Abstract
The Stem Cell Ophthalmology Treatment Study (SCOTS) is currently the largest-scale stem cell ophthalmology trial registered at ClinicalTrials.gov (identifier: NCT01920867). SCOTS utilizes autologous bone marrow-derived stem cells (BMSCs) to treat optic nerve and retinal diseases. Treatment approaches include a combination of retrobulbar, subtenon, intravitreal, intra-optic nerve, subretinal, and intravenous injection of autologous BMSCs according to the nature of the disease, the degree of visual loss, and any risk factors related to the treatments. Patients with Leber's hereditary optic neuropathy had visual acuity gains on the Early Treatment Diabetic Retinopathy Study (ETDRS) of up to 35 letters and Snellen acuity improvements from hand motion to 20/200 and from counting fingers to 20/100. Visual field improvements were noted. Macular and optic nerve head nerve fiber layer typically thickened. No serious complications were seen. The increases in visual acuity obtained in our study were encouraging and suggest that the use of autologous BMSCs as provided in SCOTS for ophthalmologic mitochondrial diseases including Leber's hereditary optic neuropathy may be a viable treatment option.
Keywords: nerve regeneration, Leber's hereditary optic neuropathy, mitochondrial disease, optic neuropathy, bone marrow derived stem cells, blindness, visual loss, neural regeneration
Introduction
Leber's hereditary optic neuropathy (LHON) is a mitochondrial disorder leading to the death of the retinal ganglion cells and is the most common primary mitochondrial disorder. Visual loss typically occurs in early adulthood although with ready availability of genetic testing, patients are being identified as LHON even into the seventh decade of life. Decreased vision in one or both eyes is an early symptom with the interval between visual loss occurrences ranging from days to months. According to Moster et al. (2016), the degree of visual loss correlates with loss of ganglion cell and inner plexiform layer thickness on optical coherence tomography (OCT).
There are several mitochondrial DNA (mtDNA) mutations correlating with the development of LHON. The three most common (“primary”) LHON mutations are: G11778A nucleotide change within the MT-ND4 gene, G3460A in MT-ND1 gene, and 14484T>C in the MT-ND6 gene. There are at least 13 more “secondary” LHON mutations, such as the mutation at position 4917 which codes for a part of the MT-ND2 subunit. These four mutations lead to a reduction of Nicotinamide Adenine Dinucleotide Coenzyme Q (NADH-CoQ) reductase activity, an enzyme crucial for oxidative phosphorylation, present in the inner membrane of the mitochondria which are located in the cytoplasm of human cells (Wallace et al., 1988; Howell et al., 1991; Brown et al., 2000; Man et al., 2002; Chinnery et al., 2011). There is variability in clinical severity due to mtDNA heteroplasmy in the retinal ganglion cell mitochondria. Increased oxidative stress and apoptosis within the optic nerve system has also been proposed as an instigating factor in the development of LHON as reported by Koilkonda and Guy (2011).
There are no proven treatments for this condition. Therapy has centered on an attempt to reduce oxidative stress leading to free radical formation and possible apoptosis, and to increase mitochondrial respiration. These treatments include: vitamins and cofactors such as coenzyme Q10, folic acid, vitamin B12, riboflavin, L-carnitine, and creatine, electron acceptors including vitamin C, free radical scavengers like idebenone, EPI-743, alpha lipoic acid, curcumin, and vitamin E, inhibitors of toxic metabolites like dichloroacetate and customized treatment for the individual patient (DiMauro and Mancuso, 2007; Parikh et al., 2009). Idebenone has been used since 1992 and may increase mitochondrial complex 1 activity, but may produce variable effects on respiration (Angebabault et al., 2011).
RHODOS, the Rescue of Hereditary Optic Disease Outpatient Study, was a placebo controlled trial that utilized idebenone, a free radical scavenger, to treat LHON. Angebabault et al. (2011) reported that 20% of treated patients demonstrated an improvement in vision. However, the European Medicines Agency and the Committee for Medicinal Products for Human Use refused to approve idebenone for LHON because of lack of benefit in patients having vision loss for greater than 5 years and because of, in their view, the questionable benefit in patients with vision loss of less than 1 year. EPI-743, an FDA approved drug similar to coenzyme Q10 and idebenone, was used to treat five patients with different mtDNA mutations. Four of five patients demonstrated an improvement in visual function (Klopstock et al., 2011).
Definitive treatment of the multiple mtDNA mutations may theoretically be accomplished by replacement of the mutated mitochondrial genomes (Koilkonda and Guy, 2011). Sadun et al. (2012) felt the mtDNA mutation alone did not account for LHON manifestation because there is variation in age of onset and only approximately 50% of males and 10% of females with one of the three primary mutations actually develop optic neuropathy.
Bone marrow-derived stem cells (BMSCs) have been used to rescue photoreceptors in rhodopsin knockout mice with retinal degeneration (Arnhold et al., 2007). Carelli et al. (2013) have noted advances in animal modeling for mitochondrial diseases. Avraham-Lubin et al. (2012) reported that vascular endothelial growth factor (VEGF) played a key role in neuroglial transdifferentiation of BMSCs. Three patients with retinitis pigmentosa and two patients with cone-rod dystrophy underwent implantation of BMSCs in a safety study by Otani et al. (2004).
SCOTS, the Stem Cell Ophthalmology Treatment Study, is the largest ophthalmology stem cell study registered with the National Institutes of Health (identifier: NCT01920867). SCOTS is an open label, non-randomized, efficacy study. There is no placebo or sham arm. All patients meeting eligibility criteria and enrolled in the study receive active treatment. SCOTS is Institutional Review Board approved and provides informed consent for all enrolled patients (Weiss et al., 2016).
There are three arms of SCOTS with the type of treatment chosen based on the degree of visual loss, etiology of visual loss, associated risk factors for the treatment arms and the patient's medical risk status. Bilateral treatment is provided assuming both eyes meet eligibility requirements. As these are autologous stem cells, no immunosuppression is required. An FDA cleared class 2 medical device is used to separate the bone marrow aspirate into a stem cell concentrate. Minimum manipulation is used. This concentrate has averaged 1.2 billion total nucleated cells including mesenchymal stem cells in approximately 14 –15 cm3 of concentrate. Retrobulbar injection consists of 3 cc of concentrate; subtenon injection of 1 cm3; intravitreal injection of 0.05 cm3; subretinal injection of approximately 0.1 cm3 and intra-optic nerve injection of approximately 0.2 cm3. The intravenous injection is provided from the remainder of the concentrate which is approximately 6 cm3.
Arm 1 consists of retrobulbar and subtenon injection of, followed by intravenous infusion of, stem cell concentrate. Patients with ophthalmic conditions which preclude safe or effective utilization of intravitreal injection of concentrate, such as the presence of silicon oil, may be offered Arm 1 if meeting inclusion criteria. Arm 2 consists of retrobulbar, subtenon and intravitreal administration of, followed by intravenous infusion of, stem cell concentrate. Patients meeting inclusion criteria with visual acuity between 20/40 and 20/200 in one or both eyes and/or visual field loss may be offered Arm 1 or 2. Arm 3 is reserved for retinal and optic nerve patients with severe visual loss (generally visual acuity of 20/200 or worse). Monocular patients are not eligible for Arm 3. Arm 3 patients’ fellow eyes have better vision and may have either Arm 1 or Arm 2 treatment. The Arm 3 eye receives a core pars plana vitrectomy with subretinal or intra-optic nerve injection of BMSC concentrate followed by intravenous infusion of stem cells. Follow up is required at 1, 3, 6 and 12 months post treatment with reporting of the eye exam results to the Principal Investigator and Study Director.
The SCOTS procedure is patient funded and performed under general anesthesia, as described by Weiss et al. (2015a). Treatment is provided in a fully licensed ambulatory surgical center in Coconut Creek, Florida.
We have previously reported treatment with BMSCs in two patients with optic nerve disease. The first patient, a 32-year-old female with a 5 year history of visual loss secondary to idiopathic optic atrophy was followed at the Wilmer Eye Institute at the Johns Hopkins Hospital. Pre-treatment best-corrected visual acuity was 20/800 in the right eye (OD) and 20/4,000 in the left eye (OS) with severe visual field loss. Four months after treatment, Weiss et al. (2015a) reported that her visual acuity had improved to 20/100 in the right eye and 20/40 in the left eye with visual field improvement in both eyes. 2 years post original treatment, with an additional second treatment in SCOTS at 13 months, vision was 20/40–2 OD and 20/30+2 OS.
The second patient, a 54-year-old female with relapsing auto-immune optic neuropathy, was followed in Columbus, OH, USA. Before treatment, the visual acuity was 20/350 in the right eye and 20/70 in the left eye with severe visual field loss in both eyes. Six months following treatment, the central visual acuity had improved to 20/150 in the right eye and 20/15 in the left eye with visual field improvement in both eyes as noted by Weiss et al. (2015b).
The aim of the SCOTS study is to ascertain the effectiveness of BMSC treatment as provided in the manner indicated for ophthalmologic mitochondrial diseases including LHON.
Case Presentation
Patient 1
The first patient was a 71-year-old male with LHON 4917, a mutation of mitochondrial DNA at position 4917. In March, 1982, he awoke with sudden painless visual loss in the left eye. The family medical history was remarkable for a brother who had lost the vision in one eye and had been diagnosed with optic neuritis. The patient's neurology work up was negative for infections and lupus erythematosus. Visual evoked potential was normal in the right eye and “profoundly abnormal” in the left eye with prolongation. The brainstem auditory evoked responses were normal. He received intravenous steroids followed by a 14 day taper of oral decadron. In July, 1983, he had a second attack in the left eye reducing his vision to 20/200 with pain. It was treated as acute optic neuritis with intravenous methylprednisolone for 3 days at the Cleveland Clinic. On August 4, 1983, he experienced the loss of vision in the opposite eye with visual acuity 20/50 in the right eye and 20/200 in the left eye. The diagnosis of Leber's hereditary optic neuropathy was considered. In 1984, the patient suffered additional visual loss in the right eye. Blood testing was negative for 11778 (mitochondrial DNA position 11778) Leber's mutation.
In 1994, the patient's blood and that of his mother, were tested positive for the newly recognized LHON4917 mutation (The patient's mother was blind with a combination of glaucoma, Graves’ orbitopathy and a stepwise optic neuropathy in both eyes by 90 years of age).
The patient was referred for SCOTS evaluation. The past ocular history was significant for argon laser trabeculoplasty, followed by trabeculectomy for glaucoma in the right eye in 1990 and an uneventful cataract surgery in 2004. The left eye had no light perception (NLP) in 1984 after a central retinal artery occlusion. A conjunctival biopsy in 1989 showed numerous plasma cells and histiocytes with periodic acid-Schiff (PAS)-positive inclusion bodies.
The best-corrected visual acuity was counting fingers (CF) at 1 foot in the right eye, and NLP in the right eye. The visual field in the right eye was almost extinguished. Both optic nerves were atrophic and surrounded by peripapillary retinal pigment epithelial scars. The left eye had atretic arteries following the central retinal artery occlusion 29 years ago. On intravenous fluorescein angiography the right eye had spotty microaneurysms consistent with background diabetic retinopathy and non-leaking telangiectatic microangiopathy consistent with Leber's optic neuropathies.
On October 15, 2013, the patient underwent the SCOTS procedure in both eyes (Arm 3 in the left eye, Arm 2 in the right eye) without complications. At 6 months after treatment, the visual acuity was CF at 2 feet in the right eye and NLP in the left eye. His eye pressures at 6 months were 10 mm Hg in the right eye and 11 mm Hg in the left eye and he used Istalol twice daily.
Patient 2
This was a 23-year-old female with LHON 3460, who first developed headaches, cervical numbness, and papilledema in February, 2013. An MRI of the brain showed 8 mm of cerebellar tonsillar ectopia (mild Chiari malformation type I). A lumbar puncture found intracranial hypertension (opening pressures of 36 cmH2O before acetazolamide administration, 26.5 cmH2O afterward). Other risk factors for papilledema were positive hypercoagulability blood tests: two heterozygous methylenetetrahydrofolate reductase (MTHFR) mutations and a heterozygous positive Leidin Factor V. The peripheral visual loss in both eyes improved with acetazolamide treatment. In April, 2013, despite reduction of headaches with acetazolamide and normalized intracranial pressure, she started to lose her central vision with a cecocentral scotomata in both eyes, leading to complete loss of central vision in both eyes over 8 months and the Leber 3460 optic neuropathy diagnosis. With hand motion (HM) vision in both eyes, she was followed with Goldmann perimetry.
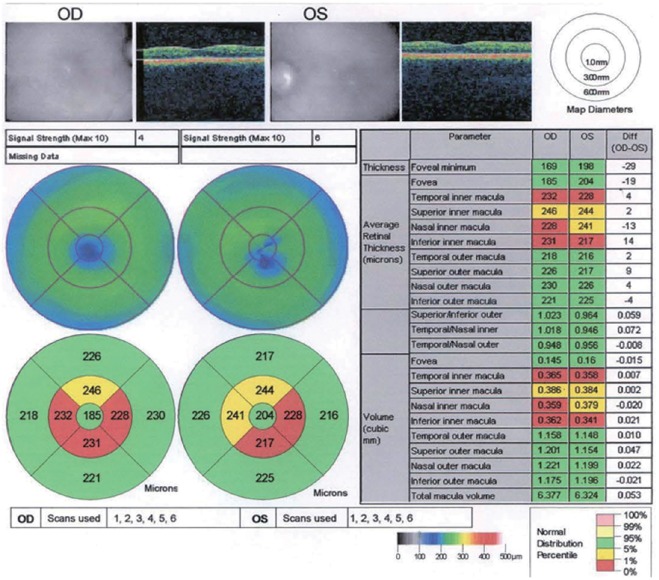
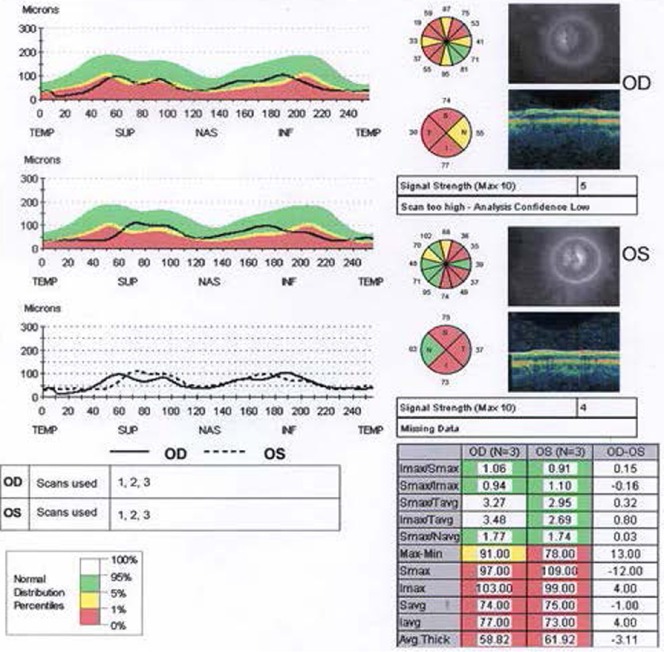
The treatments of idebenone, B12 and folic acid for the MTHFR mutation had not prevented the visual decline. Serial ocular coherence tomography macula studies showed a progressive macula and retinal nerve fiber layer (RNFL) thinning as shown in the studies performed on August 19, 2013 (Figures 1, 2).
Figure 1.
Optical coherence tomography of thinned macula in patient 2 (August 19, 2013).
Figure 2.
Optical coherence tomography of thinned retinal nerve fiber layer in patient 2 (August 19, 2013).
At presentation to SCOTS, the visual acuity was HM at 2 feet in the right eye and HM at 2 inches in the left eye. The optic nerves were pale in both eyes. She underwent the SCOTS procedure on December 10, 2013 (Arm 3 in the left eye, Arm 2 in the right eye). On day 1 post-treatment, she reported the left eye could already see better, CF at 2 feet. At 1 month, the left eye could see color in her lower left for the first time in a year, see Jaeger (J) 20/800 at near, and could decipher the difference between one dollar and ten dollar bills. Her left eye had two shunt-like vessels in the nasal half that did not leak on fluorescein angiography.
At 3 months post-treatment, her RNFL circular scan had thickened in both eyes.
At 6 months post-treatment, the visual acuity was CF at 2 feet and J16 (near vision) in each eye. She was able to guide a shopping cart down isles for the first time in a year, chose cereal boxes by color and design, and saw the design of granite for her new kitchen. At 9 months post-treatment, on Early Treatment Diabetic Retinopathy Study (ETDRS) charts, she had improved from 0 letters at 1 meter in both eyes and pre-treatment to 8 letters at 1 meter OU (her peak). At 1 year post-treatment, the visual acuity had improved to CF at 7 feet in the right eye and 20/400 in the left eye. At 16 months post-treatment, the ETDRS acuity was 6 in the right eye and 7 in the left eye at 1 meter. Visual fields by Humphrey and Goldmann perimetry continued to improve at 16 months post SCOTS.
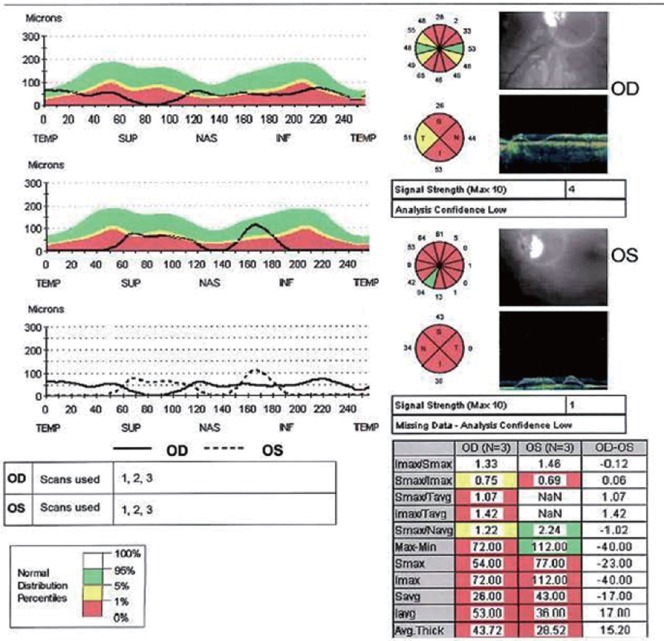
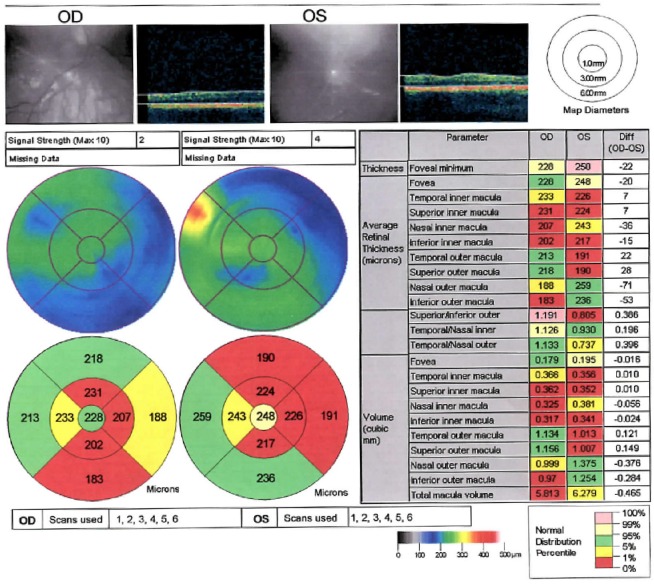
Humphrey Visual Fields continued to show improvement at 16 months post treatment. The RNFL of the optic nerve head appeared to modestly increase temporally in the right eye but decrease in the left eye when comparing the studies from December 4, 2013 to April 29, 2015. Macular OCT appeared to modestly increase temporally in the right eye and nasally in the left eye when comparing the studies from August 19, 2013 (Figures 1, 2) to April 29, 2015 (Figures 3, 4).
Figure 3.
Optical coherence tomography of thickened retinal nerve fiber layer in patient 2 (April 29, 2015).
Figure 4.
Optical coherence tomography of thickened macula in patient 2 (April 29, 2015).
Patient 3
This was a 65-year-old male with LHON 3460 and advanced chronic open angle glaucoma. He had phthisis in the right eye and good pressure control in the left eye. The patient's history also included congenital cataracts, congenital esotropia, congenital nystagmus, axial myopia with staphyloma in the left eye, chronic retinal detachment and phthisis in the right eye since age 12, and aphakia in both eyes. At presentation, his visual acuity was NLP in the right eye and HM in the left eye. The patient underwent Arm 2 of SCOTS in the left eye only on April 1, 2014. On January 12, 2015, approximately 9 months post-treatment, the visual acuity in the right eye remained NLP and that in the left eye had improved to 20/200. Vision remained stable at 20/200 at the 12 months visit. The ETDRS chart acuities peaked in improvement at 9 months: pre-treatment 30 letters at 1 meter, then 36, 41, 44, 40 at 3, 6, 9, and 12 months post-treatment respectively. The intraocular pressures of the unoperated phthisical right eye rose from 4 mmHg pre-treatment to 13.5 mmHg 3 months post-treatment. The pressure in the right eye gradually diminished to 8 mmHg at 6 months, 7 mmHg at 9 months, and 3 mmHg at 12 months post-treatment. The eye pressure in the right eye before treatment varied from 12 to15 mmHg. Following treatment, the ocular tensions remained stable in the 12 to 17 mmHg range during the follow up eye exams. Goldmann visual fields were improved, demonstrating larger superior and inferior islands.
With only a limited view of his posterior segment in the left eye through an unreactive 3 mm aphakic pupil, we used B-scan ultrasound to evaluate the posterior segment. The view in a scan from 2001 showed a long axial length, staphyloma and concave nerve head with an eye pressure of 15 mm Hg on the day of examination. After SCOTS, the vitreous body contained a significant number of cells on day 1. At 3 weeks, the vitreous body was clear, but the left nerve head had a “soft mound” appearance, and by 3 months the mound was slightly thinned.
Patient 4
This was a 72-year-old male who had childhood polio with permanent left facial paralysis. In 2006, he had a radical neck dissection for esophageal cancer complicated by C5–7 osteomyelitis. In early 2013, he aspirated, developed pneumonia, then septic shock and coma. He emerged from the coma with blurred vision in both eyes. His left eye then suffered a subacute visual loss from May to September, 2013, followed by progressive visual loss in the right eye from September to November, 2013. He began falling to the left and developed nystagmus. An MRI showed a cerebellar stroke. B6 and B12 deficiencies were found and addressed. On testing the LHON, 14484 mutation was identified. He was treated with idebenone with no significant response over 7 months.
The presenting visual acuity was CF at 6 feet in each eye within small islands remaining in the visual fields. He underwent Arm 3 in the left eye and Arm 2 in the right eye of SCOTS on June 3, 2014. At 3 months pos-treatment, his acuity had improved to 20/150 in the right eye and CF at 12 feet in the left eye. On January 17, 2015, approximately 7 months post-treatment, the visual acuity had improved to 20/100 in the right eye and 20/350 in the left eye. Visual acuity using the ETDRS chart pre-treatment was 18 letters at 1 meter in the right eye and 2 letters at 1 meter in the left eye. At 7 months post SCOTS, ETDRS visual acuity had improved to 42 letters in the right eye and 28 letters OS at 1 meter. The visual fields also improved.
Between the 3 and 7 months post-treatment, vitreous bands were observed with traction on each retina, thickening macula in the right eye and tenting macula in the left eye. Follow-up OCT showed the traction spontaneously improved at 7 months in both eyes.
In addition to the objective improvement in visual acuity and visual fields, the patient reported significant improvements in the performance of his activities of daily living. Before treatment, he had to be led when he was outside his home, but after SCOTS, he could find his way without collisions in restaurants, stores, doctors’ offices, etc. He remarked he could see terrain change when outdoors, avoiding tripping.
Patient 5
This was a 20-year-old male with LHON 14484 diagnosed at age 14. The patient's brother was also diagnosed with LHON. The medical history was significant for renal disease. Medications included daily Idebenone and Vitamin B2. The patient's presenting visual acuity was 6/270 in the right eye and 6/270 in the left eye. He underwent Arm 3 in the right eye and Arm 2 in the left eye on August 19, 2014. On April 14, 2015, approximately 8 months post-treatment, the visual acuity had improved to 6/240 in the right eye and 6/210 in the left eye.
Discussion
BMSCs have been used for treatment of orthopedic, cardiovascular, neurologic and ocular diseases. BMSCs are capable of differentiating into neuron-like cells. They have been demonstrated to provide neurotrophic factors that promote the regeneration of axons and protect retinal ganglion cells and to integrate into existing neural networks thus re-establishing neural connections (Dezawa et al., 2001; Weiss et al., 2015a, b). However, according to Monzka et al. (2009), some neural related transcription factors could be found in undifferentiated mesenchymal stem cells. Treatment with autologous BMSCs precludes immunosuppression, do not result in teratoma formation, and has no ethical or moral objections (Zarzeczny and Caulfield, 2009).
Wilkins et al. (2009) confirmed that BMSCs were neuroprotective, secreting trophic factors, such as brain derived neurotrophic factor, that protect cortical neurons from death. In a rat model of glaucoma used by Yu et al. (2006), the glial fibrillary protein expressing BMSCs were mostly present on the inner limiting membrane following transplantation into the vitreous cavity, but there was a 10–20% increase in the number of surviving retinal ganglion cells. However Johnson et al. (2010) showed both a statistically significant increase in overall retinal ganglion cell survival following intravitreal BMSC implantation in their glaucoma model, and discrete cells migrating into the host retina. In a transection/crush model of the optic nerve, BMSC doubled the number of regenerating axons 100–1,200 μm distal to the lesion site compared to a control group; in addition, retinal ganglion cell survival increased by 15–28% 8–28 days after injury (Levkovitch-Verbin et al., 2010). They concluded that the neuroprotective effects were achieved through retinal glia activation and glia-mediated neuroprotection or through signaling between the stem cells and the damaged reginal ganglion cells.
BMSCs have been detected months after implantation into the vitreous cavity of the eye (Yu et al., 2006). Mesentier-Louro et al. (2014) determined that the retinal glia is the barrier to retinal engraftment indicating that the direct placement of BMSCs into the retina or optic nerve may be of benefit. Mead et al. (2014) has argued that engrafting the BMSCs in the retina may increase the neuroprotective and axogenic effects while improving the propensity of differentiating into glia.
Mitochondrial transfer is postulated to be a mechanism by which improvement may be obtained in mitochondrial dysfunction. In a report by Liu et al. (2014), mesenchymal stem cells were observed using laser scanning confocal microscopy to transfer their mitochondria via a tunneling nanotube-like structure. They showed that mitochondrial transfer was frequent and essentially one way from the mesenchymal stem cells (MSCs) to endothelial cells, protecting them from apoptosis. Las and Shirihai (2014) showed that mitochondrial transfer was dependent on levels of Miro 1, a mitochondrial Rho-GTPase that regulates mitochondrial movement within the cells.
Mitochondrial transfer has also been shown to occur from MSCs to epithelial cells as are present in the lungs (Ahmad et al., 2014). Mitochondrial transfer from MSCs has been shown to attenuate cigarette smoke-induced respiratory damage (Li et al., 2014). They also showed that inhibition of tunneling nanotube formation blocked mitochondrial transfer. In a murine acute lung injury model, Islam et al. (2012) showed that BMSCs transferred mitochondria, protecting the pulmonary alveoli. They were able to observe the BMSC mitochondria in the epithelial cells and the resultant increased alveolar ATP concentrations.
In a chemically induced rotenone murine model of LHON, Mansergh et al. (2014) suggested that the use of stem cells would be capable of protecting visual function. They noted that cultured retinal progenitor cells can integrate close to the ganglia cell layer and maintain retinal function as ascertained by manganese-enhanced magnetic resonance imaging.
There have been a number of mechanisms identified for the effects of BMSCs including MSC-derived exosomes providing microRNA (Fernandez-Messina et al., 2009; Kordelas et al., 2014), presence of growth factors including brain-derived neurotrophic growth factor (Wilkins et al., 2009). Chen et al. (2005) have found nerve growth factor and glial cell line-derived neurotrophic factor, providing protection for injured rodent brain tissue. Paracrine effects and transdifferentiation of the stem cells have been shown to be useful in treating degenerative eye disease (Mead et al., 2015) and promoting astrocyte survival (Huang et al., 2015). Mitochondrial transfer may be a contributor to the positive effects of BMSCs and therefore a means by which patients with hereditary mitochondrial diseases including Leber's hereditary optic neuropathy may improve visual function. Depending on the disease mechanisms, it is our opinion that one or more of these methods may predominate and provide a beneficial outcome in various retinal and optic nerve diseases.
In the SCOTS study, BMSCs are being utilized in a number of different retinal and optic nerve diseases. The approach utilized in SCOTS for optic nerve disease provides transfer of the fraction of bone marrow containing BMSCs to either the optic nerve directly or to close proximity of the optic nerve and retinal ganglion cell layer. A number of preclinical studies provide evidence that mitochondrial transfer can take place between BMSCs, including mesenchymal stem cells, and tissue having undergone injury with the resultant improvement in ATP production allowing for increased survival of the injured cells. The mechanism of this transfer via a nanotube like structure has been delineated and blockage of this process has been shown to interrupt mitochondrial transfer. Both epithelial and endothelial cells have been shown to accept mitochondria and neural tissues such as the retinal ganglion cell layer and optic nerve are likely capable of participating in this receipt of mitochondria.
In five LHON patients who underwent SCOTS, there were improvements in visual acuity and peripheral vision. Several of the eyes experienced dramatic, persistent increases in visual acuity attributable to the BMSC treatment in SCOTS including CF to 20/100 and HM to 20/200. The progressive improvements on ETDRS and corresponding Snellen visual acuities with LHON genetic type are shown in Figure 5. Visual field improvements were noted. Macular thickness and optic nerve head thickness varied and did not appear correlated with vision improvements. No adverse events or serious adverse events were seen. These improvements could be a result of revitalization of existing mitochondrial function and the transfer of more viable mitochondria in existing neurons and glial cells, as well as transdifferentiation of the BMSCs and incorporation of newly developed cells in the existing ganglion and optic nerve cell layers. Further exploration of BMSC treatment in mitochondrial disease appears warranted.
Figure 5.
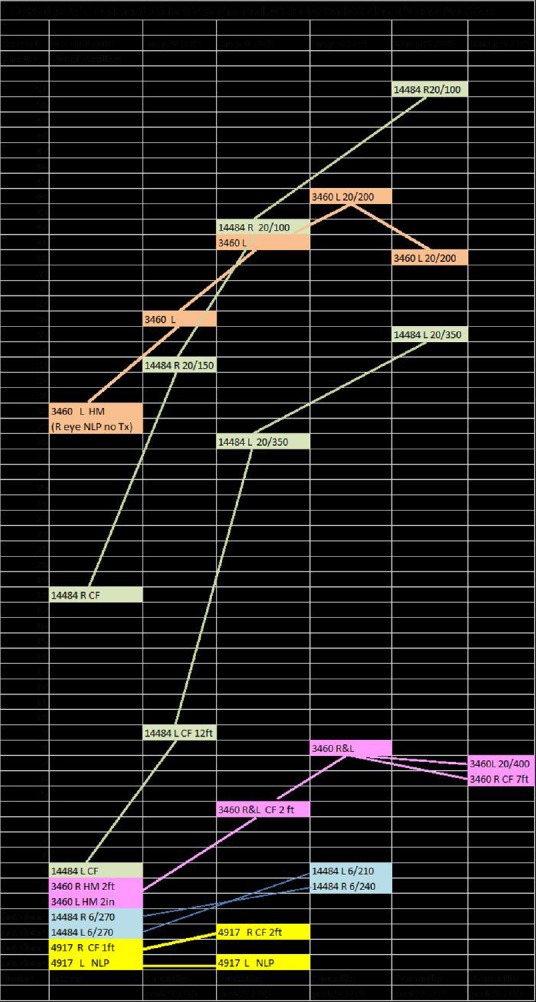
Early Treatment Diabetic Retinopathy Study (ETDRS) reported improvement in Leber's hereditary optic neuropathy genetic type and Snellen visual acuities.
L: Left; R: right; NLP: no light perception; CF: counting fingers; HM: hand motion.
Acknowledgments
We wish to thank the other ophthalmologists, neuro-ophthalmologists, retina specialists, glaucoma specialists, and molecular genetic specialists who participated in the care of these five patients: patient 1: Elmer Collins (M.D.), Robert Tomsak (M.D., Cleveland Clinic), Norman Schatz (M.D., Wills Eye), Jon Currie (M.D., National Institutes of Health, NIH), J. Lawton Smith (M.D., University of Miami), Sohan Singh Hayreh (M.D., University of Iowa), Paul Weber (M.D., Ohio State University), Donald Johns (M.D., Johns Hopkins University, JHU), Avrom Epstein (M.D., The Eye Center of Columbus); patient 2: Rob Bloomberg (M.D.), Greg Kosmorsky (D.O., Cleveland Clinic), Marc Criden (M.D., Ohio State University), Thomas W. Prior (Ph.D., Ohio State University); patient 3: William Havener (M.D., Ohio State University), Fred Kapetansky (M.D., The Eye Center of Columbus and Ohio State University), Edwin Stone (M.D., University of Iowa); patient 4: E. Mitchell Opremcak (M.D., The Eye Center of Columbus and Ohio State University), Avrom Epstein (M.D., The Eye Center of Columbus), Thomas W. Prior (Ph.D., Ohio State University).
Footnotes
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angebabault C, Gueguen N, Desquiret-Dumas V, Chevrollier A, Guillet V, Verny C, Cassereau J, Ferre M, Milea D, Amati-Bonneau P, Bonneau D, Procaccio V, Reynier P, Loiseau D. Idebenone increases mitochondrial complex I activity in fibroblasts from LHON patients while producing contradictory effects on respiration. BMC Res Notes. 2011;4:557. doi: 10.1186/1756-0500-4-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold S, Absenger Y, Klein H, Addicks K, Schraemeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414–422. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- Avraham-Lubin BC, Goldenberg-Cohen N, Sadikov T, Askenasy N. VEGF induces neuroglial differentiation in bone marrow-derived stem cells and promotes microglia conversion following mobilization with GM-CSF. Stem Cell Rev. 2012;8:1199–1210. doi: 10.1007/s12015-012-9396-1. [DOI] [PubMed] [Google Scholar]
- Brown MD, Trounce IA, Jun AS, Allen JC, Wallace DC. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber's hereditary optic neuropathy mitochondrial DNA mutation. J Biol Chem. 2000;275:39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- Carelli V, La Morgia C, Sadun AA. Mitochondrial dysfunction in optic neuropathies: animal models and therapeutic options. Curr Opin Neurol. 2013;26:52–58. doi: 10.1097/WCO.0b013e32835c5f0b. [DOI] [PubMed] [Google Scholar]
- Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, Perez-Polo JR, Yang K. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: Synthesis of neurotrophic factors. J Neurosci Res. 2005;80:611–619. doi: 10.1002/jnr.20494. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Brown DT, Andrews RM, Singh-Kler R, Riordan-Eva P, Lindley J, Applegarth DA, Turnbull DM, Howell N. The mitochondrial ND6 gene is a hot spot for mutations that cause Leber's hereditary optic neuropathy. Brain. 2011;124(Pt 1):209–218. doi: 10.1093/brain/124.1.209. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiate bone marrow stromal cells. Eur J Neurosci. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Mancuso M. Mitochondrial diseases: therapeutic approaches. Biosci Rep. 2007;27:125–137. doi: 10.1007/s10540-007-9041-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Messina L, Gutiérrez-Vázquez C, Rivas-García E, Sánchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107:61–77. doi: 10.1111/boc.201400081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N, Bindoff LA, McCullough DA, Kubacka I, Poulton J, Mackey D, Taylor L, Turnbull DM. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991;49:939–950. [PMC free article] [PubMed] [Google Scholar]
- Huang W, Lv B, Zeng H, Shi D, Liu Y, Chen F, Li F, Liu X, Zhu R, Yu L, Jiang X. Paracrine factors secreted by MSCs promote astrocyte survival associated with GFAP downregulation after ischemic stroke via p38 MAPK and JNK. J Cell Physiol. 2015;230:2461–2475. doi: 10.1002/jcp.24981. [DOI] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci. 2010;51:960–970. doi: 10.1167/iovs.09-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopstock T, Yu-Wai-Man P, Dimitriadis K, Rouleau J, Heck S, Bailie M, Atawan A, Chattopadhyay S, Schubert M, Garip A, Kernt M, Petraki D, Rummey C, Leinonen M, Metz G, Griffiths PG, Meier T, Chinnery PF. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134(Pt 9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koilkonda RD, Guy J. Leber's hereditary optic neuropathy-gene therapy: from benchtop to bedside. J Ophthalmol 2011. 2011:179412. doi: 10.1155/2011/179412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- Las G, Shirihai OS. Miro1: new wheels for transferring mitochondria. EMBO J. 2014;33:939–941. doi: 10.1002/embj.201488441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51:6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, Lian Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51:455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube likestructure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–18. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Man PY, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet. 2002;39:162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh FC, Chadderton N, Kenna PF, Gobbo OL, Farrar GJ. Cell therapy using retinal progenitor cells shows therapeutic effect in a chemically-induced rotenone mouse model of Leber hereditary optic neuropathy. Eur J Hum Genet. 2014;22:1314–1320. doi: 10.1038/ejhg.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Scheven BA. Mesenchymal stem cell therapy for retinal ganglion cell neuroprotection and axon regeneration. Neural Regen Res. 2015;10(3):371–373. doi: 10.4103/1673-5374.153681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015;14:243–257. doi: 10.1016/j.scr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9:e110722. doi: 10.1371/journal.pone.0110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R, Smeets R, Brook GA, Wöltje M. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci. 2009;10:16. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AT. Cone and cone-rod dystrophies. J Med Genet. 1992;29:289–290. doi: 10.1136/jmg.29.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster SJ, Moster ML, Scannell BM, Sergott RC. Retinal ganglion cell and inner plexiform layer loss correlate with visual acuity loss in LHON: a longitudinal, segmentation OCT analysis. Invest Ophthalmol Vis Sci. 2016;57:3872–3883. doi: 10.1167/iovs.15-17328. [DOI] [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Saneto R, Falk MJ, Anselm I, Cohen BH, Haas R, Medicine Society TM. A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol. 2009;11:414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadun AA, Chicani CF, Ross-Cisneros FN, Barboni P, Thoolen M, Shrader WD, Kubis K, Carelli V, Miller G. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol. 2012;69:331–338. doi: 10.1001/archneurol.2011.2972. [DOI] [PubMed] [Google Scholar]
- Siqueria RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a Phase I trial. Retina. 2011;31:1207–1214. doi: 10.1097/IAE.0b013e3181f9c242. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Benes SC, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment. Neural Regen Res. 2016;11:1512–1516. doi: 10.4103/1673-5374.191229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Malkin A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a preliminary report. Neural Regen Res. 2015a;10:982–988. doi: 10.4103/1673-5374.158365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen Res. 2015b;10:1507–1515. doi: 10.4103/1673-5374.165525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006;344:1071–1079. doi: 10.1016/j.bbrc.2006.03.231. [DOI] [PubMed] [Google Scholar]
- Zarzeczny A, Caulfield T. Emerging ethical, legal and social issues associated with stem cell research and the current role of the moral status of the embryo. Stem Cell Rev. 2009;5:96–101. doi: 10.1007/s12015-009-9062-4. [DOI] [PubMed] [Google Scholar]