Summary
Sclerosing bone dysplasias are a series of clinically and genetically heterogeneous diseases characterized by functional failure of the osteoclasts in bone resorption, leading to an excessive amount of bone mineral density (BMD) which could have serious clinical consequences. We treated three children affected with seriously high levels of BMD with acetazolamide, with the intention of inducing metabolic acidosis, thus increasing bone resorption and reducing BMD. All our patients tolerated and followed the treatment well and the clinical response was satisfactory in all cases.
Keywords: Acetazolamide, osteopetrosis, carbonic anhydrase inhibitor, craniometaphyseal dysplasia, osteochondrodysplasia, sclerosing bone dysplasia
1. Introduction
Sclerosing bone dysplasias are a series of clinically and genetically heterogeneous diseases characterized by functional failure of the osteoclasts in bone resorption, giving rise to anomalies in bone formation and modelling, leading to an excessive amount of bone mineral density (BMD). This defect makes the bones brittle and can cause bone marrow failure, delayed eruption of permanent teeth, nerve entrapment syndrome and growth deficiencies (1).
Osteopetrosis is a rare disease, affecting 1:20,000 live births in its dominant form and 1:250,000 in its recessive form. It is characterized by the presence of pathological fractures. Although osteopetrosis comprises a heterogeneous group of conditions, encompassing a range of molecular lesions and clinical signs, all of its forms share the hallmark of osteoclast dysfunction (2,3).
Craniometaphyseal dysplasia is a form of osteochondrodysplasia characterized by hyperostosis and sclerosis of the base of the skull, cranial vault and facial bones, as well as metaphyseal widening of the long bones. Cranial sclerosis may cause mandibular asymmetry and compression of the cranial nerves, which may eventually lead to hearing loss and facial paralysis (4). Most cases display a pattern of dominant autosomal pedigree (1:20,000) with mutations in the ANKH gene, which codifies a protein that regulates intracellular to extracellular movement of pyrophosphate (PP), although there is also a more severe recessive autosomal form (1:250,000), the gene for which has not yet been identified. Accordingly, its diagnosis is essentially clinical and based on radiology findings (5).
Although there has been some success with the use of calcitriol and interferon gamma in treating osteopetrosis, bone marrow transplant is the only cure for more serious forms of the disease (6).
Acetazolamide is a carbonic anhydrase (CA) inhibitor that suppresses the activity of carbonic anhydrase type IV, which is associated with the membranes. Acetazolamide interferes with the tubular resorption of bicarbonate (HCO3−), inducing bicarbonate diuresis and metabolic acidosis (7).
However, bone marrow transplant involves considerable risks, requiring heavy doses of immunosuppressants and involving the possibility of graft vs host disease. As there is no treatment for craniometaphyseal dysplasia (CMD) or osteopetrosis, we treated three children affected with seriously high levels of BMD with acetazolamide, after approval of off-label use, with the intention of inducing metabolic acidosis, thus increasing bone resorption and reducing BMD.
2. Clinical cases
Case 1. Three-year-old male referred due to his unusual phenotype and the fortuitous detection of cranial sclerosis during radiography performed after a mild head injury. No personal or perinatal antecedents of interest were seen. Patient had normal psychomotor development, and was performing well at school. Parents young and healthy, not consanguineous. Brother aged six years, healthy. No family antecedents of interest.
Physical examination showed normal weight (89th percentile) and height (96th percentile). Prominent forehead with normal superciliary arches, orbital hypertelorism, large ears and a small nose, with flat root and bridge and progressive lateral widening, anteverted nostrils and short columella (Figure 1). Wide philtrum, large mouth with normal palate and teeth, mouth breathing were seen. Mammary hypertelorism. No evident abnormalities of spinal column. Extremities normal, single transverse palmar crease, articulations normal, articular mobility conserved, no cramping. The feet showed clinodactyly of the 5th toe and 1/3 soft-tissue syndactyly of the 2nd and 3rd toes. Neurological examination showed only unstable gait; the rest, including the cranial nerves, tested within normal range.
Figure 1.
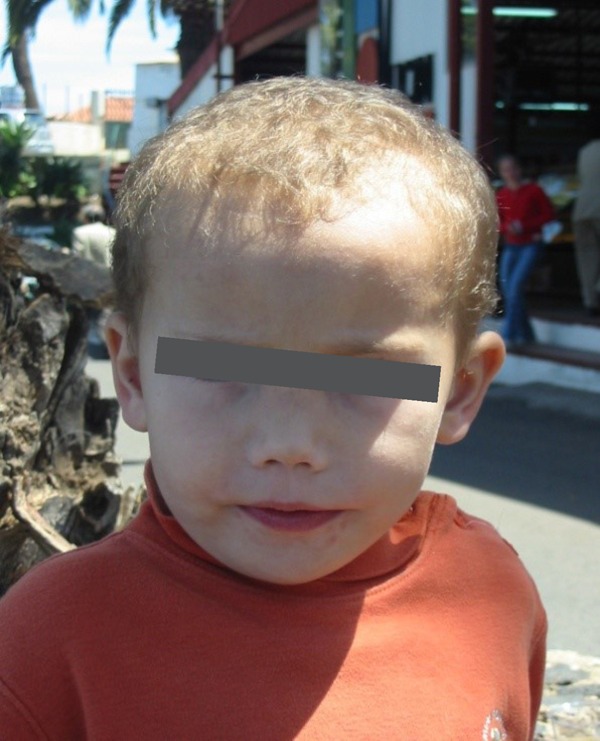
Patient's craniometaphyseal dysplasia on diagnosis, age 3 years: orbital hypertelorism, flat root and bridge of nose, anteverted nostrils and macrocephaly.
Complementary testing returned normal blood count, acid basic balance and elementary biochemistry and erythrocyte sedimentation rate (ESR) values. Karyotype 46XY. Blood and urine test results are given in Table 1.
Table 1. Clinical, analytical and radiology variables before and after treatment.
| Items | Case 1. Craniometaphyseal dysplasia | Case 2. Female, Dominant Osteopetrosis | Case 3. Male. Dominant Osteopetrosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 3 | Year 7 | Baseline | Year 1 | Year 6 | Baseline | Year 1 | Year 6 | |
| Z Score BMD | +6.83 (170%) | +3.9 (142%) | +1.21 (114%) | +0.7 | +6.25 (300%) | +7.91 (277%) | T score + 20.5 (305%) | +7.55 | +5.37 (295%) | +6.5 (221%) |
| pH/HCO3−/SBE (mmol/L) | 7.35/22/−3 | 7.29/18/−7.7 | 7.32/22/−9.3 | 7.28/24/−3.2 | 7.38/23.5/−1 | 7.34/21.4/−2.7 | 7.34/23.5/−1.4 | 7.39/24/−0.1 | 7.33/22.5/−0.4 | 7.29/20.9/−4.6 |
| Cit/Cr urine (mmol/mol) | 98.5 | 70.4 | 123.2 | 218.4 | - | 42.4 | 322.4 | - | 239.2 | 578.6 |
| Calcium/IPh (mmol/L) | 2.4/1.58 | 2.4/1.87 | 2.2/1.49 | 2.5/1.65 | 2.4/1.58 | 2.3/1.78 | 2.2/1.58 | 2.2/1.74 | 2.2/1.74 | 2.2/1.74 |
| Ca/Cr urine (mol/mol) | 116.7 | 183.9 | 95.5 | 84.9 | 0.18 | 14.1 | 31.8 | 0.18 | 28.3 | 53.0 |
| Ca/Cit urine (mol/mol) | 1.22 | 2.63 | 0.75 | 0.40 | - | 0.06 | 0.10 | - | 0.12 | 0.06 |
| iPTH (pmol/L) | 3.17 | 1.19 | 2.07 | 2.08 | 5.79 | 5.71 | 3.18 | 5.79 | 5.71 | 3.82 |
| β Crosslaps (mmol/L) | - | - | - | - | - | 0.21 | 0.16 | - | 0.26 | 0.50 |
| DP/Cr urine (nmol/mmol) | 86.29 | 52.03 | 27.32 | 37.2 | - | - | - | - | - | - |
| ALP (µkat/L) | 7.78 | 4.86 | - | 5.06 | 6.08 | 3.0 | 2.37 | 4.18 | 3.72 | 4.51 |
| Osteocalcin (mmol/L) | 10.26 | 3.59 | 1.84 | 7.86 | - | 5.71 | 4.83 | - | 11.03 | 14.59 |
| Renal ultrasound | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| BAEP | Mild bilateral sn hypoacusis | Mild bilateral sn hypoacusis | Normal | Mild bilateral sn hypoacusis | - | - | - | - | - | - |
| Acetazolamide | No | 9.9 mg/kg/day 425 mg/day/1.73 m2 | 8 mg/kg/day 375 mg/day/1.73 m2 | 7.2 mg/kg/day 366 mg/day/1.73 m2 | No | 5 mg/kg/day 300 mg/day/1.73 m2 | 12.3 mg/kg/day 816 mg/day/ 1.73 m2 | No | 7 mg/kg/day 375 mg/day/1.73 m2 | 8.6 mg/kg/day 534 mg/day/1.73 m2 |
| Height/GR | p93 | p87/−1.81 SD | p83/+1.83 SD | P67/−2.26 SD | p4 /−0.14 SD | p6/1.16 SD | P3/0 SD | p4/0.13 SD | p3/−2.43 SD | P12/+0.10 SD |
ALP: Alkaline phosphatase; BAEP: Brainstem auditory evoked potentials; BMD: Bone mineral density in lumbar region; Ca/Cit: Calcium/Citrate ratio; Ca/Cr: Calcium/Creatinine ratio; Cit/Cr: Citrate/Creatinine ratio; DP/Cr: Deoxypyridinoline/Creatinine ratio; GR: Growth rate; IPh: Inorganic phosphorus; iPTH: Intact parathyroid hormone; p: Percentile; SBE: Standard base excess; SD: Standard deviation; sn: sensorineural.
Radiology showed a marked sclerosis at the base of the skull and frontal region (Figure 2). The long bones of the lower limbs showed evidence of sclerosis of the diaphysis, the metaphysis being relatively radiolucent. A cranial computed tomography showed, in addition to sclerosis of the base of the skull, thickening of the bone in the nasal pyramid and of the lamina cribrosa (Figure 3). Neurophysiology testing showed discreet impairment of bilateral sight and mild bilateral cochlear sensorineural hypoacusis. Bone densitometry taken at lumbar level showed a significant increase in BMD (0.718 g/cm2; z-BMD + 6.83).
Figure 2.
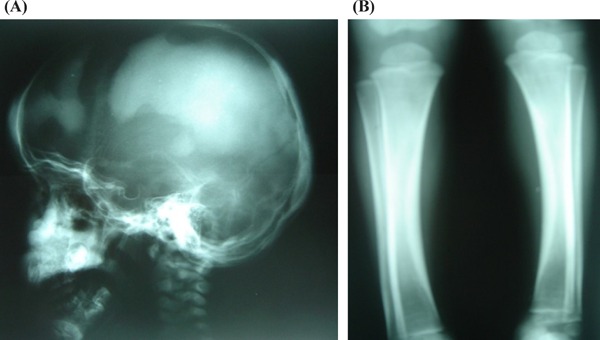
Patient's craniometaphyseal dysplasia. (A), Cranial radiography on diagnosis: Irregular cranial sclerosis. (B), Radiography of lower limb on diagnosis: Metaphyseal widening with areas of hyperdensity and hypodense zones in diaphysis
Figure 3.
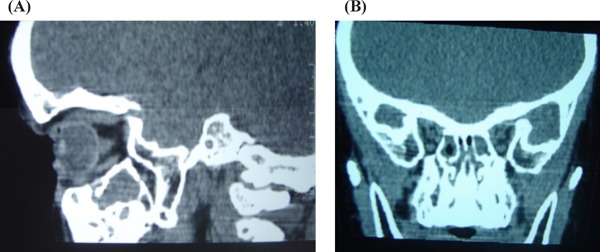
Patient's craniometaphyseal dysplasia. Cranial computed tomography on diagnosis: Notable abnormalities in bone density and thickness of cranial vault, differentiated abnormalities in bone layers. Bone thickening around nasal pyramid and lamina cribrosa. (A), Sagittal plane; (B), Coronal plane.
Physical examination of the parents returned normal results. Molecular tests for dominant autosomal craniometaphyseal dysplasia returned negative, so the child was diagnosed with autosomal recessive craniometaphyseal dysplasia and began treatment with acetazolamide, maintained over seven years to the present day. In the third year of therapy, the auditory evoked potentials had normalized, although at the following check-up the patient once again presented with mild bilateral sensorineural hypoacusis as well as a mild demyelinating lesion of both optic nerves, not affecting his day-to-day activity. In the fourth year, ultrasonography detected calyceal microlithiasis (left side), and so the patient began treatment with oral potassium citrate; and the lesions were observed to disappear over subsequent check-ups.
Case 2. Female aged 11.5 years, from Ecuador, with a clinical diagnosis of osteopetrosis and compatible radiology findings. The patient has a history of multiple pathological fractures since she was five years old (wrist, coccyx, hip, forearm (twice) and fingers), as well as recurring bone pain requiring regular painkillers; she also tires during moderate physical exercise. Patient had normal psychomotor development, and school performance. Menarche at age 10. Parents young and healthy, consanguineous. Sister aged 7 years, healthy. No family antecedents of interest, except for 2nd degree nephrourolithiasis.
Physical examination: Weight in 42nd percentile, height in 4th percentile, head circumference in 68th percentile, blood pressure 110/60 mmHg (p50–90), peculiar phenotype with bulging forehead, large ears and narrow thorax. No further abnormalities were found, except limited abduction of the left hip and a level II/VI protomesosystolic murmur in the mesocardium, which normalized at later check-ups.
Complementary testing returned normal blood count, ESR, acid basic balance, elementary biochemistry, immunoglobulin, proteinogram, complement test and urine values. Test results given in Table 1.
Dual-energy X-Ray absorptiometry (DXA) revealed a BMD of 2.578 g/cm2 at lumbar level (Z score + 6.25); no auditory or visual deficiencies. Normal abdominal ultrasound. Normal baseline and stress spirometry normal. No electrocardiographic (ECG) abnormalities, although echocardiography showed findings compatible with dilated cardiomyopathy, with ventricular function preserved. This was treated with Enalapril and in subsequent check-ups the values had returned to normal. Habitual toxicity tests were conducted, as well as extended blood testing (including the Trypanosoma cruzi serology) and ultrasound testing of the family, all returning normal values. A molecular study of gene ClCN7 was also conducted, revealing a heterozygous p.Leu213Phe (c.637C>T) mutation, confirming the diagnosis of dominant osteopetrosis.
After the good results obtained in treating the patient's CMD, we began treatment with acetazolamide. The patient's BMD levels stabilized and she reacted extremely well to therapy over six years of follow-up, to the present day, reducing her reliance on analgesics. She had suffered no new fractures, although in this past year she has had two fractures of her fingers, associated with increased BMD and requiring a higher acetazolamide dose. Patient followed and tolerated therapy well, presenting no adverse effects, except for the expected mild metabolic acidosis and hypocitraturia (Table 1).
Case 3. Male aged 9.5 years, brother of Patient 2, with radiology findings compatible with osteopetrosis and a history of suffering fractures from mild knocks since age six years (right wrist (twice), hip), frequent bone pain and tiring when performing vigorous physical activity. Normal psychomotor development, performing well at school.
Physical examination: Weight in 25th percentile, height in 4th percentile, head circumference in 40th percentile, blood pressure 90/50 mmHg (< p50), peculiar phenotype with bulging forehead, large ears and narrow thorax. The rest of the physical examination likewise evidenced limited abduction of the left hip and a level I–II/VI systolic murmur in the mesocardium, resolving over time.
Complementary testing returned normal blood count, ESR, acid basic balance, elementary biochemistry, immunoglobulin, proteinogram, complement test and urine values. Test values given in Table 1.
DXA showed BMD of 1.969 g/cm2 at lumbar level (Z score + 7.55); without auditory or visual deficiencies. Normal abdominal ultrasound, ECG and spirometry. Echocardiography showed a dilated cardiomyopathy, like his sister, with negative serology and toxicology results. Treatment commenced with enalapril, and values returned to normal over time.
Molecular analysis of the ClCN7 gene also revealed a heterozygous p.Leu213Phe (c.637C>T) mutation, which was also confirmed in the mother of the patients who, having remained asymptomatic to date, under DXA had a BMD at lumbar level in the normal range (T score 1.71).
Like his sister, the patient commenced treatment with acetazolamide. His BMD and bone pain decreased after the same follow-up period. His incidence of fractures has decreased, although in the past year he broke a bone in a toe, related to increased BMD, requiring a higher dose of the drug.
Patient followed and tolerated therapy well, presenting mild compensated metabolic acidosis and hypocitraturia, as well as reduced growth rate (Table 1). Because of this, his acetazolamide dose was reduced in the third year of therapy, after his BMD Z score had been reduced to + 3.73 (243%) (Figure 4).
Figure 4.
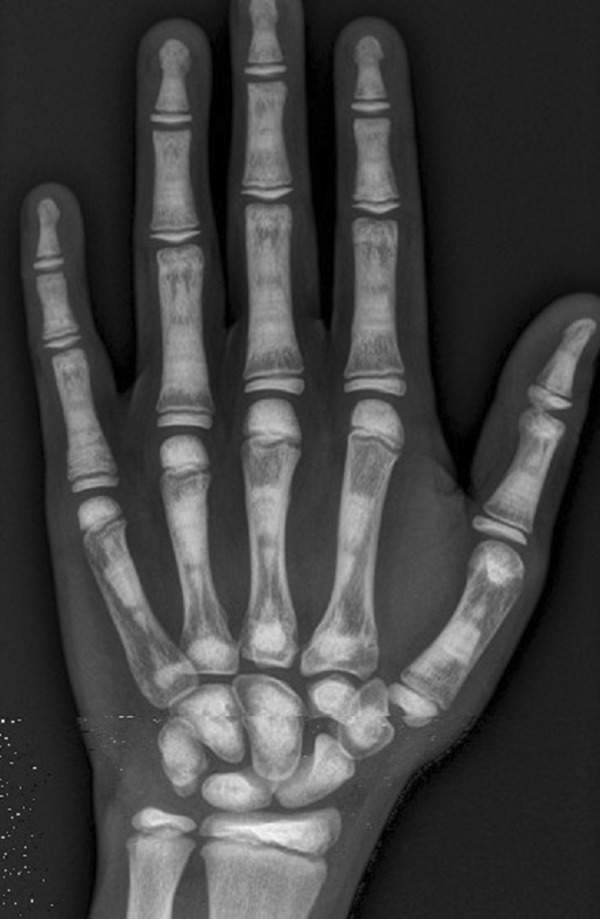
Osteopetrosis. Mottled sclerotic pattern in bones of left hand of male patient.
3. Discussion
Bone is a metabolically-dynamic, constantly-changing tissue. Bone formation is a complex process, requiring the sequential intervention of a large number of local and systemic factors, and the simultaneous and balanced participation of two types of cells: osteoclasts, giant TRAP-rich multinucleate cells which derive from myeloid processes and break down organic bone matrix; and osteoblasts, which derive from pluripotent mesenchymal cells, synthesize bone matrix and determine the eventual activation of the osteoclasts via several mediators, RANKL/RANK activating interactions and osteoprotegerin-blocking inhibitors. Mature osteoclasts adhere to the bone surface using their ruffled border, breaking down the bone matrix by acidifying the bone surface and secreting proteolytic enzymes such as Cathepsin K. Dysfunctional acidification of the resorption lacunae of the osteoclasts, caused by type II intracellular CA deficiency, proton pump deficiency and/or abnormalities in the chloride channels, is associated with several forms of osteopetrosis (2,8). On the other hand, the dominant autosomal form of CMD is associated with mutations in ANHK, leading to increased mineralization due to decreased transport of intracellular PP into the extracellular matrix (7).
Moreover, studies have shown that the skeleton contains a massive reserve of alkaline mineral (hydroxyapatite) and that, during periods of metabolic acidosis, bone acts as a buffer, helping to stabilize extracellular pH, reducing mineralization and stimulating the osteoclasts. This reduces bone calcium deposits and increases its elimination in the urine, although this mechanism has not been reproduced in all studies on humans, which show a reduction in the number of osteoblasts and osteoclasts when the body suffers from chronic metabolic acidosis (9,10).
In our cases, we decided on treatment with acetazolamide in order to induce mild metabolic acidosis, principally by inhibiting membrane-bound CA in the brush border of the proximal tubule, thus favoring bone resorption via the well-known buffer effect of the bone (11,12). Previous publications have described attempts to treat these diseases with calcitriol, calcium restriction, steroids or parathyroid hormone, all with the intention of increasing bone resorption, but with no conclusive findings in the majority of cases; likewise interferon therapy prior to hematopoietic stem cell transplantation in severe forms of the disease has been used (2). There have also been attempts made with calcitonin, which inhibits osteoclast activity, reducing bone formation by negative feedback, as osteoblast differentiation is influenced by proteins secreted during bone resorption (8). RANKL and the activation of alternative osteoclast acidification mechanisms, including the Na+/H+ antiporter, have also been proposed as potential therapeutic targets (2). In our group, we had previously trailed treatment with ammonium chloride, also attempting to generate acidosis and thereby bone resorption, but it was not tolerated well by patients (results not published).
All our patients treated with acetazolamide tolerated and followed the treatment well, in spite of it being necessary to increase the dose-probably due to its action decreasing during chronic acidosis. The proper dosage was determined by clinical outcome, patient tolerance without side effects and the findings in DXA and laboratory testing desired (metabolic acidosis). The clinical response was satisfactory in all cases, with improvements in visual and auditory disorders in the case with CMD, and a notable reduction in fractures and pain in patients with osteopetrosis. Once treatment commenced, all patients showed mild metabolic acidosis and hypocitraturia, with an increased urine calcium/citrate ratio. The patient with CMD began treatment with potassium citrate to treat an episode of lithiasis, resolving spontaneously with no increase in BMD.
Hypocitraturia in the three patients would seem to suggest intracellular acidosis (13). This contrasts with previous basic investigation work and those performed on adult women, which show the existence of osteoclast membrane-bound CA, as in the proximal renal tubule, which would act like a metabolon with intracellular CA, reducing bone resorption and increasing intracellular pH and osteoclast apoptosis after treatment with a CA inhibitor (14–16). Nevertheless, not all cases show the same results when acetazolamide is administered specifically, and in some, decreased osteoblast activity is also observed (14,16).
In our patients, the effect on BMD determined by DXA, was a reduction in the Z score in the male patient with CMD and the male patient with osteopetrosis, as reflected in Table 1. In spite of inducing acidosis and a good clinical evolution, the same effect was not achieved in the female patient with osteopetrosis, although, as the BMD was not adjusted to her bone size, due to a lack of standardization in this regard, and as BMD measured by DXA is influenced by the size of the bone, the increased Z score attained in the first year of treatment could be falsely magnified by growth (17). Nevertheless, the subsequent reduction in BMD was not as noteworthy as in the male patients, which could also be affected by not being adjusted to pubertal stage and/or body composition, and by the influence of estrogen on RANKL (8).
With regard to bone remodelling biochemical markers, Cases 1 and 2 showed a tendency towards reduction of both formation parameters (Alkaline phosphatase and osteocalcin) and resorption parameters (β Crosslap and urine Deoxypyridinoline/Creatinine ratio), compatible with the findings of Domrongkitchaiporn et al. in acidosis, and those of Shinohara et al. after administering acetazolamide (10,16). On the other hand, the male patient with osteopetrosis (Case 3) showed increased absolute values for both parameters in the third year after treatment commenced. This could be influenced by pubertal development, or the wide variation and analytical interference of these markers (18); these markers showed a reduction in the last check-up over the previous.
Also worthy of note was the absence of symptoms and bone anomalies in the mother of the two patients with osteopetrosis, even though she is a carrier of the same heterozygous mutation. This situation has already been described in the literature with the variable penetrance of the disease and its modulation by modifying factors, seen only in 66% of patients with mutations in the ClCN7 gene (19).
Finally, and within the complex equilibrium of bone formation, clearly displaced in favor of appositional growth during childhood, puberty and adolescence, we believe that the predominant beneficial effect of acetazolamide in our patients may be due to a reduction in bone formation and resorption secondary to metabolic acidosis induced by inhibiting membrane-bound CA in the proximal renal tubule, because otherwise there should also be osteoblast dysfunction in sclerosing dysplasias caused by primary defects in osteoclast resorption (1–3).
In any case, in view of the lack of effective treatment for most sclerosing bone dysplasias, acetazolamide is presented as a new form of therapy that may improve patients' prognosis and quality of life, circumventing the need for surgery. However, analytical and ultrasound controls are necessary once treatment has commenced, due to the risk of metabolic alteration and kidney stones. Nevertheless, further studies will be required at both the clinical and experimental level, to extract more solid conclusions and determine the exact action of treatment on bone metabolism and even assess the association with other treatments.
References
- 1. Warman ML, Cormier-Daire V, Hall C, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. 2011; 155A:943-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009; 4:5-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004; 351:2839-2849. [DOI] [PubMed] [Google Scholar]
- 4. Faden MA, Krakow D, Ezgu F, Rimoin DL, Lachman RS. The Erlenmeyer flask bone deformity in the skeletal dysplasias. Am J Med Genet A. 2009; 149A:1334-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reichenberger E, Tiziani V, Watanabe S, et al. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet. 2001; 68:1321-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazzolari E, Forino C, Razza A, Porta F, Villa A, Notarangelo LD. A single-center experience in 20 patients with infantile malignant osteopetrosis. Am J Hematol. 2009; 84:473-479. [DOI] [PubMed] [Google Scholar]
- 7. Maren TH. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977; 232:F291-F297. [DOI] [PubMed] [Google Scholar]
- 8. Drezner MK. Normal skeletal development and regulation of bone formation and resorption. In: UpToDate, Mulder JE. (Ed). UpToDate, Walthman MA, 2011. http://www.uptodate.com (accessed June 24, 2016). [Google Scholar]
- 9. Arnett TR. Extracellular pH regulates bone cell function. J Nutr. 2008; 138:S415-418. [DOI] [PubMed] [Google Scholar]
- 10. Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, Stitchantrakul W, Leeprasert V, Ongphiphadhanakul B, Radinahamed P, Rajatanavin R. Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int. 2002; 62:2160-2166. [DOI] [PubMed] [Google Scholar]
- 11. Sly WS, Whyte MP, Krupin T, Sundaram V. Positive Renal response to Intravenous Acetazolamide in Patients with Carbonic anhydrase II Deficiency. Pediatr Res. 1985; 19:1033-1036. [DOI] [PubMed] [Google Scholar]
- 12. Lucci MS, Tinker JP, Weiner IM, DuBose TD., Jr. Function of proximal tubule carbonic anhydrase defined by selective inhibition. Am J Physiol. 1983; 45:F443-F449. [DOI] [PubMed] [Google Scholar]
- 13. Hamm LL. Renal handling of citrate. Kidney Int. 1990; 38:728-735. [DOI] [PubMed] [Google Scholar]
- 14. Riihonen R, Supuran CT, Parkkila S, Pastorekiva S, Väänänen HK, Laitala-Leinonen T. Membrane-bound carbonic anhydrases in osteoclasts. Bone. 2007; 40:1021-1031. [DOI] [PubMed] [Google Scholar]
- 15. Pierce WM, Jr, Nardin GF, Fuqua MF, Sabah-Maren E, Stern SH. Effect of Chronic Carbonic Anhydrase Inhibitor Therapy on Bone Mineral Density in White Women. J Bone Miner Res. 1991; 6:347-354. [DOI] [PubMed] [Google Scholar]
- 16. Shinohara C, Yamashita K, Matsuo T, Kitamura S, Kawano F. Effects of carbonic anhydrase inhibitor acetazolamide (AZ) on osteoclasts and bone structure. Journal of Hard Tissue Biology. 2007; 16:115-123. [Google Scholar]
- 17. Specker BL, Schoenau E. Quantitative bone analysis in children: Current methods and recommendations. J Pediatr. 2005; 146:726-731. [DOI] [PubMed] [Google Scholar]
- 18. Robins SP. Biochemical markers for assessing skeletal growth. Eur J Clin Nutr. 1994; 48:S199-S209. [PubMed] [Google Scholar]
- 19. Waguespack SG, Hui SL, Dimeglio LA, Econs MJ. Autosomal dominant osteopetrosis: Clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab. 2007; 92:771-778. [DOI] [PubMed] [Google Scholar]


