SUMMARY
The antibody response plays a key role in protection against viral infections. While antiviral antibodies may reduce the viral burden via several mechanisms, the ability to directly inhibit (neutralize) infection of cells has been extensively studied. Eliciting a neutralizing-antibody response is a goal of many vaccine development programs and commonly correlates with protection from disease. Considerable insights into the mechanisms of neutralization have been gained from studies of monoclonal antibodies, yet the individual contributions and dynamics of the repertoire of circulating antibody specificities elicited by infection and vaccination are poorly understood on the functional and molecular levels. Neutralizing antibodies with the most protective functionalities may be a rare component of a polyclonal, pathogen-specific antibody response, further complicating efforts to identify the elements of a protective immune response. This review discusses advances in deconstructing polyclonal antibody responses to flavivirus infection or vaccination. Our discussions draw comparisons to HIV-1, a virus with a distinct structure and replication cycle for which the antibody response has been extensively investigated. Progress toward deconstructing and understanding the components of polyclonal antibody responses identifies new targets and challenges for vaccination strategies.
INTRODUCTION
In the evolutionary arms race between viruses and their hosts, viruses exploit comparatively high mutation rates, short generation times, and large population sizes to adapt to antiviral defenses. In jawed vertebrates, the immune response to infection includes the production of antibodies (Abs) capable of recognizing an extremely diverse array of antigens, including proteins and carbohydrates that decorate virus particles. Antibodies are glycoproteins of the humoral immune system with an antigen recognition surface typically composed of two polyprotein chains encoded by a complex and dynamic array of gene segments. The extraordinarily broad and adaptive binding specificities of antibodies are achieved through allelic, combinatorial, and junctional diversity of antibody gene segments (reviewed in reference 1). Additionally, following infection or vaccination, iterative rounds of somatic mutation of antibody genes and an affinity-based selection process produce antibodies that bind with high affinity to viral antigens.
Antibody-mediated neutralization of viruses is the direct inhibition of viral infectivity resulting from antibody docking to virus particles (reviewed in reference 2). The elicitation of a neutralizing-antibody (NAb) response is a correlate of protection for many vaccines and contributes to long-lived protection against many viral infections (3). A potent antiviral response may select for variants that allow escape from antibody neutralization and/or effector functions. Neutralization escape mechanisms are diverse and include the selection of amino acid variation in antibody epitopes directly as well as the modulation of structural features to prevent antibody binding. Several exciting experimental approaches to overcome these challenges were recently described for several viral systems (reviewed in reference 4). Defining the specificities of NAbs elicited by infection or vaccination allowed the identification of B cell lineages that lead to the production of potent antibodies (reviewed in reference 5). Additionally, careful study of anti-human immunodeficiency virus type 1 (HIV-1) antibodies with an ability to recognize a broad range of diverse viral isolates identified conserved structures of the viral envelope protein targeted by human NAbs (6). Insights into epitopes bound by functionally desirable antibodies may guide the design of immunogens that can elicit these responses (7).
Significant progress toward understanding the molecular and structural basis for antibody-mediated neutralization has been made for several clinically important viruses through studies of monoclonal antibodies (MAbs) (4, 8–11). How antibodies with different functional properties act in concert in human sera is poorly understood. Despite the fundamental and translational importance of characterizing polyclonal antibodies, many important questions remain: (i) how many epitopes on a virus are targeted by the neutralizing-antibody response, (ii) how many B cell clonal lineages are expanded during infection and vaccination, and (iii) how does the complexity of polyclonal antibody mixtures correlate with protection against diverse and rapidly mutating viruses? In this review, we focus on advances in efforts to translate reductionist concepts arising from studies of MAbs toward deconstructing the polyclonal neutralizing-antibody response to flaviviruses.
FLAVIVIRUSES AND HIV-1: CLINICALLY SIGNIFICANT CONTEXTS FOR STUDYING THE HUMORAL IMMUNE RESPONSE
The viruses targeted by antibodies come in many shapes and sizes and replicate within the host via numerous strategies. In many respects, these differences are reflected in the details of antibody-virus interactions or mechanisms of inhibition. For example, the neutralization potency of antibodies against some nonenveloped viruses is enhanced by interactions with TRIM21 in the cytoplasm of infected cells (12). In contrast, this mechanism of inhibition is unlikely to play a significant role in potentiating antibody function for viruses with a lipid envelope on which the principal targets of NAbs are topologically inaccessible to TRIM21. While exciting advances have been made toward understanding how antibodies protect against infection by many different viruses, this review focuses on the neutralizing-antibody response against members of the flavivirus genus. To identify general concepts, selected insights into the anti-HIV-1 antibody response are presented; excellent detailed reviews of the HIV-1 antibody response were reported previously (10, 13–15). Both groups of viruses have a global impact on public health and vary considerably in structure and replication strategies.
Flaviviruses
The Flavivirus genus of the family Flaviviridae consists of a diverse group of positive-stranded RNA viruses transmitted to vertebrate hosts principally by mosquito or tick vectors. This genus of ∼70 species includes several viruses of considerable clinical importance, including dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV), Zika virus (ZIKV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus (TBEV). An estimated 390 million humans are infected each year with the four serotypes of DENV alone (16). Flaviviruses are capable of rapid emergence and spread in nonimmune populations, as illustrated by the extremely rapid spread of ZIKV through South America following its introduction in 2015 (17). The relatively recent introduction and spread of WNV throughout the Western hemisphere are well documented (18). Flaviviruses were initially classified by using serological studies, which were subsequently confirmed and extended via phylogenetic analyses (19, 20). Viral species within the flavivirus genus generally share over 84% nucleotide sequence identity (20). However, although the four serotypes of dengue virus (DENV1 to -4) share a species classification, they differ by ~25% to ~40% in their amino acid sequences (Fig. 1A) (21).
FIG 1.
Structure and diversity of the surface glycoproteins of flaviviruses. (A) Dendrogram depicting the relatedness of selected flavivirus E proteins (the bar represents 0.1 amino acid substitutions per site). JEV, Japanese encephalitis virus; MVEV, Murray Valley encephalitis virus; WNV, West Nile virus; SLEV, Saint Louis encephalitis virus; TBEV, tick-borne encephalitis virus; POWV, Powassan virus; YFV, yellow fever virus; DENV, dengue virus. (B) Structure of the ectodomain of the flavivirus E protein dimer (PDB accession number 1OAN) from a side view (top) and top view (bottom). Domains I, II, and III are shown in red, yellow, and blue, respectively. The fusion loop in domain II is shown in green. (C) Structure of a mature flavivirus virion (PDB accession number 4CCT). The E protein is arranged as antiparallel homodimers that densely coat the virion surface. (D) The two possible glycans on the E protein are highlighted in red in the E protein dimer (PDB accession number 1OAN) from a side view (top) and top view (bottom).
Flavivirus virions are small, spherical, enveloped particles roughly 50 nm in diameter that are composed of three structural proteins: capsid (C), premembrane (prM), and envelope (E). The assembly of new virions is directed by prM and E proteins at the endoplasmic reticulum (22–24). On immature virions, these glycoproteins are incorporated as 60 icosahedrally arranged heterotrimeric spikes of three prM-E dimers (25–28). The role of prM on immature virions is to prevent low-pH-triggered conformational changes in the E proteins that drive the fusion of viral and cellular membranes (29). As immature virions traffic through the secretory pathway, they undergo a maturation process defined by the cleavage of prM by cellular furin-like proteases (30–32). This cleavage results in an ∼75-amino-acid M peptide that remains associated with the mature virion and an ∼90-amino-acid, soluble “pr” portion that disassociates from virus particles upon release from cells (25). The function of M on the mature, infectious virion is unknown. On mature virions, E proteins exist in a dense herringbone arrangement of antiparallel E protein homodimers (Fig. 1B and C) (33–38). In this configuration, E proteins lie flat against the surface of the viral membrane, in contrast to HIV-1 and many other viruses, whose envelope proteins exist as spikes that project away from the virion surface (Fig. 2C). Critically, the flavivirus maturation process is not efficient, resulting in the production of a heterogeneous population of virions. Thus, in addition to infectious, fully mature virions (no prM) and noninfectious, immature virions (180 uncleaved prM molecules), cells produce partially mature viruses that retain structural features of both mature and immature virus particles (39). Partially mature virions can be infectious, although the extent of prM cleavage required for infectivity is not known (reviewed in reference 39). The efficiency of virion maturation has the potential to impact virus binding, environmental conditions required to trigger membrane fusion, cellular tropism, and sensitivity to antibody-mediated neutralization (40–44).
FIG 2.
Structure and diversity of the surface glycoproteins of HIV-1. (A) Dendrogram depicting the relatedness of Env from HIV-1 groups M, N, O, and P and SIVcpz (CPZ) (the bar represents 0.1 amino acid substitutions per site). (B) Structure of the ectodomain of the HIV-1 Env trimeric spike (PDB accession number 4TVP), consisting of trimers of gp120/gp41 heterodimers, from a side view (left) and top view (right). gp120 and gp41 are shown in gray and cyan, respectively. On gp120, variable loops are shown in green, while the CD4-binding site is in purple. (C) Schematic of the HIV-1 virion. Env exists as trimeric spikes that sparingly populate the virion surface (shown in red and gray). A variety of cellular proteins are incorporated into the virion (shown in blue) (91). (D) The HIV glycan shield is highlighted in red on the Env trimer (PDB accession number 4TVP) from a side view (left) and top view (right). The model was generated by using GlyProt (298).
The E protein is the main target of NAbs (45). This elongated protein is composed of three domains (domain I [DI] to DIII) connected to the viral membrane by a helical stem and two transmembrane domains (Fig. 1B) (46). A highly conserved, hydrophobic fusion loop composed of 13 amino acids is located at the tip of DII (DII-FL). On mature virus particles, the fusion loop is buried in a fold composed of DI and DIII of the opposing E protein in the dimer (47). E proteins may contain up to two N-linked glycosylation sites (either on DI only or on both DI and DII) (Fig. 1D); some strains of WNV are nonglycosylated (48, 49). Neutralizing antibodies have been mapped to all three E protein domains and, in many instances, bind epitopes composed of residues from multiple domains (50–61). Antibodies that recognize prM have also been identified, but they have limited neutralization potential (53, 62–64).
The E proteins orchestrate the entry of flaviviruses into target cells, which occurs through receptor-mediated endocytosis, followed by pH-dependent fusion that typically occurs in late endosomes, although the relatively high pH threshold of ∼6.6 suggests that fusion may already occur in early endosomes (65–68). Cellular factors that mediate virus entry are not completely defined, although attachment factors that enhance virion binding to cells have been identified, including the C-type lectins DC-SIGN and DC-SIGNR, mannose receptor, heparin sulfate, and phosphatidylserine receptors of the TIM (T cell/transmembrane, immunoglobulin, and mucin) and TAM (TYRO3, AXL, and MER) protein families (40, 69–75). The role that these molecules play in the cell biology of virus entry is incompletely understood and may extend beyond simply facilitating virus attachment. To date, cellular proteins absolutely required for the low-pH-mediated conformational change in the virus have not been identified; flaviviruses are capable of fusing directly with synthetic membrane preparations (66, 76). Single-particle tracking studies suggest that virus entry occurs within 17 min of stable attachment of the virion (67). In vitro studies clearly demonstrate that viral fusion occurs very rapidly (within seconds) after exposure to mildly acidic conditions (58, 66, 76).
Human Immunodeficiency Virus Type 1
HIV-1, a lentivirus in the Retroviridae family, is the causative agent of AIDS. Developing an effective vaccine against HIV-1 has been challenging because of the extensive diversity of the virus, which results from the rapid turnover of a large number of infected cells (77, 78), an error-prone viral reverse transcriptase (79), and a high frequency of recombination owing to template switching between two copackaged RNA genomes during reverse transcription (80). HIV-1 originated from simian immunodeficiency virus in chimpanzees (SIVcpz) and is divided into several groups (groups M, N, O, and P), each of which resulted from an independent cross-species transmission event (81). Group M, which is responsible for the AIDS pandemic, is further divided into 9 subtypes (subtypes A to D, F to H, J, and K) and a number of circulating recombinant forms (82). These subtypes differ in geographical distribution (83) and can be distinguished by sequence variation in the envelope gene, which differs by ∼20% at the amino acid level within subtypes and up to 35% between subtypes (Fig. 2A) (82, 84). Remarkably, it is estimated that the diversity of HIV-1 strains found within a single individual at one time point can rival that of worldwide influenza viruses within a year (85).
HIV-1 virions are spherical particles that are ∼120 nm in diameter and display a low density of envelope (Env) spikes on their surface (∼14 Env trimers/virion) (Fig. 2B and C) (86–88). Env incorporation into virions is regulated by interactions with other viral proteins and host factors (reviewed in references 89 and 90). While HIV-1 virions incorporate a large number of host proteins (91), the sole viral protein complex on the surface is Env, which mediates entry into host cells and is the target of NAbs. Following translation in the endoplasmic reticulum, the gp160 polyprotein precursor of Env is processed by a furin-like protease in the trans-Golgi network, resulting in the gp120 surface subunit and the gp41 transmembrane subunit that remain noncovalently attached to each other (92). On the virion surface, Env exists as trimeric spikes of gp120 and gp41 heterodimers (Fig. 2B). As discussed below, both the weak association of gp120 with gp41 and the low density of Env spikes on the virion surface impact antibody recognition.
gp120 consists of five conserved regions (C1 to C5) and five highly variable regions (V1 to V5) and is structurally defined by two domains: one oriented toward the center of the trimer (inner domain) and the other oriented toward the periphery (outer domain) (93–98). The native structure of gp41 has not been solved at high resolution (95, 96, 99), but this subunit is functionally defined by six major regions: the fusion peptide, heptad repeat region 1 (HR1), HR2, the membrane-proximal external region (MPER), the transmembrane anchor, and the cytoplasmic tail. On gp120, variable regions form heavily glycosylated disulfide-linked loops that shield more conserved surfaces (Fig. 2D) (100). Indeed, a high-resolution crystal structure of the HIV-1 Env trimer suggests that due to the dense array of glycans that decorate Env, only 3% of the peptidic surface is accessible to antibodies, compared to 14% and 48% of the glycoproteins of influenza virus and respiratory syncytial virus, respectively (99). Nevertheless, NAbs against HIV-1 have been mapped to both gp120 and gp41, and remarkably, glycans themselves are also important targets for many broadly neutralizing antibodies (98, 101). Despite reaching high titers, the initial wave of NAbs elicited by infection is typically strain specific, targeting exposed variable loops in gp120 and selecting for mutations that lead to NAb escape (102–107). To date, NAbs targeting gp41 are less common than those targeting gp120, possibly due to steric hindrance afforded by the close proximity of gp41 to the viral membrane (108), although NAbs that bind gp41, including the fusion peptide, and the gp41/gp120 interface were characterized recently (109–114). The latter NAbs are analogous to antiflavivirus NAbs that target quaternary epitopes spanning multiple E protein monomers (described below).
HIV-1 primarily infects CD4+ T cells and cells of the monocyte/macrophage lineage in vivo. Entry is pH independent and occurs mainly at the plasma membrane following Env-receptor interactions. Other entry pathways, including endocytosis or macropinocytosis, may also be involved (reviewed in reference 115). Binding of gp120 to CD4 is thought to trigger conformational changes that enhance the affinity of gp120 for its coreceptor (92), although a recent study from the Mothes laboratory suggests that the ensemble of structures sampled by gp120 includes those similar to the CD4-triggered state (116). Interaction with a coreceptor (G protein-coupled receptor seven-transmembrane domain protein CCR5 or CXCR4) triggers further conformational changes, leading to a fusogenic state in which gp120 dissociates, allowing the fusion peptide of gp41 to extend outward into the target cell membrane. Next, rearrangements in HR1 and HR2 facilitate the refolding of gp41 into a six-helix bundle that brings together the cellular and viral membranes, creating a pore that allows the viral core to enter cells (117). The number of functional Env trimers needed to interact with target cell receptors and mediate virus entry has not been precisely determined and may vary by strain but has been estimated to be between one and eight (88, 118–121).
HUMORAL IMMUNE RESPONSE
A principal component of the humoral immune response is the repertoire of antibody molecules secreted by B lymphocytes. Antibodies are Y-shaped glycoproteins composed of two identical heavy chain/light chain heterodimers linked by disulfide bonds (reviewed in reference 122). The arms of antibody molecules (or Fabs) are connected to the remainder of the protein by flexible hinges, which diversify the angles with which antibodies may bind antigens. The distal end of each arm forms the antigen-binding site of the molecule, called the variable (V) region. Both heavy and light chains contain three hypervariable loops (called complementarity-determining regions [CDRs]) that form the V structural region. The constant (Fc) portion of the antibody is modified by an N-linked oligosaccharide that contributes to interactions with molecules and cells of the immune system to mediate a range of effector functions (discussed below).
Diversity of the Humoral Immune Response
The humoral response is capable of producing an incredibly diverse repertoire of antibody molecules with unique antigen-binding properties. In part, this diversity is encoded directly by the germ line. Genes encoding the variable heavy chain (VH) and variable light chain (VL) exist as multiple gene segments. The heavy chain is encoded by multiple variable (V), joining (J), and diversity (D) gene segments. Two chromosomes encode the V and J gene segments that form the light chain (the κ and λ loci). Full-length antibody molecules are assembled from these gene segments in developing B lymphocytes by a process called V(D)J recombination (reviewed in reference 1). This mechanism creates combinatorial diversity through the random pairing of VDJ gene segments (at the VH loci) or VJ gene segments (at the Vκ or Vλ loci) during somatic gene rearrangement. Additional diversity during V(D)J recombination arises by the introduction or deletion of nucleotides at the junction of segments as they are linked together. The recombined variable region of the heavy chain is then joined to μ and δ constant gene segments (to make IgM or IgD antibody classes, respectively). Random pairing of heavy and light chains results in the formation of an intact antibody molecule (and B cell receptor [BCR]). The process of allelic exclusion ensures that each lymphocyte produces only a single antibody molecule (reviewed in reference 123).
Germinal center formation and affinity maturation.
The antibody repertoire produced by B lymphocytes is refined and diversified further upon exposure to an antigen. With appropriate T cell help, B cell recognition of an antigen results in cellular activation, extensive proliferation, and potentially Ig class switching, during which the Fc portion of the antibody gene can be exchanged for another with different functional properties (124). Antigen-primed B cells can develop into short-lived plasma cells (PCs), which are terminally differentiated cells characterized by high-level antibody secretion and low-level BCR expression. PCs and their proliferating precursors, plasmablasts, are responsible for the production of the early antibody response (Fig. 3). Antigen-primed B cells can also participate in the formation of germinal centers (GCs) in lymph nodes, along with follicular helper T (TFH) cells and follicular dendritic cells (FDCs). GC reactions result in the production of long-lived plasma cells (LLPCs) and memory B cells (MBCs) (discussed further below) (125), although memory B cells may also arise from GC-independent mechanisms (126).
FIG 3.
Sources of antibodies. Upon naive B cell recognition of an antigen and activation by a cognate T cell, activated B cells are characterized by extensive proliferation. Activated B cells can then follow one of several paths: (i) they may terminally differentiate into short-lived plasma cells (PCs), which have low surface Ig levels and high Ig secretion rates; (ii) they may differentiate into memory B cells (MBCs), which retain BCR expression but do not constitutively secrete antibody; and (iii) they can participate in the formation of germinal centers (GCs), along with follicular helper T (TFH) cells and follicular dendritic cells (FDC). In GCs, B cells undergo rapid proliferation, further diversification of their antibody gene through somatic hypermutation, and class switch recombination (CSR), during which the Fc region of the Ig gene may be exchanged for another to determine antibody effector function. Selected GC B cells receive signals to differentiate into PCs or MBCs; other GC B cells undergo apoptosis. PCs may be short-lived and remain in the lymphoid organs or become long-lived plasma cells (LLPCs) and migrate to the bone marrow, where they continue to secrete antibody independent of the presence of the antigen. LLPCs are most likely responsible for the long-lived, pathogen-specific antibody titers in serum that can last years or decades following infection or vaccination. Distinct from LLPCs, MBCs are long-lived cells that remain in circulation and peripheral lymphoid tissue. Through expression of their BCR, they can be reactivated by an antigen. Upon restimulation, they may set up germinal centers, undergo further somatic hypermutation and class switching, and differentiate into antibody-secreting plasmablasts and PCs.
In GCs, B cells refine the antibody response via the process of affinity maturation. This process occurs through iterative rounds of somatic hypermutation, during which point mutations are introduced into the antibody V regions, followed by TFH cell-based selection of clones with the highest antibody affinity. One study estimated that the average number of mutations in the VH region of IgG among memory B cells and germinal center cells was 14, with 88% of the sequenced VH genes encoding between 3 and 29 mutations (127). GCs are sites of competition among B cell clones for T cell help. After multiple rounds of affinity-based selection, GCs may undergo “monoclonalization,” with one high-affinity B cell clone beginning to dominate any given mature GC (reviewed in reference 128). In GCs, TFH cells also signal B cells to initiate class switching (129–131).
Effector Functions of Antibodies
While the Fab region of an antibody defines its specificity, the invariant Fc portion of the heavy chain determines its effector function. The antibody class-switching mechanism of B cells has the potential to generate antibodies with a similar specificity capable of orchestrating diverse immune responses. The Fc regions of antibody heavy chains interact with Fc receptors (FcRs) on immune effector cells or soluble immune molecules such as those in the complement system (122). The strengths of these interactions vary among antibody classes and can be influenced by the particular carbohydrate modification on the antibody molecule (132, 133). All IgG subclasses encode an N-linked glycosylation site at residue 297 of the heavy chain. While the position of the glycosylation site is conserved, the composition and structure of the oligosaccharide added to the antibody are influenced by the host immune activation state (134, 135). Variation in the antibody glycoform provides a mechanism by which antibody effector function can be fine-tuned beyond class switch recombination. For example, the IgG repertoire of HIV-1-infected individuals is modified by sugars with an agalactosylated, proinflammatory glycan profile compared to those of uninfected individuals; this is particularly pronounced in elite controllers of HIV-1 (136). The skewing toward agalactosylated antibodies in infected individuals and elite controllers was correlated with increased antibody-dependent cellular viral inhibition (ADCVI) activity in vitro.
Nonneutralizing antibodies elicited by immunization or infection may offer protection from viral infection through Fc-mediated effector functions, including antibody-dependent cellular cytotoxicity (ADCC), opsonization, mast cell activation, and complement activation. Studies using murine models of both WNV and HIV-1 infection have demonstrated the importance of the Fc effector functions of antibodies in mediating protection in vivo (137–140). For example, anti-WNV MAbs with poor in vitro neutralizing abilities can mediate in vivo protection in mice in a manner dependent on IgG binding to complement component C1q and Fcγ receptors via the N-linked glycan at N297 (137). Conversely, the inability of strongly neutralizing MAbs with mutations at N297 to interact with Fcγ receptors may be exploited for the development of DENV therapeutics, as these nonglycosylated NAbs do not support (and may competitively inhibit) the antibody-dependent enhancement processes thought to contribute to severe disease outcomes (141, 142).
Where Do Antiviral Antibodies Come from?
Long-term humoral immunity results from at least two distinct cell populations (Fig. 3). Terminally differentiated LLPCs predominantly reside in the bone barrow and constitutively secrete antibody independently of the presence of an antigen. LLPCs do not possess antigen receptors, are not reactivated upon antigen reexposure, and are most likely responsible for the long-lived, pathogen-specific serum antibodies that can last years or decades following infection or vaccination (143, 144). In contrast, MBCs remain in circulation and peripheral lymphoid tissue, where they may reencounter antigen (145). MBCs express BCR on their surface but do not constitutively secrete antibody. Upon restimulation by antigen, MBCs may differentiate into antibody-secreting plasma cells and may form germinal centers to undergo further affinity maturation and class switching (146). MBCs are responsible for the anamnestic antibody response that occurs upon secondary exposure to an antigen, responding more rapidly and at a greater magnitude to antigenic stimulation than their naive predecessors.
LLPCs and MBCs differ with respect to the extent of the affinity maturation of the antibodies that they express (147). Only B cells capable of producing antibodies with a high affinity for the antigen are selected for LLPC formation and persistent antibody production, whereas B cells that produce antibodies with a lower affinity for the antigen may survive as MBCs (147, 148). Antibodies from these two compartments may differentially contribute to protection from infection. Purtha et al. (149) demonstrated that following infection of mice with WNV, antibodies from MBCs were able to recognize not only the infecting strain of WNV but also a variant encoding a mutation in a known neutralizing-antibody epitope. In contrast, the LLPC antibody response was specific for the infecting strain. Thus, while the LLPC-derived antibody response was of a higher affinity and capable of conferring immediate protection against homotypic viral reinfection, the MBC compartment may be critical for the recognition of a genetically diverse secondary challenge.
ANTIBODY-MEDIATED NEUTRALIZATION
How antibodies block infection has been studied extensively for decades (reviewed in references 150 and 151). Because NAbs have great potential as antiviral therapeutics, and NAb titers often correlate with protection following vaccination (3), structural and molecular insights into mechanisms of neutralization have considerable translational value. Recent technical advances that enable the isolation and characterization of human monoclonal antibodies have not only accelerated the development of therapeutics and diagnostics but also directed mechanistic studies toward antibodies with the most relevant specificities in vivo (4, 152).
Neutralization by the Numbers
How many antibodies are required to neutralize a virus? The stoichiometry of antibody-mediated neutralization has been intensely debated (reviewed in references 2 and 153). A “single-hit” model suggests that antibody binding to a virus particle in the right location is sufficient to inactivate the virion. This hypothesis was supported largely by kinetic neutralization studies of poliovirus, Western equine encephalitis virus, and influenza virus (154, 155). However, there are significant limitations of this model, and they have been reviewed in detail (151). An alternative “multiple-hit” model proposes that the neutralization of an individual virus particle requires engagement by numerous antibody molecules (153). An interesting extension of this model suggests that the number of antibodies necessary for neutralization is correlated with the size of the virion, which reflects a requirement to fully occlude its surface (reviewed in reference 2). Estimates of the stoichiometry of neutralization have been determined for multiple viruses, including phage MS2 (156), poliovirus (157–159), WNV (160), papillomavirus (161), influenza virus A (155), and rabies virus (162, 163). While in many cases, the neutralization threshold for structurally distinct groups of viruses correlates positively with virion size, in agreement with the “coating theory” (2), factors that determine the number of antibodies required for neutralization may vary among viruses with different structures, compositions, and entry mechanisms. For example, the small number of functional trimers on the HIV-1 surface allows neutralization with a stoichiometry much lower than that predicted for a virion of this size (119, 121). Relationships between the number and oligomeric state of functional viral protein complexes required to drive virus entry and the stoichiometric requirements of neutralization are not well understood (86–88, 118, 119, 121).
For WNV, experiments with MAbs that bind the lateral ridge of DIII indicate that binding of ∼30 MAbs to the virion is required for neutralization (Fig. 4, inset) (160, 164). Whether the stoichiometric requirements for the neutralization of flaviviruses vary among antibodies that bind different epitopes, engage the virion bivalently, or inhibit infection via distinct mechanisms is unknown (165). In support of these caveats, the numbers of antibodies required to neutralize flavivirus infection by blocking attachment or membrane fusion appear to differ (61, 166).
FIG 4.
Flavivirus entry and mechanisms of antibody-mediated neutralization. (A) Flavivirus entry occurs following virus interaction with attachment factors such as the C-type lectins DC-SIGN and DC-SIGNR, mannose receptor, glycosaminoglycans (GAGS), and phosphatidylserine receptors of the TIM and TAM protein families. (B) Following virus attachment, flaviviruses undergo clathrin-mediated endocytosis. (C) Flaviviruses can then enter cells by pH-dependent fusion, typically in the late endosome for dengue viruses (67). Antibody-mediated neutralization of flaviviruses may be achieved by inhibiting virus infectivity at a number of virus entry steps such as (i) preventing virus attachment to the cell surface, (ii) promoting virus detachment from cells, and (iii) inhibiting virus fusion with endosomal membranes. (Inset) Neutralization occurs when antibodies bind flaviviruses with a stoichiometry that exceeds a particular threshold (160). Antibody-dependent enhancement of infection (ADE) can occur if the number of antibodies bound to the virion does not reach the stoichiometric threshold for neutralization. The number of antibodies bound per virion is modulated by antibody affinity as well as by epitope accessibility. Therefore, antibodies that bind cryptic epitopes that are poorly accessible for antibody recognition may not be able to achieve a stoichiometry sufficient to exceed the threshold requirements for neutralization despite high affinity for the epitope. In contrast, antibodies that bind highly accessible epitopes can exceed the stoichiometric threshold for neutralization at low occupancy.
Epitope occupancy and neutralization.
At least two factors determine the number of antibodies bound to a virus particle at any given concentration of antibody (reviewed in reference 165). Antibody affinity defines the fraction of viral epitopes bound by antibody under steady-state conditions, as expressed by the equation fraction of epitopes bound = [Ab]/([Ab] + Kd) (where Kd is the dissociation constant). Thus, high-affinity antibodies bind a larger fraction of their epitopes than do lower-affinity antibodies of the same specificity at a defined antibody concentration. More critically, the number of accessible epitopes on the intact infectious virion provides the “denominator” for this relationship, as epitope accessibility ultimately governs the number of antibody molecules capable of binding the virus particle at saturation. Antibodies may bind epitopes defined by amino acid contacts from a single viral structural protein (167) or engage residues found on adjacent proteins or carbohydrates (61, 110–113, 168–172). Complex or quaternary epitopes have been defined for many viruses, including flaviviruses (see below).
The fraction of epitopes that antibodies must occupy in order to exceed a stoichiometric neutralization threshold (the fractional “occupancy”) may differ among antibodies as a function of the number or accessibility of epitopes on the virus particle. For flaviviruses, epitope accessibility may vary as a function of the position of a particular E protein on the pseudoicosahedral virus particle (167, 173). All else being equal, antibodies that bind abundant targets need to bind a smaller fraction of these epitopes in order to inhibit infection; these antibodies have a relatively low occupancy requirement for neutralization. For example, antibodies that bind the exposed lateral ridge of WNV E protein DIII neutralize infection more efficiently than antibodies that bind less accessible structures with an equivalent affinity (160). In contrast, antibodies that bind infrequent or inaccessible determinants are characterized by high fractional occupancy requirements for neutralization. Not all epitopes are equally accessible for binding, and many factors with the potential to impact epitope accessibility have been described (for flaviviruses, see reference 165). Reduced epitope exposure imposes a requirement for a higher fractional occupancy of the remaining exposed epitopes in order to exceed the stoichiometric threshold and thus have the potential to contribute to immune evasion and antibody-dependent enhancement of infection (Fig. 4, inset).
Critically, the relationships between antibody occupancy and neutralization may be complicated by factors beyond the number of epitopes displayed by the virion. For example, the binding of a single antibody to one protomer of an HIV-1 envelope trimer may be sufficient to render that complex incapable of promoting virus entry (119, 121), and thus, the contribution of additional events of binding to that same trimer may be diminished. Antibody binding itself may modulate the accessibility of surrounding epitopes in unpredictable ways through direct steric mechanisms (174) or by trapping alternative antigenic states of the virion (175).
Mechanisms of Neutralization
Antibodies have the ability to block viral infection at any number of steps in the process of virus entry (reviewed in reference 151). These steps include virus attachment to the cell surface, virus interactions with receptors or coreceptors, fusion with the host membrane (for enveloped viruses), membrane penetration or genome injection (for nonenveloped viruses), or viral genome uncoating (Fig. 4). Blocking of cell surface attachment or receptor engagement through steric hindrance may be a common mechanism and has been suggested to explain the activity of DENV-immune sera (176). However, many antibodies are also capable of blocking infection at a postattachment step (51, 59, 167, 177–179). These NAbs may inhibit conformational changes of a viral protein required to mediate virus entry. For example, structural analyses revealed that WNV-specific MAb E16 traps E proteins in a radially extended intermediate at low pH (180). Like E16, MAb CR4354 inhibits WNV at a postattachment step and can inhibit virus fusion with synthetic liposomes (59, 170). The structure of the CR4354 Fab bound to WNV revealed a discontinuous epitope that spanned neighboring E proteins, suggesting that this MAb and others that bind complex quaternary epitopes might block fusion by cross-linking E proteins on the virion (61, 170–172). While the multiple-hit hypothesis assumes that neutralization is a reversible process (181), in some cases, antibody binding results in an irreversible change in virion infectivity that persists upon the reversal of binding. Some anti-HIV-1 MAbs, such as those that bind the MPER on gp41 and the CD4-binding site on gp120, induce the shedding of viral glycoprotein gp120 from the virion, which renders virions noninfectious even when the antibody disassociates from the virus particle (182).
While Fab fragments of neutralizing antibodies can block infection (150), the Fc region of antibodies also plays a role in neutralization. Although the surface area buried by an epitope/paratope interaction is relatively small, generally <900 Å2 (183), the intact antibody molecule may occupy a larger area due to its structural flexibility. The antiviral activity of antibodies likely reflects the contribution of the entire molecule, which has the potential not only to influence the number of antibody molecules docked on the virion (relative to Fab fragments) but also to interfere with many processes that occur during virus entry (184, 185). For example, the orientation of the Fc region of virus-bound antibodies has been hypothesized to control the number of WNV DIII-reactive MAb E33 molecules docked onto viruses in different maturation states (174). Thus, the large size of the antibody molecule has the potential to influence the accessibility of surrounding epitopes and thereby impact conditions that support antibody-mediated neutralization.
VIRUS EVASION OF ANTIBODY-MEDIATED NEUTRALIZATION
Sequence Variation and Antigenic Diversity
Viral genomes mutate at a relatively high rate, allowing the selection of mutations within antibody epitopes (186, 187). Viruses that can tolerate a large number of mutations in their structural proteins, such as HIV-1, hepatitis C virus (HCV), influenza virus, and noroviruses, undergo rapid and substantial antigenic drift in the presence of immune pressure (4, 188–191). This is evident in the yearly requirement for a reformulation of the seasonal influenza vaccine, as influenza viruses are extremely adept at acquiring antibody escape mutations (192, 193). For viruses that cause chronic infections, intrahost generation of antigenic diversity is observed over time as new viral variants emerge to escape the NAbs generated early in the immune response. As the antibody response evolves to recognize poorly neutralized variants, new viral variants emerge to escape these antibodies. This iterative selection of neutralization escape variants constantly elicits the production of new antibodies (103, 104, 194). Rapid antigenic evolution is not apparent for all viruses, even among RNA viruses with error-prone RNA polymerases. For example, due to a lack of significant antigenic drift, there has not been a need to reformulate the monovalent YFV 17D vaccine, even after 60+ years of use (195). Many factors may modulate viral antigenic evolution, including differences in virus host range (196) and the inherent mutational tolerance of virus surface glycoproteins (197, 198).
Conformational Masking of Conserved Regions
Conserved structural features that play important roles in the viral life cycle may be less tolerant of sequence variation and thus represent sites of vulnerability to antibodies. Many viruses have therefore evolved mechanisms to conceal conserved regions of their structural proteins. For example, the “canyon hypothesis” speculates that the receptor-binding domain (RBD) of some picornaviruses is buried in a canyon on the surface of the viral capsid to evade immune recognition (199), although this strategy is imperfect (200). Likewise, the HIV-1 gp120 RBDs are positioned in recessed pockets of the envelope spike that are not accessible to most antibodies (100, 201). DII-FL of flaviviruses is critical for mediating fusion during virus entry, is highly conserved among distantly related flaviviruses, and is frequently targeted by the antibody response (discussed below) (202–204). However, antibodies with this specificity are typically characterized by limited neutralizing activity because the fusion loop is poorly accessible due to the proximity to residues of the opposing E protein on the antiparallel dimer (57, 205). Numerous other examples of cryptic flavivirus epitopes have been reported (166, 175, 206, 207).
Regulation of epitope accessibility by flaviviruses.
At least two factors have the potential to modulate flavivirus epitope accessibility. While steric constraints limit antibody accessibility on the mature virion, inefficient maturation of flaviviruses results in the release of partially mature virions on which epitope accessibility may differ (41, 42, 44, 205). Decreasing the efficiency of virion maturation results in an increase in sensitivity to neutralization by many antibodies (41). The conformational dynamics of E proteins incorporated into the virion also modulate epitope accessibility. Viral “breathing” has been shown in several systems to impact neutralization sensitivity (166, 208–211). Antibodies have the potential to trap transiently exposed epitopes, as flaviviruses sample an ensemble of structures at steady state. Recent studies have demonstrated that the reversible exposure of cryptic epitopes by WNV and DENV contributes to time-dependent patterns of neutralization (166, 212). In these experiments, an increase of the incubation time of antibody-virus immune complexes prior to infection of cells results in corresponding increases in neutralization potency. Similar observations have been made with antibodies targeting internal components of the capsid of some picornaviruses (208). Conformational dynamics of Env may also impact epitope exposure and neutralization sensitivity of HIV-1. When the conformational dynamics of two HIV-1 strains were compared by single-molecule fluorescence resonance energy transfer (smFRET), the laboratory-adapted, neutralization-sensitive strain demonstrated increased dynamics and transitioned more frequently between closed and open conformational states than the clinically isolated, neutralization-resistant strain (116).
Low Density of Surface Glycoproteins
The dense arrangement of flavivirus E proteins may impose steric constraints on antibody epitope accessibility (57, 160, 180, 206) (see Fig. 6). While flaviviruses incorporate a fixed number of E proteins into the virus particle, other viruses may limit the number of epitopes available for antibody binding by directly reducing the number of surface proteins incorporated into virions that are targeted by NAbs, thus preventing antibody engagement from exceeding the stoichiometric threshold required for neutralization. For example, human cytomegalovirus (HCMV) has been shown to reduce the incorporation of the surface glycoprotein gH under selective pressure by antibodies in vitro, resulting in resistance to neutralization by MAbs targeting gH (213).
FIG 6.
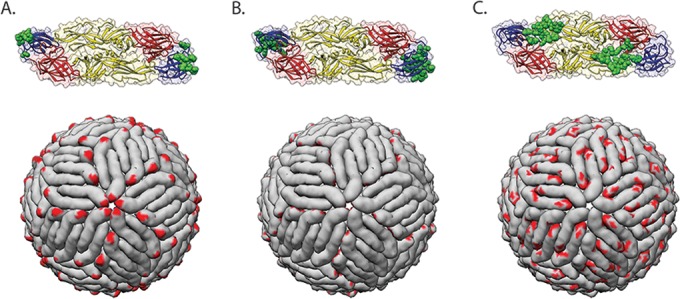
Examples of epitopes targeted by antiflavivirus monoclonal antibodies. Epitopes of selected MAbs are shown in green on the E protein dimer (PDB accession number 1OAN) (top) or in red on the mature virion (PDB accession number 4CCT) (bottom). (A) Monoclonal antibody E16 targets the DIII lateral ridge, a highly accessible epitope on the virion surface (180). (B) Monoclonal antibody E111 recognizes a cryptic epitope on DIII that is poorly accessible on the mature virion (207). This MAb may rely on virus breathing for epitope accessibility. (C) Monoclonal antibody EDE2-B7 targets a quaternary epitope that spans adjacent E protein monomers at the dimer interface (169).
A relatively low density of structural proteins on the surface of virions may also prevent bivalent engagement of the virus particle by the antibody, which in turn limits antibody avidity and impacts antibody occupancy at any given concentration of antibody. The low density of Env on HIV-1 virions has been suggested to limit the neutralizing activity of some HIV-1-reactive MAbs (reviewed in reference 214). Biochemical and structural studies have estimated that the average HIV-1 virion has a small number of Env spikes on its surface (∼14) (86–88). Comparisons of the neutralization potencies of IgG and Fab fragments of anti-HIV-1 antibodies revealed similar potencies, suggesting that bivalent recognition is uncommon (214). However, an elegant study by Galimidi et al. using engineered antibody-based molecules capable of intraspike bivalent binding demonstrates greatly increased neutralization potencies for antibodies with certain epitope specificities (215). While the densely packed, pseudoicosahedral arrangement of E proteins on flavivirus virions may allow bivalent engagement by antibodies, only one DENV E protein DIII-reactive antiflavivirus MAb that requires bivalent binding for its neutralizing activity has been characterized to date (216).
Glycan Shields
Although flavivirus E proteins contain only a limited number of glycans (1, 2), many viral structural proteins contain multiple glycosylation sites, which can mediate immune evasion (reviewed in reference 217). The presence of N- and O-linked glycans may decrease the immunogenicity of particular regions of viral glycoproteins, as carbohydrate structures on virions may be recognized as “self” by the immune system (100). Several viruses, including HIV-1, Ebola virus, and HCV, utilize a “glycan shield” to avoid antibody recognition (103, 218). For example, the HCV envelope glycoproteins E1 and E2 together have up to 16 N-linked glycosylation sites, most of which are highly conserved. Several of the glycans on the E2 glycoprotein have been shown to influence the susceptibility of HCV virions to neutralization (218). Additionally, alterations in the number, placement, and type of glycans on HIV-1 and simian immunodeficiency virus gp120 occur in order to mask NAb epitopes (98, 103, 219, 220). How the E protein glycosylation status of flaviviruses affects antibody recognition is not well understood, although antibodies that make contacts with both glycans on DENV have been reported (169).
IDENTIFYING THE FUNCTIONAL COMPONENTS OF THE POLYCLONAL ANTIBODY RESPONSE
The humoral response to viral infection has the potential to yield large numbers of B cells that produce antibodies with various specificities, antigen affinities, and functional properties. Considerable insight into how antibodies protect against viral infection, via direct neutralization of infection or antibody effector function, has come from studies of MAbs (60, 137, 160, 167, 170, 171, 177, 207, 221, 222). However, how antibodies function in concert as part of a polyclonal response is not well understood, nor is the breadth of the functionally significant components. For example, it is unclear why infection or vaccination may elicit antibodies that display neutralizing activity in vitro but contribute only modestly, if at all, to protection in the host. Two complementary approaches have been used successfully to deconstruct the functional complexity of the polyclonal response. First, large panels of human MAbs have been created for many pathogens, allowing a detailed analysis in vitro and in vivo of the individual components of an antibody response. For flaviviruses, this approach has provided insights into how antibodies engage the many surfaces of the virion (53, 62, 63, 223). Second, several groups have developed molecular and biochemical approaches to identify epitopes recognized by NAbs within sera (57, 224–228).
Neutralizing Antibodies Are a Rare Component of the Humoral Response against Viral Infection
Virus-specific human MAbs are identified by screening B cells for their ability to produce antibodies with desired functional properties. In many instances, candidate B cells, either MBCs or LLPCs, are screened for the ability to bind recombinant viral proteins (53, 229–231), virus-like particles (232, 233), or intact virions (60, 234) and then immortalized. The use of relatively high-throughput neutralization assays has allowed functional screens to identify B cell clones that produce potent NAbs directly (168). The complexity of the antigenic surface of viruses suggests that the nature of the antigen and screening strategy used will almost certainly impact the repertoire of MAbs identified (62, 168, 223).
In most instances, analyses of human antiflavivirus MAbs suggest that antibodies with limited in vitro neutralizing activity comprise a large fraction of the virus-specific antibody response (53, 60, 62, 63, 235). For example, <5% of the antibodies isolated from flavivirus-infected or vaccinated humans displayed potent (50% effective concentration [EC50] of <0.5 μg/ml) neutralizing activity (204, 235). A significant portion (∼45 to 60%) of the total E protein-specific response appears to be directed against the highly conserved fusion loop in domain II (DII-FL) (204, 236). Antibodies that target prM are also frequently isolated (53, 62, 63, 235). However, the majority of both DII-FL- and prM-reactive antibodies inhibit infection only weakly (53, 203, 204, 206, 235). These antibodies bind epitopes that are either poorly accessible for antibody recognition (57) or not present on virions with a stoichiometry sufficient to exceed the threshold requirements for neutralization (41, 57, 160). Similarly, although broad and potent NAbs against HIV-1 have been isolated from infected individuals (6, 114, 237, 238), these NAbs represent a rare component of the overall humoral immune response. For example, a screen of 25 million peripheral blood mononuclear cells (PBMCs) from an individual whose serum displayed cross-reactivity against diverse HIV-1 strains identified only 3 somatically related MAbs targeting the CD4-binding site that recapitulated the broad and potent neutralizing activity of the polyclonal serum (230).
Part of the challenge of eliciting effective NAbs against structurally complex viruses may be a requirement to engage a specific subset of the B cell repertoire. In support of this hypothesis, bNAbs against the HIV-1 CD4-binding site isolated from multiple donors appear to arise from a common, limited subset of germ line genes that remarkably converge in sequence and structure during the affinity maturation process (231, 239–242). Antibodies elicited following acute DENV infection or influenza vaccination among unrelated individuals also display convergent sequence signatures (243, 244).
Neutralizing Antibodies Target a Limited Number of Specificities
To complement studies of human MAb specificities, several groups have developed genetic and biochemical methods to investigate the contribution of particular epitopes to the NAb response to flavivirus infection or vaccination. One approach is to compare the neutralizing activity of sera preincubated with soluble recombinant antigens representing various E protein domains to that of untreated sera. Such serum depletion studies have shown that DIII is not a major target of human NAbs following vaccination or natural infection with DENV (227), WNV (236), YFV (245), and TBEV (246) despite being the target of very potent murine neutralizing MAbs (50–52, 55, 59, 247, 248). These findings are supported by genetic approaches demonstrating that mutations in DIII epitopes do not result in a significant reduction in the neutralization potency of flavivirus immune human sera (227, 236). Most of the serum neutralizing activity following YFV or DENV infection or vaccination is not affected by the depletion by soluble E protein, suggesting that quaternary epitopes may be important targets for NAbs (227, 245), as discussed below. In general, most studies characterizing antiflavivirus NAb specificities in human polyclonal sera have largely ruled out the importance of particular epitopes, such as those in DIII (227, 236, 249).
Only two studies have identified residues on the E protein targeted by NAbs in polyclonal DENV-reactive sera (228, 250). In one study, we analyzed the ability of serum samples from recipients of a monovalent DENV1 vaccine candidate (251–254) to neutralize a comprehensive panel of DENV1 variants in which surface-accessible residues of the E protein were replaced with corresponding sequences of a DENV2 strain (228). This panel was screened to identify mutations that reduced sensitivity to neutralization by DENV1-immune, but not DENV2-immune, sera. Our analysis identified mutations at just two residues in DI and DII that, when combined, ablated the DENV1 serotype-specific response to vaccination (Fig. 5A and B) (228). The observation that the antiflavivirus immune response is focused on just a few specificities aligns with data from recent studies on HIV-1, in which some (255–258) but not all (259–262) analyses of polyclonal sera with broadly neutralizing activity suggest that just one to two specificities can recapitulate the breadth and potency of the overall serum neutralizing activity. Follow-up studies with multiple strains from each DENV serotype will be needed to explore the fine specificity of serotype-specific NAbs.
FIG 5.

Epitopes targeted by antiflavivirus neutralizing antibodies in polyclonal sera. (A) The two residues highlighted in the E protein dimer, E126 in green (DII) and E157 in cyan (DI), were found to be major targets of the neutralizing-antibody response to DENV1 vaccination (228). A DENV1 variant with mutations E126K and E157K was not sensitive to neutralization by type-specific antibodies in sera from recipients of a DENV1 vaccine candidate. (B) The E126 and E157 variants were selected from a large panel of DENV1 variants, with mutations at each of the residues highlighted in purple. (C) The 40 residues highlighted in green on the E protein dimer were found to be the main targets of neutralizing antibodies in DENV2 primary sera (250). Transplantation of this DIII epitope from DENV2 into DENV4 resulted in a chimeric DENV4/2 virus that was neutralized by DENV2 primary sera with a potency similar to that measured with DENV2 (250). (D) The epitope of MAb 2D22 (172) is highlighted in purple. This potent human MAb was used to guide the construction of the DENV4/2 chimeric virus in panel C.
Another recent study investigated the epitope specificity of the polyclonal neutralizing-antibody response following DENV2 natural infection and vaccination. By using the quaternary epitope footprint of 2D22, a potently neutralizing DENV2-specific MAb (172), to guide the selection of residues for mutagenesis, Gallichotte and colleagues engineered a recombinant infectious virus in which the entire DENV2 DIII sequence was transplanted into a DENV4 molecular clone (Fig. 5C and D) (250). This chimeric virus, termed rDENV4/2, conferred neutralization sensitivity to DENV2 polyclonal sera, demonstrating that transplantation of DENV2 DIII was sufficient to recapitulate the majority of DENV2-neutralizing epitopes recognized by sera. Further fine mapping within this region may reveal a smaller subset of residues that are critical for DENV2 neutralization by polyclonal sera. Chimeric rDENV4/2 remained sensitive to neutralization by DENV4-immune sera, indicating that DENV2 and DENV4 type-specific NAbs target distinct epitopes on the E protein. Similarly, our analysis of the DENV1 and DENV2 polyclonal response showed that mutations at the two residues that are the focus of the DENV1 polyclonal response did not alter the neutralization potency of DENV2-immune sera (228). Thus, it is possible that NAbs responsible for serotype-specific responses target distinct epitopes on all four DENV serotypes.
Characterization of the kinetics and components of polyclonal sera over time may help define pathways to highly functional antibodies targeting critical epitopes. For example, there is increasing evidence that the continuous cycle of HIV-1 neutralization and escape from early strain-specific antibodies in sera often indirectly results in the exposure of epitopes that gradually lead to the induction of bNAbs during chronic infection (238, 263–267). It is possible that the initial binding of antibodies to a particular epitope affects the subsequent binding of other serum antibodies on the virion surface (185). This phenomenon may play a role in shaping the antibody repertoire that mediates potent neutralization.
Epitope Specificities of Neutralizing Antibodies Inform Vaccine Design
The characterization of large panels of potently neutralizing MAbs has greatly improved our understanding of the molecular basis of antibody neutralization and has the potential to inform vaccine design (7, 268–270). Because of the complex and dense arrangement of flavivirus E proteins on the virion surface, not all epitopes are equally accessible to antibodies (173). Antibodies that bind poorly accessible or cryptic epitopes are characterized by greater occupancy requirements for neutralization and are capable of enhancing infection of FcR-expressing cells at subneutralizing concentrations (53, 63, 160, 271) (Fig. 4, inset). This enhancement of infection has been linked mechanistically to more severe clinical outcomes of DENV infection (272, 273). Thus, a flavivirus vaccine that elicits antibodies targeting accessible epitopes may be more optimal for providing protection. However, as discussed below, depending on the biology and pathogenesis of the virus, highly accessible epitopes may not always be ideal targets for the most protective antibodies.
Accessible epitopes: easy targets.
Neutralization of flaviviruses is governed by a requirement for “multiple hits,” whereby antibodies that target highly accessible epitopes can achieve neutralization at a lower occupancy than those targeting poorly exposed epitopes (160). In support of this, many of the most potent MAbs characterized to date bind highly accessible epitopes on DIII (50, 52, 274). For example, the murine WNV-specific MAb E16 binds a discontinuous epitope in the lateral ridge of DIII (DIII-LR), neutralizes WNV at picomolar concentrations in vitro (248), and prevents WNV-induced mortality in animals when given as a single dose after infection (248, 275). Cryo-electron microscopy studies demonstrate that E16 can bind its epitope on 120 out of 180 E proteins on the mature virion (180), allowing antibody binding to exceed the stoichiometric threshold for neutralization at low occupancy (160) (Fig. 6A).
Cryptic epitopes: dynamic targets.
In contrast to highly potent antibodies that target accessible epitopes, antibodies that bind hidden epitopes often mediate neutralization only at very high concentrations. However, viral factors that modulate epitope accessibility, such as the efficiency of virion maturation and viral breathing, contribute significantly to the ability of antibodies to recognize cryptic epitopes (41, 43, 116, 166, 207, 276). For example, DENV1 MAb E111 recognizes an epitope on DIII predicted to be inaccessible on the mature virion (Fig. 6B) and is weakly neutralizing against the majority of DENV1 strains tested under standard neutralization assay conditions (207). Despite this, E111 can achieve potent neutralization of DENV1 following an increase in the time or temperature of virus-antibody incubation, which allows virus breathing to expose the E111 epitope for antibody binding (207, 277). Two additional DENV-specific MAbs that similarly recognize cryptic epitopes are murine MAbs 4E11 (278) and 1A1D-2 (54). Both MAbs target an epitope centered on the A-strand of DIII-LR that is not predicted to be accessible for antibody recognition on mature virions (175), as the fusion loop is inserted into the hydrophobic pocket near the DIII A-strand on the partner E protein in the dimer, thus theoretically blocking the binding site of 4E11 and 1A1D-2 (279). Presumably, these MAbs bind and stabilize the A-strand epitope as it becomes transiently available on the breathing virion. In support of this, 1A1D-2 has been shown to bind to DENV virions only at 37°C and not at room temperature, suggesting that temperature-dependent increases in the mobility of E proteins on the virion surface promote the accessibility of the A-strand epitope (175).
The virion maturation state also plays a role in modulating the exposure of cryptic epitopes. For example, murine MAb E53 binds an epitope that includes residues in DII-FL and the adjacent BC loop (206). E53 has almost no neutralizing activity against fully mature WNV virions but displays increased neutralization potency against virions that retain uncleaved prM on the surface (41). Cryo-electron microscopy studies revealed that the E53 Fab binds preferentially to spikes on immature flavivirus virions, providing a structural basis for the maturation state dependence of the E53 neutralizing activity (205).
Although the presence of a stoichiometric threshold of neutralization suggests that highly accessible epitopes are ideal NAb targets, for viruses with extensive genetic and antigenic heterogeneity, such as HIV-1 and influenza virus, epitopes that are highly exposed are often also highly variable. Potent NAbs can develop against hypervariable loops that shield more conserved regions in the HIV-1 envelope (100), but these antibodies tend to be highly strain specific and rapidly select for escape variants (reviewed in reference 15). An enormous challenge in developing an effective vaccine against rapidly evolving viruses is the requirement to elicit potent antibodies against conserved sites that are often occluded and are thus poorly immunogenic (reviewed in reference 280). Nonetheless, a number of conserved vulnerable sites on the HIV-1 envelope that are recognized by broad and potent NAbs have been identified. These sites include the CD4-binding site (230, 239, 281), glycan-dependent epitopes in V1/V2 (168) and V3 (282) of gp120, the MPER and fusion peptide of gp41 (109, 114), and epitopes spanning both gp120 and gp41 subunits (110–113).
In contrast to the apparent accessibility of DIII-LR epitopes that elicit strongly neutralizing antibodies against flaviviruses, epitopes that induce broad and potent NAbs against HIV-1 are often buried under a dense shield of glycans or are deeply recessed on the native trimer (283, 284). However, the remarkable potency of some bNAbs against HIV-1 suggests that such epitopes are exposed frequently enough to allow antibody binding to exceed the neutralization threshold. Alternatively, such potency may reflect the unusual characteristics displayed by most HIV-1 bNAbs identified to date, such as extensive somatic hypermutation, long CDR-H3 loops, and the ability to recognize glycans (reviewed in reference 13). For example, the broadly neutralizing HIV-1 MAb VRC01 harbors 41 VH gene alterations and 25 Vκ gene alterations from the germ line sequence (284). In contrast, a separate study found that only 1% of VH genes from IgG memory and GC B cells had >40 mutations (127). However, more recent studies of HIV-1 bNAbs suggest that these unusual characteristics are not absolutely required to achieve broad and potent neutralization (237, 238).
Quaternary epitopes: complex targets.
Many NAbs target complex, conformational, surface-accessible epitopes that are optimally exposed only on an intact virion and not on soluble envelope proteins. For example, a recent analysis of 145 human MAbs isolated from seven DENV-infected patients found that over 40% of the MAbs were reactive only against whole virions and not against recombinant E protein (169, 223). Most of these virion-specific antibodies target a conserved epitope that overlaps the DII-FL epitope and bridges the E dimer interface (E dimer-dependent epitope [EDE]) (Fig. 6C) (169, 223). Remarkably, these MAbs display very potent neutralization not only across all four DENV serotypes (169, 223) but also against the more distantly related Zika virus (285). The specificity of the EDE MAbs differs from that of most of the other potently neutralizing, antiflavivirus MAbs targeting quaternary epitopes across adjacent E protein dimers. The latter antibodies often recognize epitopes that overlap the DI/DII hinge and include human anti-WNV MAb CR4354 (170) and anti-DENV MAbs 5J7, 1F4 (60), and HM14c10 (61). The prevalence of NAbs targeting complex epitopes is not limited to those against flaviviruses, as many of the most potent HIV-1 bNAbs have been found to recognize such epitopes (110–113, 168). It is possible that the neutralization potency of antibodies targeting these complex epitopes is due to their ability to bind to sites that are exposed only on the most functional configuration of the viral envelope.
The discovery of potent NAbs that recognize quaternary epitopes exposed only on the virion surface has important implications for the identification and analysis of functional components of the polyclonal antibody response against viruses. In addition to the apparent rarity of B cells that contribute to the neutralizing component of the antibody response, one possible explanation for the discrepancy between dominant binding and neutralizing epitope specificities is that many antiviral NAbs target quaternary epitopes that are optimally exposed only on the virion surface and are thus not faithfully represented on soluble recombinant proteins that are often used in binding studies. Thus, it is important to recognize how a particular screening or selection strategy may bias the repertoire of antibodies that are recovered.
CONCLUDING REMARKS
Much of our mechanistic understanding of virus-antibody-host interactions has been gleaned from studies of MAbs. However, a polyclonal antibody response against a particular pathogen may contain a diverse array of antibodies that can differ drastically with regard to their epitope specificity, neutralizing potency, effector function, and breadth of recognition (narrow type specificity versus broad cross-reactivity). Within this diverse repertoire of a polyclonal response, the fraction of antibodies that contribute substantially to protection may be small.
As different viruses employ multiple distinct antibody evasion strategies, the diversity of antibody epitope specificities required for optimal protection may vary among viruses. Because of the potential for non- or weakly neutralizing antibodies to contribute to the pathogenesis of DENV infection (286), eliciting antibodies against accessible epitopes (including quaternary determinants) that allow high-occupancy binding at low concentrations may be ideal (160). In contrast, for viruses that exhibit more extensive antigenic variability, such as HIV-1 and influenza virus, an effective vaccine may need to elicit potent NAbs against hidden epitopes that are conserved across multiple variants. The ability of these viruses to rapidly escape from immune pressure may suggest a role for NAbs with multiple specificities in affording protection. Indeed, many studies have shown that no single antibody specificity is likely to be effective in providing protection against diverse HIV-1 variants, but combining antibodies that target distinct neutralization epitopes increases overall neutralization coverage (282, 287–293). Currently, it is unclear whether eliciting broad and potent NAbs against multiple epitopes via vaccination is feasible, although there is evidence that such NAbs can develop within a single individual during natural HIV-1 infection (256, 259, 294).
Identifying the specificities of NAbs elicited following vaccination or natural infection may guide immunogen design to more effectively engage the subset of naive B cell receptors that lead to broadly protective functional responses. This information is especially relevant given recent advances in deep-sequencing technology coupled with proteomic analysis of antigen-specific IgG that allow the isolation and characterization of functionally relevant antibodies from polyclonal sera (295, 296). Thus, it has become possible to enrich for epitope-specific NAbs and characterize their ontogeny to inform vaccine design (231, 239). Finally, there is growing evidence that functionally relevant epitopes grafted onto molecular scaffolds can serve as promising immunogens (7, 297).
ACKNOWLEDGMENTS
This work was supported by the Division of Intramural Research, National Institute for Allergy and Infectious Diseases.
We thank Christina DeMaso for assisting with figure preparation and Kimberly Dowd and Rebecca Pelc for their comments on the manuscript.
REFERENCES
- 1.Schroeder HW Jr, Cavacini L. 2010. Structure and function of immunoglobulins. J Allergy Clin Immunol 125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton DR, Saphire EO, Parren PW. 2001. A model for neutralization of viruses based on antibody coating of the virion surface. Curr Top Microbiol Immunol 260:109–143. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin Vaccine Immunol 17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton DR, Poignard P, Stanfield RL, Wilson IA. 2012. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doria-Rose NA, Joyce MG. 2015. Strategies to guide the antibody affinity maturation process. Curr Opin Virol 11:137–147. doi: 10.1016/j.coviro.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wibmer CK, Moore PL, Morris L. 2015. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS 10:135–143. doi: 10.1097/COH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V, Connell MJ, Stevens E, Schroeter A, Chen M, Macpherson S, Serra AM, Adachi Y, Holmes MA, Li Y, Klevit RE, Graham BS, Wyatt RT, Baker D, Strong RK, Crowe JE Jr, Johnson PR, Schief WR. 2014. Proof of principle for epitope-focused vaccine design. Nature 507:201–206. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corti D, Lanzavecchia A. 2013. Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 9.Diamond MS, Pierson TC, Fremont DH. 2008. The structural immunology of antibody protection against West Nile virus. Immunol Rev 225:212–225. doi: 10.1111/j.1600-065X.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West AP Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. 2014. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell 156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cashman SB, Marsden BD, Dustin LB. 2014. The humoral immune response to HCV: understanding is key to vaccine development. Front Immunol 5:550. doi: 10.3389/fimmu.2014.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. 2010. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouquet H. 2014. Antibody B cell responses in HIV-1 infection. Trends Immunol 35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Kwong PD, Mascola JR, Nabel GJ. 2013. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol 13:693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 15.Overbaugh J, Morris L. 2012. The antibody response against HIV-1. Cold Spring Harb Perspect Med 2:a007039. doi: 10.1101/cshperspect.a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 7 April 2013. The global distribution and burden of dengue. Nature doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wikan N, Smith DR. 2016. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis 16:e119–e126. doi: 10.1016/S1473-3099(16)30010-X. [DOI] [PubMed] [Google Scholar]
- 18.Roehrig JT. 2013. West Nile virus in the United States—a historical perspective. Viruses 5:3088–3108. doi: 10.3390/v5123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calisher CH, Gould EA. 2003. Taxonomy of the virus family Flaviviridae. Adv Virus Res 59:1–19. doi: 10.1016/S0065-3527(03)59001-7. [DOI] [PubMed] [Google Scholar]
- 20.Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. 1998. Phylogeny of the genus Flavivirus. J Virol 72:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. 2012. Virus taxonomy. Classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 22.Mackenzie JM, Westaway EG. 2001. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J Virol 75:10787–10799. doi: 10.1128/JVI.75.22.10787-10799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz IC, Kartenbeck J, Mezzacasa A, Allison SL, Heinz FX, Helenius A. 2003. Intracellular assembly and secretion of recombinant subviral particles from tick-borne encephalitis virus. J Virol 77:4370–4382. doi: 10.1128/JVI.77.7.4370-4382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz IC, Allison SL, Heinz FX, Helenius A. 2002. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol 76:5480–5491. doi: 10.1128/JVI.76.11.5480-5491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. 2008. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 319:1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. 2007. Structure of immature West Nile virus. J Virol 81:6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. 2003. Structures of immature flavivirus particles. EMBO J 22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allison SL, Stadler K, Mandl CW, Kunz C, Heinz FX. 1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J Virol 69:5816–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinz FX, Stiasny K, Puschner-Auer G, Holzmann H, Allison SL, Mandl CW, Kunz C. 1994. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology 198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 30.Elshuber S, Allison SL, Heinz FX, Mandl CW. 2003. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J Gen Virol 84:183–191. doi: 10.1099/vir.0.18723-0. [DOI] [PubMed] [Google Scholar]
- 31.Stadler K, Allison SL, Schalich J, Heinz FX. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol 71:8475–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. 2003. Structure of West Nile virus. Science 302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725. doi: 10.1016/S0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. 8 April 2013. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1304300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. 2016. The 3.8 A resolution cryo-EM structure of Zika virus. Science 352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. 2016. Structure of the thermally stable Zika virus. Nature 533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. 2013. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat Struct Mol Biol 20:105–110. doi: 10.1038/nsmb.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierson TC, Diamond MS. 2012. Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol 2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol 80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, Pierson TC. 2008. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog 4:e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee S, Dowd KA, Manhart CJ, Ledgerwood JE, Durbin AP, Whitehead SS, Pierson TC. 2014. Mechanism and significance of cell type-dependent neutralization of flaviviruses. J Virol 88:7210–7220. doi: 10.1128/JVI.03690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. 2014. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol 88:11726–11737. doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guirakhoo F, Bolin RA, Roehrig JT. 1992. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology 191:921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roehrig JT. 2003. Antigenic structure of flavivirus proteins. Adv Virus Res 59:141–175. doi: 10.1016/S0065-3527(03)59005-4. [DOI] [PubMed] [Google Scholar]
- 46.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 47.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 48.Winkler G, Heinz FX, Kunz C. 1987. Studies on the glycosylation of flavivirus E proteins and the role of carbohydrate in antigenic structure. Virology 159:237–243. doi: 10.1016/0042-6822(87)90460-0. [DOI] [PubMed] [Google Scholar]
- 49.Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, Takashima I. 2004. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol 85:3637–3645. doi: 10.1099/vir.0.80247-0. [DOI] [PubMed] [Google Scholar]
- 50.Shrestha B, Brien JD, Sukupolvi-Petty S, Austin SK, Edeling MA, Kim T, O'Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. 2010. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog 6:e1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, Fremont DH, Diamond MS. 2010. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol 84:9227–9239. doi: 10.1128/JVI.01087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. 2007. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol 81:12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roehrig JT, Bolin RA, Kelly RG. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 55.Crill WD, Roehrig JT. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 75:7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crill WD, Chang GJ. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol 78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stiasny K, Kiermayr S, Holzmann H, Heinz FX. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol 80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stiasny K, Brandler S, Kossl C, Heinz FX. 2007. Probing the flavivirus membrane fusion mechanism by using monoclonal antibodies. J Virol 81:11526–11531. doi: 10.1128/JVI.01041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, Diamond MS. 2009. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol 83:6494–6507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4:139ra83. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 62.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE Jr. 2012. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol 86:2665–2675. doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stiasny K, Heinz FX. 2006. Flavivirus membrane fusion. J Gen Virol 87:2755–2766. doi: 10.1099/vir.0.82210-0. [DOI] [PubMed] [Google Scholar]
- 66.Corver J, Ortiz A, Allison SL, Schalich J, Heinz FX, Wilschut J. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37–46. doi: 10.1006/viro.1999.0172. [DOI] [PubMed] [Google Scholar]
- 67.van der Schaar HM, Rust MJ, Chen C, van der Ende-Metselaar H, Wilschut J, Zhuang X, Smit JM. 2008. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog 4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaitseva E, Yang ST, Melikov K, Pourmal S, Chernomordik LV. 2010. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog 6:e1001131. doi: 10.1371/journal.ppat.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 70.Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. 2012. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. 2008. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog 4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carnec X, Meertens L, Dejarnac O, Perera-Lecoin M, Hafirassou ML, Kitaura J, Ramdasi R, Schwartz O, Amara A. 2016. The phosphatidylserine and phosphatidylethanolamine receptor CD300a binds dengue virus and enhances infection. J Virol 90:92–102. doi: 10.1128/JVI.01849-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gollins SW, Porterfield JS. 1986. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J Gen Virol 67(Part 1):157–166. [DOI] [PubMed] [Google Scholar]
- 77.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 78.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, Saag MS, Shaw GM. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 79.Mansky LM, Temin HM. 1995. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol 69:5087–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu WS, Hughes SH. 2012. HIV-1 reverse transcription. Cold Spring Harb Perspect Med 2:a006882. doi: 10.1101/cshperspect.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharp PM, Hahn BH. 2011. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med 1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson DL, Anderson JP, Bradac JA, Carr JK, Foley B, Funkhouser RK, Gao F, Hahn BH, Kalish ML, Kuiken C, Learn GH, Leitner T, McCutchan F, Osmanov S, Peeters M, Pieniazek D, Salminen M, Sharp PM, Wolinsky S, Korber B. 2000. HIV-1 nomenclature proposal. Science 288:55–56. [DOI] [PubMed] [Google Scholar]
- 83.Hemelaar J. 2013. Implications of HIV diversity for the HIV-1 pandemic. J Infect 66:391–400. doi: 10.1016/j.jinf.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 84.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 85.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. 2001. Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull 58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 86.Zhu P, Liu J, Bess J Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 87.Chojnacki J, Staudt T, Glass B, Bingen P, Engelhardt J, Anders M, Schneider J, Muller B, Hell SW, Krausslich HG. 2012. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science 338:524–528. doi: 10.1126/science.1226359. [DOI] [PubMed] [Google Scholar]
- 88.Brandenberg OF, Magnus C, Rusert P, Regoes RR, Trkola A. 2015. Different infectivity of HIV-1 strains is linked to number of envelope trimers required for entry. PLoS Pathog 11:e1004595. doi: 10.1371/journal.ppat.1004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tedbury PR, Freed EO. 2014. The role of matrix in HIV-1 envelope glycoprotein incorporation. Trends Microbiol 22:372–378. doi: 10.1016/j.tim.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Postler TS, Desrosiers RC. 2013. The tale of the long tail: the cytoplasmic domain of HIV-1 gp41. J Virol 87:2–15. doi: 10.1128/JVI.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ott DE. 2008. Cellular proteins detected in HIV-1. Rev Med Virol 18:159–175. doi: 10.1002/rmv.570. [DOI] [PubMed] [Google Scholar]
- 92.Freed EO, Martin MA. 1995. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J Biol Chem 270:23883–23886. doi: 10.1074/jbc.270.41.23883. [DOI] [PubMed] [Google Scholar]
- 93.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, Parks R, Eudailey J, Lloyd KE, Blinn J, Alam SM, Haynes BF, Amin MN, Wang LX, Burton DR, Koff WC, Nabel GJ, Mascola JR, Bewley CA, Kwong PD. 2013. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol 20:804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, Kong R, Thomas PV, Wagh K, Zhou T, Behrens AJ, Bylund T, Choi CW, Davison JR, Georgiev IS, Joyce MG, Kwon YD, Pancera M, Taft J, Yang Y, Zhang B, Shivatare SS, Shivatare VS, Lee CC, Wu CY, Bewley CA, Burton DR, Koff WC, Connors M, Crispin M, Baxa U, Korber BT, Wong CH, Mascola JR, Kwong PD. 2016. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell 165:813–826. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 101.Doores KJ. 2015. The HIV glycan shield as a target for broadly neutralizing antibodies. FEBS J 282:4679–4691. doi: 10.1111/febs.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Albert J, Abrahamsson B, Nagy K, Aurelius E, Gaines H, Nystrom G, Fenyo EM. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 104.Richman DD, Wrin T, Little SJ, Petropoulos CJ. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A 100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, Robinson JE, Gnanakaran S, Derdeyn CA. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 5:e1000594. doi: 10.1371/journal.ppat.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore PL, Ranchobe N, Lambson BE, Gray ES, Cave E, Abrahams MR, Bandawe G, Mlisana K, Abdool Karim SS, Williamson C, Morris L, CAPRISA 002 Study, NIAID Center for HIV/AIDS Vaccine Immunology . 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog 5:e1000598. doi: 10.1371/journal.ppat.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, Graham BS, Keefer MC, Pinter A, Morris L, Hahn BH, Shaw GM. 2009. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology 387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klein JS, Gnanapragasam PN, Galimidi RP, Foglesong CP, West AP Jr, Bjorkman PJ. 2009. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc Natl Acad Sci U S A 106:7385–7390. doi: 10.1073/pnas.0811427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scharf L, Scheid JF, Lee JH, West AP Jr, Chen C, Gao H, Gnanapragasam PN, Mares R, Seaman MS, Ward AB, Nussenzweig MC, Bjorkman PJ. 2014. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Rep 7:785–795. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Pena AT, Cupo A, Julien JP, van Gils M, Lee PS, Peng W, Paulson JC, Poignard P, Burton DR, Moore JP, Sanders RW, Wilson IA, Ward AB. 2014. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang CH, McBride R, von Bredow B, Shivatare SS, Wu CY, Chan-Hui PY, Liu Y, Feizi T, Zwick MB, Koff WC, Seaman MS, Swiderek K, Moore JP, Evans D, Paulson JC, Wong CH, Ward AB, Wilson IA, Sanders RW, Poignard P, Burton DR. 2014. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity 40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kong R, Xu K, Zhou T, Acharya P, Lemmin T, Liu K, Ozorowski G, Soto C, Taft JD, Bailer RT, Cale EM, Chen L, Choi CW, Chuang GY, Doria-Rose NA, Druz A, Georgiev IS, Gorman J, Huang J, Joyce MG, Louder MK, Ma X, McKee K, O'Dell S, Pancera M, Yang Y, Blanchard SC, Mothes W, Burton DR, Koff WC, Connors M, Ward AB, Kwong PD, Mascola JR. 2016. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science 352:828–833. doi: 10.1126/science.aae0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melikyan GB. 2014. HIV entry: a game of hide-and-fuse? Curr Opin Virol 4:1–7. doi: 10.1016/j.coviro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB III, Kwong PD, Blanchard SC, Mothes W. 2014. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346:759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klasse PJ. 2012. The molecular basis of HIV entry. Cell Microbiol 14:1183–1192. doi: 10.1111/j.1462-5822.2012.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sougrat R, Bartesaghi A, Lifson JD, Bennett AE, Bess JW, Zabransky DJ, Subramaniam S. 2007. Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog 3:e63. doi: 10.1371/journal.ppat.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang X, Kurteva S, Lee S, Sodroski J. 2005. Stoichiometry of antibody neutralization of human immunodeficiency virus type 1. J Virol 79:3500–3508. doi: 10.1128/JVI.79.6.3500-3508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Magnus C, Rusert P, Bonhoeffer S, Trkola A, Regoes RR. 2009. Estimating the stoichiometry of human immunodeficiency virus entry. J Virol 83:1523–1531. doi: 10.1128/JVI.01764-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klasse PJ. 2007. Modeling how many envelope glycoprotein trimers per virion participate in human immunodeficiency virus infectivity and its neutralization by antibody. Virology 369:245–262. doi: 10.1016/j.virol.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woof JM, Burton DR. 2004. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol 4:89–99. doi: 10.1038/nri1266. [DOI] [PubMed] [Google Scholar]
- 123.Bergman Y, Cedar H. 2004. A stepwise epigenetic process controls immunoglobulin allelic exclusion. Nat Rev Immunol 4:753–761. doi: 10.1038/nri1458. [DOI] [PubMed] [Google Scholar]
- 124.Chaudhuri J, Alt FW. 2004. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol 4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 125.Shlomchik MJ, Weisel F. 2012. Germinal center selection and the development of memory B and plasma cells. Immunol Rev 247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 126.Taylor JJ, Pape KA, Jenkins MK. 2012. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med 209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Victora GD, Mesin L. 2014. Clonal and cellular dynamics in germinal centers. Curr Opin Immunol 28:90–96. doi: 10.1016/j.coi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. 2001. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med 193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jefferis R. 2009. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 133.Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. 2014. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. 2007. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 135.Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, Hokke CH, Yazdanbakhsh M, Wuhrer M. 2012. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics 11:M111.014563. doi: 10.1074/mcp.M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. 2013. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vogt MR, Dowd KA, Engle M, Tesh RB, Johnson S, Pierson TC, Diamond MS. 2011. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J Virol 85:11567–11580. doi: 10.1128/JVI.05859-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chung KM, Nybakken GE, Thompson BS, Engle MJ, Marri A, Fremont DH, Diamond MS. 2006. Antibodies against West Nile virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol 80:1340–1351. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 141.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. 2010. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog 6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A 104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Slifka MK, Antia R, Whitmire JK, Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363–372. doi: 10.1016/S1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 144.Manz RA, Thiel A, Radbruch A. 1997. Lifetime of plasma cells in the bone marrow. Nature 388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 145.McHeyzer-Williams LJ, McHeyzer-Williams MG. 2005. Antigen-specific memory B cell development. Annu Rev Immunol 23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 146.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. 2009. Multiple layers of B cell memory with different effector functions. Nat Immunol 10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 147.Smith KG, Light A, Nossal GJ, Tarlinton DM. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J 16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. 2006. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. 2011. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 208:2599–2606. doi: 10.1084/jem.20110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parren PW, Burton DR. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol 77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klasse PJ, Sattentau QJ. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol 83:2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- 152.Lanzavecchia A, Corti D, Sallusto F. 2007. Human monoclonal antibodies by immortalization of memory B cells. Curr Opin Biotechnol 18:523–528. doi: 10.1016/j.copbio.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Della-Porta AJ, Westaway EG. 1978. A multi-hit model for the neutralization of animal viruses. J Gen Virol 38:1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- 154.Dulbecco R, Vogt M, Strickland AG. 1956. A study of the basic aspects of neutralization of two animal viruses, Western equine encephalitis virus and poliomyelitis virus. Virology 2:162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- 155.Taylor HP, Armstrong SJ, Dimmock NJ. 1987. Quantitative relationships between an influenza virus and neutralizing antibody. Virology 159:288–298. doi: 10.1016/0042-6822(87)90466-1. [DOI] [PubMed] [Google Scholar]
- 156.Rappaport I. 1970. An analysis of the inactivation of MS2 bacteriophage with antiserum. J Gen Virol 6:25–32. doi: 10.1099/0022-1317-6-1-25. [DOI] [PubMed] [Google Scholar]
- 157.Icenogle J, Shiwen H, Duke G, Gilbert S, Rueckert R, Anderegg J. 1983. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology 127:412–425. doi: 10.1016/0042-6822(83)90154-X. [DOI] [PubMed] [Google Scholar]
- 158.Colonno RJ, Callahan PL, Leippe DM, Rueckert RR, Tomassini JE. 1989. Inhibition of rhinovirus attachment by neutralizing monoclonal antibodies and their Fab fragments. J Virol 63:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith TJ, Olson NH, Cheng RH, Liu H, Chase ES, Lee WM, Leippe DM, Mosser AG, Rueckert RR, Baker TS. 1993. Structure of human rhinovirus complexed with Fab fragments from a neutralizing antibody. J Virol 67:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roden RB, Weissinger EM, Henderson DW, Booy F, Kirnbauer R, Mushinski JF, Lowy DR, Schiller JT. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J Virol 68:7570–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Flamand A, Raux H, Gaudin Y, Ruigrok RW. 1993. Mechanisms of rabies virus neutralization. Virology 194:302–313. doi: 10.1006/viro.1993.1261. [DOI] [PubMed] [Google Scholar]
- 163.Raux H, Coulon P, Lafay F, Flamand A. 1995. Monoclonal antibodies which recognize the acidic configuration of the rabies glycoprotein at the surface of the virion can be neutralizing. Virology 210:400–408. doi: 10.1006/viro.1995.1356. [DOI] [PubMed] [Google Scholar]
- 164.Mehlhop E, Nelson S, Jost CA, Gorlatov S, Johnson S, Fremont DH, Diamond MS, Pierson TC. 2009. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe 6:381–391. doi: 10.1016/j.chom.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pierson TC, Diamond MS. 2015. A game of numbers: the stoichiometry of antibody-mediated neutralization of flavivirus infection. Prog Mol Biol Transl Sci 129:141–166. doi: 10.1016/bs.pmbts.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. 2011. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog 7:e1002111. doi: 10.1371/journal.ppat.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. 2005. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, Girard-Blanc C, Petres S, Shepard WE, Despres P, Arenzana-Seisdedos F, Dussart P, Mongkolsapaya J, Screaton GR, Rey FA. 2015. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 170.Kaufmann B, Vogt MR, Goudsmit J, Holdaway HA, Aksyuk AA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG. 2010. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proc Natl Acad Sci U S A 107:18950–18955. doi: 10.1073/pnas.1011036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fibriansah G, Tan JL, Smith SA, de Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, de Silva AM, Crowe JE, Lok SM. 2015. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 6:6341. doi: 10.1038/ncomms7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, de Silva AM, Harris E, Crowe JE Jr, Lok SM. 2015. Dengue virus cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 349:88–91. doi: 10.1126/science.aaa8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee PD, Mukherjee S, Edeling MA, Dowd KA, Austin SK, Manhart CJ, Diamond MS, Fremont DH, Pierson TC. 2013. The Fc region of an antibody impacts the neutralization of West Nile viruses in different maturation states. J Virol 87:13729–13740. doi: 10.1128/JVI.02340-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, Kuhn RJ, Rossmann MG. 2008. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol 15:312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- 176.He RT, Innis BL, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R. 1995. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J Med Virol 45:451–461. doi: 10.1002/jmv.1890450417. [DOI] [PubMed] [Google Scholar]
- 177.Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. 2009. A therapeutic antibody against West Nile virus neutralizes infection by blocking fusion within endosomes. PLoS Pathog 5:e1000453. doi: 10.1371/journal.ppat.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lai CJ, Goncalvez AP, Men R, Wernly C, Donau O, Engle RE, Purcell RH. 2007. Epitope determinants of a chimpanzee dengue virus type 4 (DENV-4)-neutralizing antibody and protection against DENV-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody. J Virol 81:12766–12774. doi: 10.1128/JVI.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gollins SW, Porterfield JS. 1986. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature 321:244–246. doi: 10.1038/321244a0. [DOI] [PubMed] [Google Scholar]
- 180.Kaufmann B, Nybakken GE, Chipman PR, Zhang W, Diamond MS, Fremont DH, Kuhn RJ, Rossmann MG. 2006. West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody. Proc Natl Acad Sci U S A 103:12400–12404. doi: 10.1073/pnas.0603488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mandel B. 1961. Reversibility of the reaction between polio-virus and neutralizing antibody of rabbit origin. Virology 14:316–328. doi: 10.1016/0042-6822(61)90317-8. [DOI] [PubMed] [Google Scholar]
- 182.Ruprecht CR, Krarup A, Reynell L, Mann AM, Brandenberg OF, Berlinger L, Abela IA, Regoes RR, Gunthard HF, Rusert P, Trkola A. 2011. MPER-specific antibodies induce gp120 shedding and irreversibly neutralize HIV-1. J Exp Med 208:439–454. doi: 10.1084/jem.20101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Davies DR, Cohen GH. 1996. Interactions of protein antigens with antibodies. Proc Natl Acad Sci U S A 93:7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kiermayr S, Stiasny K, Heinz FX. 2009. Impact of quaternary organization on the antigenic structure of the tick-borne encephalitis virus envelope glycoprotein E. J Virol 83:8482–8491. doi: 10.1128/JVI.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Heinz FX, Mandl C, Berger R, Tuma W, Kunz C. 1984. Antibody-induced conformational changes result in enhanced avidity of antibodies to different antigenic sites on the tick-borne encephalitis virus glycoprotein. Virology 133:25–34. doi: 10.1016/0042-6822(84)90422-7. [DOI] [PubMed] [Google Scholar]
- 186.Renzette N, Bhattacharjee B, Jensen JD, Gibson L, Kowalik TF. 2011. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog 7:e1001344. doi: 10.1371/journal.ppat.1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sanjuan R, Nebot MR, Chirico N, Mansky LM, Belshaw R. 2010. Viral mutation rates. J Virol 84:9733–9748. doi: 10.1128/JVI.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A 110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lindesmith LC, Donaldson EF, Baric RS. 2011. Norovirus GII.4 strain antigenic variation. J Virol 85:231–242. doi: 10.1128/JVI.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Das SR, Hensley SE, Ince WL, Brooke CB, Subba A, Delboy MG, Russ G, Gibbs JS, Bennink JR, Yewdell JW. 2013. Defining influenza A virus hemagglutinin antigenic drift by sequential monoclonal antibody selection. Cell Host Microbe 13:314–323. doi: 10.1016/j.chom.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Overbaugh J, Bangham CR. 2001. Selection forces and constraints on retroviral sequence variation. Science 292:1106–1109. doi: 10.1126/science.1059128. [DOI] [PubMed] [Google Scholar]
- 192.Yewdell JW. 2011. Viva la revolucion: rethinking influenza A virus antigenic drift. Curr Opin Virol 1:177–183. doi: 10.1016/j.coviro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2004. Mapping the antigenic and genetic evolution of influenza virus. Science 305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 194.Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropoulos CJ, Little SJ, Richman DD. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A 102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Monath TP. 2005. Yellow fever vaccine. Expert Rev Vaccines 4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 196.Coffey LL, Forrester N, Tsetsarkin K, Vasilakis N, Weaver SC. 2013. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol 8:155–176. doi: 10.2217/fmb.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fulton BO, Sachs D, Beaty SM, Won ST, Lee B, Palese P, Heaton NS. 2015. Mutational analysis of measles virus suggests constraints on antigenic variation of the glycoproteins. Cell Rep 11:1331–1338. doi: 10.1016/j.celrep.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thyagarajan B, Bloom JD. 2014. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. eLife 3:e03300. doi: 10.7554/eLife.03300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rossmann MG. 1989. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem 264:14587–14590. [PubMed] [Google Scholar]
- 200.Smith TJ, Chase ES, Schmidt TJ, Olson NH, Baker TS. 1996. Neutralizing antibody to human rhinovirus 14 penetrates the receptor-binding canyon. Nature 383:350–354. doi: 10.1038/383350a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 202.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, Wu YC, Lai CY, Lu CH, Huang JH, Chang GJ, Wu HC, Wang WK. 2012. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS Negl Trop Dis 6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK. 2008. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol 82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Throsby M, Geuijen C, Goudsmit J, Bakker AQ, Korimbocus J, Kramer RA, Clijsters-van der Horst M, de Jong M, Jongeneelen M, Thijsse S, Smit R, Visser TJ, Bijl N, Marissen WE, Loeb M, Kelvin DJ, Preiser W, ter Meulen J, de Kruif J. 2006. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus. J Virol 80:6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG, Fremont DH. 2009. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J 28:3269–3276. doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. 2006. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J Virol 80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. 2012. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog 8:e1002930. doi: 10.1371/journal.ppat.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li Q, Yafal AG, Lee YM, Hogle J, Chow M. 1994. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol 68:3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sabo MC, Luca VC, Ray SC, Bukh J, Fremont DH, Diamond MS. 2012. Hepatitis C virus epitope exposure and neutralization by antibodies is affected by time and temperature. Virology 422:174–184. doi: 10.1016/j.virol.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lindesmith LC, Donaldson EF, Beltramello M, Pintus S, Corti D, Swanstrom J, Debbink K, Jones TA, Lanzavecchia A, Baric RS. 2014. Particle conformation regulates antibody access to a conserved GII.4 norovirus blockade epitope. J Virol 88:8826–8842. doi: 10.1128/JVI.01192-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Davenport TM, Guttman M, Guo W, Cleveland B, Kahn M, Hu SL, Lee KK. 2013. Isolate-specific differences in the conformational dynamics and antigenicity of HIV-1 gp120. J Virol 87:10855–10873. doi: 10.1128/JVI.01535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sukupolvi-Petty S, Brien JD, Austin SK, Shrestha B, Swayne S, Kahle K, Doranz BJ, Johnson S, Pierson TC, Fremont DH, Diamond MS. 2013. Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection. J Virol 87:8826–8842. doi: 10.1128/JVI.01314-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li L, Coelingh KL, Britt WJ. 1995. Human cytomegalovirus neutralizing antibody-resistant phenotype is associated with reduced expression of glycoprotein H. J Virol 69:6047–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klein JS, Bjorkman PJ. 2010. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog 6:e1000908. doi: 10.1371/journal.ppat.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Galimidi RP, Klein JS, Politzer MS, Bai S, Seaman MS, Nussenzweig MC, West AP Jr, Bjorkman PJ. 2015. Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell 160:433–446. doi: 10.1016/j.cell.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Edeling MA, Austin SK, Shrestha B, Dowd KA, Mukherjee S, Nelson CA, Johnson S, Mabila MN, Christian EA, Rucker J, Pierson TC, Diamond MS, Fremont DH. 2014. Potent dengue virus neutralization by a therapeutic antibody with low monovalent affinity requires bivalent engagement. PLoS Pathog 10:e1004072. doi: 10.1371/journal.ppat.1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vigerust DJ, Shepherd VL. 2007. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol 15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck ZY, Foung S, Penin F, Dubuisson J, Voisset C. 2007. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol 81:8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sagar M, Wu X, Lee S, Overbaugh J. 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol 80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chackerian B, Rudensey LM, Overbaugh J. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J Virol 71:7719–7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, Austin SK, Sukupolvi-Petty S, Diamond MS, Crowe JE Jr. 2013. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 4:e00873-13. doi: 10.1128/mBio.00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Williams KL, Sukupolvi-Petty S, Beltramello M, Johnson S, Sallusto F, Lanzavecchia A, Diamond MS, Harris E. 2013. Therapeutic efficacy of antibodies lacking Fcgamma receptor binding against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies. PLoS Pathog 9:e1003157. doi: 10.1371/journal.ppat.1003157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR. 2015. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lin TY, Dowd KA, Manhart CJ, Nelson S, Whitehead SS, Pierson TC. 2012. A novel approach for the rapid mutagenesis and directed evolution of the structural genes of West Nile virus. J Virol 86:3501–3512. doi: 10.1128/JVI.06435-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Williams KL, Wahala WM, Orozco S, de Silva AM, Harris E. 2012. Antibodies targeting dengue virus envelope domain III are not required for serotype-specific protection or prevention of enhancement in vivo. Virology 429:12–20. doi: 10.1016/j.virol.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. 2013. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog 9:e1003761. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, Cutrell E, Seaman MS, Mascola JR, Wyatt RT, Wardemann H, Nussenzweig MC. 2009. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods 343:65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hicar MD, Chen X, Briney B, Hammonds J, Wang JJ, Kalams S, Spearman PW, Crowe JE Jr. 2010. Pseudovirion particles bearing native HIV envelope trimers facilitate a novel method for generating human neutralizing monoclonal antibodies against HIV. J Acquir Immune Defic Syndr 54:223–235. doi: 10.1097/QAI.0b013e3181dc98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Scherer EM, Smith RA, Simonich CA, Niyonzima N, Carter JJ, Galloway DA. 2014. Characteristics of memory B cells elicited by a highly efficacious HPV vaccine in subjects with no pre-existing immunity. PLoS Pathog 10:e1004461. doi: 10.1371/journal.ppat.1004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Smith SA, de Alwis AR, Kose N, Jadi RS, de Silva AM, Crowe JE Jr. 2014. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J Virol 88:12233–12241. doi: 10.1128/JVI.00247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Smith SA, de Alwis R, Kose N, Durbin AP, Whitehead SS, de Silva AM, Crowe JE Jr. 2013. Human monoclonal antibodies derived from memory B cells following live attenuated dengue virus vaccination or natural infection exhibit similar characteristics. J Infect Dis 207:1898–1908. doi: 10.1093/infdis/jit119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, Throsby M, Fremont DH, Pierson TC, Diamond MS. 2007. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol 81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Simonich CA, Williams KL, Verkerke HP, Williams JA, Nduati R, Lee KK, Overbaugh J. 2016. HIV-1 neutralizing antibodies with limited hypermutation from an infant. Cell 166:77–87. doi: 10.1016/j.cell.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.MacLeod DT, Choi NM, Briney B, Garces F, Ver LS, Landais E, Murrell B, Wrin T, Kilembe W, Liang CH, Ramos A, Bian CB, Wickramasinghe L, Kong L, Eren K, Wu CY, Wong CH, IAVI Protocol C Investigators and the IAVI African HIV Research Network, Kosakovsky Pond SL, Wilson IA, Burton DR, Poignard P. 2016. Early antibody lineage diversification and independent limb maturation lead to broad HIV-1 neutralization targeting the Env high-mannose patch. Immunity 44:1215–1226. doi: 10.1016/j.immuni.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang ZY, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, NISC Comparative Sequencing Program, Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Briney BS, Willis JR, Crowe JE Jr. 2012. Human peripheral blood antibodies with long HCDR3s are established primarily at original recombination using a limited subset of germline genes. PLoS One 7:e36750. doi: 10.1371/journal.pone.0036750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Scharf L, West AP Jr, Gao H, Lee T, Scheid JF, Nussenzweig MC, Bjorkman PJ, Diskin R. 2013. Structural basis for HIV-1 gp120 recognition by a germ-line version of a broadly neutralizing antibody. Proc Natl Acad Sci U S A 110:6049–6054. doi: 10.1073/pnas.1303682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, Kwon YD, Longo NS, Louder MK, Luongo T, McKee K, Schramm CA, Skinner J, Yang Y, Yang Z, Zhang Z, Zheng A, Bonsignori M, Haynes BF, Scheid JF, Nussenzweig MC, Simek M, Burton DR, Koff WC, NISC Comparative Sequencing Program, Mullikin JC, Connors M, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2013. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity 39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Parameswaran P, Liu Y, Roskin KM, Jackson KK, Dixit VP, Lee JY, Artiles KL, Zompi S, Vargas MJ, Simen BB, Hanczaruk B, McGowan KR, Tariq MA, Pourmand N, Koller D, Balmaseda A, Boyd SD, Harris E, Fire AZ. 2013. Convergent antibody signatures in human dengue. Cell Host Microbe 13:691–700. doi: 10.1016/j.chom.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jackson KJ, Liu Y, Roskin KM, Glanville J, Hoh RA, Seo K, Marshall EL, Gurley TC, Moody MA, Haynes BF, Walter EB, Liao HX, Albrecht RA, Garcia-Sastre A, Chaparro-Riggers J, Rajpal A, Pons J, Simen BB, Hanczaruk B, Dekker CL, Laserson J, Koller D, Davis MM, Fire AZ, Boyd SD. 2014. Human responses to influenza vaccination show seroconversion signatures and convergent antibody rearrangements. Cell Host Microbe 16:105–114. doi: 10.1016/j.chom.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vratskikh O, Stiasny K, Zlatkovic J, Tsouchnikas G, Jarmer J, Karrer U, Roggendorf M, Roggendorf H, Allwinn R, Heinz FX. 2013. Dissection of antibody specificities induced by yellow fever vaccination. PLoS Pathog 9:e1003458. doi: 10.1371/journal.ppat.1003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jarmer J, Zlatkovic J, Tsouchnikas G, Vratskikh O, Strauss J, Aberle JH, Chmelik V, Kundi M, Stiasny K, Heinz FX. 2014. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol 88:13845–13857. doi: 10.1128/JVI.02086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gromowski GD, Barrett ND, Barrett AD. 2008. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J Virol 82:8828–8837. doi: 10.1128/JVI.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wahala WM, Huang C, Butrapet S, White LJ, de Silva AM. 2012. Recombinant dengue type 2 viruses with altered E protein domain III epitopes are efficiently neutralized by human immune sera. J Virol 86:4019–4023. doi: 10.1128/JVI.06871-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gallichotte EN, Widman DG, Yount BL, Wahala WM, Durbin A, Whitehead S, Sariol CA, Crowe JE Jr, de Silva AM, Baric RS. 2015. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 6:e01461-15. doi: 10.1128/mBio.01461-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Durbin AP, McArthur J, Marron JA, Blaney JE Jr, Thumar B, Wanionek K, Murphy BR, Whitehead SS. 2006. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin 2:167–173. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 252.Durbin AP, Whitehead SS, Shaffer D, Elwood D, Wanionek K, Thumar B, Blaney JE, Murphy BR, Schmidt AC. 2011. A single dose of the DENV-1 candidate vaccine rDEN1Delta30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl Trop Dis 5:e1267. doi: 10.1371/journal.pntd.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, Tibery C, Sabundayo BP, Shaffer D, Talaat KR, Hynes NA, Wanionek K, Carmolli MP, Luke CJ, Murphy BR, Subbarao K, Whitehead SS. 2013. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 207:957–965. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. 2011. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 29:7242–7250. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dhillon AK, Donners H, Pantophlet R, Johnson WE, Decker JM, Shaw GM, Lee FH, Richman DD, Doms RW, Vanham G, Burton DR. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J Virol 81:6548–6562. doi: 10.1128/JVI.02749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog 6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Abdool Karim S, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L. 2011. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol 85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR, Wyatt R. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol 83:1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 260.Mouquet H, Klein F, Scheid JF, Warncke M, Pietzsch J, Oliveira TY, Velinzon K, Seaman MS, Nussenzweig MC. 2011. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One 6:e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goo L, Chohan V, Nduati R, Overbaugh J. 2014. Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat Med 20:655–658. doi: 10.1038/nm.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cortez V, Wang B, Dingens A, Chen MM, Ronen K, Georgiev IS, McClelland RS, Overbaugh J. 2015. The broad neutralizing antibody responses after HIV-1 superinfection are not dominated by antibodies directed to epitopes common in single infection. PLoS Pathog 11:e1004973. doi: 10.1371/journal.ppat.1004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, Lambson BE, Ranchobe N, Ping L, Ngandu N, Abdool Karim Q, Abdool Karim SS, Swanstrom RI, Seaman MS, Williamson C, Morris L. 2012. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med 18:1688–1692. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wibmer CK, Bhiman JN, Gray ES, Tumba N, Abdool Karim SS, Williamson C, Morris L, Moore PL. 2013. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS Pathog 9:e1003738. doi: 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Murphy MK, Yue L, Pan R, Boliar S, Sethi A, Tian J, Pfafferot K, Karita E, Allen SA, Cormier E, Goepfert PA, Borrow P, Robinson JE, Gnanakaran S, Hunter E, Kong XP, Derdeyn CA. 2013. Viral escape from neutralizing antibodies in early subtype A HIV-1 infection drives an increase in autologous neutralization breadth. PLoS Pathog 9:e1003173. doi: 10.1371/journal.ppat.1003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, Lynch RM, Alam SM, Moody MA, Ferrari G, Berrong M, Kelsoe G, Shaw GM, Hahn BH, Montefiori DC, Kamanga G, Cohen MS, Hraber P, Kwong PD, Korber BT, Mascola JR, Kepler TB, Haynes BF. 2014. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kwong PD, Mascola JR, Nabel GJ. 2011. Rational design of vaccines to elicit broadly neutralizing antibodies to HIV-1. Cold Spring Harb Perspect Med 1:a007278. doi: 10.1101/cshperspect.a007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Widman DG, Baric RS. 2015. Dengue virus envelope protein domain I/II hinge: a key target for dengue virus vaccine design? Expert Rev Vaccines 14:5–8. doi: 10.1586/14760584.2015.961431. [DOI] [PubMed] [Google Scholar]
- 270.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.de Alwis R, Williams KL, Schmid MA, Lai CY, Patel B, Smith SA, Crowe JE, Wang WK, Harris E, de Silva AM. 2014. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog 10:e1004386. doi: 10.1371/journal.ppat.1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40:444–451. [DOI] [PubMed] [Google Scholar]
- 273.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38:411–419. [DOI] [PubMed] [Google Scholar]
- 274.Gromowski GD, Barrett AD. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 275.Watanabe K, Hess A, Bloch W, Michel O. 2000. Inhibition of inducible nitric oxide synthase lowers the cochlear damage by lipopolysaccharide in guinea pigs. Free Radic Res 32:363–370. doi: 10.1080/10715760000300361. [DOI] [PubMed] [Google Scholar]
- 276.Guttman M, Cupo A, Julien JP, Sanders RW, Wilson IA, Moore JP, Lee KK. 2015. Antibody potency relates to the ability to recognize the closed, pre-fusion form of HIV Env. Nat Commun 6:6144. doi: 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dowd KA, DeMaso CR, Pierson TC. 2015. Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. mBio 6:e01559-15. doi: 10.1128/mBio.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Thullier P, Lafaye P, Megret F, Deubel V, Jouan A, Mazie JC. 1999. A recombinant Fab neutralizes dengue virus in vitro. J Biotechnol 69:183–190. doi: 10.1016/S0168-1656(99)00037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cockburn JJ, Navarro Sanchez ME, Fretes N, Urvoas A, Staropoli I, Kikuti CM, Coffey LL, Arenzana Seisdedos F, Bedouelle H, Rey FA. 2012. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 20:303–314. doi: 10.1016/j.str.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 280.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Diskin R, Scheid JF, Marcovecchio PM, West AP Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA. 23 June 2016. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 286.Halstead SB. 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467. doi: 10.1016/S0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 287.Doria-Rose NA, Louder MK, Yang Z, O'Dell S, Nason M, Schmidt SD, McKee K, Seaman MS, Bailer RT, Mascola JR. 2012. HIV-1 neutralization coverage is improved by combining monoclonal antibodies that target independent epitopes. J Virol 86:3393–3397. doi: 10.1128/JVI.06745-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Goo L, Jalalian-Lechak Z, Richardson BA, Overbaugh J. 2012. A combination of broadly neutralizing HIV-1 monoclonal antibodies targeting distinct epitopes effectively neutralizes variants found in early infection. J Virol 86:10857–10861. doi: 10.1128/JVI.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Long WS, Seashore MR, Siegel NJ, Bia MJ. 1990. Idiopathic Fanconi syndrome with progressive renal failure: a case report and discussion. Yale J Biol Med 63:15–28. [PMC free article] [PubMed] [Google Scholar]
- 290.Bouvin-Pley M, Morgand M, Moreau A, Jestin P, Simonnet C, Tran L, Goujard C, Meyer L, Barin F, Braibant M. 2013. Evidence for a continuous drift of the HIV-1 species towards higher resistance to neutralizing antibodies over the course of the epidemic. PLoS Pathog 9:e1003477. doi: 10.1371/journal.ppat.1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O'Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. 2014. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 88:7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kong R, Louder MK, Wagh K, Bailer RT, deCamp A, Greene K, Gao H, Taft JD, Gazumyan A, Liu C, Nussenzweig MC, Korber B, Montefiori DC, Mascola JR. 2015. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol 89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, Rist M, Rademeyer C, Yoon H, Lapedes A, Gao H, Greene K, Louder MK, Kong R, Karim SA, Burton DR, Barouch DH, Nussenzweig MC, Mascola JR, Morris L, Montefiori DC, Korber B, Seaman MS. 2016. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog 12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, Poignard P, Feizi T, Morris L, Walker BD, Fatkenheuer G, Seaman MS, Stamatatos L, Nussenzweig MC. 2012. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med 209:1469–1479. doi: 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Cheung WC, Beausoleil SA, Zhang X, Sato S, Schieferl SM, Wieler JS, Beaudet JG, Ramenani RK, Popova L, Comb MJ, Rush J, Polakiewicz RD. 2012. A proteomics approach for the identification and cloning of monoclonal antibodies from serum. Nat Biotechnol 30:447–452. doi: 10.1038/nbt.2167. [DOI] [PubMed] [Google Scholar]
- 296.Wine Y, Boutz DR, Lavinder JJ, Miklos AE, Hughes RA, Hoi KH, Jung ST, Horton AP, Murrin EM, Ellington AD, Marcotte EM, Georgiou G. 2013. Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. Proc Natl Acad Sci U S A 110:2993–2998. doi: 10.1073/pnas.1213737110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science 340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bohne-Lang A, von der Lieth CW. 2005. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res 33:W214–W219. doi: 10.1093/nar/gki385. [DOI] [PMC free article] [PubMed] [Google Scholar]






