SUMMARY
During the asexual cycle, Plasmodium falciparum extensively remodels the human erythrocyte to make it a suitable host cell. A large number of exported proteins facilitate this remodeling process, which causes erythrocytes to become more rigid, cytoadherent, and permeable for nutrients and metabolic products. Among the exported proteins, a family of 89 proteins, called the Plasmodium helical interspersed subtelomeric (PHIST) protein family, has been identified. While also found in other Plasmodium species, the PHIST family is greatly expanded in P. falciparum. Although a decade has passed since their first description, to date, most PHIST proteins remain uncharacterized and are of unknown function and localization within the host cell, and there are few data on their interactions with other host or parasite proteins. However, over the past few years, PHIST proteins have been mentioned in the literature at an increasing rate owing to their presence at various localizations within the infected erythrocyte. Expression of PHIST proteins has been implicated in molecular and cellular processes such as the surface display of PfEMP1, gametocytogenesis, changes in cell rigidity, and also cerebral and pregnancy-associated malaria. Thus, we conclude that PHIST proteins are central to host cell remodeling, but despite their obvious importance in pathology, PHIST proteins seem to be understudied. Here we review current knowledge, shed light on the definition of PHIST proteins, and discuss these proteins with respect to their localization and probable function. We take into consideration interaction studies, microarray analyses, or data from blood samples from naturally infected patients to combine all available information on this protein family.
INTRODUCTION
Malaria is an infectious disease caused by the protozoan parasite Plasmodium and is transmitted by female Anopheles mosquitoes to humans during a blood meal. Of the five Plasmodium species that cause human malaria, two are of major public health interest: P. falciparum causes the most severe form of malaria, while P. vivax is the most widespread Plasmodium species (1, 2). The success of P. vivax is due to the presence of undetectable hypnozoites, which represent dormant stages in the liver and pose a huge problem for malaria control. With half of the human population at risk, roughly 200 million malaria cases, and an estimated 438,000 deaths in 2015, malaria still remains a major threat to public health (3).
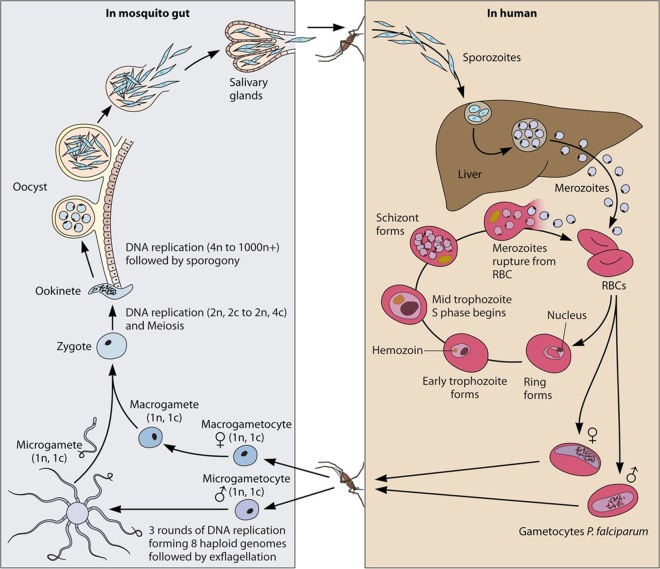
Plasmodium has a complex life cycle, alternating between the arthropod vector and its vertebrate host (Fig. 1) (1). During the bite of an infected female Anopheles mosquito, sporozoites are injected into dermal tissue and transported through the bloodstream to the liver. There, the sporozoites penetrate and invade hepatocytes, which is followed by several rounds of asexual replication. Subsequently, thousands of merozoites are released into the bloodstream through so-called merosomes, starting the blood stage cycle (4, 5).
FIG 1.
Life cycle of P. falciparum. (Right) Upon the bite of a Plasmodium falciparum-infected female Anopheles mosquito, sporozoites are injected into the dermal tissue of the human host. Sporozoites quickly enter the bloodstream and are transported to the liver, where they invade liver cells and develop into liver schizonts. Through merosomes, thousands of merozoites are released into the bloodstream, where they invade erythrocytes, starting the asexual replication cycle. Each cycle, a few parasites cease replicating, commit to the sexual cycle, and develop into gametocytes. (Left) Mature gametocytes are taken up by a mosquito during a blood meal; rapidly develop into male and female gametes, which fuse together in the gut of the mosquito; form an ookinete that penetrates the gut wall; and undergo sexual replication in the oocyst, producing thousands of sporozoites. At the end, sporozoites migrate to the salivary gland and are ready to be transmitted to a human host during the next blood meal. (Adapted from reference 148.)
Once in the bloodstream, merozoites invade erythrocytes and multiply during an ∼48-h intraerythrocytic development cycle. At the end of the cycle, ∼16 to 32 merozoites are released and reinvade new erythrocytes, starting the cycle anew. Once a merozoite has invaded the erythrocyte, it extensively refurbishes and remodels the host cell. This remodeling of the cell ensures parasite ion homeostasis within the cell, allows nutrient uptake, and is accompanied by changes in host cell membrane structure and rigidity. All this is achieved by the export of a large number of proteins from the parasite into the host cell, leading to dramatic changes of infected erythrocytes. Since such a remodeled erythrocyte would be eliminated in the spleen, the parasite also exports the major virulence factor P. falciparum erythrocyte membrane protein 1 (PfEMP1), which conveys binding of infected cells to endothelial receptors such as CD36, intracellular adhesion molecule (ICAM), or endothelial protein C receptor (EPCR) (6, 7). PfEMP1 is displayed on the surface of the infected red blood cell (iRBC) and is considered to be the key factor for morbidity and mortality. Overall, these refurbishing processes are considered to be responsible for most symptoms and the pathology of malaria (1, 8). Hence, recently, research focusing on exported proteins and processes involved in this host cell remodeling has attracted increased attention.
Protein Export
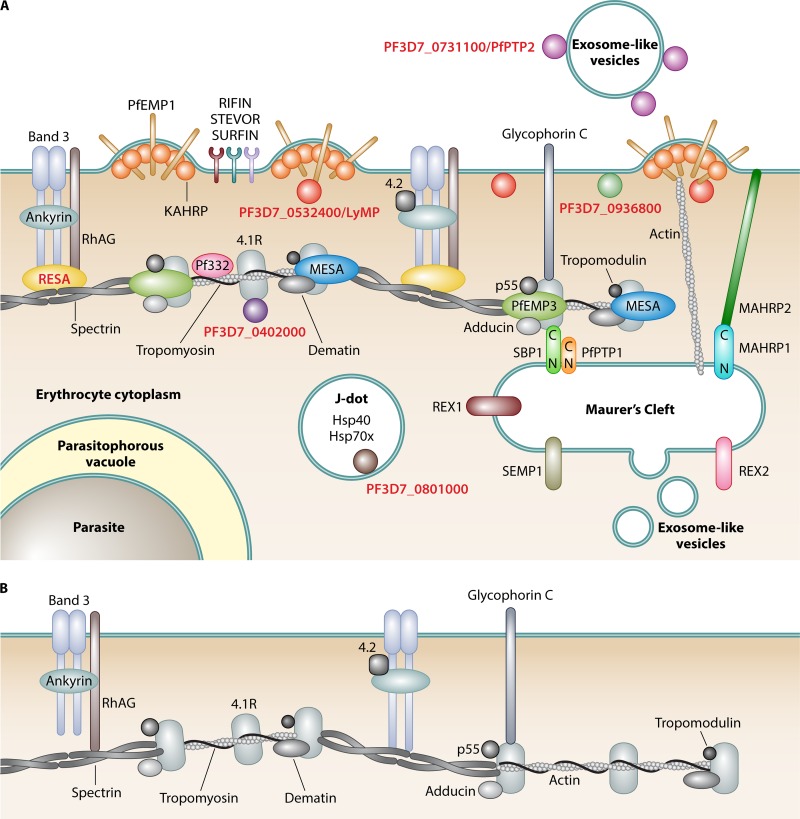
Because exported proteins of P. falciparum play such a central role in the remodeling of infected red blood cells, the description of the pentameric amino acid motif RxLxE/Q/D, called the Plasmodium export element (PEXEL) (9, 10), was a breakthrough that allowed the prediction of ∼400 exported proteins. The PEXEL motif was recently expanded into a more relaxed PEXEL motif (RxLxxE) (11) or a noncanonical motif (K/HxL/IxE/Q/D) (12), resulting in a total number of >460 proteins predicted to be exported in P. falciparum. This “exportome” provides the basis to study exported proteins and their involvement in the pathology of remodeled infected erythrocytes much more specifically. Besides the defined PEXEL exportome, there are additional exported proteins that lack a known or discernible export element, referred to as PEXEL-negative proteins (PNEPs) (13, 14). Although the definitive number of proteins exported into the erythrocyte is unknown, it is without doubt that they play important roles in pathology through the significant changes that they induce in infected erythrocytes and that they are major contributors to disease. Figure 2 shows a schematic representation of differences in cytoskeleton organization between uninfected and infected erythrocytes and the localization of exported proteins during the asexual replicative blood stage of P. falciparum.
FIG 2.
PHIST proteins in the remodeled iRBC. (A) Remodeled iRBC. During the asexual blood stage of P. falciparum, human erythrocytes are subject to extensive remodeling. All erythrocyte proteins are shown in gray (reviewed in reference 59). Parasite structures, compartments, and organelles are labeled in boldface type. PHIST proteins are labeled in red boldface type, and all parasite proteins are represented by colored shapes. References for PHIST proteins are indicated in the text. Knobs are parasite-derived protrusions in the host cell membrane, with knob-associated histidine-rich protein (KAHRP) being a prominent protein of these structures (149, 150). Maurer's clefts (reviewed in reference 151) are parasite-derived membranous structures in the iRBC cytoplasm involved in protein trafficking and are connected with knobs via actin filaments (152, 153). Maurer's cleft-associated histidine-rich protein 1 (MAHRP1) is a Maurer's cleft-resident protein (154) that potentially interacts with MAHRP2, the tether protein anchoring Maurer's clefts to the iRBC membrane (155). REX1 (156), REX2 (107), SEMP1 (157), SBP1 (158), and PfPTP1 (159) are other exported parasite proteins that localize to Maurer's clefts, with the latter two being located in a high-molecular-weight complex (159) and SBP1 interacting with the erythrocyte cytoskeleton proteins spectrin and band 4.1R (160). J dots are mobile, dot-like structures in iRBCs (161, 162). PfPTP2 is associated with exosomes, which are parasite-derived vesicles that are involved in cell-cell communication between iRBCs (43). RhAG, rhesus-associated antigen. (B) Cytoskeleton of an uninfected red blood cell (reviewed in reference 59). (Inspired by references 42 and 100.)
PHIST PROTEINS
Methodology for Review
We focused on P. falciparum and included all Plasmodium helical interspersed subtelomeric (PHIST) proteins and genes that were either identified by Sargeant and colleagues (15) or later identified as PHIST proteins by Frech and Chen (16). We did not attempt to identify new PHIST proteins but compiled only data already reported. Gene identifications in PlasmoDB have changed over time; therefore, some PHIST proteins have two or even three gene identifications, and various articles use different gene identifications, although they refer to the same protein or gene. We also scanned supplemental material for information on PHISTs. which in some instances was found only there and would not be available through a standard literature search. We compiled any available information on each member of the PHIST family by using all gene identifications or names used now or previously. Complete lists of PHIST proteins or genes found in the literature and reviewed here are shown in Tables 1 to 5 and represent an in-depth description of this important protein family.
TABLE 1.
PHISTa genes
| Gene identificationa | Other gene identification(s) or name(s)b | Presence of PEXELc | Gene statusd | Molecular mass (kDa)e | Length (aa)e | KOf | Description (source or reference[s])g |
|---|---|---|---|---|---|---|---|
| PF3D7_0102000 | PFA0100c, MAL1P1.11 | PG | 30.4 | 252 | |||
| PF3D7_0115100 | PFA0735w, MAL1P4.22 | x | 28.6 | 239 | Only 5-aa difference compared to PF3D7_0800600 (PlasmoDB) | ||
| PF3D7_0402000 | PFD0090c, MAL4P1.18 | x | 41.7 | 357 | x | Interaction between band 4.1R (58), not silenced in the 3D7 strain (28), overexpressed in samples from patients with cerebral malaria (34) | |
| PF3D7_0424900 | PFD1185w, MAL4P1.232 | x | 25.3 | 216 | |||
| PF3D7_0425300 | PFD1210w, MAL4P1.237 | PG | 27.5 | 232 | Exported despite the lack of any export signal (79) | ||
| PF3D7_0425400 | PFD1215w, MAL4P1.238 | x | 19.5 | 164 | |||
| PF3D7_0601700 | PFF0085w, MAL6P1.21 | x | PG | 26.3 | 222 | ||
| PF3D7_0800600 | MAL8P1.163 | x | 28.3 | 237 | Adjacent to a PfEMP1 variant on the gene locus; weak binding to the ATS domain of PfEMP1 compared to other PHIST proteins tested (19), only 5-aa difference compared to PF3D7_0115100 (PlasmoDB) | ||
| PF3D7_1001100.1 | PF10_0014, PF10_0015, acyl-CoA binding protein | 36.5 | 313 | x | Grouped as PHISTa (15), deleted in parasites with PfCRT mutations, annotated as a putative lipid transporter (80), upregulated under lumefantrine pressure and deletion of the gene found under chloroquine pressure (81), unusual PHIST member with an N-terminal acyl-CoA binding protein domain (16) | ||
| PF3D7_1001100.2 | PF10_0014, PF10_0015, acyl-CoA binding protein | 31.0 | 267 | x | Same as PF3D7_1001100.1 | ||
| PF3D7_1001300 | PF10_0017 | x | 22.8 | 190 | Diffuse localization in the RBC cytosol (20), differentially expressed in parasites under lumefantrine pressure (81) | ||
| PF3D7_1100600 | PF11_0012 | PG | 33.3 | 281 | |||
| PF3D7_1149700 | PF11_0514 | 10.5 | 85 | Once mentioned in relation to PHISTa structure (58) | |||
| PF3D7_1253100 | PFL2555w, MAL12P1.506 | x | 26.5 | 221 | HSP40 chaperone with a DnaJ domain (82), used as a negative control in an interaction study (58) | ||
| PF3D7_1253300 | PFL2565w, MAL12P1.508 | x | PG | 18.3 | 157 | Not silenced in 3D7 (28) | |
| PF3D7_1253800 | PFL2590w, MAL12P1.513 | x | 26.2 | 219 | |||
| PF3D7_1253900 | PFL2595w, 2600w, MAL12P1.514 | x | PG | 24.3 | 207 | Once mentioned in relation to PHISTa structure (58) | |
| PF3D7_1301100 | MAL13P1.11, MAL13P1.11a | x | PG | 24.6 | 203 | ||
| PF3D7_1301500 | MAL13P1.59 | 37.5 | 308 | Downstream of a clag gene, and its expression is affected by H3K9me3 acetylation (83); not located in the telomeric region (27) | |||
| PF3D7_1372000 | MAL13P1.470 | x | 41.0 | 349 | String database mining suggested interaction with PFI1785w, PFB0115w, and PFL0050c (84) | ||
| PF3D7_1400900 | PF14_0009 | x | PG | 24.6 | 209 | ||
| PF3D7_1477700 | PF14_0748, Pfg14-748 | 34.5 | 291 | Highest transcript concn in bone marrow comparing different organs (37); marker for early and midgametocytes, and its promoter can drive gametocyte-specific gene expression (36, 37); expressed in sexually committed schizonts with >100-fold upregulation (27); used as a marker for sexual commitment (85) | |||
| PF3D7_1478000 | PF14_0752, GEXP17 | x | 25.5 | 215 | Overexpressed in field samples compared to 3D7, suggested surface protein (30); differentially expressed in different 3D7 clones (33) | ||
| PF3D7_1478500 | PF14_0757 | x | PG | 25.1 | 215 | ||
| PF3D7_1479200 | PF14_0763 | x | 26.2 | 219 | |||
| PF3D7_1479300 | PF14_0764 PF14_0765 | x | PG | 24.6 | 209 |
Current gene identification from PlasmoDB.
Other/previous names.
Annotated as a pseudogene (PG) in PlasmoDB.
For proteins with a PEXEL motif, molecular mass and length were calculated for the PEXEL-cleaved form of the protein (the molecular mass for PEXEL-cleaved proteins was calculated by using the ExPASy Web tool [http://web.expasy.org/compute_pi/]). aa, amino acids.
Existing viable knockout (KO) parasites or natural gene deletions are indicated by “x” (see reference 44 or the reference[s] indicated).
Information or references referring to the corresponding gene or protein.
TABLE 5.
PHISTa-like and PHIST proteins
| Gene identificationa | Other gene identification(s) or name(s)b | Presence of PEXELc | Gene statusd | Molecular mass (kDa)e | Length (aa)e | KOf | Description (reference[s] or source)g |
|---|---|---|---|---|---|---|---|
| PF3D7_0831300 | MAL8P1.205, GEXP13 | (x) | 93.0 | 770 | Classified as a PHIST protein (16), although no PHIST domain identified in InterPro; detected in early gametocytes (35); has a PEXEL motif (RKLSE), although this is not yet indicated in any publication | ||
| PF3D7_0831500 | 39.1 | 335 | |||||
| PF3D7_0831750 | PG | 34.0 | 288 | ||||
| PF3D7_0831900 | MAL7P1.230 | PG | 33.5 | 287 | x | Deletion in field samples from South America (116), no deletion in samples from Central America (117) | |
| PF3D7_0832200.1 | MAL7P1.225 | 33.5 | 286 | One of the earliest-upregulated genes in the asexual cycle (28), one splice form of MAL7P1.225 (GeneDB) | |||
| PF3D7_0832200.2 | MAL7P1.225 | 34.3 | 293 | Same as PF3D7_0832200.1 | |||
| PF3D7_0832300 | MAL7P1.224 | 30.8 | 259 | Upregulated during the commitment phase for the sexual developmental cycle (53, 145) | |||
| PF3D7_0832700 | MAL7P1.220 | PG | 30.8 | 260 | |||
| PF3D7_1000700 | PF10_0007, PF10_0008 | x | PG | 26.1 | 209 | Both old gene identifications have different domains (27), PHISTa protein with N-terminal PHIST domain (15) | |
| PF3D7_1201200 | PFL0060w, MAL12P1.12 | x | 16.0 | 134 | Downregulated in several strains (28), PHISTa-like protein (15) | ||
| PF3D7_1301300 | MAL13P1.58 | PG | 28.9 | 247 | Not located in telomeric region (27) | ||
| PF3D7_1372300 | 24.2 | 206 | |||||
| PF3D7_1477300 | PF14_0744, Pfg14-744 | x | 22.8 | 196 | Strongly upregulated at gametocytogenesis (27); expression peak in stage I gametocytes but also present at stage II (85, 145, 146); grouped as PHIST or PHISTa-like protein (1, 15, 16); tested as a gametocyte marker (129); expressed as early as in committed schizont stage, regulated by AP2-G, and HP1 is reported to have a silencing effect (54) | ||
| PF3D7_1477400 | PF14_0745 | 36.2 | 303 | Expressed in parasites committed to the sexual cycle (27), found in the nuclear proteomic fraction (147), classified as a PHIST protein (16) |
Current gene identification from PlasmoDB.
Other/previous names.
The presence of a PEXEL motif is indicated by “x,” as identified in reference 15 or 1. A newly identified PEXEL is indicated by “(x).”
Annotated as a pseudogene (PG) in PlasmoDB.
For proteins with a PEXEL motif, molecular mass and length were calculated for the PEXEL-cleaved form of the protein (the molecular mass for PEXEL-cleaved proteins was calculated by using the ExPASy Web tool [http://web.expasy.org/compute_pi/]).
Existing viable knockout (KO) parasites or natural gene deletions are indicated by “x” (see reference 44 or the reference[s] indicated).
Information or references referring to the corresponding gene or protein.
The PHIST Protein Family
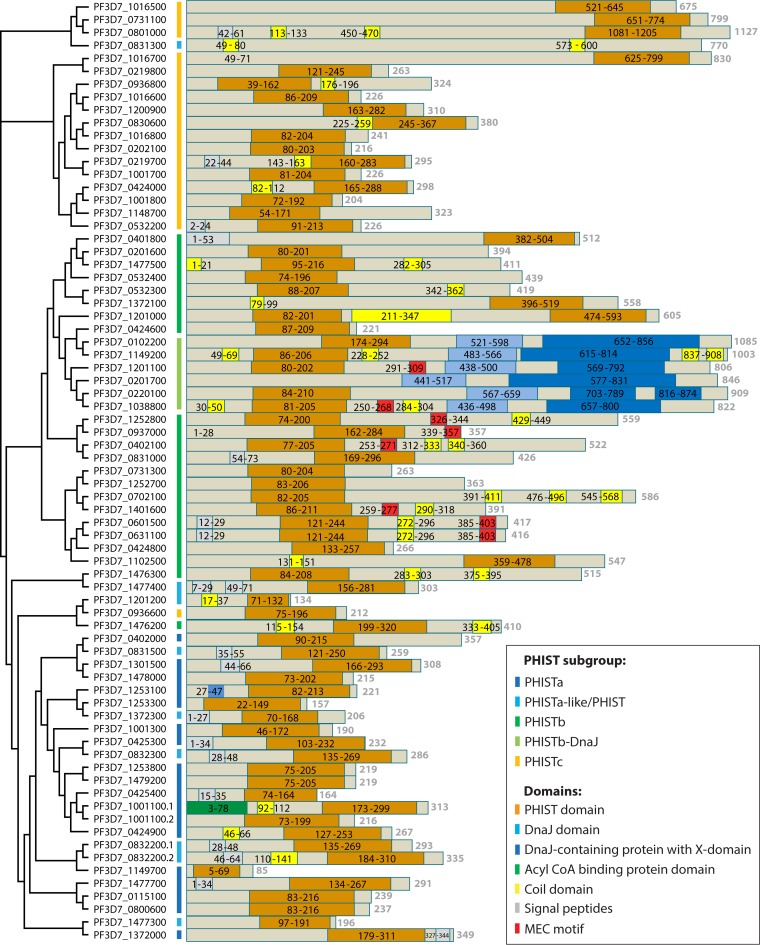
In the large number of exported proteins, Sargeant and colleagues (15) identified a new protein family, which they termed the PHIST protein family. This protein family is characterized by a conserved domain of ∼150 amino acids predicted to form four consecutive alpha helices (determined by Fugue [17]). Some members of this family comprise little more than an export signal sequence, the PEXEL motif, and the PHIST domain, whereas other members are substantially elongated and include additional domains, such as a DnaJ domain (Fig. 3). Based on the presence and position of several conserved tryptophan residues within the PHIST domain, the PHIST protein family has been further divided into three subgroups: PHISTa, PHISTb, and PHISTc (15). With more recent data included, PlasmoDB has annotated additional PHIST domains in proteins, resulting in a total of 89 currently known PHIST proteins in P. falciparum (15, 16). Some of the newly added PHIST proteins were not grouped into the already existing subgroups but were classified as PHISTa-like or simply PHIST proteins. Thus, PlasmoDB annotations give the impression that there are more than three PHIST subgroups. However, a phylogenetic tree based on a multiple-sequence alignment shows that PHISTa-like and PHIST proteins cluster with the PHISTa subgroup and the PHISTb-DnaJ protein group among the PHISTb subgroup, giving rise to three distinct PHIST subgroups (Fig. 3).
FIG 3.
Phylogenetic tree and protein domain prediction for PHIST proteins. The amino acid sequences of all 89 PHIST proteins were obtained from PlasmoDB. Of 19 PHIST proteins annotated as pseudogenes, 16 contained one or more premature stop codons in the amino acid sequence and were removed from the list. All those of the remaining 73 PHIST proteins with a PEXEL motif were PEXEL cleaved in silico by using the PEXEL motifs provided by Sargeant et al. (15), Boddey et al. (11), or Schulze et al. (12). The amino acid sequences were aligned with MUSCLE (163). The alignment is represented in a phylogenetic tree using the phylogenic tree tool built into MUSCLE at the EMBL-EBI website (164). The branch lengths are drawn in cladogram style and do not represent actual phylogenetic distances. The colored bars next to the gene identifications represent different PHIST subgroups, as indicated. The structure of the amino acid sequences was then analyzed with InterPro (https://www.ebi.ac.uk/interpro/) (165). A schematic representation of the results for each PHIST protein is shown next to its respective gene identification. The following different domains are highlighted: the PHIST domain, the DnaJ domain as defined by Pfam (family PF00226), the DnaJ-containing protein with an X domain as defined by InterPro (accession number IPR026894) or Pfam (family PF14308), the acyl coenzyme A (CoA) binding protein domain as defined by Pfam (family PF00887), coil domains as identified by InterPro, signal peptides as defined by SignalP or transmembrane domains, and the MEC motif as defined by Kilili and LaCount (39).
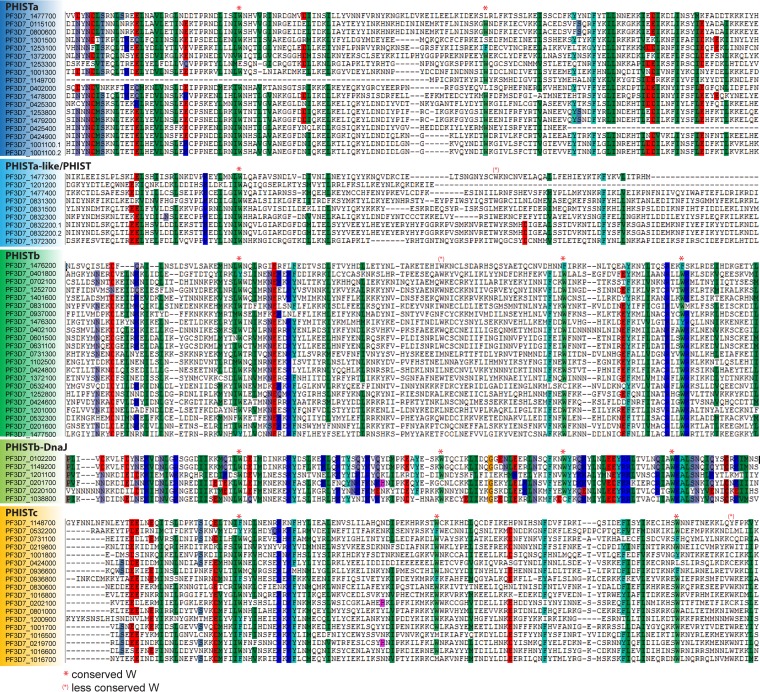
When the protein family was first described, Sargeant and colleagues used the presence and position of conserved tryptophan residues in the amino acid sequence of the PHIST domain to distinguish between the three subgroups (15). Comparison of multiple-sequence alignments for each subgroup in which PHISTb-DnaJ and PHISTa-like/PHIST proteins were treated as individual subgroups reveals a unique pattern of the conserved tryptophan residues for each of the subgroups (Fig. 4 and 5A). For each subgroup, a different positional pattern of conserved tryptophan residues was found, with PHISTa and PHISTa-like/PHIST proteins possessing only two conserved tryptophan residues within the PHIST domain and the remaining subgroups having four. There is only slight variation in the positions of these residues between the PHISTb and PHISTb-DnaJ subgroups and between the PHISTa and PHISTa-like/PHIST subgroups. We therefore use the original three subgroups introduced by Sargeant and colleagues unless otherwise stated.
FIG 4.
Sequence alignment of PHIST proteins. The processed amino acid sequences as described in the legend of Fig. 3 were sorted into PHIST subgroups, and individual alignments for each subgroup were obtained with CLUSTAL (166). The output was analyzed by using BioEdit (167). Conserved amino acids at a threshold of 83% are highlighted. Shown is the core of the alignment using the position of the first conserved tryptophan to align the different alignment blocks of the subgroups. Tryptophan residues conserved above or below the 83% threshold are marked as indicated.
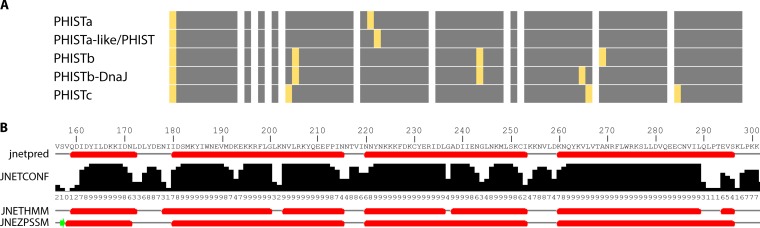
FIG 5.
PHIST domain modeling. (A) Visual representation of the positions of the conserved tryptophan residues of the PHIST subgroups, treating PHISTb and PHISTb-DnaJ as well as PHISTa and PHISTa-like/PHIST domains as separate subgroups to show the slight variations between them. A consensus sequence was generated for each subgroup alignment from Fig. 4. The consensus sequence was then split into blocks of 10 residues represented by gray boxes (boxes containing no tryptophans were shortened). The positions of the tryptophan residues conserved within a subgroup are marked by yellow bars. (B) To exemplify the differences in structure prediction, the Jpred4 (168, 169) output is shown for the PHIST protein PF3D7_0532400. The top sequence is the consensus sequence used for PHIST identification by Sargeant et al. (15). JNetPRED displays the consensus prediction: helices are marked with red tubes. JNetCONF provides confidence estimates for the prediction. High values mean high confidence. For the JNetHMM profile-based prediction, the six predicted helices are marked as red tubes. For the JNETPSSM-based prediction, the four helices are marked as red tubes, and sheets are marked with a green arrow.
Tables 1 to 5 list all 89 proteins that we identified as PHIST proteins in P. falciparum, 64 of which contain a classical PEXEL motif and are thus predicted to be exported (11, 15). Although PlasmoDB lists 19 phist genes as pseudogenes in reference strain 3D7, some have been found to be present as proteins or transcripts in other studies. Thus, PHISTs represent a substantial group of exported proteins comprising ∼14% of all PEXEL proteins or nearly 2% of the complete P. falciparum proteome. Despite their potentially important role in host cell remodeling and (in)direct involvement in pathogenicity, most PHIST proteins remain completely uncharacterized, rendering it difficult to assign specific functions or roles to the PHIST protein family and/or the three subgroups.
Comparison of PHIST and PRESAN Domains
There has been confusion in the literature on the definition of PHIST and Plasmodium ring-infected erythrocyte antigen (RESA) N-terminal (PRESAN) domains, which in fact are virtually identical. The confusion was generated when, independent of the sequence analysis reported by Sargeant and colleagues (15), transcriptome analysis of P. falciparum parasites grown at elevated temperatures mimicking febrile conditions revealed a number of upregulated genes that coded for proteins containing a DnaJ domain (Pfam family PF00226) (18). Subsequent alignments identified an extended protein family with at least 67 members, all sharing a particular N-terminal domain, with a DnaJ domain being present in only some members. Because some of the DnaJ domain-containing proteins showed similarity to the RESA, this N-terminal domain was termed the PRESAN domain (Pfam family PF09687). Examination of the sequence alignments by Sargeant and colleagues (15) and Oakley and colleagues (18) revealed that the domain boundaries of the PHIST and PRESAN domains are virtually identical. Secondary-structure predictions by Oakley and colleagues (18) suggested the presence of six α-helices in PRESAN domains; however, these α-helices overlapped the predicted four α-helices of the PHIST domain (15). Thus, the PRESAN domain can be regarded as being highly similar to the PHIST domain, with differences depending mainly on the prediction algorithms. An example of such close similarity is shown with the PHIST/PRESAN protein PF3D7_0532400 (Fig. 5B). The overall domain structure of all expressed PHIST proteins is shown in Fig. 3. Crystallographic and nuclear magnetic resonance (NMR) studies provided clear evidence that the PHIST/PRESAN domain forms a four-helix bundle (19). Subsequently, Tarr and colleagues (20) defined an “extended PRESAN” domain, which was N terminal to the original PHIST domain and which was thought to comprise a domain targeting membranes. This extended domain includes additional helix-forming sequences.
Confusingly, the current InterPro database uses the term “PHIST domain” (accession number IPR006526) to refer to a small protein fragment spanning approximately the N-terminal half of the PHIST/PRESAN domains, as defined by the original authors (15, 18). In contrast, the InterPro (accession number IPR019111) and Pfam (family PF09687) entries for the PRESAN domain are identical to these original alignments. This discrepancy needs correction urgently. In this review, we refer to this conserved domain type as the PHIST domain.
PHIST Proteins in Other Plasmodium Species
PHIST proteins are found exclusively in the genus Plasmodium, and the protein family has been significantly expanded in P. falciparum and in the laveranian species P. reichenowi, in which there is a one-to-one representation of the PHIST genes (21). While little is known about PHIST proteins in P. falciparum, even less is known about PHIST proteins in other Plasmodium species. There are fewer PHIST proteins in other plasmodia, but their exact number is not yet clear, and different publications report various counts. Initially, Sargeant et al. reported 39 PHIST proteins for P. vivax (15), but the complete analysis of the P. vivax genome (22) revealed the presence of a gene family (Pv_fam_e), which contained 44 rad genes and 21 phist genes, with both groups showing structural similarities. However, PlasmoDB currently has only 18 genes annotated as phist (PlasmoDB) for P. vivax. Supplemental material in two publications provided expression data for P. vivax PHIST proteins (23, 24). For P. knowlesi, Sargeant and colleagues (15) initially reported 27 PHIST proteins, but this has been reannotated to 38 proteins by Pain et al. (25), and PlasmoDB currently lists 39 records. For the monkey malaria parasite P. cynomolgi, 21 PHIST genes were identified, while the number of PHIST genes in the rodent malaria parasite is unclear and varies in different publications or databases. For P. berghei, 1 to 3 phist genes have been found, and 1 to 2 have been found in both P. chabaudi and P. yoelii (15, 16, 26). Moreira et al. recently characterized two PHIST proteins in P. berghei and demonstrated potentially similar roles for these PHIST proteins, as has been attributed to P. falciparum PHIST proteins (26). So far, to our knowledge, no phist genes have been identified in the avian Plasmodium lineage. Whether there are other gene families with a similar structure or function in these species remains to be seen.
Except for CVC-8195, a PHIST protein of P. vivax that has been investigated in more detail, we do not further discuss PHIST proteins in other plasmodia.
The PHIST Subfamilies
PHISTa.
Proteins of the PHISTa subgroup are very short and besides the PHIST domain consist of only a signal sequence and a PEXEL motif if present. PHISTa proteins are found exclusively in P. falciparum (15) and currently amount to 26 different proteins (Table 1). In contrast to the other subgroups, PHISTa and PHISTa-like proteins possess only two conserved tryptophan residues (Fig. 4). These proteins were previously grouped together and described as a subtelomeric protein superfamily (27), although two of them, PF3D7_1301500 and PF3D7_1301300, are not located subtelomerically and are referred to as PHISTa-like proteins today (Table 5).
Except for PF3D7_0402000 and PF3D7_1253300, PHISTa proteins have been reported to be transcriptionally silent in reference strain 3D7 (15, 28). Initially, it was proposed that this transcriptional silencing might be caused by mutually exclusive expression (15). However, as shown in the heat map in Fig. 6, several studies recently showed that other PHISTa proteins are upregulated in pregnancy-associated malaria or in cerebral malaria (29–34). Additional studies reported that members of the PHISTa group are differentially expressed in parasites committed to becoming gametocytes (27, 35–37). Whether the transcriptional silencing of PHISTa proteins in 3D7 is an adaptation to in vitro growth remains to be tested, but PHISTa proteins in natural infections seem to play an important role in pathogenesis.
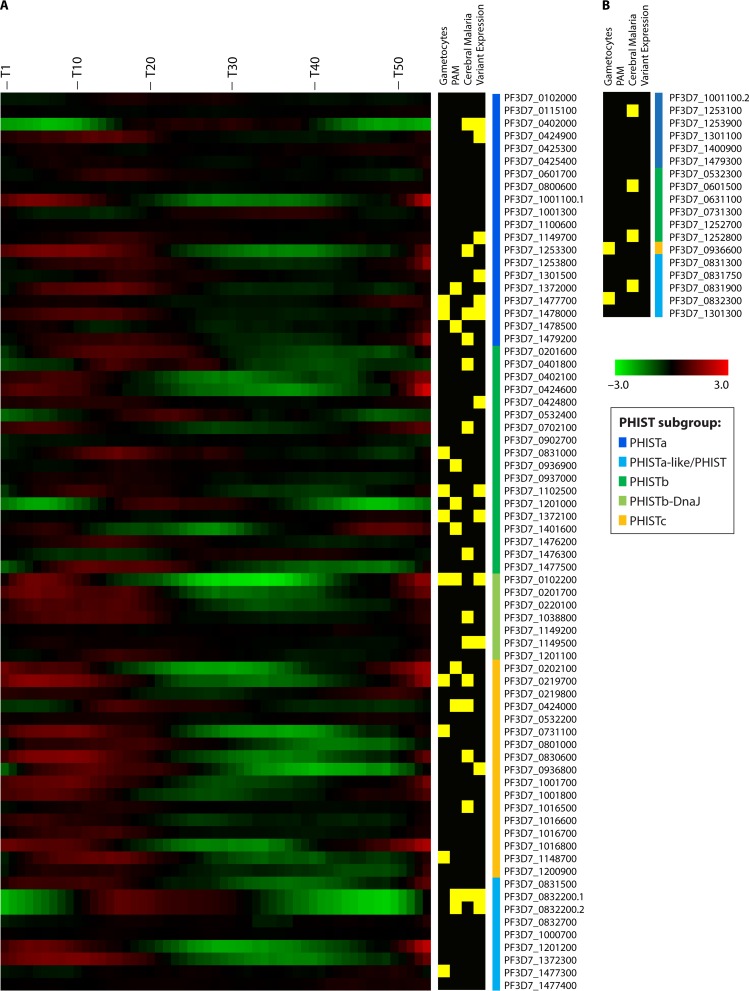
FIG 6.
Heat maps. (A) Heat map of phist gene expression of the P. falciparum 3D7 strain over the course of 53 h. Microarray data on transcript abundance were reported previously (46) (accessed through PlasmoDB, release 28, 31 March 2016). The heat map was constructed by using TMeV4.9 (170). phist genes were clustered into their respective subgroups and were then ordered by gene identification. Genes annotated “phista-like” or as “phist” genes were grouped together as “phist others.” Yellow boxes to the right of the heat map indicate PHIST proteins present in the early gametocyte proteome (35), differentially expressed phist genes in pregnancy-associated malaria (29, 49, 50, 84, 92, 112) and in cerebral malaria (32, 34), or if variant expression has been reported (55). Green to red colors represent fold changes (log fold changes from −3 to 3). (B) For a number of phist genes, no expression data were available in the data set, and these genes were grouped first by PHIST subgroup and then by gene identification. Additional information for selected PHIST proteins is provided in Tables 1 to 5. The colored bars next to the gene identifications indicate the PHIST subgroup.
PHISTb.
With 24 members, PHISTb proteins make the second largest subgroup (Table 2). Members of this subgroup are slightly longer than PHISTa proteins, with 300 to 600 residues. Characteristic of the PHISTb subgroup is a unique, long, C-terminal amino acid stretch that follows the PHIST domain (15) and that might indicate a unique and different function of these proteins compared to that of proteins of the other PHIST subgroups. It is conceivable that the PHIST domain provides a general binding motif, while the C terminus might provide a more specific interaction domain. Such a dual binding capacity was recently shown by Oberli and colleagues (38) for the PHIST protein PF3D7_0532400. C-terminal interaction motifs have indeed been identified in several other PHISTb proteins (39). In this respect, it is important to note that all PHISTb proteins characterized today were localized at and might interact with the host cell cytoskeleton (19, 20, 39, 40).
TABLE 2.
PHISTb genes
| Gene identificationa | Other gene identification(s) or name(s)b | Presence of PEXELc | Gene statusd | Molecular mass (kDa)e | Length (aa)e | KOf | Description (reference[s] or source)g |
|---|---|---|---|---|---|---|---|
| PF3D7_0201600 | PFB0080c, PF02_0016 | x | 48.0 | 394 | x | Affected by large chromosome break, which causes a loss of cytoadherence and permanent expression of var2csa, regulates var2csa and var gene expression (86); displayed on iRBC surface (86); cytosolic localization with weak accumulation at the erythrocyte periphery (20); PHISTb domain-containing RESA-like protein 1 (PlasmoDB); overexpressed in samples from patients with cerebral malaria (34) | |
| PF3D7_0401800 | PFD0080c, MAL4P1.16, PfD80 | x | 54.8 | 512 | Upregulated within 90 min after artesunate treatment (87), suggested to interact with PFD0985w based on data mining (88), putative Maurer's cleft protein (89, 90), differentially expressed in Pf-FRC parasites selected for CSA or CD36 binding (91), among the most significantly upregulated genes in children with P. falciparum malaria (92), discrepancy observed between RNA and protein levels (93, 94) | ||
| PF3D7_0402100 | PFD0095c, MAL4P1.19 | x | 61.7 | 522 | var gene chr4var7 showed recombination with PFD0100c (95), essential for in vitro growth (44), apparently linked to invasion ligand RH1 abundance in FCR3 (80, 95), contains a MEC motif (39) | ||
| PF3D7_0424600 | PFD1170c, MAL4P1.229 | x | 26.2 | 221 | x | Required for correct KAHRP transport and knob formation, and deletion has an effect similar to that in KAHRP KO parasites but does not affect PfEMP1 transport (44); protein shows peripheral localization (20); no interaction with the ATS of PfEMP1 (19); involved in knob formation and cytoadherence (96); present in peripheral blood of malaria patients (77) | |
| PF3D7_0424800 | PFD1180w, MAL4P1.231 | x | 31.1 | 266 | |||
| PF3D7_0532300 | PFE1600w, MAL5P1.314 | x | 49.9 | 419 | Exported and tyrosine phosphorylated in the RBC cytoplasm (97), shown on immunoblots with candidate vaccine antigens (98), shown in an interaction cluster with PFE1605w (99) | ||
| PF3D7_0532400 | PFE1605w, MAL5P1.315, LyMP | x | 50.6 | 439 | Showed interaction with ATS of PfEMP1 variants localized to the knobs (19); localization at membrane cytoskeleton between knobs (40); recently reviewed, indicating both knob and membrane localizations (100); referred to as Hsp40 protein, with interaction with MSP1 (101); HSP40 protein with J domain (102); yeast two-hybrid interaction with SBP1 and PFE1600w (102); also computationally predicted to be a nuclear pore protein and to be part of a signaling pathway in the FIKK protein family (103) | ||
| PF3D7_0601500 | PFF0075c, MAL6P1.19 | x | 50.0 | 417 | Has two MEC motifs, with only a 1-aa difference compared to PF3D7_0631100 (39) | ||
| PF3D7_0631100 | PFF1510w | x | 49.9 | 416 | Has two MEC motifs, with only a 1-aa difference compared to PF3D7_0601500 (39) | ||
| PF3D7_0702100 | MAL7P1.7 | x | PG | 69.1 | 586 | Part of an interaction network with SBP in the center (99), hexadecyl-trimethyl-ammonium bromide treatment changed its expression (104), identified as a RESA-like protein (82), identified as a RESA-like protein but not HSP40 (105) | |
| PF3D7_0731300 | MAL7P1.174, Pfg174 | x | 31.6 | 263 | x | Putative Maurer's cleft protein (89, 90), suggested location of a surface protein (106), soluble protein (107) | |
| PF3D7_0831000 | MAL8P1.2, GEXP09 | 51.0 | 426 | Listed as an HSP40 chaperone with a J domain (102) | |||
| PF3D7_0902700 | PFI0130c | x | PG | 44.0 | 372 | Silencing of this gene resulted in inhibition of apoptosis without affecting parasite growth (108), potentially links an unknown surface protein to the iRBC cytoskeleton (109), contains a MEC motif (39) | |
| PF3D7_0936900 | PFI1785w | x | PG | 32.9 | 274 | Particularly abundant protein in samples from pregnant women but not in samples from children (49, 110, 111), affected by deletions on chromosome 9 (112, 113), String database mining suggested interaction with var2csa and MAL13P1.470-1 (84), one of the earliest-upregulated genes in the asexual cycle (28), often mentioned together with PFD1140w | |
| PF3D7_0937000 | PFI1790w | 43.0 | 357 | x | Yeast two-hybrid interaction with band 4.1R (99, 114), potential involvement in host cytoskeleton remodeling (39), located in a region prone to deletion in P. falciparum strains IT and FCR3 (113), contains a MEC motif (39) | ||
| PF3D7_1102500 | PF11_0037, GEXP2 | x | 64.6 | 547 | x | Immunoprecipitation using this protein pulled down Plasmodium translocon of exported proteins (PTEX) components HSP101, PTEX150, and EXP2 (45); differentially expressed in HP1-depleted parasites (53); involved in cytoadherence (109) | |
| PF3D7_1201000 | PFL0050c, MAL12P1.10 | x | 71.7 | 605 | x | Putative Maurer's cleft protein (89, 90), String database mining suggests interaction with var2csa and MAL13P1.470-1 (84) | |
| PF3D7_1252700 | PFL2535w, MAL12P1.502 | x | 42.1 | 363 | RESA-like protein (115) | ||
| PF3D7_1252800 | PFL2540w, MAL12P1.503 | x | 66.6 | 559 | Contains a MEC motif (39) | ||
| PF3D7_1372100 | MAL13P1.475, GEXP04 | x | 67.0 | 558 | x | Frequently deleted in field samples, affected by the same deletion as HRP2 and HRP3 (116, 117) | |
| PF3D7_1401600 | PF14_0018 | x | 45.7 | 391 | x | Knockout resulted in less rigid but viable parasites (39, 44), putative TM domain or GPI anchor (118), contains a MEC motif (39) | |
| PF3D7_1476200 | PF14_0731 or PF14_0730 | x | 49.3 | 410 | Upregulated in vitro when cultured with human serum compared to AlbuMAX (119) | ||
| PF3D7_1476300 | PF14_0732 | x | 61.5 | 514 | x | Frequently deleted in HB3 and other strains (120) | |
| PF3D7_1477500 | PF14_0746 | x | 49.9 | 411 |
Current gene identification from PlasmoDB.
Other/previous names.
Annotated as a pseudogene (PG) in PlasmoDB.
For proteins with a PEXEL motif, molecular mass and length were calculated for the PEXEL-cleaved form of the protein (the molecular mass for PEXEL-cleaved proteins was calculated by using the ExPASy Web tool [http://web.expasy.org/compute_pi/]).
Existing viable knockout (KO) parasites or natural gene deletions are indicated by “x” (see reference 44 or the reference[s] indicated).
Information or references referring to the corresponding gene or protein. TM, transmembrane; GPI, glycosylphosphatidylinositol.
The non-PHIST protein mature parasite-infected erythrocyte surface antigen (MESA) (PF3D7_0500800) interacts via an N-terminal 19-amino-acid motif with band 4.1R. This motif has been termed the MESA erythrocyte cytoskeleton binding (MEC) motif (39) and has been found in 14 other exported P. falciparum proteins, 9 of which belong to the PHISTb/PHISTb-DnaJ subfamily (Tables 2 and 3). Each of these 9 PHIST proteins has a MEC motif in the C terminus downstream of the PHIST domain and in two cases is followed by a DnaJ domain. Three of these PHIST proteins also have been found to bind to inside-out vesicles (IOVs), suggesting an interaction between the MEC motif and a cytoskeleton interaction partner. Coprecipitation with the MEC motif of the PHISTb-DnaJ protein PF3D7_1038800 revealed band 4.1R as an interaction partner (39). Although these PHIST proteins were not further functionally characterized, the presence of a MEC motif, with its capacity to bind to band 4.1R, suggests their involvement in the remodeling of the iRBC cytoskeleton and, thus, a possible contribution to the pathology of malaria.
TABLE 3.
PHISTb genes with a DnaJ domain
| Gene identificationa | Other gene identification(s) or name(s)b | Presence of PEXELc | Gene statusd | Molecular mass (kDa)e | Length (aa)e | KOf | Description (reference[s] or source)g |
|---|---|---|---|---|---|---|---|
| PF3D7_0102200 | PFA0110w, MAL1P1.13, Pf155, RESA | 126.5 | 1,085 | x | RESA-KO iRBCs have reduced cell rigidity (44, 65), RESA stiffens iRBCs and protects them from cell damage at febrile temperatures (71), binds to spectrin (68), expressed in ring stages of early gametocytes (51), part of an interaction network with SBP in the center (99), found in peripheral blood of malaria patients (77), RESA-positive and parasite-free RBCs observed (121), subject of research in vaccine development (search term, Plasmodium Pf155 vaccine) | ||
| PF3D7_0201700 | PFB0085c, PF02_0017 | x | 99.7 | 846 | x | Part of an upregulated gene cluster in C4S binding parasites but suggested to be nonessential for PfEMP1 expression (86); HSP40 chaperone with a DnaJ domain, RESA-like protein (82); chromosomal deletion also affecting KAHRP and resulting in the absence of knobs and reduced cytoadherence (15, 122) | |
| PF3D7_0220100 | PFB0920w, PF02_0188 | x | 106.8 | 909 | x | Identified as type III HSP40 (105, 123), deletion leads to increased rigidity and increased CS2 binding (44), putative DnaJ protein (PlasmoDB) | |
| PF3D7_1038800 | PF10_0378 | x | 96.8 | 822 | x | Coprecipitated full-length band 4.1R (39), identified as type III HSP40 (123), RESA-like protein with PHIST and DnaJ domains (PlasmoDB) | |
| PF3D7_1149200 | PF11_0509, RESA 3 | x | 117.2 | 1,003 | Part of an interaction network with SBP in the center but also present in a different interaction network with RESA, SBP1, and other PHISTs (99); identified as type IV HSP40 (105); absent in parasites treated with T4, which causes arrest of the cell cycle (124); less abundant in proteomic data than RESA (65); described as an essential gene by Maier et al. (44); expression peak in late ring, early trophozoite stage (125); high level of sequence homology with RESA (126) | ||
| PF3D7_1149500 | PF11_0512, RESA 2 | x | PG | 89.0 | 839 | x | Although annotated as a pseudogene, it has been found to be poorly translated, with very low protein levels, function is not known, protein might be degraded (127); identified as RESA2 (65); mutation T1526C frequently found in cases of severe malaria, upregulated in vivo (128); identified as type IV HSP40 (105); not expressed in laboratory strains according to Maier et al. (42) but highly upregulated when two isogenic 3D7 strains were compared (33); peak expression in trophozoite stage (125); overexpressed in in vivo samples (30); transcript found at high levels in samples from patients with cerebral malaria from Malawi (129) (data not shown) |
| PF3D7_1201100 | PFL0055c, MAL12P1.11 | x | 96.2 | 806 | Identified as type III HSP40 (105, 123), RESA-like protein with PHIST and DnaJ domains (PlasmoDB), contains a MEC motif (39) |
Current gene identification from PlasmoDB.
Other/previous names.
Annotated as a pseudogene (PG) in PlasmoDB.
For proteins with a PEXEL motif, molecular mass and length were calculated for the PEXEL-cleaved form of the protein (the molecular mass for PEXEL-cleaved proteins was calculated by using the ExPASy Web tool [http://web.expasy.org/compute_pi/]).
Existing viable knockout (KO) parasites or natural gene deletions are indicated by “x” (see reference 44 or the reference[s] indicated).
Information or references referring to the corresponding gene or protein.
Seven PHISTb members (Table 3), including the well-known RESA, also comprise a DnaJ domain referred to as PHISTb-DnaJ. Proteins with a DnaJ domain belong to the HSP40 family. The J domains can act as cochaperones for proteins of the DnaK/HSP70 families and can associate with unfolded polypeptide chains to prevent their aggregation (18, 41). PHISTb and PHISTb-DnaJ differ in only one of the four conserved tryptophan positions (Fig. 4 and 5A), indicating their close relatedness. Another characteristic that sets the PHISTb-DnaJ proteins apart from the PHISTb subgroup is the extended length ranging from ∼800 to 1,100 amino acids (15).
Several PHISTb proteins have been shown to localize at the host cell periphery, and solubility assays with green fluorescent protein (GFP)-tagged PHISTb proteins suggested an interaction with components of the host cytoskeleton (20). The authors of this study also investigated sequence requirements for peripheral localization and showed that the PHIST domain or the N-terminal region of the PHIST domain alone is not sufficient for peripheral localization. The PHIST domain together with parts of the N-terminal region, however, conferred peripheral localization (20). This was shown for the PHISTb protein PF3D7_0401800 and the PHISTb-DnaJ protein PF3D7_0102200, and it remains to be confirmed whether this applies to all PHISTb proteins and the precise sequence or structural requirements that are necessary. It also needs to be shown whether PHIST proteins of the other subfamilies have similar requirements for correct localization. Although localization does not predict function, these observations in general confirm that PHISTb proteins tend to associate with the iRBC cytoskeleton.
PHISTc.
Most information is available for the PHISTc subgroup, which is entirely shared with P. vivax and P. knowlesi (15), indicating that the expansion of this subgroup occurred before the lineage diverged. The PHISTc subgroup is also the most diverse group in length, with lengths varying from <200 to >1,200 amino acids. In most of the 18 PHISTc proteins (Table 4), the PHIST domain is found very near the C terminus of the protein (Fig. 3), similarly to the PHISTa subgroup. In contrast to PHISTb proteins, which are associated mostly with the iRBC cytoskeleton, several PHISTc proteins have been found at structures such as Maurer's clefts (19, 42) and exosome-like vesicles (43) and are thought to be involved in protein trafficking (44, 45). There is recent evidence for PF3D7_0936800 (PFI1780w) to also be localized at the host cell membrane (19).
TABLE 4.
PHISTc genes
| Gene identificationa | Other gene identification(s) or name(s)b | Presence of PEXELc | Gene statusd | Molecular mass (kDa)e | Length (aa)e | KOf | Description (reference[s] or source)g |
|---|---|---|---|---|---|---|---|
| PF3D7_0202100 | PFB0105c, LSAP2 | x | 25.4 | 216 | Not recognized by pooled immune sera (98); continuously upregulated, with predicted TM, granular pattern in immunofluorescence assays (IFA) of older parasites, expressed in liver stages (130); located at periphery in liver stage parasites similarly to circumsporozoite protein (CSP) (131); polymorphic gene with SNPs (80); differentially expressed in parasites under lumefantrine pressure (81) | ||
| PF3D7_0219700 | PFB0900c, PF02_0184, GEXP20 | 35.3 | 295 | ||||
| PF3D7_0219800 | PFB0905c, PF02_0185 | x | 31.9 | 263 | |||
| PF3D7_0424000 | PFD1140w, MAL4P1.223 | x | 35.2 | 296 | x | Antigenic properties, antibody recognition in several reports, potential vaccine candidate (29, 49, 111, 132, 133); stronger expression in culture supplemented with albumin instead of human serum (119); String database mining suggests interaction with var2csa and PFB0115w (84); often referred to together with PFI1785w | |
| PF3D7_0532200 | PFE1595c, MAL5P1.313 | 27.3 | 226 | Involved in response to chloroquine treatment (134, 135) | |||
| PF3D7_0731100 | MAL7P1.172, GEXP11 PfPTP2 | x | 92.5 | 799 | x | Involved in cell-cell communication and plasmid transfer, located on budding vesicles from Maurer's clefts (43); knockout with very reduced levels of surface PfEMP1, which was trapped in Maurer's clefts, no cytoadherence to CSA (44); indirect evidence for PfPTP2 being located inside the lumen of Maurer's clefts (44); coprecipitated with plasmepsin V (136); RESA-like protein found among soluble proteins from parasitophorous vacuole (PV) lumen and iRBC cytosol (82) | |
| PF3D7_0801000 | PF08_0137 | x | 147.1 | 1,127 | Found among soluble proteins from PV lumen and iRBC cytosol (82), coprecipitated PTEX components such as HSP101 and PTEX150 (45), associated with J dots (J. Przyborski, personal communication), P. yoelii orthologue is annotated as a putative dentin phosphoryn protein possibly involved in mineral nucleation or functions as an extracellular matrix protein (137), contains a relaxed PEXEL motif (1), elicits an immune response (138) | ||
| PF3D7_0830600 | MAL8P1.4 | x | 45.1 | 380 | Located in Maurer's clefts, no interaction with the ATS of PfEMP1 (19); expression downregulated in HB3, 3D7, and Dd2 (28); expression upregulated with treatment with histone deacetylase inhibitors (trichostatin A, suberoylanilide hydroxamic acid) (139) | ||
| PF3D7_0936600 | PFI1770w, GEXP5 | x | 25.2 | 212 | Originally classified as PHISTb (15); reclassified as PHISTc (16); sole “PHISTb” that showed only cytosolic localization (20); upregulated in gametocytes (107, 140); earliest-known postinvasion gametocyte marker, expressed at 14 h postinvasion and independent of major gametocyte marker, cannot promote gametocyte maturation alone when other factors are not present (51) | ||
| PF3D7_0936800 | PFI1780w | x | 38.6 | 324 | Protein of unknown function, several TM domains predicted (15); interacts with the ATS of PfEMP1 (56); located at the iRBC periphery with PfEMP1 but not cotransported with PfEMP1 (19); stuttering motif identified not found in any other PHIST protein (141); noncanonical PEXEL motif that is correctly cleaved (12); suggested presence in figure displaying exported proteins (100) | ||
| PF3D7_1001700 | PF10_0021 | x | 27.6 | 226 | Significantly downregulated after chloroquine treatment (80) | ||
| PF3D7_1001800 | PF10_0022 | x | 23.8 | 204 | Corrected gene model with adjusted exon and intron sizes (142, 143) | ||
| PF3D7_1016500 | PF10_0161 | x | 81.3 | 675 | Wrongly annotated as PF3D7_1016600 previously (15), evidence for sumoylation (144) | ||
| PF3D7_1016600 | PF10_0161 or PF10_0161a | x | 27.8 | 226 | Had been annotated together with PF3D7_1016500 (15), evidence for sumoylation (144), IFA shows bright punctuate pattern in iRBCs (20) | ||
| PF3D7_1016700 | PF10_0162 | 98.8 | 830 | ||||
| PF3D7_1016800 | PF10_0163 | x | 30.0 | 241 | |||
| PF3D7_1148700 | PF11_0503, GEXP12 | x | 38.1 | 323 | |||
| PF3D7_1200900 | PFL0045c, MAL12P1.9 | x | 37.6 | 310 | Noncanonical PEXEL, localizes to small dotted structures in iRBCs (12) |
Current gene identification from PlasmoDB.
Other/previous names.
Annotated as a pseudogene (PG) in PlasmoDB.
For proteins with a PEXEL motif, molecular mass and length were calculated for the PEXEL-cleaved form of the protein (the molecular mass for PEXEL-cleaved proteins was calculated by using the ExPASy Web tool [http://web.expasy.org/compute_pi/]).
Existing viable knockout (KO) parasites or natural gene deletions are indicated by “x” (see reference 44 or the reference[s] indicated).
Information or references referring to the corresponding gene or protein. LSAP2, liver stage-associated protein 2; SNPs, single-nucleotide polymorphisms.
Other PHIST proteins.
PlasmoDB lists 14 additional proteins that are annotated as PHISTa-like or simply PHIST proteins but originally were not included in the PHIST family (Table 5). Some of the PHISTa-like proteins were included in the subtelomeric protein superfamily identified by Eksi et al. (27), most of which have now been included in the PHISTa subgroup. There seems to be a close relationship between the PHISTa and the PHISTa-like proteins, indicated by sequence alignment and the pattern of conserved tryptophan residues (Fig. 4 and 5A).
PHIST Gene Expression
Transcriptome analysis of the P. falciparum 3D7 strain showed that most phist genes were expressed at an early stage during the intraerythrocytic development cycle. Throughout the second half of the cycle, almost all phist genes were switched off, while some were upregulated again toward the very end of the cycle (Fig. 6) (46). Genes with similar expression patterns were described to be involved in parasite-specific processes such as host cell invasion (47). In Plasmodium, the presence of the protein is often delayed after transcription, and the appearance of PHIST proteins was delayed on average by ∼11 h (48). Thus, most PHIST proteins are present and exported during the first half of the intraerythrocytic development cycle, again strongly suggesting an important role of PHIST proteins in host cell remodeling.
Some phist genes have been reported to be differentially expressed, for example, during gametocytogenesis (35), in pregnancy-associated malaria (29, 49), or in cerebral malaria (32). Variable expression generally has also been observed in field isolates (50).
Figure 6 presents expression data obtained only from asexual blood stage parasites, but a proteomic study revealed that a number of PHIST proteins are enriched in early gametocytes, the sexual blood stage of P. falciparum. Of 26 putatively exported proteins enriched during early gametocyte stages, 9 belong to the PHIST protein family (35) (Fig. 6, yellow boxes). Two of these proteins, namely, GEXP5 (PF3D7_0936600) and PfEMP1-trafficking protein 2 (PfPTP2) (PF3D7_0731100), have been shown by microscopy to localize to gametocytes, with the former having been shown to be expressed exclusively in gametocytes (51). A function during sexual development remains to be shown (43, 51). The presence of some PHIST proteins in gametocytes suggests transcriptional upregulation, and indeed, the expression of ∼20 phist genes, including GEXP5 (PF3D7_0936600), was shown to be under PfHP1 regulation (52). HP1 is a negative regulator of AP2-G, a transcription factor needed for sexual conversion, which binds to a promoter motif common to several early gametocyte genes, including the phist gene PF3D7_1477300 (53, 54). This provides strong evidence that gene regulation by HP1 affects the expression of phist genes and their involvement in gametocytogenesis.
Almelli et al. (32) compared transcriptional differences of samples from patients with cerebral malaria and samples from asymptomatic malaria cases from the 3D7 reference strain. A number of phist genes from all subfamilies were differentially either up- or downregulated (Fig. 6).
Mackinnon et al. (50) compared gene expression levels of the P. falciparum 3D7 strain with those of field isolates and also showed that a number of genes were differentially expressed. Among the 20 most regulated genes were also 7 phist genes (PF3D7_0202100, PF3D7_0424000, PF3D7_0702100, PF3D7_0832200.1, PF3D7_0936600, PF3D7_0936800, and PF3D7_1477700), suggesting that some PHIST proteins might be dispensable in culture but not for in vivo growth.
It has also been shown that 14 phist genes were variably expressed in P. falciparum 3D7 (Fig. 6) (55), indicating an active role in adaptation to changing environments such as heat shock (febrile illness) or nutrient depletion.
All of these studies were performed on the transcriptome or proteome level and reported general patterns and trends in gene expression or protein abundance. These studies repeatedly showed that phist genes or proteins are involved in different processes and support the notion that PHIST proteins play a central role in host cell remodeling. However, the functional and physical characterization of these proteins lags far behind. So far, we know that PHIST proteins are involved in cellular processes in iRBCs and in disease-associated functions, but our understanding of their actual function and interactions is very limited, at least for the large majority of them.
Below, we review and discuss individual PHIST proteins for which more detailed information is available, with most of them belonging to the PHISTb or PHISTc subgroup. Further information on other PHIST proteins is summarized in Tables 1 to 5.
PF3D7_0532400 (LyMP)
The PHISTb lysine-rich membrane-associated protein (LyMP) (PF3D7_0532400 or PFE1605w) is a PEXEL-containing PHISTb protein that is exported during the first half of the intraerythrocytic development cycle to the erythrocyte membrane, where it can localize at parasite-induced protrusions on the red blood cell membrane called knobs. Its transient localization at Maurer's clefts prior to its final destination correlates in space and time with that of PfEMP1 (19). The PHIST domain of LyMP (amino acids 122 to 335) interacts with the intracellular acidic terminal segment (ATS) of PfEMP1. Conditional downregulation of LyMP reduced binding to CD36 by >60%, indicating that the interaction between the PHIST domain of LyMP and the ATS domain of PfEMP1 is important for the cytoadhesive properties of iRBCs (19, 38, 40, 56). A similar conditional downregulation of LyMP in iRBCs preselected for binding to different adhesion receptors displaying different PfEMP1 variants on the surface strongly differed in the reduction of cytoadherence (38), which led to the hypothesis that different PHIST proteins might be responsible for anchoring different PfEMP1 variants to the cytoskeleton.
It was further shown that the C-terminal segment of LyMP (amino acids 319 to 528) was able to bind IOVs, which are used to study protein interactions involving cytoskeletal proteins (40). Recently, we were able to show that this part of LyMP binds directly to human membrane protein band 3, which is linked to the cytoskeleton via ankyrin (38). Together with the ATS interaction mediated by the LyMP PHIST domain (19), it is evident that LyMP can act as a linker between the virulence complex of P. falciparum PfEMP1 and the host cytoskeleton.
PF3D7_0936800
PF3D7_0936800 (PFI1780w) is a PHISTc protein that was also shown to interact with the ATS domain of PfEMP1 albeit much more weakly than LyMP (56). A crystallographic structure of its PHIST domain (residues 85 to 247) has been obtained and is the first available structure for any PHIST protein. It confirms the predicted four-alpha-helix structure of the PHIST domain with a very short first alpha helix. The remaining three helices form a three-helix bundle with weak structural similarity to spectrin (19, 56).
PF3D7_0936800 has been classified as a noncanonical PEXEL protein with the first position of its PEXEL motif rendered from K to R, which was recently shown to be correctly cleaved and N-acetylated, confirming that this PHIST protein is correctly exported from parasites into iRBCs (12).
PF3D7_0936800 localizes underneath the iRBC membrane but is absent from knobs (19). It is speculated that it contains an interaction epitope for binding of iRBC membrane/cytoskeleton components (12). Here it is also noteworthy that PF3D7_0936800 has been found to be variantly expressed in 3D7 (55) and that there has been no reported PF3D7_0936800 knockout (PF3D7_0936800-KO) parasite.
PF3D7_0731100 (PfPTP2)
The PHISTc protein PF3D7_0731100 (PfEMP1 trafficking protein 2, PfPTP2) seems to play a role in PfEMP1 trafficking or surface display. Depletion of this protein leads to PfEMP1 accumulation in Maurer's clefts and its absence from knobs. Under flow conditions, PfPTP2-KO parasites do not adhere to chondroitin sulfate A (CSA), suggesting that the main role of PfPTP2 might be the trafficking of PfEMP1 from Maurer's clefts to knobs (42, 44).
Recently, PfPTP2 was also described to be located at exosome-like vesicles of ∼70 nm that are involved in cell-cell communication and allow nucleic acid transfer between Plasmodium parasites. PfPTP2-deficient parasites showed reduced cell-cell communication, and Regev-Rudzki et al. (43) suggested that PfPTP2 is essential for cell-cell communication between P. falciparum-infected erythrocytes, leading to increased gametocytogenesis in vitro. PfPTP2 was the only protein involved in knob formation or PfEMP1 display (besides skeleton binding protein 1 [SBP1] [57]) but was also found to be enriched in early gametocytes (35, 43). In contrast, transcriptional analysis shows that PfPTP2 is downregulated in gametocytes; thus, PfPTP2 abundance in early gametocytes might be acquired through exosome-like vesicles, and this might in turn induce sexual conversion. Such a hypothetical model would accommodate the fact that PfPTP2 is upregulated in asexual stages but also enriched in gametocytes.
Immunoelectron microscopy and proteinase K assays revealed the localization of PfPTP2 on the cytosolic face of Maurer's clefts, on budding vesicles, and on the surface of exosome-like vesicles (43, 44).
PF3D7_0402000
The PHISTa protein PF3D7_0402000 was identified in a yeast two-hybrid assay to determine potential interaction partners of the human erythrocyte cytoskeleton protein band 4.1R. The protein interacted with the N and alpha lobes of the FERM (four-point-one, ezrin, radixin, and moesin) domain (Pfam family PF00373) of band 4.1R through helices 2 and 3 of the PHIST domain, but the entire domain was required for maximal interaction (58). The host cytoskeleton protein band 4.1R binds to actin filaments and links these to membrane proteins such as band 3 and glycophorin C, maintaining the biconcave shape (reviewed in references 58 and 59). In this context, it is noteworthy that other P. falciparum proteins, e.g., MESA (PF3D7_0500800), which is not a PHIST protein, bind to this host cell cytoskeleton interaction hub (60). In immunofluorescence imaging analyses, subpopulations of both band 4.1R and PF3D7_0402000 were shown to colocalize at the parasitophorous vacuole membrane (58). The role that PF3D7_0402000 plays in changes of membrane rigidity or host actin recruitment remains unclear.
PF3D7_0936600 (GEXP5)
GEXP5 (gametocyte exported protein 5) was originally classified as PHISTb (15) but is now grouped among the PHISTc proteins (16), which is confirmed by the position of its conserved tryptophan residues (Fig. 4). GEXP5 was the first PHIST protein detected in gametocytes. A GEXP5-GFP fusion protein, when episomally expressed under the control of the endogenous promoter in asexual stages, was not detected by fluorescence imaging (51) but could be detected when expressed under the pfcam promoter, confirming a cytosolic localization (20). This indicated that endogenous GEXP5 is expressed and exported only in sexual stages and is found in the iRBC cytosol of stage I to IV gametocytes. Its uniform distribution throughout the iRBC and its presence in the soluble protein fraction suggest that it is a soluble protein (51). GEXP5 is already found at 14 h postinvasion in ring stage parasites committed to gametocytogenesis and is now considered to be the earliest gametocyte marker (51). This early appearance of GEXP5 in committed parasites might explain the presence of transcripts in the expression data sets in PlasmoDB.
The expression and export of GEXP5 are independent of transcription factors normally associated with gametocytogenesis, such as PfAP2-G, but GEXP5 is not able to drive gametocyte maturation alone in the absence of these transcription factors (51). It is therefore assumed that GEXP5 is not involved in processes driving gametocytogenesis but is used to remodel or prepare the host cell to accommodate sexual development.
PF3D7_0102200 (RESA)
RESA (ring-infected erythrocyte surface antigen) contains both PHIST and DnaJ domains and is one of seven members of the PHISTb-DnaJ subgroup. Its name is a misnomer since RESA is an intracellular exported protein (20).
RESA is expressed in mature-stage parasites (61) and is stored in the dense granules of newly formed merozoites (62). Within minutes after the invasion of a merozoite into an erythrocyte, the contents of the dense granules, including RESA, are discharged into the parasitophorous vacuole (63–65). From there, RESA is then exported to the iRBC and localizes at the iRBC cytoskeleton, where it remains for ∼24 h (64, 65). At the cytoskeleton, RESA is phosphorylated (66, 67) and interacts with spectrin (68). A fragment of 108 amino acids has been identified to interact with repeat 16 of the β-chain of spectrin (here it is important to note that residues 663 to 770 correspond to only a partial DnaJ X domain and not to the DnaJ J domain). As a result of this interaction, the spectrin tetramers are stabilized and rigidify the iRBC (69); however, it needs to be proven whether this interaction and the accompanying rigidification would also occur with the full-length domain or full-length RESA protein.
There is controversy regarding whether this interaction impairs the invasion of merozoites, since one study with erythrocytes prepacked with a recombinant form of RESA showed that the RESA-spectrin interaction had no significant effect on invasion (70). The recombinant RESA used in this study contained residues 322 to 1073 and lacked the entire PHIST domain (residues 174 to 294) (70) (Fig. 3). However, using a similar setup, Pei et al. showed that an even shorter fragment of RESA (residues 663 to 770) was able to reduce the invasion efficiency (69). Importantly, this recombinant peptide lacked both the PHIST domain and the DnaJ domain and hence might have created an artificial inhibitory interaction.
A domain of 70 amino acids found in RESA shares 39% homology with the DnaJ heat shock protein of Escherichia coli (41). Proteins with a DnaJ domain belong to the HSP40 family and act as cochaperones to the DnaK/HSP70 chaperones, which are involved in protein assembly and trafficking (18, 41). Proteins with a DnaJ domain have been described to be membrane associated (41), a feature shared by RESA. Initially, it was proposed that RESA destabilizes the RBC cytoskeleton (64), but now there is evidence that RESA in fact does the contrary, namely, stabilizing the cytoskeleton with the effect that it rigidifies ring stage infected parasites (65, 69, 71). It has been shown that the RESA-spectrin interaction protects iRBCs from thermal damage (65, 69, 70), and it has been speculated that the DnaJ domain of RESA might prevent spectrin from unfolding at elevated temperatures, or it might directly bind to hydrophobic regions of partially unfolded spectrin molecules (70). This rigidification of ring stage parasite-infected erythrocytes, potentially impairing passage through the spleen (72), is rather surprising, but this might simply be a side effect of the protective function of RESA against thermal damage to the host cell.
At the end of each 48-h cycle, the iRBC ruptures and releases new merozoites. This event is accompanied by fever peaks that last for a few hours (73, 74). During these fever episodes, the parasites are in ring stages, and this correlates with the time when RESA interacts with the cytoskeleton, protecting it from thermal damage caused by fever. After ∼24 h, RESA dissociates from spectrin (61), and other proteins might be responsible for further rigidification of the cytoskeleton (71). This apparent functional contradiction might be an evolutionary tradeoff between the need for survival during fever and costs of increased cell rigidity to a level that still allows passage through the spleen. The fact that less deformable ring stage parasite-iRBCs are cleared by the spleen supports this idea of a tradeoff (72).
While the function of the DnaJ domain in RESA has been described in much detail, the function of the PHIST domain still remains enigmatic.
PVX_093680 (CVC-8195)
The only PHIST protein not from P. falciparum that has been further characterized is the P. vivax PHIST CVC-8195 (PVX_093680). Caveola-vesicle complexes (CVCs) are parasite-induced indentations in P. vivax-infected erythrocyte cell membranes. The exact structure, protein composition, and function are not yet fully understood, but CVC-8195 is exported, is a predominant protein in these CVCs, and is found on tubular extensions going inwards from the CVC (75, 76). Its orthologue in P. cynomolgi shows a similar localization (75). CVC-8195 was found by Acharya et al. (77) in P. vivax-infected peripheral blood, and another study showed that over 80% of patient sera reacted positively with CVC-8195 (78). Based on these findings, CVC-8195 was suggested to be involved in immune evasion (78), as the PHIST protein family was previously suggested to be involved in this process (24). Therefore, in P. vivax, PHIST proteins also seem to be localized to parasite-induced structures in iRBCs and might be essential for host-parasite interactions. However, more functional analyses are required to fully understand the role of this PHIST protein in the CVC of P. vivax.
phist Gene Knockout Study
In a large gene knockout study in P. falciparum to functionally characterize exported proteins, 17 phist genes were targeted for deletion (44). These genes included 1 phista gene, 7 phistb genes, 6 phistb-dnaJ genes, and 3 phistc genes. Of these, all but four (PF3D7_0936800, PF3D7_0401800, PF3D7_0402100, and PF3D7_1149000) were successfully disrupted, indicating that the majority was dispensable for in vitro growth. Except for PF3D7_0731100 (PfPTP2), none of the phist knockout parasites from this study were analyzed in greater detail. Some were reported to have an influence on altered knob morphology (PF3D7_0424600-KO) or reduced (PF3D7_0102200/RESA-KO) or increased (PF3D7_0220100-KO) iRBC rigidity (44). Further phenotypic characterization of these parasite lines is urgently needed to better understand the role of PHIST proteins in host cell remodeling.
Structure and Function
It is highly conceivable that PHIST proteins might fulfill similar roles as chaperones, since a number of chaperones also contain DnaJ domains. They may also be responsible for the close association with the host cytoskeleton and cell membrane due to the intrinsic positive charge of many PHIST proteins. The few studies on PHISTs have looked mostly at the PHIST domain only; however, Tarr et al. (20) suggested that the PHIST domain alone is not sufficient to correctly target the protein to its destination, at least for those PHIST proteins investigated. Mayer et al. (56) analyzed the binding affinities of various PHIST domains for a number of ATS domains, but only the PHIST protein PF3D7_0532400 has been studied in greater detail with respect to structure and function (19, 56). This is rather surprising for a protein family comprising a large proportion of the complete exportome of P. falciparum.
CONCLUSION
The three major PHIST subfamilies not only differ in sequence but also seem to differ in function. PHISTb proteins mostly interact with and localize to the iRBC cytoskeleton and seem to be involved in changes of cell properties such as rigidity. PHISTc proteins seem to be involved mostly in protein trafficking of other exported proteins, while the PHISTa subfamily remains enigmatic, and there is not yet sufficient information available to draw a conclusion on function.
The presence of PHIST proteins at various localizations in the host cell makes them highly interesting candidates as interaction partners for other exported proteins, and therefore, it would be important to understand what determines the destination of PHIST proteins. The identification of export requirements for PHIST proteins would increase our understanding of the interaction network of exported proteins.
It seems that some PHIST proteins have at least two binding domains, the PHIST domain and a second one toward the C terminus, e.g., the MEC motif, the spectrin binding site, the band 3 interaction epitope, or a DnaJ domain. This would make PHIST proteins ideal molecules for multifunctional interactions at the iRBC cytoskeleton or in protein export with the PHIST domain serving as a general adaptor, while the C-terminal domain could function as a highly specific binding site. However, only a few additional binding motifs have been identified in some PHIST proteins, and more PHIST proteins remain to be studied.
Our knowledge of PHIST proteins is still very limited and based mainly on studies of the 3D7 laboratory strain, which does not suffice to understand the full extent of PHIST protein functions for in vivo parasite survival and malaria-associated disease and pathology. PHIST proteins should be studied in various disease presentations from mild to severe malaria as well as in pregnancy-associated malaria. Furthermore, the role of PHIST proteins in gametocytogenesis and also in mosquito stages remains completely enigmatic. Since many PHIST proteins seem to be central for host cell remodeling, it is essential to understand their function in this crucial process.
ACKNOWLEDGMENTS
We thank Beatrice Schibler and Alexander Oberli for carefully reading and commenting on the manuscript. We acknowledge Christoph Schmid and Anita Lerch for their help with the alignments and phylogenetic tree construction.
J.D.W. conducted the literature review. J.D.W., I.V., and H.-P.B. wrote the manuscript.
We claim no conflicting interests.
J.D.W. and H.-P.B. were supported by Swiss National Science Foundation grant number 31003A_149297/1. I.V. was supported by a Wellcome Trust RCD fellowship (088497/Z/09/Z). The funders had no role in determining the content of the paper or in the decision to submit this work for publication.
Biographies

Jan D. Warncke obtained his B.Sc. in biology with an emphasis on microbiology from Brigham Young University—Idaho, Rexburg, ID. He then moved on to the Swiss Tropical and Public Health Institute and the University of Basel, Switzerland, where he received his M.Sc. in Infection Biology. Currently, he is enrolled in a Ph.D. program at the Swiss Tropical and Public Health Institute, working in the Molecular Parasitology and Epidemiology unit under the supervision of Professor Hans-Peter Beck. His interest is in exported proteins of P. falciparum and their role in host cell remodeling during the asexual blood stage of malaria.

Ioannis Vakonakis has a B.Sc. in Biology from the University of Crete. He obtained his Ph.D. in Biochemistry from Texas A&M University, where he pioneered the structural analysis of circadian clock proteins from cyanobacteria. His postdoctoral work at the University of Oxford focused on the mechanisms behind cell adhesion and the formation of the extracellular matrix in animals. He was trained in X-ray crystallography during a second postdoc at the Swiss Light Source prior to starting his own laboratory in Oxford Biochemistry as a Wellcome Trust Career Development Fellow. He is now an Associate Professor in Structural Biology and Biophysics at the University of Oxford. Over the last 6 years, his research has aimed to understand how large molecular machines form in cells, such as the cytoadherent assemblies created upon P. falciparum infection of human erythrocytes.

Hans-Peter Beck obtained his M.Sc. and Ph.D. in cell biology at the University of Tübingen. After his postdoc position at the Wellcome Centre of Molecular Parasitology in Edinburgh-Glasgow, where he worked on the bovine parasite Theileria annulata, he moved as a visiting scientist to the Walter and Eliza Hall Institute in Melbourne before he took up the position as group leader in Molecular Parasitology at the Papua New Guinean Institute of Medical Research. During this period, he pioneered molecular epidemiology for P. falciparum infections and established a research group working on the cell biology of these parasites. After 5 years in Papua New Guinea, he moved to the University of Witten-Herdecke, Germany, as group leader and subsequently moved to the Swiss Tropical and Public Health Institute, where he holds the position of unit head of a research group and holds a Professorship for Molecular Parasitology at the University of Basel. His research focus is on the molecular epidemiology and cell biology of the human malaria parasite P. falciparum. His research group in particular focuses on the interaction network of exported proteins of the parasite.
REFERENCES
- 1.Boddey JA, Cowman AF. 2013. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu Rev Microbiol 67:243–269. doi: 10.1146/annurev-micro-092412-155730. [DOI] [PubMed] [Google Scholar]
- 2.Tuteja R. 2007. Malaria—an overview. FEBS J 274:4670–4679. doi: 10.1111/j.1742-4658.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO. 2015. World malaria report 2015. WHO, Geneva, Switzerland. [Google Scholar]
- 4.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prevost MC, Ishino T, Yuda M, Menard R. 2008. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3:88–96. doi: 10.1016/j.chom.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 6.Gardner JP, Pinches RA, Roberts DJ, Newbold CI. 1996. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci U S A 93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, Magistrado P, Lusingu J, Smith JD, Higgins MK, Theander TG. 2013. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister L, Mitchell G. 2003. The ins, outs and roundabouts of malaria. Trends Parasitol 19:209–213. doi: 10.1016/S1471-4922(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 9.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 10.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K. 2004. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 11.Boddey JA, Carvalho TG, Hodder AN, Sargeant TJ, Sleebs BE, Marapana D, Lopaticki S, Nebl T, Cowman AF. 2013. Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic 14:532–550. doi: 10.1111/tra.12053. [DOI] [PubMed] [Google Scholar]
- 12.Schulze J, Kwiatkowski M, Borner J, Schluter H, Bruchhaus I, Burmester T, Spielmann T, Pick C. 2015. The Plasmodium falciparum exportome contains non-canonical PEXEL/HT proteins. Mol Microbiol 97:301–314. doi: 10.1111/mmi.13024. [DOI] [PubMed] [Google Scholar]
- 13.Spielmann T, Gilberger TW. 2010. Protein export in malaria parasites: do multiple export motifs add up to multiple export pathways? Trends Parasitol 26:6–10. doi: 10.1016/j.pt.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Heiber A, Kruse F, Pick C, Gruring C, Flemming S, Oberli A, Schoeler H, Retzlaff S, Mesen-Ramirez P, Hiss JA, Kadekoppala M, Hecht L, Holder AA, Gilberger TW, Spielmann T. 2013. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog 9:e1003546. doi: 10.1371/journal.ppat.1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sargeant TJ, Marti M, Caler E, Carlton JM, Simpson K, Speed TP, Cowman AF. 2006. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol 7:R12. doi: 10.1186/gb-2006-7-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frech C, Chen N. 2013. Variant surface antigens of malaria parasites: functional and evolutionary insights from comparative gene family classification and analysis. BMC Genomics 14:427. doi: 10.1186/1471-2164-14-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J, Blundell TL, Mizuguchi K. 2001. FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J Mol Biol 310:243–257. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- 18.Oakley MS, Kumar S, Anantharaman V, Zheng H, Mahajan B, Haynes JD, Moch JK, Fairhurst R, McCutchan TF, Aravind L. 2007. Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect Immun 75:2012–2025. doi: 10.1128/IAI.01236-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberli A, Slater LM, Cutts E, Brand F, Mundwiler-Pachlatko E, Rusch S, Masik MF, Erat MC, Beck HP, Vakonakis I. 2014. A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface. FASEB J 28:4420–4433. doi: 10.1096/fj.14-256057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarr SJ, Moon RW, Hardege I, Osborne AR. 2014. A conserved domain targets exported PHISTb family proteins to the periphery of Plasmodium infected erythrocytes. Mol Biochem Parasitol 196:29–40. doi: 10.1016/j.molbiopara.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto TD, Rayner JC, Bohme U, Pain A, Spottiswoode N, Sanders M, Quail M, Ollomo B, Renaud F, Thomas AW, Prugnolle F, Conway DJ, Newbold C, Berriman M. 2014. Genome sequencing of chimpanzee malaria parasites reveals possible pathways of adaptation to human hosts. Nat Commun 5:4754. doi: 10.1038/ncomms5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang'a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. 2008. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westenberger SJ, McClean CM, Chattopadhyay R, Dharia NV, Carlton JM, Barnwell JW, Collins WE, Hoffman SL, Zhou Y, Vinetz JM, Winzeler EA. 2010. A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis 4:e653. doi: 10.1371/journal.pntd.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, Ginsburg H, Nosten F, Day NP, White NJ, Carlton JM, Preiser PR. 2008. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci U S A 105:16290–16295. doi: 10.1073/pnas.0807404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pain A, Bohme U, Berry AE, Mungall K, Finn RD, Jackson AP, Mourier T, Mistry J, Pasini EM, Aslett MA, Balasubrammaniam S, Borgwardt K, Brooks K, Carret C, Carver TJ, Cherevach I, Chillingworth T, Clark TG, Galinski MR, Hall N, Harper D, Harris D, Hauser H, Ivens A, Janssen CS, Keane T, Larke N, Lapp S, Marti M, Moule S, Meyer IM, Ormond D, Peters N, Sanders M, Sanders S, Sargeant TJ, Simmonds M, Smith F, Squares R, Thurston S, Tivey AR, Walker D, White B, Zuiderwijk E, Churcher C, Quail MA, Cowman AF, Turner CM, Rajandream MA, Kocken CH, et al. 2008. The genome of the simian and human malaria parasite Plasmodium knowlesi. Nature 455:799–803. doi: 10.1038/nature07306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira CK, Naissant B, Coppi A, Bennett BL, Aime E, Franke-Fayard B, Janse CJ, Coppens I, Sinnis P, Templeton TJ. 2016. The Plasmodium PHIST and RESA-like protein families of human and rodent malaria parasites. PLoS One 11:e0152510. doi: 10.1371/journal.pone.0152510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC. 2005. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol 143:90–99. doi: 10.1016/j.molbiopara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Scholz M, Fraunholz MJ. 2008. A computational model of gene expression reveals early transcriptional events at the subtelomeric regions of the malaria parasite, Plasmodium falciparum. Genome Biol 9:R88. doi: 10.1186/gb-2008-9-5-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuikue Ndam N, Bischoff E, Proux C, Lavstsen T, Salanti A, Guitard J, Nielsen MA, Coppee JY, Gaye A, Theander T, David PH, Deloron P. 2008. Plasmodium falciparum transcriptome analysis reveals pregnancy malaria associated gene expression. PLoS One 3:e1855. doi: 10.1371/journal.pone.0001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daily JP, Le Roch KG, Sarr O, Ndiaye D, Lukens A, Zhou YY, Ndir O, Mboup S, Sultan A, Winzeler EA, Wirth DF. 2005. In vivo transcriptome of Plasmodium falciparum reveals overexpression of transcripts that encode surface proteins. J Infect Dis 191:1196–1203. doi: 10.1086/428289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW, Turner L, Arman M, Raza A, Bozdech Z, Rowe JA. 2012. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 109:E1772–E1781. doi: 10.1073/pnas.1120461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almelli T, Nuel G, Bischoff E, Aubouy A, Elati M, Wang CW, Dillies MA, Coppee JY, Ayissi GN, Basco LK, Rogier C, Ndam NT, Deloron P, Tahar R. 2014. Differences in gene transcriptomic pattern of Plasmodium falciparum in children with cerebral malaria and asymptomatic carriers. PLoS One 9:e114401. doi: 10.1371/journal.pone.0114401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mok BW, Ribacke U, Winter G, Yip BH, Tan CS, Fernandez V, Chen Q, Nilsson P, Wahlgren M. 2007. Comparative transcriptomal analysis of isogenic Plasmodium falciparum clones of distinct antigenic and adhesive phenotypes. Mol Biochem Parasitol 151:184–192. doi: 10.1016/j.molbiopara.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Bertin GI, Sabbagh A, Argy N, Salnot V, Ezinmegnon S, Agbota G, Ladipo Y, Alao JM, Sagbo G, Guillonneau F, Deloron P. 2016. Proteomic analysis of Plasmodium falciparum parasites from patients with cerebral and uncomplicated malaria. Sci Rep 6:26773. doi: 10.1038/srep26773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joice R, Narasimhan V, Montgomery J, Sidhu AB, Oh K, Meyer E, Pierre-Louis W, Seydel K, Milner D, Williamson K, Wiegand R, Ndiaye D, Daily J, Wirth D, Taylor T, Huttenhower C, Marti M. 2013. Inferring developmental stage composition from gene expression in human malaria. PLoS Comput Biol 9:e1003392. doi: 10.1371/journal.pcbi.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, Seydel KB, Bertuccini L, Alano P, Williamson KC, Duraisingh MT, Taylor TE, Milner DA, Marti M. 2014. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med 6:244re5. doi: 10.1126/scitranslmed.3008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberli A, Zurbrugg L, Rusch S, Brand F, Butler ME, Day JL, Cutts EE, Lavstsen T, Vakonakis I, Beck HP. 24 February 2016. Plasmodium falciparum PHIST proteins contribute to cytoadherence and anchor PfEMP1 to the host cell cytoskeleton. Cell Microbiol doi: 10.1111/cmi.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilili GK, LaCount DJ. 2011. An erythrocyte cytoskeleton-binding motif in exported Plasmodium falciparum proteins. Eukaryot Cell 10:1439–1447. doi: 10.1128/EC.05180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proellocks NI, Herrmann S, Buckingham DW, Hanssen E, Hodges EK, Elsworth B, Morahan BJ, Coppel RL, Cooke BM. 2014. A lysine-rich membrane-associated PHISTb protein involved in alteration of the cytoadhesive properties of Plasmodium falciparum-infected red blood cells. FASEB J 28:3103–3113. doi: 10.1096/fj.14-250399. [DOI] [PubMed] [Google Scholar]
- 41.Bork P, Sander C, Valencia A, Bukau B. 1992. A module of the DnaJ heat-shock proteins found in malaria parasites. Trends Biochem Sci 17:129. doi: 10.1016/0968-0004(92)90319-5. [DOI] [PubMed] [Google Scholar]
- 42.Maier AG, Cooke BM, Cowman AF, Tilley L. 2009. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol 7:341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 43.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF. 2013. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. 2008. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134:48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, Sanders PR, Lundie RJ, Maier AG, Cowman AF, Crabb BS. 2009. A newly discovered protein export machine in malaria parasites. Nature 459:945–949. doi: 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llinas M, Bozdech Z, Wong ED, Adai AT, DeRisi JL. 2006. Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res 34:1166–1173. doi: 10.1093/nar/gkj517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozdech Z, Llinás M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol 1:e5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foth BJ, Zhang N, Chaal BK, Sze SK, Preiser PR, Bozdech Z. 2011. Quantitative time-course profiling of parasite and host cell proteins in the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 10:M110.006411. doi: 10.1074/mcp.M110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis SE, Malkov VA, Oleinikov AV, Rossnagle E, Wendler JP, Mutabingwa TK, Fried M, Duffy PE. 2007. Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect Immun 75:4838–4850. doi: 10.1128/IAI.00635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackinnon MJ, Li J, Mok S, Kortok MM, Marsh K, Preiser PR, Bozdech Z. 2009. Comparative transcriptional and genomic analysis of Plasmodium falciparum field isolates. PLoS Pathog 5:e1000644. doi: 10.1371/journal.ppat.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiburcio M, Dixon MW, Looker O, Younis SY, Tilley L, Alano P. 2015. Specific expression and export of the Plasmodium falciparum gametocyte exported protein-5 marks the gametocyte ring stage. Malar J 14:334. doi: 10.1186/s12936-015-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flueck C, Bartfai R, Volz J, Niederwieser I, Salcedo-Amaya AM, Alako BT, Ehlgen F, Ralph SA, Cowman AF, Bozdech Z, Stunnenberg HG, Voss TS. 2009. Plasmodium falciparum heterochromatin protein 1 marks genomic loci linked to phenotypic variation of exported virulence factors. PLoS Pathog 5:e1000569. doi: 10.1371/journal.ppat.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brancucci NM, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, Freymond C, Rottmann M, Felger I, Bozdech Z, Voss TS. 2014. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe 16:165–176. doi: 10.1016/j.chom.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Goldowitz IS. 2015. Plasmodium's crossroads: deciphering the molecular pathway that leads to malaria transmission. Doctoral dissertation. Harvard University, Boston, MA. [Google Scholar]
- 55.Rovira-Graells N, Gupta AP, Planet E, Crowley VM, Mok S, Ribas de Pouplana L, Preiser PR, Bozdech Z, Cortes A. 2012. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res 22:925–938. doi: 10.1101/gr.129692.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayer C, Slater L, Erat MC, Konrat R, Vakonakis I. 2012. Structural analysis of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) intracellular domain reveals a conserved interaction epitope. J Biol Chem 287:7182–7189. doi: 10.1074/jbc.M111.330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dixon MW, Thompson J, Gardiner DL, Trenholme KR. 2008. Sex in Plasmodium: a sign of commitment. Trends Parasitol 24:168–175. doi: 10.1016/j.pt.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Parish LA, Mai DW, Jones ML, Kitson EL, Rayner JC. 2013. A member of the Plasmodium falciparum PHIST family binds to the erythrocyte cytoskeleton component band 4.1. Malar J 12:160. doi: 10.1186/1475-2875-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fowler VM. 2013. The human erythrocyte plasma membrane: a Rosetta Stone for decoding membrane-cytoskeleton structure. Curr Top Membr 72:39–88. doi: 10.1016/B978-0-12-417027-8.00002-7. [DOI] [PubMed] [Google Scholar]
- 60.Lustigman S, Anders RF, Brown GV, Coppel RL. 1990. The mature-parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum associates with the erythrocyte membrane skeletal protein, band 4.1. Mol Biochem Parasitol 38:261–270. doi: 10.1016/0166-6851(90)90029-L. [DOI] [PubMed] [Google Scholar]
- 61.Brown GV, Culvenor JG, Crewther PE, Bianco AE, Coppel RL, Saint RB, Stahl HD, Kemp DJ, Anders RF. 1985. Localization of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum in merozoites and ring-infected erythrocytes. J Exp Med 162:774–779. doi: 10.1084/jem.162.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aikawa M, Torii M, Sjolander A, Berzins K, Perlmann P, Miller LH. 1990. Pf155/Resa antigen is localized in dense granules of Plasmodium falciparum merozoites. Exp Parasitol 71:326–329. doi: 10.1016/0014-4894(90)90037-D. [DOI] [PubMed] [Google Scholar]
- 63.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. 2011. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Coppel RL, Lustigman S, Murray L, Anders RF. 1988. MESA is a Plasmodium falciparum phosphoprotein associated with the erythrocyte membrane skeleton. Mol Biochem Parasitol 31:223–232. doi: 10.1016/0166-6851(88)90152-1. [DOI] [PubMed] [Google Scholar]
- 65.Silva MD, Cooke BM, Guillotte M, Buckingham DW, Sauzet JP, Le Scanf C, Contamin H, David P, Mercereau-Puijalon O, Bonnefoy S. 2005. A role for the Plasmodium falciparum RESA protein in resistance against heat shock demonstrated using gene disruption. Mol Microbiol 56:990–1003. doi: 10.1111/j.1365-2958.2005.04603.x. [DOI] [PubMed] [Google Scholar]
- 66.Anders R, Murray L, Thomas L, Davern K, Brown G, Kemp D. 1987. Structure and function of candidate vaccine antigens in Plasmodium falciparum. Biochem Soc Symp 53:103–114. [PubMed] [Google Scholar]
- 67.Foley M, Murray LJ, Anders RF. 1990. The ring-infected erythrocyte surface-antigen protein of Plasmodium falciparum is phosphorylated upon association with the host cell membrane. Mol Biochem Parasitol 38:69–75. doi: 10.1016/0166-6851(90)90206-2. [DOI] [PubMed] [Google Scholar]
- 68.Foley M, Tilley L, Sawyer WH, Anders RF. 1991. The ring-infected erythrocyte surface antigen of Plasmodium falciparum associates with spectrin in the erythrocyte membrane. Mol Biochem Parasitol 46:137–147. doi: 10.1016/0166-6851(91)90207-M. [DOI] [PubMed] [Google Scholar]
- 69.Pei XH, Guo XH, Coppel R, Bhattacharjee S, Haldar K, Gratzer W, Mohandas N, An XL. 2007. The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion. Blood 110:1036–1042. doi: 10.1182/blood-2007-02-076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Da Silva E, Foley M, Dluzewski AR, Murray LJ, Anders RF, Tilley L. 1994. The Plasmodium falciparum protein RESA interacts with the erythrocyte cytoskeleton and modifies erythrocyte thermal stability. Mol Biochem Parasitol 66:59–69. doi: 10.1016/0166-6851(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 71.Mills JP, Diez-Silva M, Quinn DJ, Dao M, Lang MJ, Tan KS, Lim CT, Milon G, David PH, Mercereau-Puijalon O, Bonnefoy S, Suresh S. 2007. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc Natl Acad Sci U S A 104:9213–9217. doi: 10.1073/pnas.0703433104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Safeukui I, Correas JM, Brousse V, Hirt D, Deplaine G, Mule S, Lesurtel M, Goasguen N, Sauvanet A, Couvelard A, Kerneis S, Khun H, Vigan-Womas I, Ottone C, Molina TJ, Treluyer JM, Mercereau-Puijalon O, Milon G, David PH, Buffet PA. 2008. Retention of Plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood 112:2520–2528. doi: 10.1182/blood-2008-03-146779. [DOI] [PubMed] [Google Scholar]
- 73.Crutcher JM, Hoffman SL. 1996. Malaria, chapter 83, p 997 In Baron S. (ed), Medical microbiology, 4th ed University of Texas Medical Branch at Galveston, Galveston, TX. [PubMed] [Google Scholar]
- 74.Oakley MS, Gerald N, McCutchan TF, Aravind L, Kumar S. 2011. Clinical and molecular aspects of malaria fever. Trends Parasitol 27:442–449. doi: 10.1016/j.pt.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Akinyi S, Hanssen E, Meyer EVS, Jiang J, Korir CC, Singh B, Lapp S, Barnwell JW, Tilley L, Galinski MR. 2012. A 95 kDa protein of Plasmodium vivax and P. cynomolgi visualized by three-dimensional tomography in the caveola-vesicle complexes (Schüffner's dots) of infected erythrocytes is a member of the PHIST family. Mol Microbiol 84:816–831. doi: 10.1111/j.1365-2958.2012.08060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson DC, Lapp SA, Akinyi S, Meyer EV, Barnwell JW, Korir-Morrison C, Galinski MR. 2015. Plasmodium vivax trophozoite-stage proteomes. J Proteomics 115:157–176. doi: 10.1016/j.jprot.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acharya P, Pallavi R, Chandran S, Chakravarti H, Middha S, Acharya J, Kochar S, Kochar D, Subudhi A, Boopathi AP, Garg S, Das A, Tatu U. 2009. A glimpse into the clinical proteome of human malaria parasites Plasmodium falciparum and Plasmodium vivax. Proteomics Clin Appl 3:1314–1325. doi: 10.1002/prca.200900090. [DOI] [PubMed] [Google Scholar]
- 78.Lu F, Li J, Wang B, Cheng Y, Kong DH, Cui L, Ha KS, Sattabongkot J, Tsuboi T, Han ET. 2014. Profiling the humoral immune responses to Plasmodium vivax infection and identification of candidate immunogenic rhoptry-associated membrane antigen (RAMA). J Proteomics 102:66–82. doi: 10.1016/j.jprot.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 79.Nyalwidhe J, Lingelbach K. 2006. Proteases and chaperones are the most abundant proteins in the parasitophorous vacuole of Plasmodium falciparum-infected erythrocytes. Proteomics 6:1563–1573. doi: 10.1002/pmic.200500379. [DOI] [PubMed] [Google Scholar]
- 80.Jiang H, Patel JJ, Yi M, Mu J, Ding J, Stephens R, Cooper RA, Ferdig MT, Su XZ. 2008. Genome-wide compensatory changes accompany drug-selected mutations in the Plasmodium falciparum crt gene. PLoS One 3:e2484. doi: 10.1371/journal.pone.0002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mwai L, Diriye A, Masseno V, Muriithi S, Feltwell T, Musyoki J, Lemieux J, Feller A, Mair GR, Marsh K, Newbold C, Nzila A, Carret CK. 2012. Genome wide adaptations of Plasmodium falciparum in response to lumefantrine selective drug pressure. PLoS One 7:e31623. doi: 10.1371/journal.pone.0031623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mbengue A, Berry L, Braun-Breton C. 2014. Establishment of Plasmodium falciparum extracellular compartments in its host erythrocyte, p 133–159. In Shonhai A, Blatch G (ed), Heat shock proteins of malaria. Springer, New York, NY. [Google Scholar]
- 83.Crowley VM, Rovira-Graells N, Ribas de Pouplana L, Cortes A. 2011. Heterochromatin formation in bistable chromatin domains controls the epigenetic repression of clonally variant Plasmodium falciparum genes linked to erythrocyte invasion. Mol Microbiol 80:391–406. doi: 10.1111/j.1365-2958.2011.07574.x. [DOI] [PubMed] [Google Scholar]
- 84.Bertin GI, Sabbagh A, Guillonneau F, Jafari-Guemouri S, Ezinmegnon S, Federici C, Hounkpatin B, Fievet N, Deloron P. 2013. Differential protein expression profiles between Plasmodium falciparum parasites isolated from subjects presenting with pregnancy-associated malaria and uncomplicated malaria in Benin. J Infect Dis 208:1987–1997. doi: 10.1093/infdis/jit377. [DOI] [PubMed] [Google Scholar]
- 85.Buchholz K, Burke TA, Williamson KC, Wiegand RC, Wirth DF, Marti M. 2011. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J Infect Dis 203:1445–1453. doi: 10.1093/infdis/jir037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goel S, Muthusamy A, Miao J, Cui L, Salanti A, Winzeler EA, Gowda DC. 2014. Targeted disruption of a ring-infected erythrocyte surface antigen (RESA)-like export protein gene in Plasmodium falciparum confers stable chondroitin 4-sulfate cytoadherence capacity. J Biol Chem 289:34408–34421. doi: 10.1074/jbc.M114.615393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Natalang O, Bischoff E, Deplaine G, Proux C, Dillies MA, Sismeiro O, Guigon G, Bonnefoy S, Patarapotikul J, Mercereau-Puijalon O, Coppee JY, David PH. 2008. Dynamic RNA profiling in Plasmodium falciparum synchronized blood stages exposed to lethal doses of artesunate. BMC Genomics 9:388. doi: 10.1186/1471-2164-9-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai H, Hong C, Gu J, Lilburn TG, Kuang R, Wang Y. 2012. Module-based subnetwork alignments reveal novel transcriptional regulators in malaria parasite Plasmodium falciparum. BMC Syst Biol 6(Suppl 3):S5. doi: 10.1186/1752-0509-6-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, Braun Breton C. 2005. Proteomic analysis identifies novel proteins of the Maurer's clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol Cell Proteomics 4:582–593. doi: 10.1074/mcp.M400176-MCP200. [DOI] [PubMed] [Google Scholar]
- 90.Lanzer M, Wickert H, Krohne G, Vincensini L, Braun Breton C. 2006. Maurer's clefts: a novel multi-functional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. Int J Parasitol 36:23–36. doi: 10.1016/j.ijpara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Ralph SA, Bischoff E, Mattei D, Sismeiro O, Dillies MA, Guigon G, Coppee JY, David PH, Scherf A. 2005. Transcriptome analysis of antigenic variation in Plasmodium falciparum—var silencing is not dependent on antisense RNA. Genome Biol 6:R93. doi: 10.1186/gb-2005-6-11-r93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vignali M, Armour CD, Chen J, Morrison R, Castle JC, Biery MC, Bouzek H, Moon W, Babak T, Fried M, Raymond CK, Duffy PE. 2011. NSR-seq transcriptional profiling enables identification of a gene signature of Plasmodium falciparum parasites infecting children. J Clin Invest 121:1119–1129. doi: 10.1172/JCI43457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Roch KG, Johnson JR, Florens L, Zhou Y, Santrosyan A, Grainger M, Yan SF, Williamson KC, Holder AA, Carucci DJ, Yates JR III, Winzeler EA. 2004. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res 14:2308–2318. doi: 10.1101/gr.2523904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D. 2008. Discrepancy between mRNA and protein abundance: insight from information retrieval process in computers. Comput Biol Chem 32:462–468. doi: 10.1016/j.compbiolchem.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nair S, Nkhoma S, Nosten F, Mayxay M, French N, Whitworth J, Anderson T. 2010. Genetic changes during laboratory propagation: copy number at the reticulocyte-binding protein 1 locus of Plasmodium falciparum. Mol Biochem Parasitol 172:145–148. doi: 10.1016/j.molbiopara.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sam-Yellowe TY. 2009. The role of the Maurer's clefts in protein transport in Plasmodium falciparum. Trends Parasitol 25:277–284. doi: 10.1016/j.pt.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 97.Talevich E, Tobin AB, Kannan N, Doerig C. 2012. An evolutionary perspective on the kinome of malaria parasites. Philos Trans R Soc Lond B Biol Sci 367:2607–2618. doi: 10.1098/rstb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mu J, Awadalla P, Duan J, McGee KM, Keebler J, Seydel K, McVean GA, Su XZ. 2007. Genome-wide variation and identification of vaccine targets in the Plasmodium falciparum genome. Nat Genet 39:126–130. doi: 10.1038/ng1924. [DOI] [PubMed] [Google Scholar]
- 99.LaCount DJ, Vignali M, Chettier R, Phansalkar A, Bell R, Hesselberth JR, Schoenfeld LW, Ota I, Sahasrabudhe S, Kurschner C, Fields S, Hughes RE. 2005. A protein interaction network of the malaria parasite Plasmodium falciparum. Nature 438:103–107. doi: 10.1038/nature04104. [DOI] [PubMed] [Google Scholar]
- 100.Spillman NJ, Beck JR, Goldberg DE. 2015. Protein export into malaria parasite-infected erythrocytes: mechanisms and functional consequences. Annu Rev Biochem 84:813–841. doi: 10.1146/annurev-biochem-060614-034157. [DOI] [PubMed] [Google Scholar]
- 101.Pallavi R, Acharya P, Chandran S, Daily JP, Tatu U. 2010. Chaperone expression profiles correlate with distinct physiological states of Plasmodium falciparum in malaria patients. Malar J 9:236. doi: 10.1186/1475-2875-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavithra SR, Kumar R, Tatu U. 2007. Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum. PLoS Comput Biol 3:1701–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oyelade J, Ewejobi I, Brors B, Eils R, Adebiyi E. 2011. Computational identification of signalling pathways in Plasmodium falciparum. Infect Genet Evol 11:755–764. doi: 10.1016/j.meegid.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Razak MR, Abdullah NR, Chomel R, Muhamad R, Ismail Z. 2014. Effect of choline kinase inhibitor hexadecyltrimethylammonium bromide on Plasmodium falciparum gene expression. Southeast Asian J Trop Med Public Health 45:259–266. [PubMed] [Google Scholar]
- 105.Botha M, Pesce ER, Blatch GL. 2007. The Hsp40 proteins of Plasmodium falciparum and other apicomplexa: regulating chaperone power in the parasite and the host. Int J Biochem Cell Biol 39:1781–1803. doi: 10.1016/j.biocel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 106.Florens L, Liu X, Wang Y, Yang S, Schwartz O, Peglar M, Carucci DJ, Yates JR III, Wu Y. 2004. Proteomics approach reveals novel proteins on the surface of malaria-infected erythrocytes. Mol Biochem Parasitol 135:1–11. doi: 10.1016/j.molbiopara.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 107.Spielmann T, Hawthorne PL, Dixon MW, Hannemann M, Klotz K, Kemp DJ, Klonis N, Tilley L, Trenholme KR, Gardiner DL. 2006. A cluster of ring stage-specific genes linked to a locus implicated in cytoadherence in Plasmodium falciparum codes for PEXEL-negative and PEXEL-positive proteins exported into the host cell. Mol Biol Cell 17:3613–3624. doi: 10.1091/mbc.E06-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siau A, Toure FS, Ouwe-Missi-Oukem-Boyer O, Ciceron L, Mahmoudi N, Vaquero C, Froissard P, Bisvigou U, Bisser S, Coppee JY, Bischoff E, David PH, Mazier D. 2007. Whole-transcriptome analysis of Plasmodium falciparum field isolates: identification of new pathogenicity factors. J Infect Dis 196:1603–1612. doi: 10.1086/522012. [DOI] [PubMed] [Google Scholar]
- 109.Penn WD. 2010. Yeast two-hybrid analysis of host-parasite protein interactions in falciparum malaria. Doctoral dissertation. Purdue University, West Lafayette, IN. [Google Scholar]
- 110.Fried M, Hixson KK, Anderson L, Ogata Y, Mutabingwa TK, Duffy PE. 2007. The distinct proteome of placental malaria parasites. Mol Biochem Parasitol 155:57–65. doi: 10.1016/j.molbiopara.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 111.Hviid L. 2011. The case for PfEMP1-based vaccines to protect pregnant women against Plasmodium falciparum malaria. Expert Rev Vaccines 10:1405–1414. doi: 10.1586/erv.11.113. [DOI] [PubMed] [Google Scholar]
- 112.Nunes MC, Sterkers Y, Gamain B, Scherf A. 2008. Investigation of host factors possibly enhancing the emergence of the chondroitin sulfate A-binding phenotype in Plasmodium falciparum. Microbes Infect 10:928–932. doi: 10.1016/j.micinf.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 113.Carret CK, Horrocks P, Konfortov E, Winzeler E, Qureshi M, Newbold C, Ivens A. 2005. Microarray-based comparative genomic analyses of the human malaria parasite Plasmodium falciparum using Affymetrix arrays. Mol Biochem Parasitol 144:177–186. doi: 10.1016/j.molbiopara.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 114.Prajapati SK, Singh OP. 2013. Remodeling of human red cells infected with Plasmodium falciparum and the impact of PHIST proteins. Blood Cells Mol Dis 51:195–202. doi: 10.1016/j.bcmd.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez LE, Curtidor H, Urquiza M, Cifuentes G, Reyes C, Patarroyo ME. 2008. Intimate molecular interactions of P. falciparum merozoite proteins involved in invasion of red blood cells and their implications for vaccine design. Chem Rev 108:3656–3705. doi: 10.1021/cr068407v. [DOI] [PubMed] [Google Scholar]
- 116.Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, Incardona S, Perkins M, Bell D, McCarthy J, Cheng Q. 2010. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdallah JF, Okoth SA, Fontecha GA, Torres RE, Banegas EI, Matute ML, Bucheli ST, Goldman IF, de Oliveira AM, Barnwell JW, Udhayakumar V. 2015. Prevalence of pfhrp2 and pfhrp3 gene deletions in Puerto Lempira, Honduras. Malar J 14:19. doi: 10.1186/s12936-014-0537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gelhaus C, Fritsch J, Krause E, Leippe M. 2005. Fractionation and identification of proteins by 2-DE and MS: towards a proteomic analysis of Plasmodium falciparum. Proteomics 5:4213–4222. doi: 10.1002/pmic.200401285. [DOI] [PubMed] [Google Scholar]
- 119.Singh K, Agarwal A, Khan SI, Walker LA, Tekwani BL. 2007. Growth, drug susceptibility, and gene expression profiling of Plasmodium falciparum cultured in medium supplemented with human serum or lipid-rich bovine serum albumin. J Biomol Screen 12:1109–1114. doi: 10.1177/1087057107310638. [DOI] [PubMed] [Google Scholar]
- 120.Ribacke U, Mok BW, Wirta V, Normark J, Lundeberg J, Kironde F, Egwang TG, Nilsson P, Wahlgren M. 2007. Genome wide gene amplifications and deletions in Plasmodium falciparum. Mol Biochem Parasitol 155:33–44. doi: 10.1016/j.molbiopara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 121.Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. 1997. In vivo removal of malaria parasites from red blood cells without their destruction in acute falciparum malaria. Blood 90:2037–2040. [PubMed] [Google Scholar]
- 122.Dixon MW, Kenny S, McMillan PJ, Hanssen E, Trenholme KR, Gardiner DL, Tilley L. 2011. Genetic ablation of a Maurer's cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol 81:982–993. doi: 10.1111/j.1365-2958.2011.07740.x. [DOI] [PubMed] [Google Scholar]
- 123.Rug M, Maier AG. 2011. The heat shock protein 40 family of the malaria parasite Plasmodium falciparum. IUBMB Life 63:1081–1086. doi: 10.1002/iub.525. [DOI] [PubMed] [Google Scholar]
- 124.Le Roch KG, Johnson JR, Ahiboh H, Chung DW, Prudhomme J, Plouffe D, Henson K, Zhou Y, Witola W, Yates JR, Mamoun CB, Winzeler EA, Vial H. 2008. A systematic approach to understand the mechanism of action of the bisthiazolium compound T4 on the human malaria parasite, Plasmodium falciparum. BMC Genomics 9:513. doi: 10.1186/1471-2164-9-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Acharya P, Kumar R, Tatu U. 2007. Chaperoning a cellular upheaval in malaria: heat shock proteins in Plasmodium falciparum. Mol Biochem Parasitol 153:85–94. doi: 10.1016/j.molbiopara.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 126.Badaut C, Guyonnet L, Milet J, Renard E, Durand R, Viwami F, Sagbo G, Layla F, Deloron P, Bonnefoy S, Migot-Nabias F. 2015. Immunoglobulin response to Plasmodium falciparum RESA proteins in uncomplicated and severe malaria. Malar J 14:278. doi: 10.1186/s12936-015-0799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caro F, Ahyong V, Betegon M, DeRisi JL. 2014. Genome-wide regulatory dynamics of translation in the Plasmodium falciparum asexual blood stages. eLife 3:e04106. doi: 10.7554/eLife.04106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Durand R, Migot-Nabias F, Andriantsoanirina V, Seringe E, Viwami F, Sagbo G, Lalya F, Deloron P, Mercereau-Puijalon O, Bonnefoy S. 2012. Possible association of the Plasmodium falciparum T1526C resa2 gene mutation with severe malaria. Malar J 11:128. doi: 10.1186/1475-2875-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pelle KG, Oh K, Buchholz K, Narasimhan V, Joice R, Milner DA, Brancucci NM, Ma S, Voss TS, Ketman K, Seydel KB, Taylor TE, Barteneva NS, Huttenhower C, Marti M. 2015. Transcriptional profiling defines dynamics of parasite tissue sequestration during malaria infection. Genome Med 7:19. doi: 10.1186/s13073-015-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siau A, Silvie O, Franetich JF, Yalaoui S, Marinach C, Hannoun L, van Gemert GJ, Luty AJ, Bischoff E, David PH, Snounou G, Vaquero C, Froissard P, Mazier D. 2008. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog 4:e1000121. doi: 10.1371/journal.ppat.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Speake C, Duffy PE. 2009. Antigens for pre-erythrocytic malaria vaccines: building on success. Parasite Immunol 31:539–546. doi: 10.1111/j.1365-3024.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duffy PE, Fried M. 2011. Pregnancy malaria: cryptic disease, apparent solution. Mem Inst Oswaldo Cruz 106(Suppl 1):64–69. doi: 10.1590/S0074-02762011000900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davies DH, Duffy P, Bodmer JL, Felgner PL, Doolan DL. 2015. Large screen approaches to identify novel malaria vaccine candidates. Vaccine 33:7496–7505. doi: 10.1016/j.vaccine.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Radfar A, Diez A, Bautista JM. 2008. Chloroquine mediates specific proteome oxidative damage across the erythrocytic cycle of resistant Plasmodium falciparum. Free Radic Biol Med 44:2034–2042. doi: 10.1016/j.freeradbiomed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 135.Fatumo S, Adebiyi M, Adebiyi E. 2013. In silico models for drug resistance. Methods Mol Biol 993:39–65. doi: 10.1007/978-1-62703-342-8_4. [DOI] [PubMed] [Google Scholar]
- 136.Boddey JA, Hodder AN, Gunther S, Gilson PR, Patsiouras H, Kapp EA, Pearce JA, de Koning-Ward TF, Simpson RJ, Crabb BS, Cowman AF. 2010. An aspartyl protease directs malaria effector proteins to the host cell. Nature 463:627–631. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sam-Yellowe TY, Florens L, Wang T, Raine JD, Carucci DJ, Sinden R, Yates JR III. 2004. Proteome analysis of rhoptry-enriched fractions isolated from Plasmodium merozoites. J Proteome Res 3:995–1001. doi: 10.1021/pr049926m. [DOI] [PubMed] [Google Scholar]
- 138.Baum E, Badu K, Molina DM, Liang X, Felgner PL, Yan G. 2013. Protein microarray analysis of antibody responses to Plasmodium falciparum in western Kenyan highland sites with differing transmission levels. PLoS One 8:e82246. doi: 10.1371/journal.pone.0082246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Andrews KT, Gupta AP, Tran TN, Fairlie DP, Gobert GN, Bozdech Z. 2012. Comparative gene expression profiling of P. falciparum malaria parasites exposed to three different histone deacetylase inhibitors. PLoS One 7:e31847. doi: 10.1371/journal.pone.0031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 141.Chookajorn T, Hartl DL. 2006. Position-specific polymorphism of Plasmodium falciparum Stuttering motif in a PHISTc PFI1780w. Exp Parasitol 114:126–128. doi: 10.1016/j.exppara.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 142.Otto TD, Wilinski D, Assefa S, Keane TM, Sarry LR, Böhme U, Lemieux J, Barrell B, Pain A, Berriman M. 2010. New insights into the blood-stage transcriptome of Plasmodium falciparum using RNA-Seq. Mol Microbiol 76:12–24. doi: 10.1111/j.1365-2958.2009.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carver T, Harris SR, Otto TD, Berriman M, Parkhill J, McQuillan JA. 2013. BamView: visualizing and interpretation of next-generation sequencing read alignments. Brief Bioinform 14:203–212. doi: 10.1093/bib/bbr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Issar N, Roux E, Mattei D, Scherf A. 2008. Identification of a novel posttranslational modification in Plasmodium falciparum: protein sumoylation in different cellular compartments. Cell Microbiol 10:1999–2011. doi: 10.1111/j.1462-5822.2008.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Josling GA, Llinas M. 2015. Sexual development in Plasmodium parasites: knowing when it's time to commit. Nat Rev Microbiol 13:573–587. doi: 10.1038/nrmicro3519. [DOI] [PubMed] [Google Scholar]
- 146.Haase S, Herrmann S, Gruring C, Heiber A, Jansen PW, Langer C, Treeck M, Cabrera A, Bruns C, Struck NS, Kono M, Engelberg K, Ruch U, Stunnenberg HG, Gilberger TW, Spielmann T. 2009. Sequence requirements for the export of the Plasmodium falciparum Maurer's clefts protein REX2. Mol Microbiol 71:1003–1017. doi: 10.1111/j.1365-2958.2008.06582.x. [DOI] [PubMed] [Google Scholar]
- 147.Volz J, Carvalho TG, Ralph SA, Gilson P, Thompson J, Tonkin CJ, Langer C, Crabb BS, Cowman AF. 2010. Potential epigenetic regulatory proteins localise to distinct nuclear sub-compartments in Plasmodium falciparum. Int J Parasitol 40:109–121. doi: 10.1016/j.ijpara.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 148.Lee AH, Symington LS, Fidock DA. 2014. DNA repair mechanisms and their biological roles in the malaria parasite Plasmodium falciparum. Microbiol Mol Biol Rev 78:469–486. doi: 10.1128/MMBR.00059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taylor DW, Parra M, Chapman GB, Stearns ME, Rener J, Aikawa M, Uni S, Aley SB, Panton LJ, Howard RJ. 1987. Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol 25:165–174. doi: 10.1016/0166-6851(87)90005-3. [DOI] [PubMed] [Google Scholar]
- 150.Watermeyer JM, Hale VL, Hackett F, Clare DK, Cutts EE, Vakonakis I, Fleck RA, Blackman MJ, Saibil HR. 2016. A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes. Blood 127:343–351. doi: 10.1182/blood-2015-10-674002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mundwiler-Pachlatko E, Beck HP. 2013. Maurer's clefts, the enigma of Plasmodium falciparum. Proc Natl Acad Sci U S A 110:19987–19994. doi: 10.1073/pnas.1309247110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cyrklaff M, Sanchez CP, Frischknecht F, Lanzer M. 2012. Host actin remodeling and protection from malaria by hemoglobinopathies. Trends Parasitol 28:479–485. doi: 10.1016/j.pt.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 153.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, Lanzer M. 2011. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science 334:1283–1286. doi: 10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 154.Spycher C, Rug M, Klonis N, Ferguson DJ, Cowman AF, Beck HP, Tilley L. 2006. Genesis of and trafficking to the Maurer's clefts of Plasmodium falciparum-infected erythrocytes. Mol Cell Biol 26:4074–4085. doi: 10.1128/MCB.00095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pachlatko E, Rusch S, Muller A, Hemphill A, Tilley L, Hanssen E, Beck HP. 2010. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer's cleft tethers. Mol Microbiol 77:1136–1152. doi: 10.1111/j.1365-2958.2010.07278.x. [DOI] [PubMed] [Google Scholar]
- 156.Hawthorne PL, Trenholme KR, Skinner-Adams TS, Spielmann T, Fischer K, Dixon MWA, Ortega MR, Anderson KL, Kemp DJ, Gardiner DL. 2004. A novel Plasmodium falciparum ring stage protein, REX, is located in Maurer's clefts. Mol Biochem Parasitol 136:181–189. doi: 10.1016/j.molbiopara.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 157.Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. 2014. Characterization of the small exported Plasmodium falciparum membrane protein SEMP1. PLoS One 9:e103272. doi: 10.1371/journal.pone.0103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Blisnick T, Eugenia M, Betoulle M, Barale JC, Uzureau P, Berry L, Desroses S, Fujioka H, Mattei D, Breton CB. 2000. Pfsbp 1, a Maurer's cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Mol Biochem Parasitol 111:107–121. doi: 10.1016/S0166-6851(00)00301-7. [DOI] [PubMed] [Google Scholar]
- 159.Rug M, Cyrklaff M, Mikkonen A, Lemgruber L, Kuelzer S, Sanchez CP, Thompson J, Hanssen E, O'Neill M, Langer C, Lanzer M, Frischknecht F, Maier AG, Cowman AF. 2014. Export of virulence proteins by malaria-infected erythrocytes involves remodeling of host actin cytoskeleton. Blood 124:3459–3468. doi: 10.1182/blood-2014-06-583054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kats LM, Proellocks NI, Buckingham DW, Blanc L, Hale J, Guo X, Pei X, Herrmann S, Hanssen EG, Coppel RL, Mohandas N, An X, Cooke BM. 2015. Interactions between Plasmodium falciparum skeleton-binding protein 1 and the membrane skeleton of malaria-infected red blood cells. Biochim Biophys Acta 1848:1619–1628. doi: 10.1016/j.bbamem.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Külzer S, Rug M, Brinkmann K, Cannon P, Cowman A, Lingelbach K, Blatch GL, Maier AG, Przyborski JM. 2010. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell Microbiol 12:1398–1420. doi: 10.1111/j.1462-5822.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 162.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. 2012. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol 14:1784–1795. doi: 10.1111/j.1462-5822.2012.01840.x. [DOI] [PubMed] [Google Scholar]
- 163.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R. 2015. The EMBL-EBI bioinformatics Web and programmatic tools framework. Nucleic Acids Res 43:W580–W584. doi: 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mitchell A, Chang HY, Daugherty L, Fraser M, Hunter S, Lopez R, McAnulla C, McMenamin C, Nuka G, Pesseat S, Sangrador-Vegas A, Scheremetjew M, Rato C, Yong SY, Bateman A, Punta M, Attwood TK, Sigrist CJ, Redaschi N, Rivoire C, Xenarios I, Kahn D, Guyot D, Bork P, Letunic I, Gough J, Oates M, Haft D, Huang H, Natale DA, Wu CH, Orengo C, Sillitoe I, Mi H, Thomas PD, Finn RD. 2015. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res 43:D213–D221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. [Google Scholar]
- 168.Cole C, Barber JD, Barton GJ. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36:W197–W201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cuff JA, Barton GJ. 2000. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 40:502–511. doi:. [DOI] [PubMed] [Google Scholar]
- 170.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. 2006. TM4 microarray software suite. Methods Enzymol 411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]