Summary
The World Health Organization (WHO) has emphasized that aging of the population is inextricably linked to many other global public health issues, such as universal health coverage, non-communicable diseases, and disability. However, Alzheimer's Disease International (ADI) estimates that 46.8 million elderly people worldwide were living with dementia in 2015. Alzheimer's disease (AD), the most common form of dementia, is one of the most common neurodegenerative diseases and is the main cause of cognitive impairment. AD will affect 5–7 out of every 100 older adults who are age 60 years or over. In response to the serious challenge posed by AD, governments are expected to play an important role in the prevention, diagnosis, and treatment of AD. As specific examples, i) the Japanese Government has instituted and supported regulations to encourage the development of AD drugs in order to accelerate research and development of innovative drugs; ii) the United States Government has cooperated with multiple partners such as non-governmental organizations in the response to AD; iii) Chinese governmental measures have standardized clinical diagnosis and treatment as part of the response to AD, including eligible patients, diagnostic criteria, therapeutic schedules, drug selection, and required inspections; iv) with political support from member governments, the European Union has issued guidelines and conducted clinical studies on medicines for the treatment of AD in order to ascertain the various stages of the disease and the relevance of biomarkers. AD is an intractable disease, so different countries need to share clinic trial information and cooperate in the conduct of those trials. International cooperation will play a key role in the response to other intractable and rare diseases.
Keywords: Alzheimer's disease, dementia, accelerated regulation, cooperation with multiple partners, clinical pathway
1. Introduction
As a result of aging of the population, there were 900 million people (12.2% of the population) worldwide age 60 years or over in 2015 (1). The World Health Organization (WHO) has emphasized that aging of the population is inextricably linked to many other global public health issues, such as universal health coverage, non-communicable diseases, and disability (2). Alzheimer's Disease International (ADI) estimates that 46.8 million elderly people worldwide were living with dementia in 2015, which included 22.9 million in Asia, 10.5 million in Europe, 9.4 million in the United States (US), and 4.0 million in Africa (3). Alzheimer's disease (AD), a common form of dementia, is one of the most common neurodegenerative diseases and is the main cause of cognitive impairment (4). AD will affect 5–7 out of every 100 older adults who are age 60 years or over (5).
AD is an incurable disease, and a major symptom of AD is progressive dementia that eventually results in disruption of daily life (6). Current drugs for AD target cholinergic and glutamatergic neuro transmission, thus improving symptoms, but their neuroprotective activity is still debated (7). Much effort is directed towards identifying disease-modifying therapies, as indicated by the fact that several compounds in different phases of development. Since AD can start 10–15 years before it becomes full-blown, once a person is displaying classic symptoms of dementia may be too late, so drug treatment during its early stages remains one hope to stem the disease's course (8).
In response to the serious challenge posed by AD, governments are expected to play an important role in the prevention, diagnosis, and treatment of AD. The purpose of this paper is to explore the government's role in regulation, coordination, and standardization in order to provide a reference for government support of the response to AD and to put forward several suggestions.
2. Government regulations to encourage the development of AD drugs
The Japanese Government has instituted and supported regulations to encourage the development of AD drugs in order to accelerate research and development of innovative drugs. In Japan, the elderly age 60 years or over account for 33.2% of the total population, which is the highest proportion in the world, thus underscoring the urgent need for discovery of AD drugs (9).
Overcoming the “lag in AD drugs” with government initiatives. The issue of a “lag in AD drugs” refers to the fact that the official release date of AD drugs in Japan lagged behind that in Europe and the US, mainly because of a lag in the experimental stage and the prolonged application process. In response, the Pharmaceuticals and Medical Devices Agency (PMDA), a Japanese regulatory agency, put forward appropriate countermeasures, such as increase in examinants, gradually reducing the “lag in AD drugs” (Figure 1). As a specific example, donepezil is used to improve cognition and behavior. Donepezil was the first drug approved for the treatment of AD in Japan in 1999. In 2011, 2 cholinesterase inhibitors (galantamine and rivastigmine) and an N-methyl-D-aspartate receptor inhibitor (memantine) were approved as symptomatic drugs, and the prescription of 3 drugs to patients with AD thus became possible in clinical settings, thereby overcoming the issue of the “lag in AD drugs” (10).
Figure 1.
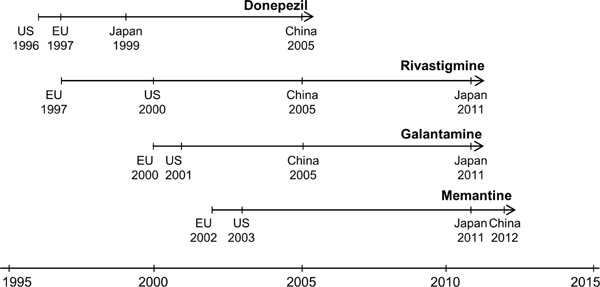
The four drugs approved for the treatment of Alzheimer's disease in Japan, the US, the EU, and China. Donepezil, rivastigmine, galantamine, and memantine are four conventional drugs for the treatment of AD. The official release date of AD drugs in Japan lagged behind that in the EU and the US, resulting in the issue of a “lag in AD drugs.” Indeed, the year of approval for Galantamine was far later China, mainly because of the lag in the experimental stage and the prolonged application process. In response, the Pharmaceuticals and Medical Devices Agency (PMDA) put forward appropriate countermeasures, such as increase in examinants, gradually reducing the “lag in AD drugs.” AD: Alzheimer's disease; EU: European Union; US: United States.
Accelerating research. The Japanese Government has implemented measures to accelerate research. According to international trends in drug development and research on AD drugs, the following three topics have passed review by the Ministry of Health, Labor and Welfare (MHLW) and will be implemented by the PMDA and University of Tokyo Hospital: i) trials examining AD drug dosage in comparison to a placebo; ii) differences in results of AD research and medical settings in Japan and overseas; iii) clinical trials of proof of concept (POC) and first in human (FIH) trials in Japan. In order to facilitate the implementation of these projects, a “Strategic discussion of pharmaceutical affairs” was instituted in 2011 with support from the MHLW, guidance from the PMDA and involvement of colleges, research institutes, and companies (11). The main purposes include i) announcement of applications for AD drugs and devices and ii) routine communication between academia and the PDMA and guidance on clinical trials.
Facilitating the development of innovative drugs. In 2012, Japan's MHLW launched a novel project entitled “Accelerating Regulatory Science Initiatives” to facilitate the development of innovative drugs by developing a guideline for innovative drugs and by promoting personnel exchanges by the PMDA and research institutions (12). The PMDA, in cooperation with the University of Tokyo Hospital, implemented a research project to establish two research groups to develop a guideline for the clinical evaluation of drugs for AD: i) the Biomarker and Clinical Evaluation Group will establish biomarker-based criteria for the clinical evaluation of drugs for AD, and ii) the Modeling and Simulation Group will create disease models of AD using these techniques. Based on the support from the MHLW and collaboration between the PMDA and University of Tokyo Hospital, issues in clinical evaluation and development have been identified for the first time, including the use of biomarkers in inclusion criteria, efficacy endpoints, and clinical data required for application in Japan.
3. Government emphasis on cooperation with multiple partners in the response to AD
The US Government cooperates with multiple partners in the response to AD. As the sixth leading cause of death in the US, AD is a terrible progressive disease that destroys brain cells. The primary federal agency engaged in AD research, the National Institute on Aging (NIA) is a division of the US National Institutes of Health (NIH). The NIA is leading a broad scientific effort to understand the nature of aging and to extend the healthy, active years of life. The NIA emphasizes cooperation with multiple partners such as non-governmental organizations (NGOs).
Global clinical trials of AD drugs. Currently, there are two types of medications approved by Food and Drug Administration (FDA), cholinesterase inhibitors (Aricept, Exelon, Razadyne, and Cognex) and memantine (Namenda), to treat cognitive symptoms such as memory loss, confusion, and problems with thinking and reasoning of early AD (13). In order to test the effectiveness of these drugs, the Alzheimer's Disease Neuroimaging Initiative (ADNI), a global research project started in the US, actively supports the investigation and development of treatments that slow or stop the progression of AD, elucidating various disease biomarkers that reflect and even predict the progression of disease (14). In addition, three clinical trials are being conducted by the Alzheimer's Prevention Initiative (API), the Dominantly Inherited Alzheimer's Network (DIAN), and the Alzheimer's Therapeutic Research Institute (ATRI) to examine the preventive effects of AD drugs in the preclinical phase (15).
The NIA and the Alzheimer's Association. The Alzheimer's Association is the leading voluntary health organization in Alzheimer's care, support, and research. The Association works at the global, national, and local level to enhance care and support for all those affected by Alzheimer's and other dementias. With the encouragement of the NIA, the Alzheimer's Association was founded on April 10, 1980 (16). The Alzheimer's Association made new investments of over $17 million in more than 80 scientific investigations, part of the over 350 ongoing research projects funded by the Association in 21 countries, totaling over $80 million. These include grant awards to 68 projects through the Association's International Research Grant Program, representing proposals ranked highest by peer reviewers from an extremely competitive field of over 1,000 proposal ideas (540 invited applications). Since 1982, the Association has invested over $350 million in more than 2,300 scientific investigations. Advancing AD research remains a core element of the Association's identity and a key facet of its mission (17).
Cooperation with other research institutes. Cooperation with other research institutes, grant partnerships, and even joint studies led by government are crucial to a project working successfully (18). As specific examples, i) Biomarkers Across Neurodegenerative Diseases (BAND) is a program to stimulate analyses across AD, to perform further analysis of existing research information to advance biomarker discovery, and to standardize assays, genetic profiles, and imaging modalities; ii) Mechanisms of Cellular Death in Neurodegeneration (MEND) is a program to discover and understand mechanisms and pathophysiological processes by which brain cell loss is mediated in AD and thereby seek insights and potential targets for therapeutic interventions that would sustain healthy brain function, and iii) Imaging Dementia Evidence for Amyloid Scanning (IDEAS) is a program to determine clinical usefulness of diagnosing AD with a brain PET scan that detects amyloid plaques, a core feature of AD.
4. Government standardization of AD treatment
4.1. The Chinese Government has implemented a clinical pathway for AD
As of the end of 2011, there were 185 million people age 60 years or over in China, accounting for 13.7% of the total population. Aging is a key issue in China nowadays, given the serious challenge posed by AD (19). AD has a similar level of prevalence in China, Japan, the US, and Europe (Table 1) (9,20–23). Although the prevalence rates are roughly the same in these countries, China accounts for the major share of AD prevalence worldwide because of China's huge population. Chan et al. estimated that there were 5.69 million people with AD (3.85–7.53) in 2010 (19).
Table 1. The prevalence of Alzheimer's disease in China, Japan, the US, and Europe.
| Year | Location | Population | Prevalence (%) | Reference |
|---|---|---|---|---|
| 2008 | Spain | n = 2.170 | 6.6 | Tola-Arribas MA, 2013 (5) |
| 2008–2009 | China | n = 10,276; Age ≥ 65 | 5.14 | Jia J, 2014 (6) |
| 2010–2012 | Japan | n = 2,922 | 5.7 | Yasue M, 2015 (7) |
| 2013 | Portugal | n = 160,287; Age ≥ 60 | 5.91 | Santana I, 2015 (8) |
| 2015 | US | Meta-Analysis | 5.5 | Steenland K, 2015 (9) |
The Chinese Government has focused on a clinical pathway for AD. Evidence that provides the basis for guidelines is mostly from trials conducted in other countries due to very limited Chinese data available for local systematic review. Thus, more local evidence on AD care is needed to develop an evidence-based guideline appropriate for people in China. The inadequate implementation of the current AD guideline has resulted in a low rate of diagnosis and high rate of missed diagnosis, representing a further obstacle for patients with AD to receive dementia care in different areas nationwide. With this in mind, Peking University developed the Clinical Pathway for Alzheimer's Disease in China (CPAD) in 2013. The pathway was developed by determining how AD was clinically diagnosed and treated by physicians in routine practice, and the pathway should help address the low rate of AD diagnosis and the low rate of prescription of anti-dementia drugs and to support guideline development (24).
Based on previous studies, the National Health and Family Planning Commission (NHFPC) implemented a clinical pathway for AD in China in April 2016; the Chinese Medical Association (CMA) oversees the administrators of health care and medical facilities that manage the quality of medical care (25–26). This pathway has standardized clinical diagnosis and treatment as part of the response to AD, including eligible patients, diagnostic criteria, therapeutic schedules, drug selection, and required inspections.
4.2. The clinical guideline on AD medicines in the European Union (EU)
In 2008, the Committee for Medicinal Products for Human Use (CHMP), a center of the European Medicines Agency (EMA), issued the “Guideline on Medicinal Products for the Treatment of Alzheimer's Disease and other Dementias”. In 2014, the CHMP released a paper entitled “Discussion Paper on the Clinical Investigation of Medicines for the Treatment of Alzheimer's Disease and Other Dementias (EMA/CHMP/539931/2014 Corr.)” (27). Given the current uncertainties regarding the pathophysiology of AD, the draft Guideline is intended to identify the various stages of the disease and the relevance of biomarkers. The draft Guideline aims at integrating the requirements for development programs which start earlier in the disease course with the necessary adaptations to the distinct manifestations of the illness at these stages.
The draft Guideline addresses: i) the impact of new diagnostic criteria for AD (including early and even symptomatic stages of disease) on clinical trial design; ii) the potential use of biomarkers and their temporal relationship to the different phases of AD in different stages of drug development (mechanism of action, target engagement, use as a diagnostic test, enrichment of study populations, stratification for subgroups, safety and efficacy markers, etc.); iii) evaluation of the efficacy and safety of AD drugs by assembling data on their long-term safety, the speed of their anticipated action, and the duration of the trial.
5. Perspectives for the future
As indicated by the specific regulations to accelerate research, cooperative efforts with multiple partners, and standardization of diagnosis and treatment, government plays an important role in the response to AD (Table 2). As specific examples, i) the Japanese Government has instituted and supported regulations to encourage the development of AD drugs in order to accelerate research and development of innovative drugs; ii) the US Government cooperates with multiple partners such as the Alzheimer's Association, BAND, MEND, IDEAS, and other NGOs in the response to AD; iii) Chinese governmental measures have standardized clinical diagnosis and treatment as part of the response to AD, including eligible patients, diagnostic criteria, therapeutic schedules, drug selection, and required inspections; and iv) with political support from member governments, the EU has issued guidelines and conducted clinical studies on medicines for the treatment of AD in order to ascertain the various stages of the disease and the relevance of biomarkers.
Table 2. Government support for treatment of Alzheimer's disease in Japan, the US, China, and the EU.
| Role | State | Measures | Results |
|---|---|---|---|
| Regulation | Japan |
i) The PMDA put forward appropriate countermeasures, such as an increase in examinants, gradually reducing the “lag in AD drugs”; ii) Three topics have passed review by the MHLW and have been implemented by the PMDA and University of Tokyo Hospital; iii) A “Strategic discussion of pharmaceutical affairs” was instituted in 2011 with support from the MHLW, guidance from the PMDA, and involvement of colleges, research institutes, and companies; iv) In 2012, Japan's MHLW launched a novel project entitled “Accelerating Regulatory Science Initiatives” to facilitate the development of innovative drugs. |
i) The prescription of 4 drugs to patients with AD thus became possible in clinical settings, thereby overcoming the issue of the “lag in AD drugs”; ii) Announcement of applications for AD drugs and devices and ii) routine communication between academia and the PDMA and guidance on clinical trials; iii) Issues in clinical evaluation and development have been identified for the first time, including the use of biomarkers in inclusion criteria, efficacy endpoints, and clinical data required for application in Japan. |
| Coordination | US |
i) In order to test the effectiveness of drugs, ADNI, which was supported by the NIA, actively supports the investigation and development of treatments that slow or stop the progression of AD; ii) With the encouragement of the NIA, the Alzheimer's Association was founded on April 10, 1980; iii) The NIA cooperates with multiple partners such as BAND, MEND, IDEAS, and other NGOs in the response to AD. |
i) Clinical trials by the API, DIAN, and ATRI are underway; ii) Since 1982, the Association has invested over $350 million in more than 2,300 scientific investigations; iii) Communication and cooperation on advance biomarker discovery, pathophysiological processes, and clinical usefulness. |
| Standardization | China |
i) Peking University develops CPAD in 2013; ii) The NHFPC implements a clinical pathway for AD in China in April 2016; the CMA oversees the administrators of health care and medical facilities that manage the quality of medical care. |
CPAD has standardized clinical diagnosis and treatment as part of the response to AD, including eligible patients, diagnostic criteria, therapeutic schedules, drug selection, and required inspections |
| EU |
i) In 2008, the CHMP issued the “Guideline on Medicinal Products for the Treatment of Alzheimer's Disease and other Dementias”; ii) In 2014, the CHMP released a paper entitled “Discussion Paper on the Clinical Investigation of Medicines for the Treatment of Alzheimer's Disease and Other Dementias”. |
i) Impact of new diagnostic criteria for AD; ii) Potential use of biomarkers and their temporal relationship with the different phases of AD in different stages of drug development; iii) Evaluation of the efficacy and safety of AD drugs. |
ATRI: Alzheimer's Therapeutic Research Institute; ADNI: Alzheimer's Disease Neuroimaging Initiative; API: Alzheimer's Prevention Initiative; CHMP: the Committee for Medicinal Products for Human Use; CMA: Chinese Medical Association; CPAD: the Clinical Pathway for Alzheimer's Disease in China; DIAN: Dominantly Inherited Alzheimer's Network; EU: European Union; MHLW: the Ministry of Health, Labor, and Welfare; NGO: non-governmental organization; NHFPC: the National Health and Family Planning Commission; NIA: the National Institute on Aging; PMDA: the Pharmaceuticals and Medical Devices Agency; US: United States.
The advantage of government support for AD research is that it accelerates new drug research and it reduces the nonstandard diagnosis of intractable diseases such as AD through regulation, coordination, and standardization. Once AD diagnosis and treatment has been nationally standardized, a clinical pathway covers all stages of AD diagnosis and treatment will guide physicians in caring for patients with AD. In addition, government support is also essential to facilitate the global establishment of common standards and guidelines for AD. AD is an intractable disease, so different countries need to share clinic trial information and cooperate in the conduct of those trials. International cooperation will play a key role in the response to other intractable and rare diseases.
Acknowledgements
This work is granted by the National Natural Science Foundation of China (71673169).
References
- 1. Jaworski T, Dewachter I, Seymour CM, Borghgraef P, Devijver H, Kügler S, Van Leuven F. Alzheimer's disease: Old problem, new views from transgenic and viral models. Biochim Biophys Acta. 2010; 1802:808-818. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. World report on Ageing and Health. 2015. http://www.who.int/ageing/publications/world-report-2015/en/ (accessed June 14, 2016).
- 3. Alzheimer's Disease International. World Alzheimer Report 2015. The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. 2015. http://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed June 14, 2016).
- 4. Dodel R, Neff F, Noelker C, Pul R, Du Y, Bacher M, Oertel W. Intravenous immunoglobulins as a treatment for Alzheimer's disease: Rationale and current evidence. Drugs. 2010; 70:513-528. [DOI] [PubMed] [Google Scholar]
- 5. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013; 9:63-75.e2. [DOI] [PubMed] [Google Scholar]
- 6. Schindler RJ. Study design considerations: Conducting global clinical trials in early Alzheimer's disease. J Nutr Health Aging. 2010; 14:312-314. [DOI] [PubMed] [Google Scholar]
- 7. Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: Clinical trials and drug development. Lancet Neurol. 2010; 9:702-716. [DOI] [PubMed] [Google Scholar]
- 8. Humbert IA, McLaren DG, Kosmatka K, Fitzgerald M, Johnson S, Porcaro E, Kays S, Umoh EO, Robbins J. Early deficits in cortical control of swallowing in Alzheimer's disease. J Alzheimers Dis. 2010; 19:1185-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yasue M, Sugiura S, Uchida Y, Otake H, Teranishi M, Sakurai T, Toba K, Shimokata H, Ando F, Otsuka R, Nakashima T. Prevalence of sinusitis detected by magnetic resonance imaging in subjects with dementia or Alzheimer's disease. Curr Alzheimer Res. 2015; 12:1006-1011. [DOI] [PubMed] [Google Scholar]
- 10. Moritoyo T. New and future treatments for neurological disorders - Knowledge essential to daily clinics and future prospects. Topics: 2. Dementia. Nihon Naika Gakkai Zasshi. 2013; 102:1916-1922. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 11. Moritoyo T. Accelerating regulatory science initiatives for the development of drugs for Alzheimer's disease in Japan. Clin Ther. 2015; 37:1622-1626. [DOI] [PubMed] [Google Scholar]
- 12. Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2012; 8(1 Suppl):1-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008; 27:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landmark 10-year Alzheimer's study increases pace of discovery. http://www.alz.org/documents_custom/071715_adni.pdf (accessed June 24, 2016)
- 15. Cooperation advances Alzheimer's disease prevention research. http://alz.org/documents_custom/cap_nature_news_release_092915.pdf (accessed June 20, 2016)
- 16. About the Alzheimer's Association. http://www.alz.org/about_us_about_us_.asp (accessed February 20, 2016)
- 17. The Alzheimer's Association. Alzheimer's Association Annual Report. 2015. https://www.alz.org/annual_report/downloads/annual-report.pdf#page=3 (accessed June 20, 2016)
- 18. The Alzheimer's Association. Transforming Alzheimer's Disease Therapies Through Collaboration. https://c-path.org/wp-content/uploads/2015/11/Hendrix-TransformingAD-Therapies.pdf (accessed March 4, 2016).
- 19. Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I; Global Health Epidemiology Reference Group (GHERG). Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet. 2013; 381:2016-2023. [DOI] [PubMed] [Google Scholar]
- 20. Tola-Arribas MA, Yugueros MI, Garea MJ, Ortega-Valín F, Cerón-Fernández A, Fernández-Malvido B, San José-Gallegos A, González-Touya M, Botrán-Velicia A, Iglesias-Rodríguez V, Díaz-Gómez B. Prevalence of dementia and subtypes in Valladolid, northwestern Spain: The DEMINVALL study. PLoS One. 2013; 8:e77688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014; 10:1-9. [DOI] [PubMed] [Google Scholar]
- 22. Santana I, Farinha F, Freitas S, Rodrigues V, Carvalho Å. The epidemiology of dementia and Alzheimer disease in Portugal: Estimations of prevalence and treatment-costs. Acta Med Port. 2015; 28:182-188. [PubMed] [Google Scholar]
- 23. Steenland K, Goldstein FC, Levey A, Wharton W. A meta-analysis of Alzheimer's disease incidence and prevalence comparing African-Americans and Caucasians. J Alzheimers Dis. 2015; 50:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clinical Pathway for Alzheimer's Disease in China (CPAD). https://clinicaltrials.gov/ct2/show/NCT01779310 (accessed June 4, 2016).
- 25. The National Health and Family Planning Commission (NHFPC) implemented the clinical pathway for Alzheimer's Disease in China in April 2016. http://www.nhfpc.gov.cn/yzygj/s3593/201604/efa3826fbde44dc789e4420c6c57daeb.shtml (accessed May 4, 2016).
- 26. The clinical pathway for Alzheimer's disease in China. http://www.cma.org.cn/attachment/2016425/1461563271534.docx (accessed May 4, 2016).
- 27. European Medicines Agency. Discussion paper on the clinical investigation of medicines for the treatment of Alzheimer's disease and other dementias. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176827.pdf (accessed May 12, 2016).


