Abstract
Background & objectives:
Scrub typhus is a major public health threat in South and Southeastern Asian countries including India. Understanding local patterns of disease and factors that place individuals at risk is pivotal to future preventive measures against scrub typhus. The primary aim of this study was to identify specific epidemiological and geographical factors associated with an increased risk of developing scrub typhus in this region.
Methods:
We mapped 709 patients from Tamil Nadu, Andhra Pradesh and Telangana who were admitted to the Christian Medical College (CMC) Hospital, Vellore, Tamil Nadu, India, for the period 2006-2011, assessed seasonality using monthly counts of scrub typhus cases, and conducted a case-control study among a subset of patients residing in Vellore.
Results:
The geographic distribution of cases at CMC Hospital clusters around the Tamil Nadu-Andhra Pradesh border. However, distinct hotspots clearly exist distal to this area, near Madurai and the coast in Tamil Nadu, and in the Northeast of Andhra Pradesh. Seasonally, the highest numbers of cases were observed in the cooler months of the year, i.e. September to January. In the case-control analysis, cases were more likely to be agricultural laborers (OR 1.79, 95% CI 1.01 - 3.15), not wear a shirt at home (OR 4.23, 95% CI 1.12 - 16.3), live in houses adjacent to bushes or shrubs (OR 1.95, 95% CI 1.08 - 3.53), and live in a single room home (OR 1.75, 95% CI 1.02 - 3.01). On binary logistic regression, the first three of these variables were statistically significant.
Interpretation & conclusions:
With the growing number of cases detected in India, scrub typhus is fast emerging as a public health threat and further research to protect the population from this deadly infection is essential. Health education campaigns focusing on the agricultural workers of Southern India, especially during the cooler months of the year, can serve as an important public health measure to control infection.
Keywords: Epidemiology, Orientia tsutsugamushi, risk factors, scrub typhus, south India
Scrub typhus is an acute febrile illness, which can involve multiple organ systems and results in significant morbidity and mortality. This zoonotic infection is caused by the obligate intracellular bacteria Orientia tsutsugamushi, which is transmitted to humans by the bite of the larval trombiculid mite. In South and Southeast Asia and the Asian Pacific rim, scrub typhus is a major public health threat. Annually, one million cases have been reported in these regions, and an estimated one billion people are at risk for contracting the illness1. Occurring in periodic outbreaks in the early 1900s, scrub typhus was classified as a typhus-like fever in 19172. In the pre-antibiotic era, case fatality rate due to this illness approached 50 per cent. Tetracycline and chloramphenicol subsequently dramatically lowered the mortality associated with this infection3. Interest in this infection generally decreased, as the reported incidence declined in the subsequent decades, potentially because of the common use of tetracycline and chloramphenicol for treating febrile illnesses, pesticide use, and limited diagnostics leading to underreporting. However, resurgence of scrub typhus with considerable morbidity and mortality has now been well documented in several States in India and neighbouring countries4,5,6.
Agricultural labourers in the endemic region are known to have a higher risk for developing scrub typhus. However, other known risk factors for this disease can be region-specific. A study in Korea found that women tended to be more at risk for scrub typhus than men7. Conversely, a study from Beijing, PR China found men to be at higher risk8. Additionally, while rice farming has traditionally been associated with scrub typhus infection, studies in Korea and Taiwan point to dry farming as a greater risk8,9. Similarly, keeping domestic animals in the yard has also been found to be a significant risk factor in northeast India, but not so in Beijing8,10.
We undertook this epidemiological study to understand the local pattern of disease in the south Indian States of Tamil Nadu (TN) and Andhra Pradesh (AP) (now including Telangana). The primary aim of this study was to identify specific epidemiological and geographical factors associated with an increased risk of developing scrub typhus in this region. The secondary aim was to map these local infections to highlight geographic areas of concern where further data are needed to ascertain disease burden and identify additional local risk factors. Finally, because prior research has also shown an increase in scrub typhus incidence before and after rains in the cooler months of the year11 in countries like India that have a monsoon season, the seasonality of these cases was also examined using the month of presentation of the patients to the hospital.
Material & Methods
Subjects: To map patients, all patients >16 yr of age admitted to the Christian Medical College (CMC) Hospital, Vellore, India, with a confirmed diagnosis of scrub typhus between January 2006 and April 2011 were included. All cases were confirmed to have scrub typhus by scrub typhus IgM ELISA and physician-adjudicated final diagnosis.
Mapping: Postal addresses obtained from official hospital records were used to assign each patient to a taluk (administrative subdivision), which was then mapped using ArcGIS 10 software (Environmental Systems Research Institute Inc., Redlands, CA, USA). State and taluk vector maps for Tamil Nadu and Andhra Pradesh (now including Telangana) were obtained from the Global Administrative Areas database, a free service that provides vector maps of the administrative divisions and sub-divisions in different countries of the world12. Scrub typhus cases were plotted using circles of varying sizes to depict the number of cases in each taluk within the States. This geographic information system (GIS) map was interpreted visually to assess potential clusters.
Seasonality analysis: In order to assess potential seasonal variations of scrub typhus, a time series was compiled using the monthly counts of scrub typhus cases reported in CMC Hospital during 2006-2011. The overall pattern of seasonality in southern India was assessed by fitting a smoothed curve of the monthly observed cases for each year, using the LOESS (locally weighted least-squares regression) smoothing technique13. The relative effect of the month of the year on the reported cases of scrub typhus was also assessed using a Poisson regression analysis, with the monthly counts of scrub typhus cases as an outcome and a dummy variable for the month of diagnosis. The results of the regression model are presented as incidence rate ratios (IRR) with 95 per cent confidence intervals (CI), which denote the relative increase in scrub typhus cases for a given month compared to a reference category, which in this case was May as it had the fewest reported cases.
Case-control analysis: To assess the risk factors associated with the disease, a case-control study was conducted among the subset of patients that resided in Vellore district. Age and sex-matched controls were selected from randomly chosen households in the same village where the case was located. Information on demographic characteristics, occupation, living environment, leisure activities, and sanitation/hygiene practices was collected from cases and controls using a pre-designed proforma. Cases and controls were also questioned about exposure to domestic animals and rodents, as well as shrubs and bushes. The presence of shrubs and bushes around the houses was assessed by the interviewers. Community outreach personnel were trained to interview the patients in the local language and record the data after obtaining informed consent. Odd ratios were subsequently calculated, and the exposures found to be significant (P<0.05) were incorporated into a binary logistic regression medel, using SPSS software, version 16 (Chicago, USA). The study was approved by the CMC Institutional Ethics Committee.
Results
Seven hundred and nine patients with a confirmed diagnosis of scrub typhus were included in the mapping portion of this study. The mean age of patients was 49.9 ± 15.7 yr and 51.3 per cent (n=364) were male. Apart from Vellore, the taluks with the highest number of reported cases were Gudiyatham (51 cases), Polur (44 cases), and Walajah (44 cases) in Tamil Nadu and Chittoor (123 cases), Tirupati (30 cases), and Porumanilla (15 cases) in Andhra Pradesh. Cases reported from Andhra Pradesh appeared to cluster around the State border with Tamil Nadu (16 taluks) and a few from Peddapuram. Cases were also reported from Nizamabad in Telangana (Fig. 1). Cases of scrub typhus from Vellore appeared dispersed; however, the high prevalence of cases around Vellore might be due to the close proximity and thus availability of CMC Hospital.
Fig. 1.
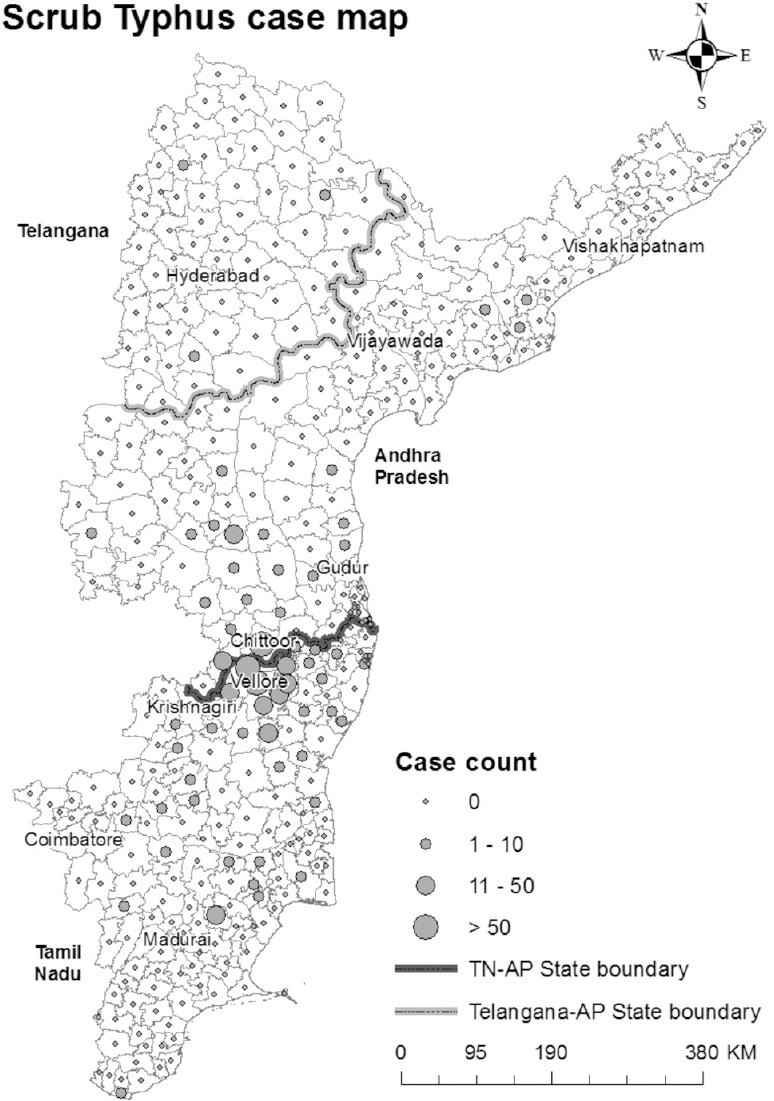
Distribution of scrub typhus cases from Tamil Nadu, Andhra Pradesh and Telangana States during the study period.
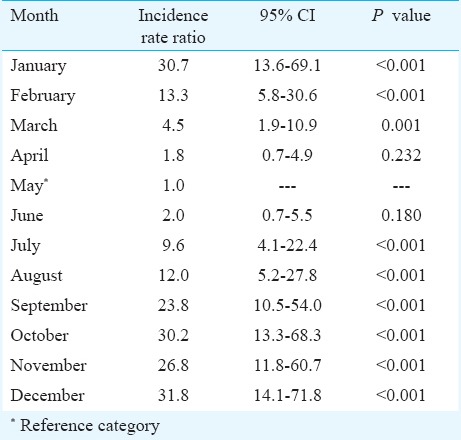
The cumulative distribution of scrub typhus cases by month of diagnosis is presented in Fig. 2, and the monthly time-series is presented in Fig. 3. The highest numbers of cases were observed during the cooler months of the year (September-January), with a peak observed during January (Fig. 2). The monthly time-series displays a similar pattern across the years 2006-2011, with distinct winter peaks. Assessing the relative effects of time in months revealed that the highest relative risk of scrub typhus was for the months of October, December and January with IRRs of 30.2 (13.3-68.3), 31.8 (14.1 - 71.8) and 30.7 (13.6 - 69.1), respectively, relative to the reference month of May (Table I).
Fig. 2.
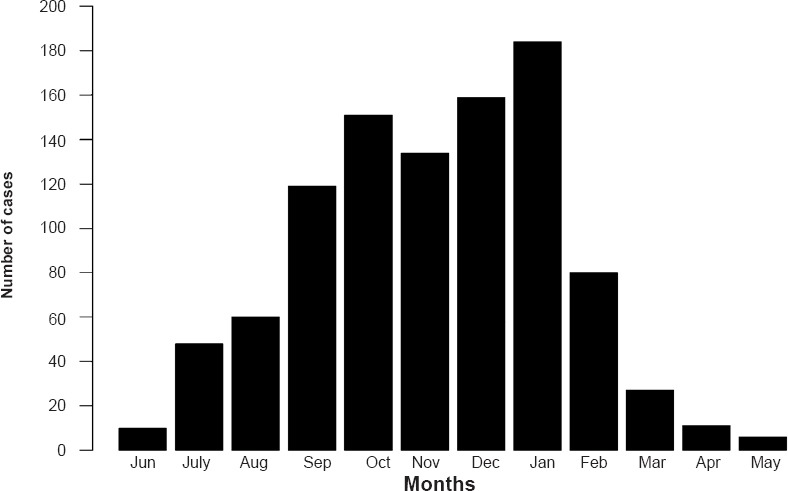
Cumulative scrub typhus case distribution by month.
Fig. 3.
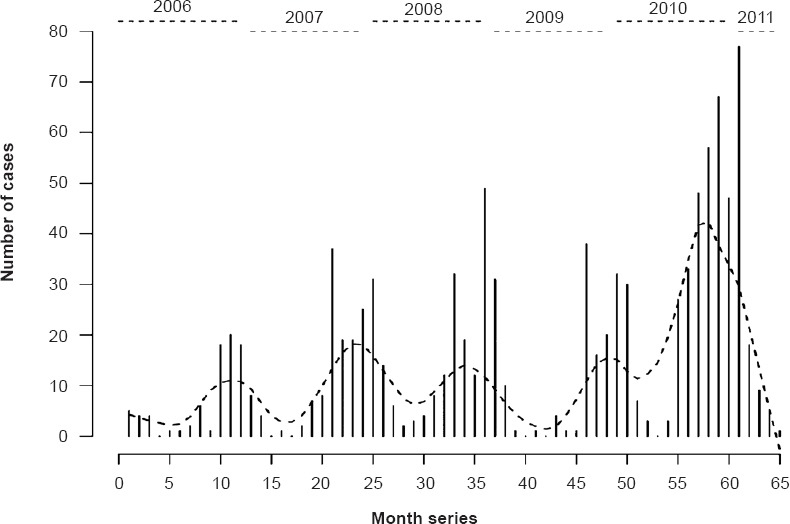
Time trend in reported cases of scrub typhus over time. The dotted line indicates the smoothed trend of observed monthly cases.
Table I.
Distribution of cases and relative risk of scrub typhus by month
To assess risk factors, 128 cases and 132 matched controls from Vellore district were analyzed in the case-control study. One hundred and thirty two cases were chosen initially, but four did not have final confirmation of diagnosis and were excluded. Compared to their controls, cases were more likely to work as agricultural labourers (OR 1.79, 95% CI 1.01 - 3.16), not wear a shirt at home (OR 4.23, 95% CI 1.12 - 16.3), live in houses surrounded by bushes or shrubs (OR 1.95, 95% CI 1.08 - 3.53), and live in a single room home (OR 1.75, 95% CI 1.02 - 3.01). Other social factors that were evaluated did not show a significant difference between cases and controls. The findings are summarized in Table II.
Table II.
Odds exposure for scrub typhus in cases and controls (n=128)
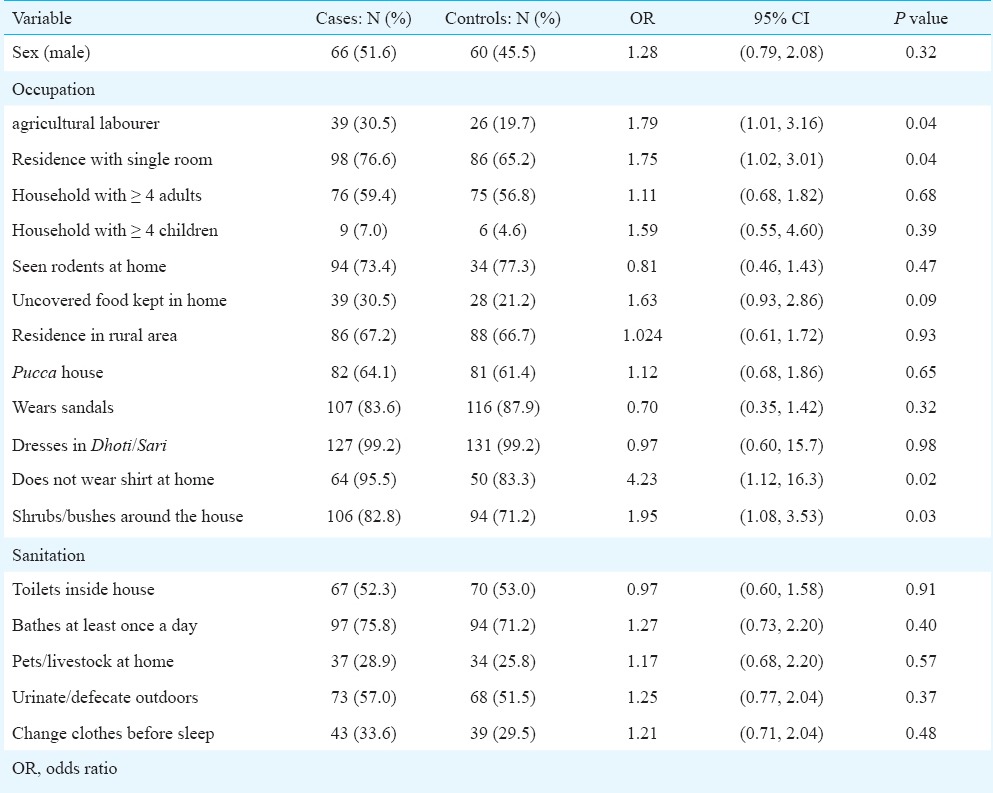
When the variables that were found to have significant odds ratios were used in a logistic regression model with “Enter” variable selection, three of the four variables remained significant at P<0.05 level. Having bushes or shrubs around the home (OR 1.95, 95% CI 1.08 - 3.53, P=0.007), working in agriculture (OR 1.75, 95% CI 1.02 - 3.01, P=0.025), and not wearing a shirt at home (OR 4.23, 95% CI 1.12 - 16.3, P=0.026) were significant.
Discussion
While scrub typhus has been extensively reported in Tamil Nadu, only one study has emerged from Andhra Pradesh, in the district of Adilabad in 200914. We observed a high prevalence of cases in and around Vellore and the State border. This could potentially be due to a case-detection bias. Alternatively, these areas could represent a scrub typhus hot-spot as denser vegetation has been observed near a river in this region. Further evaluation would be necessary to resolve this. Other areas contributing large numbers of cases distal to this border area, could also represent areas of higher scrub typhus activity in these States. In total, we reported 193 cases from AP, with more than half (54.6%) of these cases from Chittoor. Notably, the Agricultural Census by the Ministry of Agriculture, Government of India, for the year 2010-2011 reported a 9.1 per cent increase in the number of operational agricultural land holders in Andhra Pradesh compared to 2005-200615. This increase in agricultural workers in the area might be the reason for high number of cases as people working in agriculture could increase exposure to the trombiculid mites that harbour O. tsutsugamushi. Spatial analysis, i.e. hot-spot or cluster analyses could not be performed in this study as the vector maps obtained from the open-source database lacked spatial projection information. Future research examining the relationship between environmental features such as precipitation, land use, vegetation index, and relative humidity to scrub typhus incidence, as has been performed in Taiwan16,17, could be important for future efforts to control its spread in southern India.
Many studies from India have reported a “post-monsoon” or “winter” spike in the number of scrub typhus cases18,19,20. Our study showed a similar increase in cases during the cooler months. However, a drastic reduction was observed in reported cases between February and June, which was contrary to the findings of Vivekanandan et al 20 in Puducherry that showed a high number of cases reported until April. The highest risk of scrub typhus was seen in October, December and January, which coincides with the period immediately after the Southeast and Northwest monsoons, respectively in Tamil Nadu. Post-monsoon scrub typhus in India is believed to occur due to increased exposure to trombiculid mites during harvesting season as well as the growth of new vegetation, which serves as habitat for this vector18.
Our study was limited in its ability to distinguish risk factors, as there was no guarantee of a truly negative control group. Though, scrub typhus had a sero-prevalence of around 5 per cent in the Vellore area around our study period21, making it less likely that controls had the disease. These seroprevalence data were collected early during the recognition of the re-emergence of this infection, so the prevalence could be higher now. Some of the controls could have previously been infected as well and, thus, were not true controls. Furthermore, our risk assessment was a broad one, geared to cover the major classical risk factors. A more detailed analysis of factors associated with housing conditions and local vegetation around the house, insecticide spraying, personal practices such as taking a bath after outdoor activities, and personal protective measures along with the assessment of risk factors associated with farming (more detailed analysis of the type of vegetation; crops or vegetables grown) could have provided more information on the details of risk situations.
In conclusion, this study provides valuable, regional data about scrub typhus in south India, which can inform clinical and public health decisions at a local level. Though there are some weaknesses in our data, it seems reasonable for public health interventions to focus on agricultural workers, particularly in the cooler months of the year. Our study, though in some ways limited by case-detection bias, documents a growing number of cases. Further research and health interventions are warranted to protect the population from this infection.
Acknowledgmen
This work was supported by internal grants from Christian Medical College, Vellore
Footnotes
Conflicts of Interest: None.
References
- 1.Watt G. Parola P. Scrub typhus and tropical Rickettsioses. Curr Opin Infect Dis. 2003;16:429–36. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Tattersall R. Tsutsugamushi fever on the India-Burma border. Lancet. 1945;2:392–4. [Google Scholar]
- 3.Kelly D, Fuerst A, Ching W, Richards A. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48:5203–30. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 4.Varghese GM, Janardhanan J, Trowbridge P, Peter JV, Prakash JAJ, Sathyendra S, et al. Scrub typhus in South India: Clinical and laboratory manifestations, genetic variability and outcome. Int J Infect Dis. 2013;17:e981–7. doi: 10.1016/j.ijid.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Devine J. A review of scrub typhus management in 2000-2001 and implications for soldiers. J Rural Remote Environment Health. 2003;2:14–20. [Google Scholar]
- 6.Watt G. In: Scrub typhus. Oxford textbook of medicine. 4th ed. Warrel DA, Cox TM, Firth JD, Benz EJ Jr, editors. London: Oxford University Press; 2003. pp. 629–31. [Google Scholar]
- 7.Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, Ryu SY, et al. A community-based case-control study of behavioral factors associated with scrub typhus during autumn epidemic season in South Korea. Am J Trop Med Hyg. 2009;80:442–6. [PubMed] [Google Scholar]
- 8.Lyu Y, Tian L, Zhang L, Dou X, Wang X, Li W, et al. A case-control study of risk factors associated with scrub typhus infection in Beijing, China. PLoS One. 2013;8:e63668. doi: 10.1371/journal.pone.0063668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo CC, Huang JL, Ko CY, Lee PF, Want CH. Spatial analysis of scrub typhus infection and its association with environmental and socioeconomic factors in Taiwan. Acta Tropica. 2011;120:52–8. doi: 10.1016/j.actatropica.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Sharma PK, Ramakrishnan R, Hutin YJ, Barui AK, Manickam P, Kakkar M, et al. Scrub typhus in Darjeeling, India: opportunities for simple, practical prevention methods. Trop Med Hyg. 2009;103:1153–8. doi: 10.1016/j.trstmh.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Traub R, Wisseman C. Ecological considerations in scrub typhus. Bull World Health Organ. 1968;39:219–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Global Administrative Areas. Boundaries without limits. [accessed on October 8, 2014]. Available from: http://www.gadm.org/
- 13.Gijbels I, Prosdocimi I. Loess. WIREs Comp Stat. 2010;2:590–9. [Google Scholar]
- 14.Boorugu H, Dinaker M, Roy ND, Jude JA. Reporting a case of scrub typhus from Andhra Pradesh. J Assoc Physicians India. 2010;58:520. [PubMed] [Google Scholar]
- 15.Agricultural census, 2010-2011 (Phase - 1). All India Report on Number and Area of Operational Holdings. Agriculture census division department of agriculture & co-operation, Ministry of Agriculture, Government of India. New Delhi: 2014. [Google Scholar]
- 16.Tsai PJ, Yeh HC. Scrub typhus islands in the Taiwan area and the association between scrub typhus disease and forest land use and farmer population density: geographically weighted regression. BMC Infect Dis. 2013;13:191. doi: 10.1186/1471-2334-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wardrop NA, Kuo CC, Wang HC, Clements AC, Lee PF, Atkinson PM. Bayesian spatial modelling and the significance of agricultural land use to scrub typhus infection in Taiwan. Geospat Health. 2013;8:229–39. doi: 10.4081/gh.2013.69. [DOI] [PubMed] [Google Scholar]
- 18.Narvencar KP, Rodrigues S, Nevrekar RP, Dias L, Dias A, Vaz M, et al. Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Stephen S, Kandhakumari G, Pradeep J, Vinithra SM, Siva PK, Hanifah M, et al. Scrub typhus in south India: a re-emerging infectious disease. Jpn J Infect Dis. 2013;66:552–4. doi: 10.7883/yoken.66.552. [DOI] [PubMed] [Google Scholar]
- 20.Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24–8. [PubMed] [Google Scholar]
- 21.Isaac R, Varghese GM, Mathai E, Manjula J, Joseph I. Scrub typhus: prevalence and diagnostic issues in rural southern India. Clin Infect Dis. 2004;39:1395–6. doi: 10.1086/424748. [DOI] [PubMed] [Google Scholar]


