Abstract
Hydroxycitric acid (HCA) is derived primarily from the Garcinia plant and is widely used for its anti-inflammatory effects. Multiple sclerosis can cause an inflammatory demyelination and axonal damage. In this study, to validate the hypothesis that HCA exhibits therapeutic effects on multiple sclerosis, we established female C57BL/6 mouse models of multiple sclerosis, i.e., experimental autoimmune encephalomyelitis, using Complete Freund's Adjuvant (CFA) emulsion containing myelin oligodendrocyte glycoprotein (35–55). Treatment with HCA at 2 g/kg/d for 3 weeks obviously improved the symptoms of nerve injury of experimental autoimmune encephalomyelitis mice, decreased serum interleulin-6, tumor necrosis factor alpha, nitric oxide, and malondialdehyde levels, and increased superoxide dismutase and glutathione reductase activities. These findings suggest that HCA exhibits neuroprotective effects on multiple sclerosis-caused nerve injury through ameliorating inflammation and oxidative stress.
Keywords: nerve regeneration, hydroxycitric acid, multiple sclerosis, inflammation, oxidative stress, experimental autoimmune encephalomyelitis, neural regeneration
Introduction
Multiple sclerosis (MS) is a common and chronic central nervous system (CNS) demyelinating disease and a leading cause of permanent disability. MS is characterized by the destruction of the myelin sheath that surrounds neuronal axons in the CNS, a process that results in neurodegeneration and consequently the formation of sclerotic plaques in the brain (Steinman, 2001). Experimental autoimmune encephalomyelitis (EAE) is the most frequently used model for studying MS. EAE can cause inflammation and demyelination similar to disease manifestation seen in humans (Peiris et al., 2007). Adaptive and innate immune cells activate and infiltrate the CNS where they act synergistically in inducing and perpetuating local inflammation and demyelination (Huitinga et al., 1995). The activated microglia and the release of molecules such as matrix metalloproteinases (MMPs) and pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β and IL-6, which are detrimental to oligodendrocytes have been suggested as mechanisms by which innate immunity proceeds demyelination in EAE (Sriram, 2011). Another type of innate immune cell that plays a pivotal role in EAE pathogenesis is the macrophage (Huitinga et al., 1995). Macrophages contribute to demyelination by promoting immune cell infiltration and inflammation in the CNS by secreting an array of pro-inflammatory mediators, including IL-6 and TNF-α which exerts neurotoxic and chemoattractant effects on the CNS (Mirshafiey et al., 2014b). The participation of these cytokines in the demyelinating process is probably due to the perpetuation of macrophage and microglial activation (Taupin et al., 1997). In addition, oxidative stress could contribute to the pathogenesis of MS. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) have been demonstrated to be created in the CNS of MS patients mainly by activated macrophages and microglia structures responsible for demyelination and axonal disruption (Lu et al., 2000; Miller et al., 2013). Circumstantial evidence suggests that nitric oxide (NO) plays a role in several features of the MS disease, including disruption of the blood-brain barrier (BBB), oligodendrocyte injury and demyelination, and axonal degeneration, and that it contributes to the loss of function by causing impairment of axonal conduction (Smith and Lassmann, 2002). Antioxidants decrease the expression of inflammation related molecules such as inducible nitric oxide synthase (iNOS) and nitrotyrosine, a marker of peroxynitrite reactivity in the CNS of EAE mice (Moriya et al., 2008). Dietary antioxidants are well known for their antioxidant activity, but recent findings indicate that they may have additional properties independent of their roles as antioxidants and radical scavengers (Riccio et al., 2011). Antioxidants possess significant therapeutic potential for the treatment of inflammatory disorders. Antioxidants are present in a variety of plants and promote health by removing damaging free radicals from the cellular environment and inhibiting the production of inflammatory mediators (Lu et al., 2009).
Garcinia extract is an herbal preparation and a safe dietary supplement. Its active ingredient is hydroxycitric acid (HCA) which has been traditionally used for weight loss and blood cholesterol reduction in humans (Kim et al., 2011). Recently, HCA has been reported to reduce the inflammatory mediators (Sripradha and Magadi, 2015) and oxidative stress determinants (Amin et al., 2011b) in several experimental models of autoimmune and inflammatory diseases including inflammatory bowel disease (dos Reis et al., 2009), gastric ulcer (Mahendran et al., 2002), type 2 diabetes (Asghar et al., 2007), and oxidative dysfunction of the brain (Amin et al., 2011a). Therefore, the objective of this study was to investigate the anti-inflammatory and anti-oxidative effects of HCA in experimental models of MS.
Materials and Methods
Animals
Twenty-four C57BL/6 male mice, aged 10 weeks, weighing 20–22 g, were obtained from the Experimental Animal Center of Pasture Institute of Iran. The mice were randomly separated into three groups: normal intact (no EAE induction and no treatment), EAE induction (EAE induction only) and HCA treated (EAE induction followed by HCA administration), with 8 mice in each group. All mice were housed in cages under 12-hour light/dark cycle and with free access to food and water. All procedures involving animals were approved by Animal Ethics Committee of Tehran University of Medical Sciences, Iran.
EAE induction and therapeutic protocol
All mice were daily weighed starting from the first day of the adaptation to the end of the experiment, and their weights were recorded. EAE induction was performed using a Hooke Kit (Hooke Laboratories, Inc., Lawrence, MA, USA). The EAE induction kit consisted of two components: antigen (MOG35-55) in an emulsion with Complete Freund's Adjuvant (CFA) in two pre-filled syringes, and a vial of lyophilized pertussis toxin (PTX). The lyophilized PTX was dissolved in PBS on day 0. The mice were subcutaneously injected with 0.1 mL of emulsion each site on the upper back and lower back, respectively. Approximately 2 hours after injection of the emulsion, the first dose of PTX (0.1 mL/mouse) was injected intraperitoneally (IP). This was repeated 24 hours later as the second dose of PTX (0.1 mL/mouse).
The garcinia extract (SanHerb Biotech Inc., Chengdu, Sichuan Province, China) used in this study contained 50%(-)-hydroxycitric acid. The (-)-hydroxycitric acid content was determined by titration against 1 M NaOH. The mice in the EAE induction and HCA treated groups were administered orally with vehicle (sterile water) and HCA, respectively at the specified dose of 2 g/kg/mouse (0.2 mL) once daily, for 3 weeks, from day 0 after immunization through animal-feeding needles. The mice were monitored daily and assessed by clinical score (visual cumulative clinical scoring system, the mice scored higher than 3 in EAE induction group were regarded as the successful models) based on Hooke Laboratories recommendation. Clinical score of EAE mice was defined as follows: 0, no clinical sign; 0.5, paralysis of the tail tip; 1, complete paralysis of the tail; 1.5, complete paralysis of the tail and inhibition of hindlimbs; 2, complete paralysis of the tail and numbness of the hindlimbs, hindlimbs held together when lifting them by the tail tips; 2.5, complete paralysis of the tail, dragging the hindlimbs when moving; 3, complete paralysis of the tail and hindlimbs, and/or paralysis of the tail and one of the hindlimbs and one of the forelimbs; 3.5, complete paralysis of the tail and hindlimbs; 4, complete paralysis of the tail and hindlimbs and partial paralysis of the forelimb; 4.5, complete paralysis of the tail and hindlimbs and forelimbs, no movement (sacrifice at this time is proposed); 5, dead because of paralysis.
Histopathological examination
On day 21 after immunization, all mice were anesthetized and blood samples were taken from their hearts. For histopathological evaluation, mice were sacrificed following anesthesia, and the brains from normal, EAE induction, and HCA treated mice were removed and fixed in neutral 10% formalin, embedded in paraffin, sectioned (8 μm thick) and then stained with hematoxilin-eosin (H&E) for counts of meningeal and parenchymal inflammatory foci and with Luxol fast blue (LFB) stain to distinguish demyelination (Azizi et al., 2014). All sections were observed by an expert pathologist blinded to the study under optical microscopy (Olympus, Tokyo, Japan). The following criteria were used for scoring: 0 = no symptoms; 1 = very mild; 2 = mild; 3 = moderate; 4 = severe; 5 = very severe.
NO assay
NO in the serum samples was assessed by Griess method (Amin et al., 2011a) based on the assessment of the end product of nitrite. Griess method uses a colorimetric reaction for measuring the NO2– (nitrite) level in aqua solution. Griess reagent was prepared by solving 1g sulfanilamide in 100 mL 5% phosphoric acid mixed with 0.1 g naphtyl ethylene diamine-HCl (NED) in 100 mL distilled water. Serum sample (50 μL) was mixed with 50 μL of Griess reagent at room temperature for 10 minutes. Absorbance at 540 nm was measured using ELISA reader instrument (BioTek Instruments, Inc., Winooski, VT, USA). Concentration of nitrite was determined by standard curve of 0.1 M sodium nitrite in distilled water.
Total antioxidant capacity
Total antioxidant capacity was determined based on ABTS radical cation2,29-azino-bis (3-ethylbenzothiazoline-6- sulfonic acid) scavenging. ABTS radical cation is blue/green, but loses its color after reduction. ABTS (7 mM) was mixed with potassium persulfate (2.5 mM) in water and then stored in the dark at room temperature for 12 hours. Stock solution was diluted in PBS to absorbance of 0.70 ± 0.02 at 734 nm to prepare working solution. 20 μL of serum and 2 mL of ABTS radical working solution were mixed in a plastic cuvette. Reducing optical absorbance was monitored by spectrophotometry and expressed as the percentage of radical inhibition. Bocine serum albumin (BSA) instead of Trolox was used. To convert the inhibition percentage to g/dL, BSA standard curve was used.
Super oxide dismutase (SOD) activity
A ZellBio GmbH SOD kit (Biocore Diagnostik, Ulm, Germany) was used to perform SOD assay in serum of all mice. This kit can be used for SOD activity determination in the range of 5–100 U/mL with 1 U/mL sensitivity. In this assay, SOD activity unit was considered as the amount of the sample that catalyzes decomposition of 1 μmol of O2– to H2O2 and O2 in 1 minute. The final activity of SOD was determined calorimetrically at 420 nm.
Glutathione reductase (GR) assessment
A ZellBio GmbH (Biocore Diagnostik) kit was used to assay GR. This kit can be used for activity determination in the range of 10–15 U/L with 1U/L sensitivity. The GR activity was determined photometrically at 340 nm.
Quantification of malondialdehyde (MDA)
Lipid peroxidation was estimated according to a previous report (Satoh, 1978). The principle of the method was based on spectrophotometric measurement. The complex of thiobarbituric acid (TBA) dissolved in sodium sulfate and MDA was created. After heating, the chromogenic (thiobarbituric acid (TBA) reactive substances (TBARS)) complex was extracted by n-butyl alcohol and then solid phase was checked by spectrophotometry (Shimadzu, Kanagawa, Japan) at a wavelength of 530 nm. 1,1,3,3-Tetramethoxypropane in sulfuric acid was used as a standard solution. MDA concentration in nmol/mL was calculated from standard curve from 1,1,3,3 tetraethoxypropane.
Quantification of TNF-α
Serum level of TNF-α was measured in normal, EAE induction, and HCA treated mice using enzyme-linked immunosorbent assay (Azizi et al., 2014). To evaluate TNF-α, we used R&D kits (R&D Systems, Inc. Minneapolis, MN, USA). All assays were performed according to the manufacturer's instructions. Absorbance at 450 nm was read in a 96-well microplate ELISA reader (BioTek Instruments, Inc.).
Quantification of IL-6
ELISA assay was used to measure serum level of IL-6 in all groups using a LEGEND MAX™ Mouse IL-6 ELISA Kit (Biolegend, Inc. San Diego, CA, USA). The complete kit contained 96-well strip plates pre-coated with a capture antibody which was used to measure serum level of IL. ELISA assay was performed according to the manufacturer's instructions. Absorbance at 450 nm was read in a 96-well microplate ELISA reader (BioTek Instruments, Inc.).
Statistical analysis
Data were expressed as the mean ± SEM, except for histological scores, which were calculated as the mean ± SD. Statistical analysis was performed by the Mann-Whitney U test for nonparametric data whereas Student's t-test and one-way analysis of variance were used for parametric data. A P value of < 0.05 was considered statistically significant.
Results
Clinical findings
The clinical course and severity of the disease differed between HCA treated and EAE induction groups. The mean severity score of the disease was higher in the EAE induction group than in the HCA treated group (Figure 1). Also, EAE incidence was lower (7 out of 8 mice), and EAE onset was delayed in HCA treated group than in EAE induction group (8 of 8 mice; Figure 2). These effects led to significant clinical improvement and delayed disease progression during 21 days of observation, indicating that HCA can inhibit the progression of EAE.
Figure 1.
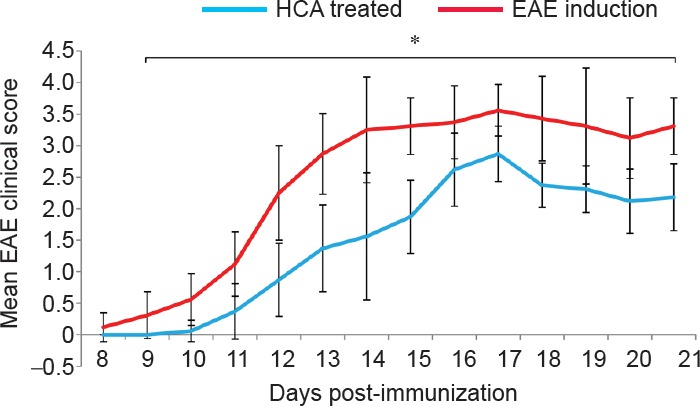
Effect of hydroxycitric acid (HCA) on clinical course and disease severity of experimental autoimmune encephalomyelitis (EAE) mice.
Female C57BL/6 mice (n = 8) were orally administered HCA (2 g/kg/d) from day 0 to day 20 after immunization in the HCA treated group. Disease severity was assessed by a visual cumulative scoring system. Lower scores indicate less severe symptoms. Cumulative scores from day 8 to day 21 after immunization are given as the mean ± SEM. *P < 0.05 at each time point was considered between HCA and EAE induction groups by Mann-Whitney U test.
Figure 2.
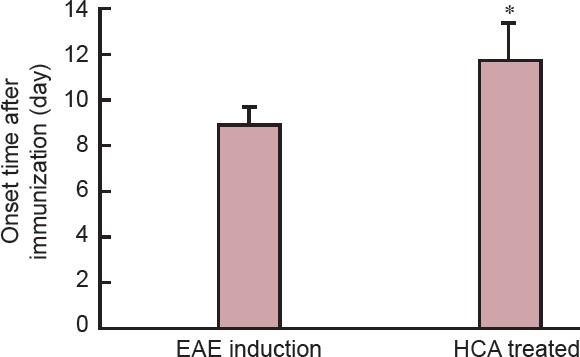
Effect of hydroxycitric acid (HCA) on experimental autoimmune encephalomyelitis (EAE) in mice.
After immunization, C57BL/6 mice (n = 8) were orally administered HCA. In the HCA treated group, the onset of EAE was significantly delayed compared with that in the EAE induction group. Data analysis was performed using Student's t-test. Data are expressed as the mean ± SEM. *P < 0.01, vs. EAE induction group.
Histological findings
Representative images of hematoxylin-eosin and Luxol fast blue-stained tissue sections from all groups demonstrated that inflammation and demyelination were significantly milder in the HCA treated group than in the EAE induction group (Figure 3). The results illustrated in Tables 1, 2 indicate that the severity of inflammation in the cerebellum and cerebrum observed by histopathology was consistent with the clinical symptoms of mice in the HCA treated and EAE induction groups.
Figure 3.
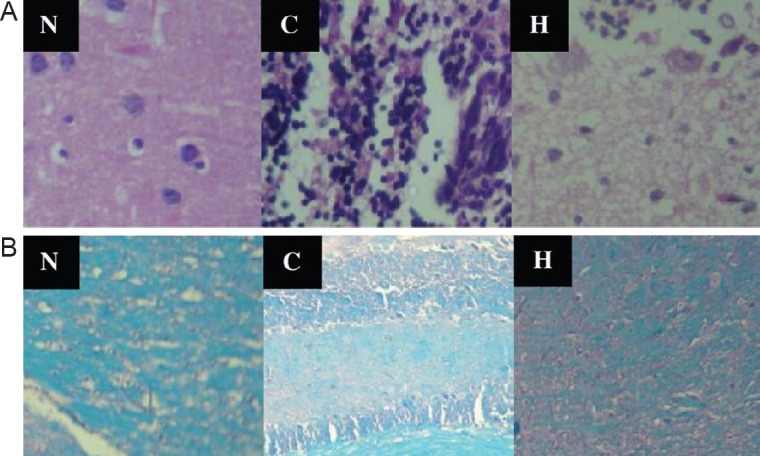
Representative optical microscopy images of the brain slices in different groups on day 21 after immunization.
(A) Hematoxylin-eosin staining of brain slices showed that HCA treatment suppressed the progression of inflammation (arrows) obviously by restricting leukocyte infiltration (magnification × 100). (B) Luxol fast blue staining showed less demyelination sites (arrows) in the HCA treated group than in the EAE induction group (magnification × 40). N: Normal group; C: control group (EAE induction only); H: HCA treated group (EAE induction followed by HCA treatment). HCA: Hydroxycitric acid; EAE: experimental autoimmune encephalomyelitis.
Table 1.
Histopathological finding in the cerebrum of experimental autoimmune encephalomyelitis mice on day 21 after immunization
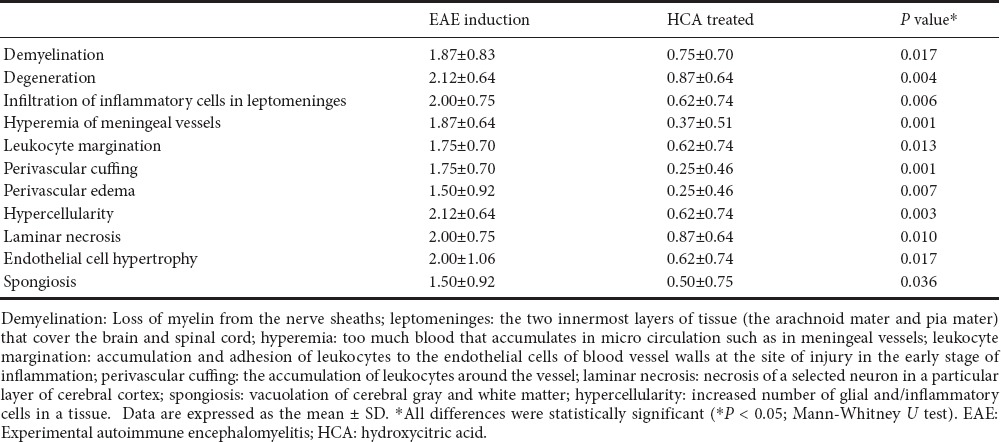
Table 2.
Histopathological findings in the cerebellum of experimental autoimmune encephalomyelitis mice on day 21 after immunization
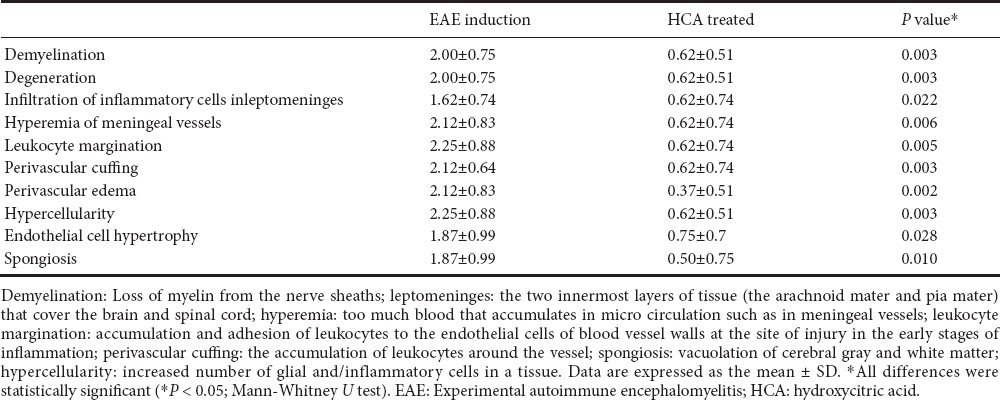
Antioxidants and oxidative stress status
At 21 days after EAE induction, as shown in Table 3, NO production was significantly reduced in HCA treated group than in EAE induction group (P = 0.025). Serum level of HCA was significantly increased in the HCA treated group than in the EAE induction group (P = 0.009). As shown in Table 3, SOD activity was increased in the HCA treated group than in the EAE induction group (P = 0.005). GR activity was also increased in the HCA treated group, however, this increase was not significant compared to that in the EAE induction group (P = 0.507). HCA treatment resulted in a decrease in serum MDA concentration. As shown in Table 3, serum MDA concentration was decreased in the HCA treated group than in the EAE induction group (P = 0.021). Therefore, HCA treatments decreased serum NO and MDA concentrations but increased serum total antioxidant capacity, which are consistent with clinical findings.
Table 3.
Serum total antioxidant capacity, super oxide dismutase and glutathione reductase activities, and malondialdehyde and nitric oxide in mice on day 21 after immunization
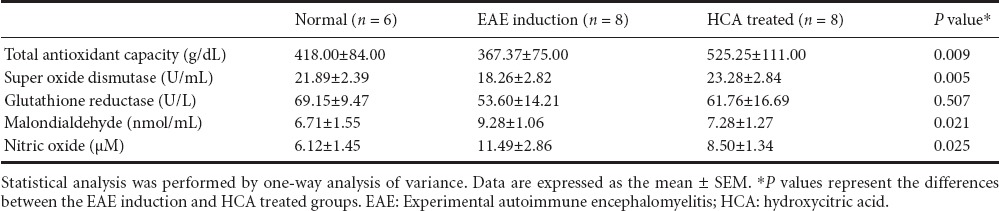
Serum levels of TNF-α and IL-6
Serum levels of TNF-α and IL-6 were significantly decreased in the HCA treated group than in the EAE induction group (P = 0.001 for TNF-α; P = 0.012 for IL-6) (Figures 4, 5).
Figure 4.
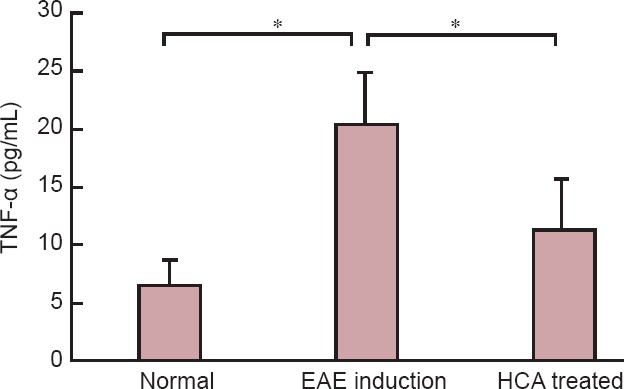
Inhibitory effects of HCA on serum TNF-α level.
TNF-α measurement was performed in normal, EAE induction, and HCA treated groups on day 21 after immunization. HCA treatment significantly reduced TNF-α production. Data are expressed as the mean ± SEM. *P < 0.01 (one-way analysis of variance). EAE: Experimental autoimmune encephalomyelitis; HCA: hydroxycitric acid; TNF-α: tumor necrosis factor alpha.
Figure 5.
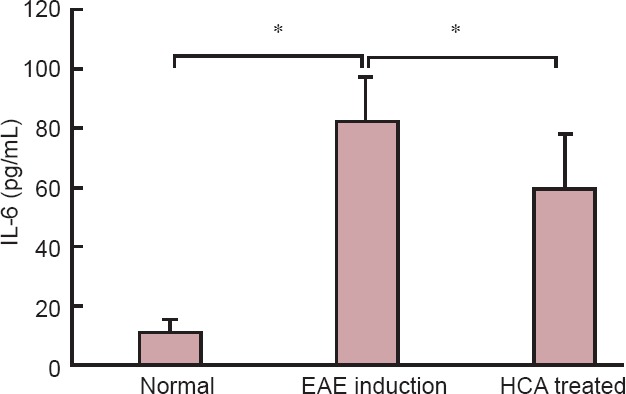
Effect of HCA on serum IL-6 level on day 21 after immunization.
Serum IL-6 level was significantly decreased in the HCA treated group than in the EAE induction group. Data are expressed as the mean ± SEM. *P < 0.01 (one-way analysis of variance). EAE: Experimental autoimmune encephalomyelitis; HCA: hydroxycitric acid; IL-6: interleukin-6.
Discussion
EAE is an animal model of MS that causes brain inflammation and demyelination mediated by immune responses to brain antigens. CD4+ T cells, especially Th1 and Th17, as well as their pro-inflammatory cytokines including IL-17, IFN-γ and TNF-α, along with myelin-specific CD8+ T cells and infiltrated macrophage within the CNS are suspected to be important in the immunoinflammatory-mediated demyelination of MS (Beck et al., 1988; Murphy et al., 2010). Immunomodulatory agents are reasonably effective in the treatment of MS and EAE, and appear to delay the time of progression of the disease to disabling stages (Lopez-Diego and Weiner, 2008; Azizi and Mirshafiey, 2013; Naddafi et al., 2013; Haghmorad et al., 2014; Mirshafiey et al., 2014a; Afraei et al., 2015; Azizi et al., 2015). In the present study, we evaluated the efficacy of HCA in animal models of MS and found that this anti-inflammatory and antioxidant agent (Clouatre and Preuss, 2013) can treat EAE by reducing the severity, lowering the incidence and delaying the onset of EAE in C57BL/6 mice.
Our findings suggest that HCA is capable of suppressing a pre-activated immune system in the late effector phase leading to disease eruption, as histopathological studies showed that the severity of demyelination, neuronal degeneration, infiltration of inflammatory cells, and perivascular cuffing in the brain and cerebellum of the mice with EAE which were treated orally with HCA was significantly milder than in vehicle treated mice.
NO may be involved in the pathogenesis of MS, the hallmark of which is the demyelinated plaque with reactive glial scar formation (Smith and Lassmann, 2002). Down-regulation of myelin gene expression in human oligodendrocytes by NO has been reported in a study by Jana and Pahan (2013). This study illustrates a novel biological role played by NO in down-regulating the expression of myelin genes preceding the death of oligodendrocytes (Jana and Pahan, 2013). Shin et al. (1998) showed that inhibition of iNOS expression and NO production prevented the development of allergic encephalomyelitis. It is suggested that HCA reduces markers of inflammation in the brain, intestines, kidney and serum (Clouatre and Preuss, 2013). HCA can also be useful as a protecting factor against diseases associated with oxidative stress (Kolodziejczyk et al., 2009). In animal models, it is demonstrated that the anti-inflammatory effects provided by HCA can ameliorate inflammation and oxidative stress in experimental colitis and type II diabetes (Asghar et al., 2007; dos Reis et al., 2009). Consistent with this data, the results of our study showed that HCA modulated EAE, at least in part, by suppressing NO production, as the serum NO level in HCA treated mice was significantly less than in the EAE induction group. However, findings from reports by Amin et al. (2011a,b) are not consistent with our data and the abovementioned data, as they suggested that HCA can ameliorate brain oxidative stress but increase serum NO level.
TNF-α, a pro-inflammatory cytokine, has been implicated in the pathology of MS and EAE. In individuals with MS, TNF-α level has been reportedly to be elevated in serum, cerebrospinal fluid and also at the site of active lesions, and this elevation correlates with the severity of the disease (Beck et al., 1988).
Our findings showed that HCA treatment could decrease serum TNF-α level, which was in agreement with the clinical and histopathological findings, and also reduce serum NO level in EAE mice. Our results also showed that high serum NO level was produced after high expression of iNOS gene. Recent studies have shown that inflammatory cytokines such as TNF-α, can increase iNOS gene expression and consequently increase NO production as a cytotoxic effector molecule (Murphy, 2000; Fonseca et al., 2003; Fereidoni et al., 2013). These findings are in accordance with our findings that HCA can reduce serum NO and TNF-α levels probably under the controlled regulation of NF-κB and MAPKs pathways in EAE mice. Masullo et al. (2014) showed that HCA was able to modulate cytokine signaling in different cultured cell lines, as induced anti-inflammatory activity in macrophages, through inhibition of NF-κB and mainly by inhibiting STAT-1 nuclear transfer and DNA binding.
In conclusion, our study demonstrated that HCA ameliorated the severity of EAE and attenuated inflammatory cellular infiltration into the CNS. In addition, we observed that HCA decreased the production of NO probably by suppressing the expression of iNOS and TNF-α (Kim et al., 2008). On the other hand, it should be noted that at the doses usually administered to patients, no side effects or adverse events have been reported in humans treated with HCA (Marquez et al., 2012). Therefore, anti-inflammatory effects along with reported safety indicate the beneficial effect of HCA on EAE which can also be applicable to the treatment of MS.
Footnotes
Funding: This work was supported by Vice Chancellor for Research, Alborz University of Medical Sciences, No. 1394-01-01-1050.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Afraei S, Azizi G, Zargar SJ, Sedaghat R, Mirshafiey A. New therapeutic approach by G2013 in experimental model of multiple sclerosis. Acta Neurol Belg. 2015;115:259–266. doi: 10.1007/s13760-014-0392-x. [DOI] [PubMed] [Google Scholar]
- Amin KA, Kamel HH, Abd Eltawab MA. The relation of high fat diet, metabolic disturbances and brain oxidative dysfunction: modulation by hydroxy citric acid. Lipids Health Dis. 2011a;10:74. doi: 10.1186/1476-511X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin KA, Kamel HH, Abd Eltawab MA. Protective effect of Garcinia against renal oxidative stress and biomarkers induced by high fat and sucrose diet. Lipids Health Dis. 2011b;10:6. doi: 10.1186/1476-511X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M, Monjok E, Kouamou G, Ohia SE, Bagchi D, Lokhandwala MF. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol Cell Biochem. 2007;304:93–99. doi: 10.1007/s11010-007-9489-3. [DOI] [PubMed] [Google Scholar]
- Azizi G, Mirshafiey A. Imatinib mesylate: an innovation in treatment of autoimmune diseases. Recent Pat Inflamm Allergy Drug Discov. 2013;7:259–267. doi: 10.2174/1872213x113079990021. [DOI] [PubMed] [Google Scholar]
- Azizi G, Goudarzvand M, Afraei S, Sedaghat R, Mirshafiey A. Therapeutic effects of dasatinib in mouse model of multiple sclerosis. Immunopharmacol Immunotoxicol. 2015;37:287–294. doi: 10.3109/08923973.2015.1028074. [DOI] [PubMed] [Google Scholar]
- Azizi G, Haidari MR, Khorramizadeh M, Naddafi F, Sadria R, Javanbakht MH, Sedaghat R, Tofighi Zavareh F, Mirshafiey A. Effects of imatinib mesylate in mouse models of multiple sclerosis and in vitro determinants. Iran J Allergy Asthma Immunol. 2014;13:198–206. [PubMed] [Google Scholar]
- Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Clouatre DL, Preuss HG. Hydroxycitric acid does not promote inflammation or liver toxicity. World J Gastroenterol. 2013;19:8160–8162. doi: 10.3748/wjg.v19.i44.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis SB, de Oliveira CC, Acedo SC, Miranda DD, Ribeiro ML, Pedrazzoli J, Jr, Gambero A. Attenuation of colitis injury in rats using Garcinia cambogia extract. Phytother Res. 2009;23:324–329. doi: 10.1002/ptr.2626. [DOI] [PubMed] [Google Scholar]
- Fereidoni M, Sabouni F, Moghimi A, Hosseini S. The effects of trypsin on rat brain astrocyte activation. Iran J Neurol. 2013;12:129–135. [PMC free article] [PubMed] [Google Scholar]
- Fonseca SG, Romao PR, Figueiredo F, Morais RH, Lima HC, Ferreira SH, Cunha FQ. TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur J Immunol. 2003;33:2297–2306. doi: 10.1002/eji.200320335. [DOI] [PubMed] [Google Scholar]
- Haghmorad D, Mahmoudi MB, Mahmoudi M, Rab SZ, Rastin M, Shegarfi H, Azizi G, Mirshafiey A. Calcium intervention ameliorates experimental model of multiple sclerosis. Oman Med J. 2014;29:185–189. doi: 10.5001/omj.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitinga I, Ruuls SR, Jung S, Van Rooijen N, Hartung HP, Dijkstra CD. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin Exp Immunol. 1995;100:344–351. doi: 10.1111/j.1365-2249.1995.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Pahan K. Down-regulation of myelin gene expression in human oligodendrocytes by nitric oxide: implications for demyelination in multiple sclerosis. J Clin Cell Immunol. 2013:4. doi: 10.4172/2155-9899.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Jeon SM, Park KH, Lee WS, Jeong TS, McGregor RA, Choi MS. Does Glycine max leaves or Garcinia Cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: a randomized control trial. Nutr J. 2011;10:94. doi: 10.1186/1475-2891-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim KY, Kim MS, Lee JH, Lee KP, Park T. A mixture of the aqueous extract of Garcinia cambogia, soy peptide and L: -carnitine reduces the accumulation of visceral fat mass in rats rendered obese by a high fat diet. Genes Nutr. 2008;2:353–358. doi: 10.1007/s12263-007-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk J, Masullo M, Olas B, Piacente S, Wachowicz B. Effects of garcinol and guttiferone K isolated from Garcinia cambogia on oxidative/nitrative modifications in blood platelets and plasma. Platelets. 2009;20:487–492. doi: 10.3109/09537100903165182. [DOI] [PubMed] [Google Scholar]
- Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis--a multifaceted adversary. Nat Rev Drug Discov. 2008;7:909–925. doi: 10.1038/nrd2358. [DOI] [PubMed] [Google Scholar]
- Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C, Kalman B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lu XY, Zeng YY, Ye YX, Zhou YY, Mu JJ, Zhao XH. Anti-inflammatory and immunosuppressive effect of phloretin. Yao Xue Xue Bao. 2009;44:480–485. [PubMed] [Google Scholar]
- Mahendran P, Vanisree AJ, Shyamala Devi CS. The antiulcer activity of Garcinia cambogia extract against indomethacin-induced gastric ulcer in rats. Phytother Res. 2002;16:80–83. doi: 10.1002/ptr.946. [DOI] [PubMed] [Google Scholar]
- Marquez F, Babio N, Bullo M, Salas-Salvado J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit Rev Food Sci Nutr. 2012;52:585–594. doi: 10.1080/10408398.2010.500551. [DOI] [PubMed] [Google Scholar]
- Masullo M, Menegazzi M, Di Micco S, Beffy P, Bifulco G, Dal Bosco M, Novelli M, Pizza C, Masiello P, Piacente S. Direct interaction of garcinol and related polyisoprenylated benzophenones of Garcinia cambogia fruits with the transcription factor STAT-1 as a likely mechanism of their inhibitory effect on cytokine signaling pathways. J Nat Prod. 2014;77:543–549. doi: 10.1021/np400804y. [DOI] [PubMed] [Google Scholar]
- Miller E, Wachowicz B, Majsterek I. Advances in antioxidative therapy of multiple sclerosis. Curr Med Chem. 2013;20:4720–4730. doi: 10.2174/09298673113209990156. [DOI] [PubMed] [Google Scholar]
- Mirshafiey A, Ghalamfarsa G, Asghari B, Azizi G. Receptor tyrosine kinase and tyrosine kinase inhibitors: new hope for success in multiple sclerosis therapy. Innov Clin Neurosci. 2014a;11:23–36. [PMC free article] [PubMed] [Google Scholar]
- Mirshafiey A, Asghari B, Ghalamfarsa G, Jadidi-Niaragh F, Azizi G. The significance of matrix metalloproteinases in the immunopathogenesis and treatment of multiple sclerosis. Sultan Qaboos Univ Med J. 2014b;14:e13–25. doi: 10.12816/0003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya M, Nakatsuji Y, Miyamoto K, Okuno T, Kinoshita M, Kumanogoh A, Kusunoki S, Sakoda S. Edaravone, a free radical scavenger, ameliorates experimental autoimmune encephalomyelitis. Neurosci Lett. 2008;440:323–326. doi: 10.1016/j.neulet.2008.05.110. [DOI] [PubMed] [Google Scholar]
- Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29:1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Naddafi F, Reza Haidari M, Azizi G, Sedaghat R, Mirshafiey A. Novel therapeutic approach by nicotine in experimental model of multiple sclerosis. Innov Clin Neurosci. 2013;10:20–25. [PMC free article] [PubMed] [Google Scholar]
- Peiris M, Monteith GR, Roberts-Thomson SJ, Cabot PJ. A model of experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice for the characterisation of intervention therapies. J Neurosci Methods. 2007;163:245–254. doi: 10.1016/j.jneumeth.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Riccio P, Rossano R, Liuzzi GM. May diet and dietary supplements improve the wellness of multiple sclerosis patients? A molecular approach. Autoimmune Dis 2010. 2011:249842. doi: 10.4061/2010/249842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Shin T, Tanuma N, Kim S, Jin J, Moon C, Kim K, Kohyama K, Matsumoto Y, Hyun B. An inhibitor of inducible nitric oxide synthase ameliorates experimental autoimmune myocarditis in Lewis rats. J Neuroimmunol. 1998;92:133–138. doi: 10.1016/s0165-5728(98)00194-5. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Sripradha R, Magadi SG. Efficacy of garcinia cambogia on body weight, inflammation and glucose tolerance in high fat fed male wistar rats. J Clin Diagn Res. 2015;9:BF01–04. doi: 10.7860/JCDR/2015/12045.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S. Role of glial cells in innate immunity and their role in CNS demyelination. J Neuroimmunol. 2011;239:13–20. doi: 10.1016/j.jneuroim.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Taupin V, Renno T, Bourbonniere L, Peterson AC, Rodriguez M, Owens T. Increased severity of experimental autoimmune encephalomyelitis, chronic macrophage/microglial reactivity, and demyelination in transgenic mice producing tumor necrosis factor-alpha in the central nervous system. Eur J Immunol. 1997;27:905–913. doi: 10.1002/eji.1830270416. [DOI] [PubMed] [Google Scholar]


