
Keywords: nerve regeneration, brain injury, repetitive transcranial magnetic stimulation, cerebral infarction, low-frequency stimulation, high-frequency stimulation, upper-limb motor function, cerebral cortex, stroke rehabilitation, motor-evoked potential, central motor conduction time, primary motor cortex, neuroplasticity, neural reorganization, neural regeneration
Abstract
Studies have confirmed that low-frequency repetitive transcranial magnetic stimulation can decrease the activity of cortical neurons, and high-frequency repetitive transcranial magnetic stimulation can increase the excitability of cortical neurons. However, there are few studies concerning the use of different frequencies of repetitive transcranial magnetic stimulation on the recovery of upper-limb motor function after cerebral infarction. We hypothesized that different frequencies of repetitive transcranial magnetic stimulation in patients with cerebral infarction would produce different effects on the recovery of upper-limb motor function. This study enrolled 127 patients with upper-limb dysfunction during the subacute phase of cerebral infarction. These patients were randomly assigned to three groups. The low-frequency group comprised 42 patients who were treated with 1 Hz repetitive transcranial magnetic stimulation on the contralateral hemisphere primary motor cortex (M1). The high-frequency group comprised 43 patients who were treated with 10 Hz repetitive transcranial magnetic stimulation on ipsilateral M1. Finally, the sham group comprised 42 patients who were treated with 10 Hz of false stimulation on ipsilateral M1. A total of 135 seconds of stimulation was applied in the sham group and high-frequency group. At 2 weeks after treatment, cortical latency of motor-evoked potentials and central motor conduction time were significantly lower compared with before treatment. Moreover, motor function scores were significantly improved. The above indices for the low- and high-frequency groups were significantly different compared with the sham group. However, there was no significant difference between the low- and high-frequency groups. The results show that low- and high-frequency repetitive transcranial magnetic stimulation can similarly improve upper-limb motor function in patients with cerebral infarction.
Introduction
Cerebral infarction is a common, and frequently genetics-based, type of ischemic stroke that has a high rate of disability and mortality, and is one of the main conditions threatening health around the world. Patients often have differing degrees of dysfunction that can make activities of daily living difficult or impossible, requiring significant family and societal input. Thus, reducing morbidity and improving the quality of life in survivors is a priority. Recovery from cerebral infarction has attracted much attention in recent years (Weaver and Liu, 2015). Currently, a combination of exercise and physical therapy is considered the gold standard treatment for motor function recovery after stroke. However, even with high-intensity rehabilitation training, 55–75% of patients are left with upper-limb motor dysfunction (Hoyer et al., 2011), which significantly affects quality of life (Levin et al., 2009). Transcranial magnetic stimulation (TMS) has been shown to be beneficial for motor function recovery in hemiplegic patients after stroke by activating the cerebral cortex (Mansur et al., 2005; Alonso-Alonso et al., 2007; Koganemaru et al., 2010), achieved mainly by changing the magnetic field to an electric field, and passing a rapid current pulse through the stimulating coil (Pell et al., 2011; Li et al., 2016). The charged coil generates an electromagnetic field, which is not attenuated through the skin and skull, causing secondary currents produced by adjacent nerve tissue to polarize cerebral cortex neurons, thereby affecting brain activity (Thickbroom, 2007). Using repetitive stimulation can lead to restructuring of cortical neurons and improvement in upper-limb function, but the exact mechanism remains unclear (Simonetta-Moreau, 2014; Michelle et al., 2015).
Presently, there are three main types of TMS stimulation: single-pulse, double-pulse, and repetitive TMS (rTMS). rTMS was developed on the basis that TMS can affect the excitability of the cerebral cortex. Low-frequency (LF)-rTMS can produce cortical inhibition, and high-frequency (HF)-rTMS can increase cortical excitability (Fitzgerald et al., 2006). Chang et al. (2010) showed that HF-rTMS combined with exercise training can improve motor dysfunction in patients with subacute stroke. After 10 days of treatment, upper-limb muscle strength, motor function score, and grip strength improved significantly, with the effects lasting for 3 months after treatment.
rTMS has been widely used in neuroscience as a neural electrophysiological stimulation technique, and has the following advantages: it is painless, non-invasive, produces localized effects, and is simple and safe to use. In recent years, rTMS has become an effective method to improve upper-limb dysfunction after stroke (Emara et al., 2010). Until now, no study has used both HF- and LF-rTMS on different hemispheres. The purpose of this study was to investigate how different frequencies of rTMS affect upper-limb motor function in patients with subacute cerebral infarction. By comparing different efficacies and side effects among different groups, we aimed to clarify which treatment scheme was more suitable for patients with upper-limb dysfunction after cerebral infarction, and to provide a basis for further research and clinical application of rTMS.
Subjects and Methods
Subjects
A total of 153 patients with cerebral infarction, who met inclusion criteria, were admitted to our hospital for hemiplegia after stroke between September 2014 and December 2015. They were equally and randomly divided into three groups according to the consecutive order of admission using a random number table: LF (1 Hz)-rTMS group, HF (10 Hz)-rTMS group, and sham group. The sham group used a false coil (only noise, but no substantial stimulus effect). All patients were treated 5 days per week for 2 weeks.
This study was approved by the Clinical Trial Committee of the Affiliated Hospital of Qingdao University of China. This study was approved by the Hospital Ethical Committee and performed according to the Declaration of Helsinki. All patients gave informed consent prior to taking part in any procedure in this study.
Inclusion criteria
(1) Age between 30 and 80 years, with upper-limb dysfunction; (2) diagnosis of cerebral infarction based on the Fourth National Cerebrovascular Disease Conference (Chinese Society of Neurological Department of Internal Medicine, 1996); confirmed using computed tomography or magnetic resonance imaging; (3) vital signs stable; consciousness clear; no cognitive deficits; cooperation with assessment and treatment; (4) successful measurement of motor-evoked potential (MEP) from the ipsilateral primary motor cortex (M1); (5) patients or their families signed informed consent before the study.
Exclusion criteria
(1) Metallic object in the head, cardiac pacemaker, or cochlear implants; (2) history of seizure or epilepsy; (3) pregnancy, severe cognitive or communication dysfunction, or inability to express feelings; (4) refusal to sign the informed consent; could not carry out training or cooperate with assessments; (5) had any unstable disease that might affect participation.
Treatment methods
Instrument parameters and false coil: LF- or HF-rTMS was conducted using a magnetic stimulator, YRD CCY-I (YRD Company, Wuhan, China), connected to a prototype round coil; 125 mm in diameter and a peak magnetic field of 3.0 T. Parameters: the frequencies used were from 0.01 to 100 Hz, continuously adjustable; wave width was 340 ± 20 μs. We used a false coil (offered by YRD Company) for the sham stimulation, which delivered negligible magnetic output with audible click-on discharge. As the patients had not previously undergone TMS treatment, they were naturally blinded as to sham or actual treatment.
Conventional rehabilitation treatment: During the study, all patients received the same conventional rehabilitation treatment including occupational therapy for 40 minutes each day. Conventional physiotherapy (40 minutes to 1 hour daily, 5 times per week) included task-oriented training that involved active participation of the affected limb and individualized motor task training. Subsequently, exercise training, including active-assistive range of motion exercise of the affected extremity, holding, moving, releasing of cups and cubes, was administered by the same therapists.
rTMS treatment: The LF-rTMS group received 1-Hz stimulation to the “hot spot” of the primary cerebral cortex, i.e., M1 region, in the contralateral hemisphere at 80% of motor threshold. “Hot spots” were found by searching for loci that triggered maximum MEP amplitude in the contralateral abductor pollicis brevis (Jung et al., 2008). The HF-rTMS group received 10-Hz stimulation to the M1 hot spot in the ipsilateral cerebral hemisphere at 80% motor threshold. The sham group was given HF stimulation (10 Hz) through an attached false coil to the real coil, with a 5-cm gap. The patients heard a “ta ta” sound, but there was no stimulation.
During stimulation, the patient sat comfortably and relaxed, with the coil tangent to the skull over M1. The coil in the HF-rTMS group was placed on the hot spot in the ipsilateral cerebral hemisphere at 10-Hz stimulation for 1.5 seconds, followed by a 10-second rest, which was repeated 90 times, for a total of 1,350 stimulation pulses. The coil center was aligned to the hot spot, and the handle was perpendicular to the occipital side. The LF-rTMS group received 1-Hz stimulation for 10 seconds, followed by a 2-second rest, which was repeated 100 times, for 1,000 stimulation pulses. The coil was placed on the contralateral side on the same site as in the HF-rTMS group. In the sham group, the false coil was placed on the same site as the HF-rTMS group at the same frequency. Patients in each group were treated once per day for 20 minutes, 5 days per week (Monday to Friday), for 2 consecutive weeks.
Outcome assessment
The following neuro-electrophysiological indices and upper-limb function scores were assessed before and after the 2-week treatment in each group.
-
(1)
Upper-limb Fugl-Meyer assessment (FMA) motor function score (Sung et al., 2013): FMA was used to assess flexion of the shoulder, elbow, wrist, and hand. FMA comprises nine categories, 33 sub-items, and three grades (0–2). The total possible score is 66, where a higher score indicates better function of the upper-limb.
-
(2)
Wolf Motor Function Test (WMFT): The Motor Assessment Scale (MAS), based on the WMFT, was used to assess upper-limb function, coarse hand movement, and fine hand movement (Wolf et al., 2005). It is divided into six grades (0–5), with each grade being recorded as one point. A perfect score is 18, where a higher score indicates better function of the upper-limb.
-
(3)
MEP latency and central motor conduction time (CMCT): The magnetic field coil was used to stimulate the motor cortex and spinal nerve root. We recorded the incubation period in the target muscle. MEP latency was recorded from electrodes placed over the contralateral abductor pollicis brevis muscle in the upper-limb, with suprathreshold intensity stimulation administered to the contralesional M1. After five repetitions that generated large amplitude waveforms, mean MEP latency was calculated. CMCT was equal to motor cortex incubation period–spinal nerve root incubation period (Ma et al., 2011).
Statistical analysis
Data are presented as the mean ± SD, and were analyzed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). The data were checked for normal distribution. Inter-group comparisons after treatment were conducted using one-way analysis of variance with the least significant difference post-hoc test. Paired t-tests were used for intra-group comparisons before and after treatment. A value of P < 0.05 was considered statistically significant.
Results
Quantitative analysis of subjects
The trial enrolled 227 patients with cerebral infarction with informed consent. According to the inclusion and exclusion criteria, some patients were excluded: inclusion criteria not met (n = 21); excluded (n = 32); refused participation (n = 10); other reasons (n = 11). Finally, 153 patients were selected, and were equally and randomly divided into three groups. Twenty-six patients were withdrawn from the trial for various reasons. Among them, seven patients received botulinum toxin injection, nine patients were discharged from hospital for personal and domestic reasons, six patients felt no effect and requested to withdraw, and four patients were unable to tolerate the pain caused by stimulation. The remaining 127 patients completed the trial and were included in statistical analyses (Figure 1). Sex, age, interval from stroke onset, and affected hemisphere did not differ between the three groups (P > 0.05; Table 1). There were 42 cases in the LF-rTMS group, 43 in the HF-rTMS group, and 42 in the sham group. No significant differences in assessment indices were found between the groups before treatment (P < 0.05; Table 2).
Figure 1.
Study flow chart.
LF-rTMS: Low-frequency repetitive transcranial magnetic stimulation; HF-rTMS: high-frequency repetitive transcranial magnetic stimulation; MEP: motor-evoked potential; CMCT: central motor conduction time; FMA: Fugl-Meyer assessment; WMFT: Wolf Motor Function Test.
Table 1.
Baseline patient data

Table 2.
Upper limb function and neuro-electrophysiological parameters in the three groups before and after treatment
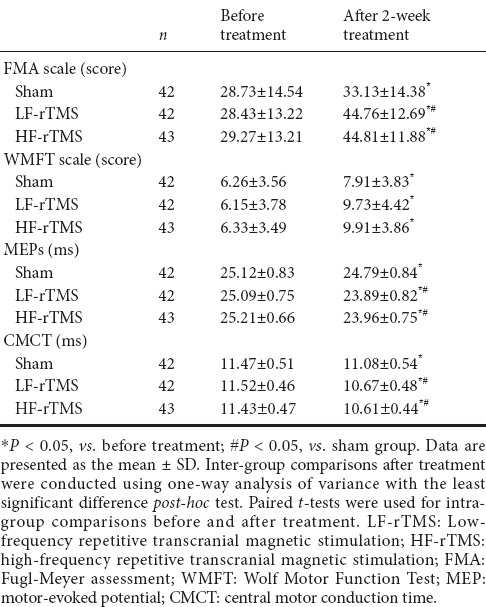
After 2 weeks of treatment, MEP latency and CMCT in the HF-rTMS and LF-rTMS groups were significantly shorter compared with pre-treatment, and significantly better compared with the sham group (P < 0.05), but not significantly different from each other (P > 0.05). In addition, MEP latency and CMCT in the sham group were significantly shorter after treatment compared with before treatment (P < 0.05).
Changes in upper-limb function
After 2 weeks of treatment, FMA scores significantly increased in the rTMS treatment groups compared with the sham group (P < 0.05). FMA scores were better in the rTMS treatment groups compared with the sham group (P < 0.05). However, there were no significant differences between the HF-rTMS and LF-rTMS groups (P > 0.05). FMA scores in the upper-limb significantly increased in all groups after treatment (P < 0.05), but did not differ from each other (P > 0.05; Table 2).
During treatment, no adverse reactions to LF-rTMS were reported, but a few patients in the HF-rTMS group experienced numbness in the scalp and facial muscles at the stimulation site during the first few sessions of therapy. Numbness was bearable, and disappeared when stimulation stopped. No special treatment was needed and patients continued until finished. No seizures occurred in any group.
Discussion
Upper-limb dysfunction is a common sequela of patients with cerebral infarction. Recovery from upper-limb dysfunction is slower compared with recovery from lower-limb dysfunction. Particularly, fine motor recovery often takes a long time, and rehabilitation is not always satisfactory (Ohman et al., 2008). Thus, patients and their families can lose confidence in the rehabilitation process. The rehabilitation sessions also tend to focus on rehabilitation of lower-limb function, ignoring upper-limb function training to some extent.
Improvement in motor dysfunction is accompanied by enhanced cortical excitability. Previous studies have demonstrated that one mechanism of motor dysfunction after stroke was due to excessive transcallosal inhibition of the affected hemisphere by the unaffected hemisphere (Demirtas-Tatlidede et al., 2015). Normally, individuals maintain a balance between the two cerebral hemispheres through interactive inhibition. After cerebral infarction, this physiological balance is disturbed and the affected hemisphere is weakened by continued inhibition from the unaffected hemisphere (Mello et al., 2015). Reciprocally, reduced inhibition by the affected hemisphere leads to enhanced excitability of the unaffected hemisphere. Increasingly, evidence suggests that disturbing the mutual inhibition of the cerebral hemispheres causes decompensation of the central nervous system (Simis et al., 2013).
rTMS is the repetition of clusters of regular TMS pulse stimulation. Previous studies have shown that rTMS can regulate the excitability of the cerebral cortex (Cassidy et al., 2005), and can rebalance cortical excitability of both hemispheres (Cassidy et al., 2005; Hsu et al., 2012), thus promoting recovery of motor function after cerebral infarction. LF-rTMS of the non-lesioned hemisphere has an inhibitory effect (Fregni et al., 2006), and HF-rTMS to the affected hemisphere has an excitatory effect (Khedr et al. 2009, 2010). In this study, we showed that cortical excitability of LF-rTMS and HF-rTMS groups was significantly improved after treatment compared with the sham group.
Because stimulation frequency is one of the most important parameters, many studies have compared low-frequency stimulation of the contralateral hemisphere with high-frequency stimulation of the affected side, and then observed efficacy and side effects of different stimulus frequencies (Mongabadi et al., 2013; Sasaki et al., 2014). Presently, many studies use low-frequency subthreshold intensity stimulation, because it not only improves motor function, but is low-risk and well tolerated (Kakuda et al., 2010). In this study, we used 1 Hz low-frequency stimulation in line with current research (Yin et al., 2014). Some reports have shown that high-frequency stimulation at certain intensities can induce seizures, and the risk of adverse events is higher with high-frequency stimulation (Lomarev et al., 2007). However, Rossi et al. (2009) demonstrated that high-frequency stimulation parameters (intensity, frequency, stimulation time, and time interval) in published safety guidelines were safe, and could increase the excitability of the cerebral cortex on the affected side.
In a prior study, one daily session of rTMS for 5 consecutive days was administered to nine patients in the high-frequency (10 Hz) group, and 11 patients in the low-frequency (1 Hz) group. The high-frequency group showed significantly greater improvement compared with the low-frequency group (Sasaki et al., 2013). Nevertheless, the present study shows that neurophysiological scores in the three groups improved after treatment, suggesting that both rTMS and conventional regular therapy are effective ways to promote functional recovery in patients with cerebral infarction. We also showed that functional recovery in the two rTMS groups improved more significantly compared with the sham group; their levels of improvement were not comparable with each other. This indicates that low-frequency contralateral rTMS and high-frequency ipsilateral rTMS can have equal effects, consistent with the results reported by Kim et al. (2014). The possible reason may be that both inhibiting contralesional M1 with low-frequency stimulation and exciting ipsilateral M1 with high-frequency stimulation can reset the balance of transcallosal inhibition, which in turn improves the function of patients with upper-limb motor dysfunction.
In addition to physiological measurements, upper-limb FMA scores improved in the three groups after treatment, and significantly improved in the actual rTMS treatment groups. The improvement was not different between the LF-rTMS and HF-rTMS groups. These findings suggest that these frequencies of rTMS promote motor function recovery of the upper-limbs with equal efficacy.
We did not find any significant differences in upper-limb MAS scores among any of the three groups. This might be because the upper-limb portion of the MAS scale requires recovery of fine motor skills, and is more difficult compared with the FMA scale portion of the upper-limb assessment. In addition, the small sample size and short treatment period may also have contributed to the lack of significant differences.
Despite the negative findings with respect to MAS scores, this study shows that the effects of high- and low-frequency rTMS on electrophysiological parameters of patients with cerebral infarction were positively correlated with upper-limb FMA scores. This result indicates that rTMS is an effective treatment method in patients with cerebral infarction (van Kuijk et al., 2009).
Both HF-rTMS and LF-rTMS therapies have a significant effect on the recovery of motor function in patients with cerebral infarction. To date, reports on the disadvantages of rTMS are few. However, in some patients, rTMS might increase the risk of seizure (Lomarevm et al., 2007). Therefore, indications and contraindications should be strictly controlled in patients receiving clinical rTMS treatment. In this study, no patient experienced seizure or aggravation during treatment, and the stimulation parameters adopted for the study were considered safe. Indeed, rTMS can be considered a safe and efficient method for treatment of cerebral infarction.
In conclusion, both HF- and LF-rTMS are equally effective methods for improving motor function in the upper-limbs of patients with cerebral infarction. As there were the limitations of small sample size, short treatment period, and differences among individual patients, further studies are required to verify the findings reported here. Additionally, further double-blinded clinical study and exploration should be undertaken to validate the therapeutic approach, and find optimal stimulation parameters.
Acknowledgments
We are very grateful to several colleague therapists of the Rehabilitation Medicine Department of the Affiliated Hospital of Qingdao University of China for their support and selfless help.
Footnotes
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Barrett R, Wysong S, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovasc Dis 24 Suppl. 2007;1:157–166. doi: 10.1159/000107392. [DOI] [PubMed] [Google Scholar]
- Cassidy JM, Chu H, Anderson DC, Krach LE, Snow L, Kimberley TJ, Carey J. A comparison of primed low-frequency repetitive transcranial magnetic stimulation treatments in chronic stroke. Brain Stimul. 2015;8:1074–1084. doi: 10.1016/j.brs.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. Rehabil Med. 2010;42:758–764. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- Chinese Society of Neurological Department of Internal Medicine. The society of the Chinese Department of Neurosurgery (1996) Diagnostic key points of all kinds of cerebral vascular diseases. Zhonghua Shenjingke Zazhi. 29:379–381. [Google Scholar]
- Demirtas-Tatlidede A, Alonso-Alonso M, Shetty RP, Ronen I, Pascual-Leone A, Fregni F. Long-term effects of contralesional rTMS in severe stroke: safety, cortical excitability, and relationship with transcallosal motor fibers. NeuroRehabilitation. 2015;36:51–59. doi: 10.3233/NRE-141191. [DOI] [PubMed] [Google Scholar]
- Emara TH, Moustafa RR, Elnahas NM, Elganzoury AM, Abdo TA, Mohamed SA, Eletribi MA. Repetitive transcranial magnetic stimulation at 1 Hz and 5 Hz produce sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17:1203–1209. doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5 day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci. 2011;29:395–409. doi: 10.3233/RNN-2011-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- Jaiser SR, Barnes JD, Baker SN, Baker MR. A multiple regression model of normal central and peripheral motor conduction times. Muscle Nerve. 2015;51:706–712. doi: 10.1002/mus.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Shin JE, Jeong YS, Shin HI. Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol. 2008;119:71–79. doi: 10.1016/j.clinph.2007.09.124. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Uruma G, Kaito N, Watanabe M. Low frequency rTMS with language therapy over a 3-month period for sensory-dominant aphasia: case series of two post-stroke Japanese patients. Brain Inj. 2010;24:1113–1117. doi: 10.3109/02699052.2010.494587. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of l and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Eur J Neurol. 2009;16:1323–1330. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121:30–37. doi: 10.1111/j.1600-0404.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- Kim C, Choi HE, Jung H, Lee BJ, Lee KH, Lim YJ. Comparison of the effects of 1 Hz and 20 Hz rTMS on motor recovery in subacute stroke patients. Ann Rehabil Med. 2014;38:585–591. doi: 10.5535/arm.2014.38.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Thabit MN, Ikkaku T, Shimada K, Kanematsu M, Takahashi K, Fawi G. Recovery of upper limb function due to enhanced use-dependent plasticity in chronic stroke patients. Brain. 2010;133:3373–3384. doi: 10.1093/brain/awq193. [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in atients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang XW, Zuo ZT, Lu J, Meng CL, Fang HY, Xue R, Fan Y, Guan YZ, Zhang WH. Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: an fmri study. CNS Neurosci Ther. 2016 doi: 10.1111/cns.12593. doi: 10.1111/cns.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomarev MP, Kim DY, Richardson SP, Voller B, Hallett M. Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clin Neurophysiol. 2007;118:2072–2075. doi: 10.1016/j.clinph.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Koblar S, Ward NS, Rothwell JC, Hordacre B, Ridding MC. An investigation of cortical neuroplasticity following stroke in adults: is there evidence for a critical window for rehabilitation? BMC Neurol. 2015;15:109. doi: 10.1186/s12883-015-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello EA, Cohen LG, Monteiro Dos Anjos S, Conti J, Andrade KN, Tovar Moll F, Marins T, Fernandes CA, Rodrigues W. Increase in short-interval intracortical facilitation of the motor cortex after low-frequency repetitive magnetic stimulation of the unaffected hemisphere in the subacute phase after stroke. Neural Plast 2015. 2015:407320. doi: 10.1155/2015/407320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongabadi S, Firoozabadi SM, Javan M, Shojaei A, Mirnajafi-Zadeh J. Effect of different frequencies of repetitive transcranial magnetic stimulation on acquisition of chemical kindled seizures in rats. Neurol Sci. 2013;34:1897–1903. doi: 10.1007/s10072-013-1401-1. [DOI] [PubMed] [Google Scholar]
- Ohman A, Kull L, Andersson J, Flygare L. Radiation doses in examination of lower third molars with computed tomography and conventional raldiography. Dentomaxillofac Radiol. 2008;37:445–452. doi: 10.1259/dmfr/86360042. [DOI] [PubMed] [Google Scholar]
- Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93:59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Kakuda W, Abo M. Bilateral high- and low-frequency rTMS in acute stroke patients with hemiparesis: a comparative study with unilateral high-frequency rTMS. Brain Inj. 2014;28:1682–1686. doi: 10.3109/02699052.2014.947626. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. Stroke Cerebrovasc Dis. 2013;22:413–418. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Simis M, Adeyemo BO, Medeiros LF, Miraval F, Gagliardi RJ, Fregni F. Motor cortex-induced plasticity by noninvasive brain stimulation: a comparison between transcranial direct current stimulation and transcranial magnetic stimulation. Neuroreport. 2013;24:973–975. doi: 10.1097/WNR.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M. Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann Phys Rehabil Med. 2014;57:530–542. doi: 10.1016/j.rehab.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Sung WH, Wang CP, Chou CL, Chen YC, Chang YC, Tsai PY. Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke. 2013;44:1375–1382. doi: 10.1161/STROKEAHA.111.000522. [DOI] [PubMed] [Google Scholar]
- Thickbroom G. Transcranial magnetic stimulation and synaptic plasticity:experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- van Kuijk AA, Pasman JW, Hendricks HT, Zwarts MJ, Geurts AC. Predicting hand motor recovery in severe stroke:the role of motor evoked potentials in relation to early clinical assessment. Neurorehabil Neural Repair. 2009;23:45–51. doi: 10.1177/1545968308317578. [DOI] [PubMed] [Google Scholar]
- Weaver J, Liu KJ. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med Gas Res. 2015;5:11. doi: 10.1186/s13618-015-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Thompson PA, Morris DM, Rose DK, Winstein CJ, Taub E, Giuliani C, Pearson SL. The excite trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- Yin ZF, Shen Y, Meng DH, Hou H, Dai WJ, Li JA. The effectiveness of low-frequency transcranial magnetic stimulation for restoring upper limb function after cerebral infarction. Zhonghua Wulixue yu Kangfu Zazhi. 2014;36:596–600. [Google Scholar]



