Abstract
Background & objectives:
CNDP1 gene, present on chromosome 18q22.3-23, encodes carnosinase, the rate-limiting enzyme in hydrolysis of carnosine to β-alanine and L-histidine. Linkage of CTG trinucleotide (leucine) repeat polymorphism in CNDP1 gene with diabetic nephropathy has been observed in several populations. However, this association is conflicting and population-dependent. We investigated this association in type 2 diabetes mellitus (T2DM) patients with and without nephropathy in north India.
Methods:
A total of 564 individuals [199 T2DM without nephropathy (DM), 185 T2DM with nephropathy (DN) and 180 healthy individuals (HC)] were enrolled. CNDP1 CTG repeat analysis was done by direct sequencing of a 377 base pair fragment in exon 2.
Results:
The most frequent leucine (L) repeats were 5L-5L, 6L-5L and 6L-6L. 5L-5L genotype frequency was reduced in DN (24.3%) as compared to DM (34.7%, P=0.035) and HC (38.4%, P=0.005). Similarly, 5L allele frequency was lower in DN (46.8%) as compared to DM (57.3%, P=0.004) and HC (60.5%, P<0.001). The genotype and allelic frequencies were similar in DM and HC groups. No gender specific difference was observed in the genotype or allelic frequencies between groups.
Interpretation & conclusions:
Compared to healthy individuals and those with diabetes but no kidney disease, patients with diabetic nephropathy exhibited lower frequencies of 5L-5L genotype and 5L allele of CNDP1 gene, suggesting that this allele might confer protection against development of kidney disease in this population.
Keywords: Carnosinase, CNDP1, CTG repeat, diabetic nephropathy, genetics, genotype, type 2 diabetes
Diabetic nephropathy (DN), a microvascular complication of diabetes, is the leading cause of end-stage renal disease (ESRD) worldwide. About 35 per cent of type 2 diabetes mellitus (T2DM) patients develop DN1. A population based study showed DN to be the commonest (44%) cause of ESRD in India2. Although the precise mechanisms leading to DN remain unclear, genetic susceptibility is considered to be an important factor in its development and/or progression3.
Several candidate genes have been evaluated for their role in DN, with conflicting results. These include ACE, AGT, APOE, CCR5, ELMO1, SLC12A3 and many more4. Carnosinase, encoded by the gene CNDP1 (carnosine dipeptidase 1) has also been shown to have a role in the genesis of DN5. This enzyme hydrolyzes carnosine into β-alanine and histidine, inhibits the advanced glycation end products (AGE) formation6 and acts as an inhibitor of angiotensin converting enzyme (ACE)7. Apart from these characteristics, carnosinasealso possesses antioxidant properties8 and inhibits hyperglycaemia induced extracellular matrix accumulation5.
Vardali et al9 discovered a susceptibility locus for DN at locus 18q22.3-q23 in Turkish families8. Later, an association of CTG trinucleotide repeat polymorphism in second exon of the CNDP1 gene at 18q with nephropathy in both type 1 and 2 diabetes was reported5. Homozygosity for 5 leucine repeats (5L) was associated with reduced risk of DN5. This finding was replicated in a cohort of European American individuals with T2DM10. In contrast, other studies have failed to show such an association. Examples include Japanese with type 2 diabetes11, type 1 diabetes in Caucasians12, and Scandinavians and African Americans with T2DM13,14. One study showed a gender specific association of 5L-5L CNDP1 in Caucasians with type 2 diabetes15. An association between this polymorphism and microvascular complications of diabetes seems to have biological plausibility, since the number of CTG repeats is associated with serum carnosinase concentrations5. Moreover, CNDP1 is differentially expressed in kidney of animal models of DM16.
With the contradictory results in various ethnic populations, the association of 5L-5L homozygous repeat in CNDP1 with DN remains uncertain. There are no studies on CNDP1-leucine repeat polymorphism in Indian population. We, therefore, investigated the association between the CTG trinucleotide (leucine) repeats in CNDP1 gene with DN in T2DM patient with and without DN in north India.
Material & Methods
This study was carried out at the Nehru Hospital, Postgraduate Institute of Medical Education and Research, Chandigarh during 2011-2012. Adult individuals aged between 18 and 70 yr, including 199 subjects patients with T2DM but no kidney disease (DM) and 185 T2DM patients with nephropathy (DN) were recruited consecutively from Endocrinology and Nephrology Clinics. A control population comprising 180 healthy adults was also included. Healthy individuals were voluntary kidney donors and relatives of the patients. The Institute Ethics Committee approved the study, and all participants provided written informed consent.
All patients and controls underwent a detailed clinical examination including ocular fundus examination for diabetic retinopathy; T2DM was diagnosed on the basis of World Health Organization guidelines17. Those with diabetic retinopathy and sustained albuminuria (>150 mg/day) for ≥3 months in the absence of any other cause were considered to have DN. Diabetics with duration of disease >5 years, normal blood pressure, and urinary albumin excretion <150 mg/day formed the group of diabetes without nephropathy (DM).
Genotype analysis: Peripheral blood (5 ml) was collected from each patient after overnight fast. Buffy coat was separated using Ficoll-histopaque density gradient method18, and genomic DNA extracted from the buffy coat using QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany) according to manufacturer's instructions. The trinucleotide CTG repeat polymorphism in exon 2 of CNDP1 gene was analyzed by direct sequencing of 377 base pair covering exon2 using forward primer 5’-GTGTTTGGGGAAGGACGTAG-3’ and reverse primer 5’-TCTCACCCAAATACCAAAGG-3’19 on ABI 3730XL DNA analyzer (Applied Biosystems, Darmstadt, Germany).
Statistical analysis: Continuous and nominal clinical variables were compared with Student's t test and χ2 test (with Yates correction where appropriate). Mann-Whitney U test was used for parameters with skewed distribution. One-way ANOVA was used for comparison of more than two groups. Hardy-Weinberg equilibrium (HWE) was tested for each population. Allelic and genotypic associations of CTG repeat were evaluated by Pearson's χ2 test and odds ratio (OR) and 95% confidence intervals (CI). Multivariate logistic regression analysis was performed to find association of DN with 5L-5L genotype and other parameters. Statistical analysis was performed using SPSS v16.0 (Chicago, IL, USA).
Results
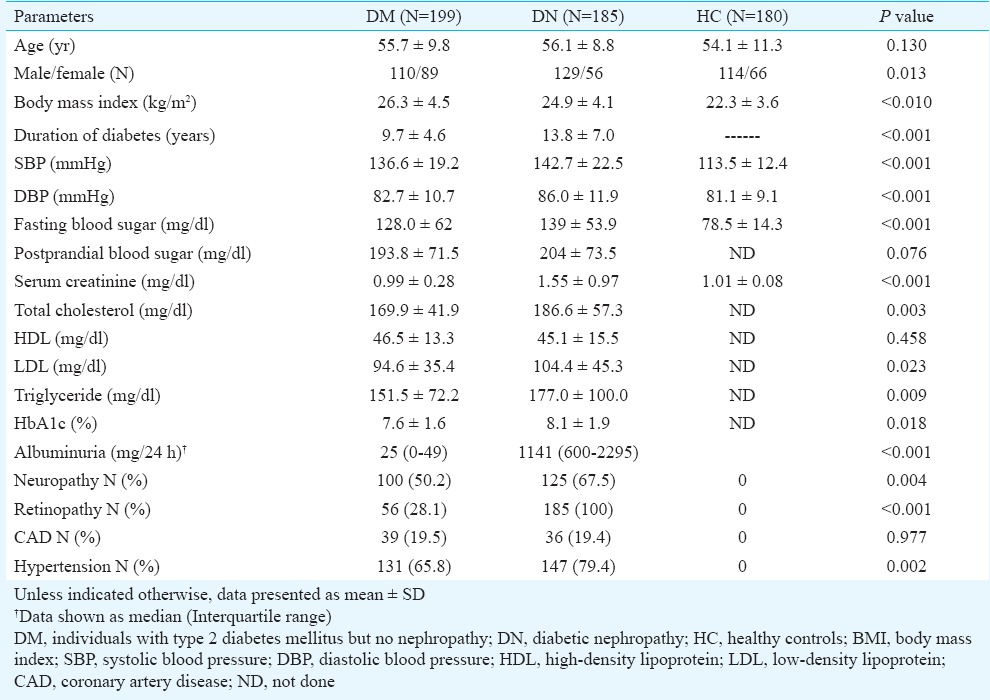
Table I shows the characteristics of study participants in different groups. There was no difference in age of the DN, DM and HC individuals. DN group had more males than DM (70 vs 55%). The duration of diabetes and prevalence of hypertension were more in DN group compared to DM. Systolic and diastolic blood pressure were higher in DN group as compared to DM and HC (P<0.001 for both), whereas BMI was higher in DM group (P<0.01). As expected, serum creatinine, total cholesterol, low-density lipoprotein cholesterol, triglyceride and HbA1c were higher in DN group compared to DM and HC groups (Table I). DN was also accompanied more frequently by diabetic neuropathy (P<0.01) and retinopathy (P<0.001) compared to DM.
Table I.
Baseline parameters of the study groups
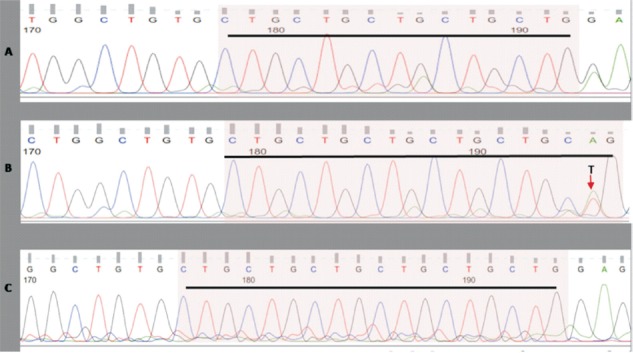
Tables II and III show the frequency of leucine repeats and comparison of genotype and allelic frequencies between groups. All populations were in the Hardy-Weinberg equilibrium (P=0.297, χ2 =1.09 for HC, P=0.284, χ2 =1.14 for DM and P=0.178, χ2 =1.87 for DN). 5L-5L, 5L-6L and 6L-6L were most common repeats found in this study. Only three other repeats, one 4L-7L and two 5L-7L repeats were noted in healthy individuals. These three were excluded from further analyses. The Figure shows representative DNA sequence chromatograph for 5 and 6 CTG repeats.
Table II.
Frequency of leucine repeat allele and genotype in study groups
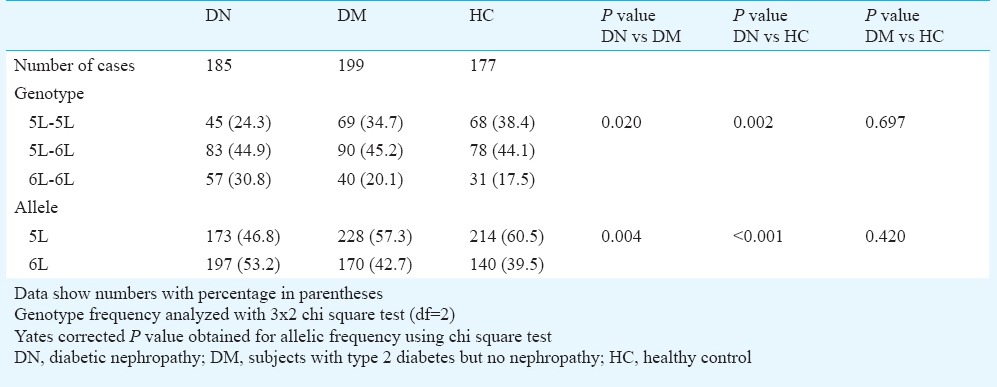
Table III.
Association of CNDP1 CTG repeat genotype and allele with diabetic nephropathy
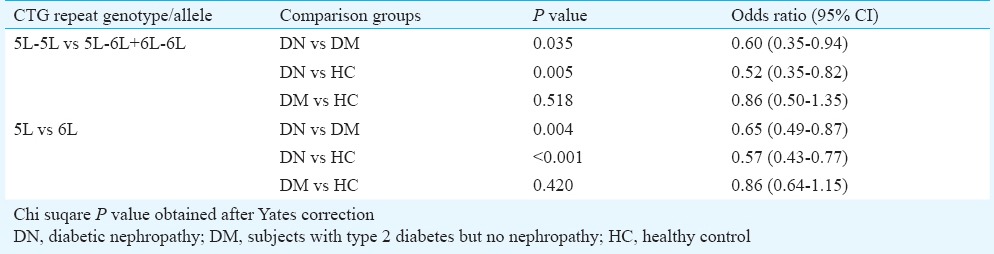
Figure.
Representative DNA sequence chromatograph showing (A) 5 -5 CTG repeats, (B) 6-6 CTG repeats, and (C) 5-6 CTG repeats. 5-5, 5-6 and 6-6 CTG repeats are highlighted and underlined.
Frequency of 5L-5L genotype was lower in DN group (24.3%) compared to DM (34.7%, P=0.035) and HC (38.4%, P=0.005) groups. Similarly, the 5L allele frequency was also reduced in patients with DN (46.8%) compared to DM (57.3%, P=0.004) and HC (60.5%, P<0.001) (Table II). Further, to assess whether the susceptibility for DN is independent of susceptibility for T2DM, the DM group was compared with HC, and similar frequencies of the 5L-5L genotypes and 5L alleles were found in the two groups (Table III).
Next, the sex-specific frequency of 5L-5L homozygous in DN, DM and HC groups was compared. Amongst women, 5L-5L genotype was noted in 28.5, 33.7 and 39.4 per cent in DN, DM and HC groups, respectively. The frequency of males with 5L-5L homozygosity was 22.5 per cent in DN, 31.8 per cent in DM and 33.3 per cent in HC group. There was no sex-specific difference in the frequencies.
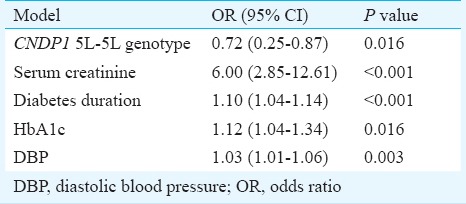
Multivariate logistic regression analysis was performed to assess the association of nephropathy with genotype and various clinical parameters. Apart from CNDP1 5L-5L genotype, serum creatinine, HbA1c, diastolic blood pressure, and duration of diabetes were found to be independently associated with DN (Table IV).
Table IV.
Multivariate logistic regression analysis of factors related to diabetic nephropathy
Discussion
Our study showed that amongst north Indian patients with diabetic nephropathy, the frequency of the 5L-5L genotype in CNDP1 gene was reduced in comparison to those with T2DM but no kidney disease and healthy non-diabetic individuals. These findings suggest the possible protective effect of this polymorphism for development of kidney disease in T2DM patients.
This finding fulfils the criterion of biological plausibility, as 5L repeats are associated with carnosinase activity. Upon transfecting COS cells with CNDP1 construct containing 4-8 CTG repeats, cells with higher number of CTG repeats showed increased carnosinase in the supernatant20. Individuals homozygous for the 5L allele (also called Mannheim allele) exhibit reduced serum carnosinase concentrations compared to those with higher number of CTG repeats15. Carnosinase hydrolyses the dipeptide carnosine, hence lower level of this enzyme is likely to result in higher renal carnosine concentrations. Carnosine has been shown to have multiple potentially renoprotective effects21. In a cell culture experiment, Janssen et al5 showed that carnosine blocked glucose-induced extracellular matrix accumulation by renal cells. Moreover, epithelial cells in glomeruli and renal tubules express CNDP1 mRNA and carnosinase protein22.
CNDP1 polymorphism has been studied in the context of diabetic kidney disease in various ethnicities, with conflicting results. Some studies5,10,15 showed an association of D18S880 microsatellite marker with DN whereas others11,12,13,14,[ 23 failed to find any such association. A meta-analysis confirmed the association of 5L-5L genotype with DN24.
The 5L-5L homozygous genotype was encountered with reduced frequency in South Asian Surinamese compared to White Dutch and African Surinamese22. The frequency of various genotypes in DM, DN and HC populations in this study was similar to those reported in the Caucasians5,10. The findings of this study were also consistent with the finding of reduced risk for developing diabetic renal disease amongst European-Americans with 5L-5L homozygous repeat polymorphism in the CNDP1 gene10. In another study, lower 5L-5L frequency was not associated with DN and in the follow up study 5L-5L was associated with higher risk of ESRD beyond six years23. They also showed an association of 5L-5L genotype with sex specific risk of mortality, with the risk being higher in women23. In another study, 5L-5L genotype was associated with DN in sex specific manner, with protection seen only in women22. We did not find any gender-specific association of 5L-5L genotype with DN.
Our study had some limitations. The number of patients in different groups was small, and only albuminuric patients were included in the DN group. The diagnosis of diabetic nephropathy was made on clinical grounds alone. In absence of kidney biopsy, it was not possible to exclude non-diabetic lesions with certainty. The strength of the study was a well selected homogenous population reducing phenotype errors and bias. The study included well matched controls of the same ethnic background which reduced the risk of population stratification.
In conclusion, our findings show an association of CTG repeat polymorphism in the CNDP1 gene with DN in north Indian population; with reduced frequency of 5L-5L repeat genotype and 5L allele in DN.
Acknowledgment
This study was funded by a grant from Indian Council of Medical Research, Government of India, New Delhi.
Footnotes
Conflicts of Interest: None.
References
- 1.Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115:69–84. doi: 10.1055/s-2007-949721. [DOI] [PubMed] [Google Scholar]
- 2.Modi GK, Jha V. The incidence of end-stage renal disease in India: a population-based study. Kidney Int. 2006;70:2131–3. doi: 10.1038/sj.ki.5001958. [DOI] [PubMed] [Google Scholar]
- 3.Maeda S. Genetics of diabetic nephropathy. Ther Adv Cardiovasc Dis. 2008;2:363–71. doi: 10.1177/1753944708094768. [DOI] [PubMed] [Google Scholar]
- 4.Mooyaart AL, Valk EJ, van Es LA, Bruijn JA, de Heer E, Freedman BI, et al. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia. 2011;54:544–53. doi: 10.1007/s00125-010-1996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, et al. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54:2320–7. doi: 10.2337/diabetes.54.8.2320. [DOI] [PubMed] [Google Scholar]
- 6.Hipkiss AR, Preston JE, Himsworth DT, Worthington VC, Keown M, Michaelis J, et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann N Y Acad Sci. 1998;854:37–53. doi: 10.1111/j.1749-6632.1998.tb09890.x. [DOI] [PubMed] [Google Scholar]
- 7.Hou WC, Chen HJ, Lin YH. Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for angiotensin converting enzyme purification. J Agric Food Chem. 2003;51:1706–9. doi: 10.1021/jf0260242. [DOI] [PubMed] [Google Scholar]
- 8.Boldyrev A, Bulygina E, Leinsoo T, Petrushanko I, Tsubone S, Abe H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp Biochem Physiol B Biochem Mol Biol. 2004;137:81–8. doi: 10.1016/j.cbpc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Vardarli I, Baier LJ, Hanson RL, Akkoyun I, Fischer C, Rohmeiss P, et al. Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3-23. Kidney Int. 2002;62:2176–83. doi: 10.1046/j.1523-1755.2002.00663.x. [DOI] [PubMed] [Google Scholar]
- 10.Freedman BI, Hicks PJ, Sale MM, Pierson ED, Langefeld CD, Rich SS, et al. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant. 2007;22:1131–5. doi: 10.1093/ndt/gfl717. [DOI] [PubMed] [Google Scholar]
- 11.Kurashige M, Imamura M, Araki S, Suzuki D, Babazono T, Uzu T, et al. The influence of a single nucleotide polymorphism within CNDP1 on susceptibility to diabetic nephropathy in Japanese women with type 2 diabetes. PLoS One. 2013;8:e54064. doi: 10.1371/journal.pone.0054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanic K, Placha G, Dunn J, Smiles A, Warram JH, Krolewski AS. Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case-control and follow-up studies. Diabetes. 2008;57:2547–51. doi: 10.2337/db07-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahluwalia TS, Lindholm E, Groop LC. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia. 2011;54:2295–302. doi: 10.1007/s00125-011-2178-5. [DOI] [PubMed] [Google Scholar]
- 14.McDonough CW, Hicks PJ, Lu L, Langefeld CD, Freedman BI, Bowden DW. The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum Genet. 2009;126:265–75. doi: 10.1007/s00439-009-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooyaart AL, Zutinic A, Bakker SJ, Grootendorst DC, Kleefstra N, van Valkengoed IG, et al. Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes. 2010;59:1555–9. doi: 10.2337/db09-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Kaisaki PJ, Argoud K, Wilder SP, Wallace KJ, Woon PY, et al. Functional annotations of diabetes nephropathy susceptibility loci through analysis of genome-wide renal gene expression in rat models of diabetes mellitus. BMC Med Genomics. 2009;2:41. doi: 10.1186/1755-8794-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Islam A. A new, fast and convenient method for layering blood or bone marrow over density gradient medium. J Clin Pathol. 1995;48:686–8. doi: 10.1136/jcp.48.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zschocke J, Nebel A, Wicks K, Peters V, El Mokhtari NE, Krawczak M, et al. Allelic variation in the CNDP1 gene and its lack of association with longevity and coronary heart disease. Mech Ageing Dev. 2006;127:817–20. doi: 10.1016/j.mad.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Riedl E, Koeppel H, Brinkkoetter P, Sternik P, Steinbeisser H, Sauerhoefer S, et al. A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7 transfected cells. Diabetes. 2007;56:2410–3. doi: 10.2337/db07-0128. [DOI] [PubMed] [Google Scholar]
- 21.Hipkiss AR. Carnosine, a protective, anti-ageing peptide? Int J Biochem Cell Biol. 1998;30:863–8. doi: 10.1016/s1357-2725(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 22.Mooyaart AL, van Valkengoed IG, Shaw PK, Peters V, Baelde HJ, Rabelink TJ, et al. Lower frequency of the 5/5 homozygous CNDP1 genotype in South Asian Surinamese. Diabetes Res Clin Pract. 2009;85:272–8. doi: 10.1016/j.diabres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Alkhalaf A, Bakker SJ, Bilo HJ, Gans RO, Navis GJ, Postmus D, et al. A polymorphism in the gene encoding carnosinase (CNDP1) as a predictor of mortality and progression from nephropathy to end-stage renal disease in type 1 diabetes mellitus. Diabetologia. 2010;53:2562–8. doi: 10.1007/s00125-010-1863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu JM, Wang B, Li J, Chen GM, Fan YG, Feng CC, et al. D18S880 microsatellite polymorphism of carnosinase gene and diabetic nephropathy: a meta-analysis. Genet Test Mol Biomarkers. 2013;17:289–94. doi: 10.1089/gtmb.2012.0341. [DOI] [PubMed] [Google Scholar]