheet N°384 Spinal cord injury. In.


