Abstract
Foundation species provide critical resources to ecological community members and are key determinants of biodiversity. The barnacle Balanus glandula is one such species and dominates space among the higher reaches of wave-swept shores (Northeastern Pacific Ocean). This animal produces a cuticular glycoprotein (named ‘MULTIFUNCin’) of 199.6 kDa, and following secretion, a 390 kDa homodimer in native form. From field and lab experiments, we found that MULTIFUNCin significantly induces habitat selection by conspecific larvae, while simultaneously acting as a potent feeding stimulant to a major barnacle predator (whelk, Acanthinucella spirata). Promoting immigration via settlement on the one hand, and death via predation on the other, MULTIFUNCin drives opposing demographic processes towards structuring predator and prey populations. As shown here, a single compound is not restricted to a lone species interaction or sole ecological function. Complex biotic interactions therefore can be shaped by simple chemosensory systems and depend on the multifunctional properties of select bioactive proteins.
Keywords: predator, prey, habitat colonization, dispersal, settlement, foundation species, population dynamics, community ecology, sensory exploitation, behavior, chemoreception, chemical cue, chemical ecology, intertidal zone, marine, wave-swept shore, barnacle, whelk, larva, glycoprotein, α2-macroglobulin, thioester-containing protein, complement factor protein, barnacle settlement-inducing protein complex
Introduction
Wave-swept shores are invaluable for developing and testing key ecological principles (Connell 1961, 1970, Dayton 1971, Paine 1974, Menge 2000, Trussell et al. 2002, Nazareth and Berlow 2006). Over the last two decades, a profusion of new molecular, biochemical, and biophysical approaches have linked physiological performances to population- and community-wide consequences (Dalhoff et al. 2001, Somero 2002, Helmuth et al. 2005, Somero 2012). Emerging paradigms recognize the impacts of even seemingly subtle changes in environmental features (such as food, temperature, or oxygen) on species distributions and abundances, as well as on individual growth, reproduction, and fitness (Sanford 1999, Dalhoff et al. 2001, Helmuth et al. 2002, Gilman et al. 2006, Sokolova 2013, Kordas et al. 2014).
A synthesis of ecological and physiological research among organisms of wave-swept shores is nonetheless missing a critical component – the sensory basis for behavioral interactions that determine population- and community-wide attributes. Predator-prey relations and habitat colonization, in particular, can structure rocky intertidal communities. Theoretically, chemical cues in association with higher-order predators (e.g., crabs) cause hiding behavior in lower-order consumers (e.g., whelks), thus freeing basal resources (e.g., barnacles) from grazing impacts (Trussell et al. 2002, Trussell et al. 2004). In this way, chemical cues and non-consumptive effects would alter considerably rates of material and energy flow across entire food webs. Previous studies have described population- and community-wide impacts of chemically mediated trophic cascades (Schmitz et al. 1997, Turner et al. 2000, Trussell et al. 2003). Although meaningful, the implications of these findings may have been limited by methodological considerations. To our knowledge, chemical stimuli were neither identified nor scaled according to natural predator or prey cue release rates. Similarly, native properties of fluid dynamic environments and mass transport were not determined in the field, or simulated in the lab. Field experiments typically involved repeated or continuous chemical exposure over days to months (Trussell et al. 2003, 2004, Edgell 2010) and/or used cages and tethers (Trussell et al. 2004, 2006, Gosnell and Gaines 2012, Kimbro 2012). Moreover, lab and field investigations did not distinguish the effects of non-specific metabolites (such as ammonium) produced by all heterotrophic organisms with those compounds of unique, predator- or prey-specific origins (Leonard et al. 1999, Grason and Miner 2012, Greig et al. 2013). New, less invasive techniques, as well as cue identifications, would promote greater realism in lab and field experiments, and further advance understanding of natural chemical-ecological processes.
Studies on relationships between chemical cues and habitat selection by marine larvae, in contrast, have linked natural products chemistry to biotic effects. Full structural elucidations of settlement inducing compounds were reported for sea urchins (Swanson et al. 2003, 2004) and honeycomb worms (Pawlik and Faulkner 1986; but, see Jensen et al. 1990), with the most complete picture for barnacle larvae (Crisp and Meadows 1963, Larman et al. 1982, Matsumura et al. 1998a, Dreanno et al. 2006a, 2007, Thiyagarajan 2010). Sharing many structural similarities to the α2-macroglobulins, barnacle settlement-inducing protein complex (SIPC) is produced by juvenile and adult conspecifics (Matsumura et al. 1998b, c, Dreanno et al. 2006a, b), synthesized by epidermal cells that secrete the cuticle (Dreanno et al. 2006c), and consists of three subunits in the native form (Matsumura et al. 1998c, Dreanno et al. 2006a). Studies on SIPC, thus far, were conducted exclusively in the lab. Here, we provide field investigations to complement and extend previous laboratory findings.
The present investigation focused on the chemical identities and ecological consequences of bioactive compound(s) in barnacles, a common community inhabitant. These animals are foundation species, and whelk predation on barnacles impacts prey population stability, species coexistence, and species diversity in the higher reaches of rocky intertidal communities (Connell 1961, 1970, Murdoch 1969, Menge 1976, West 1986, Navarrete 1996, Barnes 1999). Through field and lab tests, we found a multifunctional protein cue that evokes whelk predation and barnacle larval settlement. Opposing demographic processes (immigration via habitat colonization, death via predation) are thus linked fundamentally by a single, bioactive compound.
Methods
Overview
This study addressed the chemosensory basis for behavioral interactions within the context of populations and communities. Initial results of bioassay-guided fractionations suggested strong similarity between the bioactive materials that caused whelks (Acanthinucella spirata) to feed and barnacle larvae (B. glandula) to settle (Ferrier 2010). Consequently, we based chemical fractionation and isolation procedures on prior studies with SIPC (with Amphibalanus amphitrite) (Matsumura et al. 1998a, Dreanno et al. 2006a, Ferrier 2010, Ferrier et al. in press). In succession, we employed ammonium sulfate precipitation, size exclusion chromatography, lentil-lectin (LCA) affinity chromatography, and finally, SDS-PAGE protein separation with electroelution (Ferrier 2010). Each preparation rendered a single active fraction that was then employed in the subsequent step. Ultimately, this approach yielded a lone glycoprotein candidate (199.6 kDa, Fig. 1) for bioassay in the current study. This compound exhibits low solubility in water and is expressed in particulate form within the epidermis, cuticle, and new shell material (Ferrier et al. in press). Field assays of barnacle (B. glandula) larval settlement were run in combination with trials on faux prey selection by whelks.
Fig. 1.
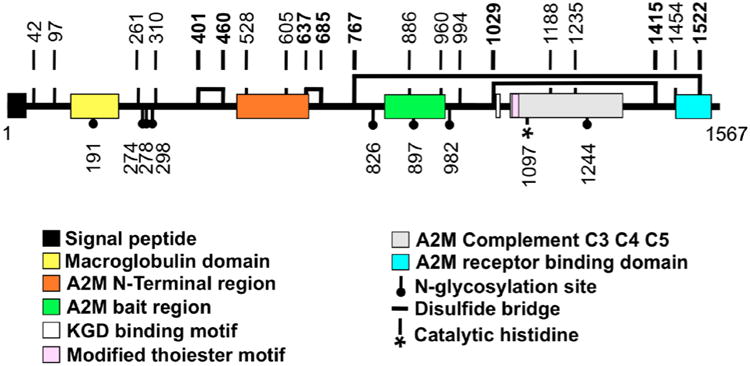
Structural architecture and domain organization of barnacle (Balanus glandula) MULTIFUNCin (from Ferrier 2010, Ferrier et al. submitted), a 199.6 kDa glycoprotein cue with 1567 amino acids (1 = amino terminus; 1567 = carboxy terminus). A signal peptide, modified thioester motif, and conserved domains and regions are denoted by color-specific rectangles. Amino acid positions of N-glycosylation sites are provided (closed circles), as well as the site of a putative catalytic histidine (*). Vertical lines with bold numbers denote cysteine positions. The estimated location of disulfide bridges are marked with horizontal lines connecting bold cysteine residues. Abbreviations: A2M, α2-macroglobulin; KGD, lysine-glycine-aspartic acid; C3, C4, and C5, complement factor proteins 3, 4 and 5.
Laboratory experiments testing a potential predatory cue
Animal maintenance and holding
Laboratory and field tests were performed during May – October (2011 and 2012) when a majority (≥ 60 %) of whelks were feeding along rocky, wave-swept shores. Whelks (Acanthinucella spirata) were collected at field sites (Broad Beach, Malibu, California; 34° 02′ 04.37″ N, 118° 51′ 42.74″ W), and held in the lab, where they fasted for 3-5 days before testing. Individuals were transported from the field in aerated containers with fresh seawater and maintained at ambient ocean temperature. The lab set-up consisted of a 28,000 L seawater reservoir (oceanic quality, 33 psu salinity) with particle filtration (5 μm cut-off), UV sterilization (as needed), and computer-controlled water/air temperature (± 1 °C off set-points) and light cycle (L:D 12:12, light on at 0630 h). The animals were held at densities of 10-15 m-2 in 280 L tanks (240 × 60 × 20 cm). Seawater in each holding tank was continuously re-circulated at 45 L min-1, sand-filtered, aerated, and cooled to site temperatures. All tanks were cleaned thoroughly and the seawater replenished once or twice per week. After being tested in a single trial, each whelk was permanently (and unobtrusively) marked and returned to the field.
Faux prey
Experiments established the chemosensory basis for prey recognition and selection by whelks. As a foraging generalist, Acanthinucella spirata feeds naturally on two foundation species (barnacle: Balanus glandula; mussel: Mytilus californianus), on a common herbivore (black turban snail: Chlorostoma funebralis), and, occasionally, on other taxa (Murdoch 1969, Menge 1974, G.A. Ferrier and R.K. Zimmer unpublished data). Whelks prefer barnacles over all other tested prey species (Ferrier 2010).
We constructed faux prey to mimic the physical and chemical properties of barnacles. Live barnacles (0.4 – 0.6 cm shell height) were collected at field sites, flash frozen in liquid nitrogen, and then transported to the lab for immediate processing. In the lab, the inner soft body parts and cuticle were removed with a sterile scalpel, spatula, and bristle/wire brush. The empty barnacle shells were combusted for 12 h at 130 °C, bathed for 15 min in a stirred, 5 % (v/v) HCl solution, and finally rinsed 3-4 times with 100 ml aliquots of Nanopure-grade deionized water to eliminate any residual organics. Cleaned, empty shells served as physical replicas of live animals.
Prey chemistry was simulated in flavored gels. Sodium carboxymethylcellulose (11.7 % w/v) was mixed with a 0.45-μm seawater extract of barnacle, mussel, or black turban snail flesh and allowed to cure for 10 min at ≤ 4 °C. Gels also were made using purified MULTIFUNCin from barnacle, or using seawater alone. The low temperature protected proteins and minimized loss of volatile organics from extracts. Protein levels were established using the Bradford method with bovine serum albumin (BSA) as a standard. Concentrations of proteins (w/w) were carefully matched between live prey tissues and gels containing extracts of prey flesh. MULTIFUNCin purity was confirmed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-ToF) (Voyager DE-STR, Applied Biosystems, Foster City, California), and its concentration matched to the level in barnacle cuticle. Once prepared, each gel was injected into a cleaned, empty barnacle shell for immediate use in an experiment.
Bioassays
Two experiments were conducted in succession. Experiment 1 determined the chemical basis for prey preference in a total of 22 replicate trials. Each trial ran for 1 h, or until an initial faux prey was chosen and eaten. A trial began when a single whelk (1.5 – 2.2 cm shell length) was placed at the center of a circular arena (10 cm diam) and presented with 12 faux prey. The twelve were divided into three sets of four that encircled the whelk. Each set contained one barnacle (purified MULTIFUNCin), one mussel, one black turban snail, and one seawater treatment. The exact position of each prey type within a given set was determined at random. Mussel and black turban snail treatments served as organic enrichment controls; they were consumed voraciously in the absence of a MULTIFUNCin-laced prey. Every arena received a constant supply of single-pass, 5 μm-filtered seawater (1 L min-1), held at ambient ocean temperature, salinity, pH, and dissolved oxygen.
Experiment 2 established the bioactivity of MULTIFUNCin as compared to a live barnacle. Here, in each trial, prey were grouped into three sets of four. Each set contained one live and three faux prey. The three were fabricated from crude barnacle extract (organic enrichment control), MULTIFUNCin, or seawater. All other bioassay procedures were identical to those described in the preceding paragraph. Equivalent bioactivity was indicated by whelks feeding equally as often on all prey types, but rejecting seawater. There were 36 replicate trials performed for Experiment 2.
Field experiments testing a potential predatory cue
Field plots
Experimental plots (10 cm long × 10 cm wide) were prepared in the high intertidal at Broad Beach. In each plot, all but 12 live barnacles (0.4 – 0.6 cm shell height) were scraped clean to expose the bare rock. Soft tissues, and cuticle, from either nine or all 12 of these barnacles, were removed with a sterile scalpel, spatula, and bristle/wire brush. The empty shells, along with the exposed rock surfaces, were then scrubbed and cleaned by applying 5 % HCl, and rinsed with Nanopure water. Each prepared plot was rinsed further by ocean water during successive flood tides over 24 h. New animal/plant recruits were scraped and removed every other day.
Faux prey
Employing methods similar to those in ‘Lab experiments testing a potential predatory cue’, faux prey were prepared in the field. At Broad Beach, extracts of barnacles, mussels, and black turban snails, purified MULTIFUNCin, and filtered (0.45 μm) seawater were held on dry ice until use. After thawing, each solution was combined, on ice, with sodium carboxymethylcellulose to produce an 11.7 % (w/v) mixture. The empty barnacle shells were cleaned once more with a wire brush, scrubbed with a 1 % (v/v) HCl solution, and rinsed with Nanopure water and then with ocean water. After curing for 10 min, each gel was injected into a cleaned, barnacle shell and used immediately in an experiment.
Bioassays
Two field experiments were performed as in the lab. Whereas Experiment 1 determined the chemical basis for prey preference, Experiment 2 established the bioactivity of MULTIFUNCin relative to a live barnacle. In each Experiment 1 trial, 12 faux prey were divided into three sets of four. They consisted of one barnacle (MULTIFUNCin), one mussel (crude extract), one black turban snail (crude extract), and one seawater (control) treatment. In Experiment 2, each set was composed of one live barnacle and three faux prey. Here, the three consisted of crude barnacle extract, MULTIFUNCin, and seawater. The exact position of each prey type within a set was determined at random. Whelks (1.5 – 2.2 cm shell length) were captured by hand after they emerged from refuges on a flooding tide. A single individual was placed at the center of each experimental plot. Once positioned, snails were free to move in any direction. Over 1 h, feeding responses were monitored visually, and undisturbed, by a snorkeler on the water surface. There were 16 and 36 replicate trials run for Experiments 1 and 2, respectively.
Field experiments testing a potential settlement cue
Field plots
Settlement preferences of barnacle (Balanus glandula) larvae were determined at Broad Beach. Each experiment was run during May – June (2011), when settlement occurred naturally at the study site. Individual plots (75 cm long × 20 cm wide) of rocky substrates were scraped clean, scrubbed with 5 % HCl, and then rinsed with Nanopure water. They were rinsed further by ocean water during subsequent flood tides over 24 h, and cleaned of new animal/plant recruits on a weekly basis. In each plot, we drilled four, 0.5 cm diam holes positioned parallel to shore at a distance of 15.0, 30.0, 45.0, and 60.0 cm from the leftmost edge. A single, stainless steel bolt (4 cm long × 0.5 cm diam) was secured in each hole, with threads extending slightly above the rock face.
Settlement substrates
Slices of barren rock (hereafter called ‘rocks’) were prepared as experimental and control substrates for presentation to potential larval colonizers. Each rock was carved from the native habitat using a hammer and chisel, and cut into a flat, 5 cm (long) × 5 cm (wide) × 0.75 cm (thick) square, with a 0.5-cm-diam hole drilled through the center. All surfaces were scrubbed and cleaned using procedures described in the preceding paragraph. The rocks then were transported to the lab, autoclaved (15 min at 150 °C and 101.3 kPa), and combusted (12 h at 130 °C) to sterilize and remove any residual organics. After cooling to room temperature, 1 ml of barnacle, mussel, or black turban snail extract, purified MULTIFUNCin, or seawater was applied by capillary action over one entire face of a given rock for 12 h at 4 °C. The water either evaporated or was absorbed, thus leaving a thin film of applied material on the rock face. Each treated rock was wrapped in sterile plastic, placed on ice, and taken to the field. The final protein concentration on a rock surface was scaled (± 25 %) to match a density of 32 adult barnacles per 25 cm2. This value was the mean density for the higher reaches of rocky intertidal habitat at Broad Beach (data not shown). Total protein levels were equivalent between coatings of barnacle, mussel, and black turban snail extracts (One-way ANOVA, df = 2/21, F = 0.21, P = 0.893).
Bioassays
As in predation assays, two experiments were performed. In Experiment 1, we determined the chemical basis for settlement preference. MULTIFUNCin was tested against seawater and against mussel and black turban snail extracts. One coated rock of each treatment was attached per plot via a stainless steel bolt. The exact position of any particular rock was selected at random. In Experiment 2, three rocks were attached per plot – one each coated with barnacle extract, MULTIFUNCin, or seawater. All rocks were placed before, and retrieved after, inundation by a single flood tide (∼ 4 h ocean submergence). Eight replicate trials were performed in each experiment.
Following collection, each rock was brought to the laboratory. Barnacle settlers (attached larvae + newly metamorphosed juveniles) were counted using a dissecting microscope (Leica S6E at 10× magnification). Select rocks were placed in aquaria with re-circulating seawater (see previous section on Animal maintenance and holding). Settlers were raised on a steady diet of brine shrimp (Artemia salina) eggs and nauplii larvae and grown for 5-6 weeks, enabling taxonomic identification by morphology. A total of 53 settlers were raised, enumerated, and classified – 90.6 % as Balanus glandula. Field experiments thus effectively targeted the species of immediate interest to this study.
Results and Discussion
Sensory exploitation, predator-prey interactions, and habitat colonization
This investigation established the behavioral and ecological effects of a multifunctional protein cue. Whelks (Acanthinucella spirata) strongly preferred faux barnacles over all other tested faux prey (Fig. 2). Shell morphology of prey contributed little or nothing to the observed predatory response. In fact, whelks chose barnacle-flavored gel over mussel- and turban snail-flavored gels whether, or not, preparations were set in barnacle, mussel, or snail shells (see Appendix S1). Laboratory and field results concurred.
Fig. 2.
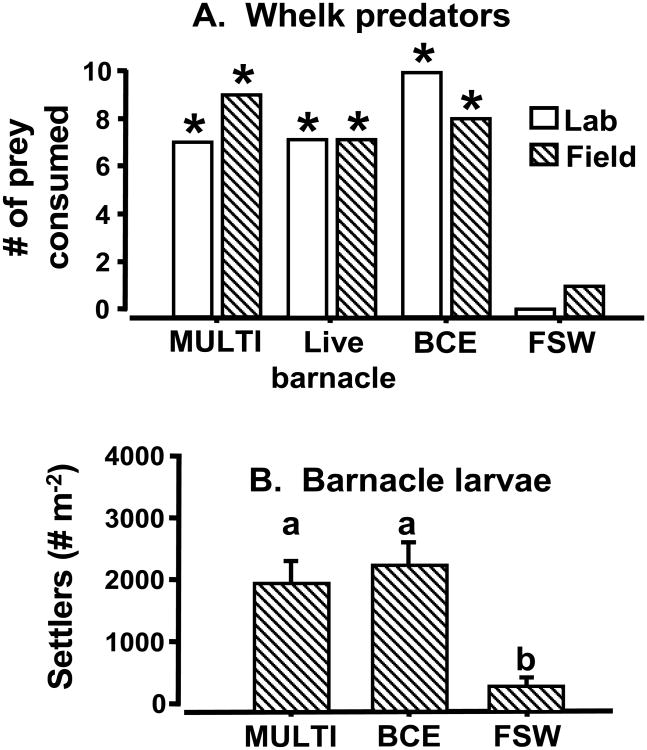
Experiment 1 results showing chemical preferences by predatory whelks (Acanthinucella spirata) and settling barnacle (Balanus glandula) larvae, respectively. Along the x-axis, ‘MULTI’ refers to a final natural product from live, adult barnacles; ‘MCE’ and ‘TCE’ denote mussel (Mytilus californianus) and black turban snail (Chlorostoma funebralis) crude tissue extracts as organic-enrichment (positive) controls; and, ‘FSW’ is a 0.45-μm filtered seawater (negative) control. (A) The y-axis shows the number of trials in which a whelk fed on a given treatment or control faux prey. Each whelk was tested only once. An asterisk (*) indicates a significant feeding response relative to filtered seawater (G-test for homogeneity with Williams' and Bonferoni's corrections: G2 ≥ 10.81, df = 1, P ≤ 0.002). Laboratory and field results across all treatments were not significantly different (G2 = 4.13, df = 3, P = 0.25). (B) Different letters indicate significant differences in settlement among treatments (two-way nested ANOVA, followed by Tukey tests with Bonferroni's correction: qs ≥ 5.063, df = 14, P ≤ 0.001). For each ANOVA, chemical treatment and Julian date were main effects; habitat position, or plot, was nested within each date.
The feeding response of whelks for barnacles is explained by the presence of a single particulate compound. When presented at a concentration typical of a barnacle (= 24 μg per animal), whelks did not distinguish between MULTIFUNCin-impregnated faux prey and their live, intact counterparts (Fig. 3). MULTIFUNCin, therefore, is necessary and sufficient as a contact cue of considerable ecological significance. An extreme environment demands extreme physiological and behavioral innovations, especially on wave-swept shores. When severe fluid-dynamic constraints dominate habitats and impede the transmission of most (e.g., waterborne), alternative sensory information, contact chemical cues become important for resource recognition, selection, and acquisition. Field experiments showed no evidence for waterborne cues eliciting a whelk predatory response even from dense populations of live, intact, barnacle prey (10,240 – 12,180 individuals m-2) (Ferrier 2010).
Fig. 3.
Experiment 2 results showing bioactivity of MULTIFUNCin (‘MULTI’) relative to a live barnacle and an extract of barnacle tissue (BCE). (A) Predation by whelks (Acanthinucella spirata) on live and faux prey. The y-axis is the number of trials in which a whelk fed on a given prey type. Each whelk was tested only once. An asterisk (*) indicates a significant response relative to filtered seawater (FSW) (G-test for homogeneity with Williams' and Bonferoni's corrections: G2 ≥ 5.49, df = 1, P ≤ 0.017). Activity evoked by a live barnacle, barnacle crude extract (BCE), and purified MULTIFUNCin (MULTI) were non-significantly different (G2 ≤ 0.93, df = 2, P ≥ 0.63). Lab and field results across all treatments did not differ significantly (G2 = 0.77, df = 3, P = 0.85). (B) Different letters indicate significant differences in settlement by barnacle larvae (Balanus glandula) between treatments (two-way nested ANOVA, followed by Tukey tests with Bonferroni's correction: qs ≥ 6.82, df = 14, P ≤ 0.0001).
Chemical cues induce larval settlement by organisms that live in coral reefs, giant kelp forests, and muddy embayments, as well as on wave-swept shores (Pawlik 1992, Steinberg et al. 2002, Dreanno et al. 2006a, Hay 2009). The current study demonstrates a significant effect of a fully purified natural product on habitat selection by larvae in the field. Here, we scaled the concentration of MULTIFUNCin (65 μg per 25 cm2 rock surface) in each trial to simulate the actual amount on exposed, adult barnacle surfaces. Wild-type barnacle larvae dispersed naturally from adult parental locations to test sites via ocean currents. Even under such challenging conditions, larvae exhibited a significant preference for the single, fully-purified, natural product as compared to bare rock or other organic coatings (Figs. 2 and 3). MULTIFUNCin thus facilitates the settlement of B. glandula larval dispersers, and promotes connectivity between demes within a metapopulation network. These field results underscore the importance of surface chemistry as a determinant of demographic processes.
MULTIFUNCin drives opposing demographic processes and structures rocky, intertidal communities
Requisite for internal (cell level) and external (organism level) communication, glycoproteins are well suited for sensory exploitation. A prime example is the targeting of host-expressed mucin and collagen by pathogenic bacteria for attachment and colony establishment (Roos and Jonsson 2002). Moreover, a single glycoprotein – e.g., MULTIFUNCin – also can convey different information to different species. Promoting habitat colonization (immigration) on the one hand, and death via predation on the other, MULTIFUNCin simultaneously mediates opposing demographic effects towards structuring both predator and prey populations (Fig. 4). These antagonistic demographic processes likely are not limited to barnacles and whelks. Surface glycoproteins are distributed widely, among diverse species, in every major biome on earth (Gagneux and Varki 1999, Mengerink and Vacquier 2001, Snell 2011).
Fig. 4.
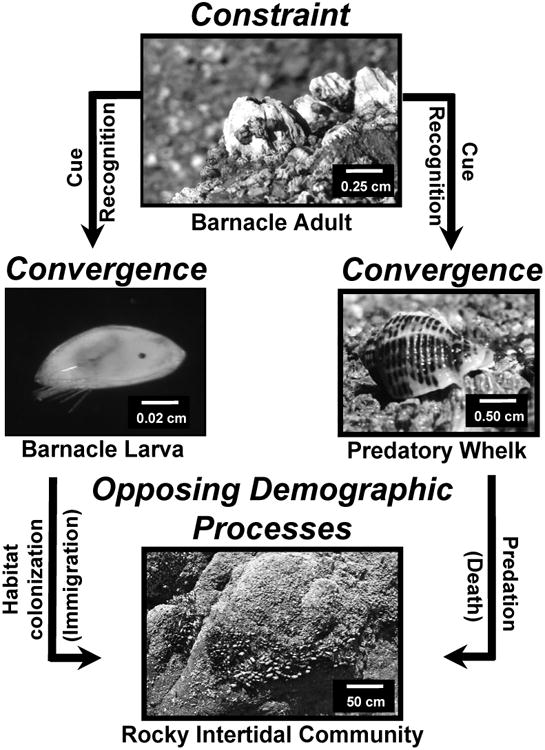
A conceptual model highlighting results from the current study. Adult barnacles (Balanus glandula) are constrained to synthesize MULTIFUNCin as a component of structural (cuticle) and/or immunological defense. Presumably, the molecule is subjected to purifying selection so as to maintain its original function and binding specificity. Over evolutionary time, the compound has become exploited as an honest and predictable chemical cue of hospitable habitat to conspecific larvae in settlement, and to a common predator (whelk, Acanthinucella spirata) having specialized weaponry for penetrating armored barnacle prey. The sensory systems of whelks and barnacle larvae thus, for different reasons, are converged to detect MULTIFUNCin as a chemosensory stimulus. Promoting habitat selection (immigration) on the one hand, and death via predation on the other, MULTIFUNCin simultaneously mediates opposing demographic processes towards structuring both prey and predator populations. Combined results establish MULTIFUNCin as a key determinant of species interactions that create patterns of biodiversity in rocky intertidal habitats.
Predation and habitat colonization are factors that limit organism abundances and distributions in marine communities. The relative magnitude of larval settlement, for example, can determine which species are common and which are rare at a particular locale (Cowan and Sponaugle 2009). Patterns of species-specific habitat selection establish population templates and strongly influence community dynamics (Gaines and Roughgarden 1987, Roughgarden et al. 1988, Morris 2003, Abrams 2007, Angelini et al. 2011). Likewise, species distributions and abundances at the metapopulation scale are a function of colonization and extinction of individual populations or patches. Through a combination of direct and indirect effects, predation also imposes community-wide impacts at multiple trophic levels (Schmitz et al. 2000, Trussell et al. 2003). Consequences manifest when either a numerically abundant, or a keystone, predator consumes preferentially a foundation species prey (Paine 1966, 1974, Navarrete and Manzur 2008). Local extinction of, or a significant decline in, a foundation species changes the availability of critical resources in a community, including food and space. This change can lead to new ‘winners’ and ‘losers’ in a sweepstakes among subordinate, or dependent, species, thus initiating a cascade of community-wide impacts (Petraitis and Dudgeon 1999).
The barnacle Balanus glandula is one such foundation species. Over 50 years ago, it was demonstrated that barnacles are superior competitors for space at higher reaches of rocky intertidal habitats (Connell 1961, 1970). Whelk predation creates gaps and enables colonization by sub-dominant competitors (Murdoch 1969, Connell 1970, Navarrete 1996). Until now, the chemosensory basis for such species interactions and community-wide impacts was unknown. Chemical stimuli evoke behavioral responses and determine species interactions that structure populations and communities (Zimmer and Ferrer 2007, Hay 2009, Raguso et al. 2015). Bioactive molecules, however, need not be restricted to a single function or pairwise species association. As shown here, single compounds link opposing demographic processes over time and in space. Complex biotic relationships thus can be shaped by simple sensory systems and depend on the multifunctional properties of select bioactive compounds.
Supplementary Material
Acknowledgments
We thank many UCLA undergraduates (Nancy Tu, Hao Dieu, Tinh Ton, Michal Ross, Margaret McGonigle, Erin Eastwood, Aleksey Kurylov, Michael Awshee, Patrick Green, Ian Jackson, Edward Lopez, Jennifer Wan, Noelle Bidegainberry, Emily Adamson, Christopher Leber and Veganeh Parhizhar) for their valuable contributions to the project. Dr. Rachel Ogorzalek Loo (UCLA) graciously assisted with the de novo peptide sequencing; Dr. Deborah Leon (UCLA) ably contributed to protein mass spectrometry analysis; and, Dr. Seth Miller (UC Davis) kindly supplied the image of a barnacle (Balanus glandula cypris stage) larva for Fig. 4. Dr. Robert Raguso (Cornell University) and Dr. Gabrielle Nevitt (UC Davis) provided many thoughtful comments that greatly improved earlier drafts of the manuscript. This research was supported by awards from the National Science Foundation (OCE 08-52361 and IOS 11-21692 to RKZ), the National Institutes of Health (R01GM104610 and R01GM103479 to JAL) and the UCLA Council on Research.
Literature Cited
- Abrams PA. Habitat choice in predator-prey systems: spatial instability due to interacting adaptive movements. American Naturalist. 2007;169:581–594. doi: 10.1086/512688. [DOI] [PubMed] [Google Scholar]
- Angelini C, Altieri AH, Silliman BR, Bertness MD. Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. BioScience. 2011;61:782–789. [Google Scholar]
- Barnes M. The mortality of intertidal cirripedes. Oceanography and Marine Biology Annual Review. 1999;37:153–244. [Google Scholar]
- Connell JH. Effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecological Monographs. 1961;31:61–104. [Google Scholar]
- Connell JH. A predator-prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecological Monographs. 1970;40:49–78. [Google Scholar]
- Cowan RK, Sponaugle S. Larval dispersal and marine population connectivity. Annual Review of Marine Science. 2009;1:443–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- Crisp DJ, Meadows PS. Adsorbed layers: the stimulus to settlement in barnacles. Proceedings of the Royal Society, London: Part B. 1963;158:364–387. [Google Scholar]
- Dahlhoff EP, Buckley BA, Menge BA. Physiology of the rocky intertidal predator Nucella ostrina along an environmental stress gradient. Ecology. 2001;82:2816–2829. [Google Scholar]
- Dayton PK. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecological Monographs. 1971;41:351–389. [Google Scholar]
- Dreanno C, Matsumura K, Dohmae N, Takio K, Hirota H, Kirby RR, Clare AS. An α2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite. Proceedings of the National Academy of Sciences, USA. 2006a;103:14396–14401. doi: 10.1073/pnas.0602763103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreanno C, Kirby RR, Clare AS. Smelly feet are not always a bad thing: the relationship between cyprid footprint protein and the barnacle settlement pheromone. Biology Letters. 2006b;2:423–425. doi: 10.1098/rsbl.2006.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreanno C, Kirby RR, Clare AS. Locating the barnacle settlement pheromone: spatial and ontogenetic expression of the settlement inducing protein complex of Balanus amphitrite. Proceedings of the Royal Society, London: Part B. 2006c;273:2721–2728. doi: 10.1098/rspb.2006.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreanno C, Kirby RR, Clare AS. Involvement of the barnacle settlement inducing protein complex (SIPC) in species recognition at settlement. Journal of Experimental Marine Biology and Ecology. 2007;351:276–282. [Google Scholar]
- Edgell TC. Past predation risk induces an intertidal whelk (Nucella lamellosa) to respond to more dilute concentrations of its predator's scent. Marine Biology. 2010;157:215–219. [Google Scholar]
- Ferrier GA. Dissertation. Los Angeles: University of California Los Angeles; 2010. The sensory basis for ecological paradigms on wave-swept shores; p. 173. [Google Scholar]
- Ferrier GA, Kim SJ, Kaddis CS, Loo JA, Zimmer CA, Zimmer RK. Identification of a multifunctional protein cue that induces habitat selection by, and predation on, barnacles. Integrative and Comparative Biology. 56 doi: 10.1093/icb/icw076. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;7:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- Gaines SD, Roughgarden J. Fish in offshore kelp forests affect recruitment to intertidal barnacle populations. Science. 1987;235:479–481. doi: 10.1126/science.235.4787.479. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Wethey DS, Helmuth B. Variation in the sensitivity of organismal body temperature to climate change over local and geographic scales. Proceedings of the National Academy of Sciences, USA. 2006;103:9560–9565. doi: 10.1073/pnas.0510992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell SJ, Gaines SD. Keystone intimidators in the intertidal: non-consumptive effects of a keystone sea star regulates feeding and growth in whelks. Marine Ecology Progress Series. 2012;450:107–114. [Google Scholar]
- Grason EW, Miner BG. Behavioral plasticity in an invaded system: non-native whelks recognize risk from native crabs. Oecologia. 2012;169:105–115. doi: 10.1007/s00442-011-2188-5. [DOI] [PubMed] [Google Scholar]
- Greig HS, Wissinger SA, McIntosh AR. Top-down control of prey increases with drying disturbance in ponds: a consequence of non-consumptive effects? Journal of Animal Ecology. 2013;82:598–607. doi: 10.1111/1365-2656.12042. [DOI] [PubMed] [Google Scholar]
- Hay ME. Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Annual Review of Marine Science. 2009;1:193–212. doi: 10.1146/annurev.marine.010908.163708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth B, Harley CDG, Halpin PM, O'Donnell M, Hofmann GE, Blanchette CA. Climate change and latitudinal patterns of intertidal thermal stress. Science. 2002;298:1015–1017. doi: 10.1126/science.1076814. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Kingsolver JG, Carrington E. Biophysics, physiological ecology, and climate change: does mechanism matter? Annual Review of Physiology. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. [DOI] [PubMed] [Google Scholar]
- Jensen RA, Morse DE, Petty RL, Hooker N. Artificial induction of larval metamorphosis by free fatty acids. Marine Ecology Progress Series. 1990;67:55–71. [Google Scholar]
- Kimbro DL. Tidal regime dictates the cascading consumptive and non-consumptive effects of multiple predators on a marsh plant. Ecology. 2012;93:334–344. doi: 10.1890/11-0596.1. [DOI] [PubMed] [Google Scholar]
- Kordas RL, Dudgeon S, Storey S, Harley CDG. Intertidal community response to field based experimental warming. Oikos. 2014 doi: 10.1111/oik.00806. [DOI] [Google Scholar]
- Larman VN, Gabbott PA, East J. Physico-chemical properties of the settlement factor proteins from the barnacle Balanus balanoides. Comparative Biochemistry and Physiology, Part A. 1982;72:329–338. [Google Scholar]
- Leonard GH, Bertness MD, Yund PO. Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology. 1999;80:1–14. [Google Scholar]
- Matsumura K, Mori S, Nagano M, Fusetani N. Lentil lectin inhibits adult extract-induced settlement of the barnacle, Balanus amphitrite. Journal of Experimental Zoology. 1998a;280:213–219. [PubMed] [Google Scholar]
- Matsumura K, Nagano M, Fusetani N. Purification of a larval settlement-inducing protein complex (SIPC) of the barnacle, Balanus amphitrite. Journal of Experimental Zoology. 1998b;281:12–20. [Google Scholar]
- Matsumura K, Nagano M, Kato-Yoshinaga Y, Yamazaki M, Clare AS, Fusetani N. Immunological studies on the settlement-inducing protein complex (SIPC) of the barnacle Balanus amphitrite and its possible involvement in larva-larva interactions. Proceedings of the Royal Society, London: Part B. 1998c;265:1825–1830. doi: 10.1098/rspb.1998.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge BA. Organization of the New England rocky intertidal community: role of predation, competition and environmental heterogeneity. Ecological Monographs. 1976;46:355–393. [Google Scholar]
- Menge BA. Top-down and bottom-up community regulation in marine rocky intertidal habitats. Journal of Experimental Marine Biology and Ecology. 2000;250:257–289. doi: 10.1016/s0022-0981(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Menge JL. Prey selection and foraging period of the predaceous rocky intertidal snail, Acanthina punctulata. Oecologia. 1974;17:293–316. doi: 10.1007/BF00345748. [DOI] [PubMed] [Google Scholar]
- Mengerink KJ, Vacquier VD. Glycobiology of sperm-egg interactions in deuterostomes. Glycobiology. 2001;11:37–43. doi: 10.1093/glycob/11.4.37r. [DOI] [PubMed] [Google Scholar]
- Morris DW. Towards an ecological synthesis: a case study for habitat selection. Oecologia. 2003;136:1–13. doi: 10.1007/s00442-003-1241-4. [DOI] [PubMed] [Google Scholar]
- Murdoch WM. Switching in general predators: experiments on predator specificity and stability of prey populations. Ecological Monographs. 1969;39:335–354. [Google Scholar]
- Navarrete SA. Variable predation: effects of whelks on a mid-intertidal successional community. Ecological Monographs. 1996;66:301–321. [Google Scholar]
- Navarrete SA, Manzur T. Individual-and population-level responses of a keystone predator to geographic variation in prey. Ecology. 2008;89:2005–2018. doi: 10.1890/07-1231.1. [DOI] [PubMed] [Google Scholar]
- Paine RT. Food web complexity and species diversity. American Naturalist. 1966;100:65–75. [Google Scholar]
- Paine RT. Intertidal community structure: Experimental studies on the relationship between a dominant competitor and its principal prey. Oecologia. 1974;15:93–120. doi: 10.1007/BF00345739. [DOI] [PubMed] [Google Scholar]
- Pawlik JR. Chemical ecology of the settlement of benthic marine invertebrates. Oceanography and Marine Biology Annual Review. 1992;30:273–335. [Google Scholar]
- Pawlik JR, Faulkner DJ. Specific free fatty acids induce larval settlement and metamorphosis of the reef building tube worm Phragmatopoma californica. Journal of Experimental Marine Biology and Ecology. 1986;102:301–310. [Google Scholar]
- Petraitis PS, Dudgeon SD. Experimental evidence for the origin of alternative communities on rocky intertidal shores. Oikos. 1999;84:239–245. [Google Scholar]
- Raguso RA, Agrawal AA, Douglas AE, Jander G, Kessler A, Poveda K, Thaler JS. The raison d'être of chemical ecology. Ecology. 2015;96:617–630. doi: 10.1890/14-1474.1. [DOI] [PubMed] [Google Scholar]
- Roos S, Jonsson H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology. 2002;148:433–442. doi: 10.1099/00221287-148-2-433. [DOI] [PubMed] [Google Scholar]
- Roughgarden J, Gaines S, Possingham H. Recruitment dynamics in complex life cycles. Science. 1988;241:1460–1466. doi: 10.1126/science.11538249. [DOI] [PubMed] [Google Scholar]
- Sanford E. Regulation of keystone predation by small changes in ocean temperature. Science. 1999;228:2095–2097. doi: 10.1126/science.283.5410.2095. [DOI] [PubMed] [Google Scholar]
- Schmitz OJ, Beckerman AP, O'Brian KM. Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology. 1997;78:1388–1399. [Google Scholar]
- Schmitz OJ, Hamäck PA, Beckerman AP. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. American Naturalist. 2000;55:141–153. doi: 10.1086/303311. [DOI] [PubMed] [Google Scholar]
- Snell TW. Contact chemoreception and its role in zooplankton mate recognition. In: Breithaupt T, Thiel M, editors. Chemical Communication in Crustaceans. Springer Science+Business Media LLC; 2011. pp. 451–466. [Google Scholar]
- Sokolova IM. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integrative and Comparative Biology. 2013;53:597–608. doi: 10.1093/icb/ict028. [DOI] [PubMed] [Google Scholar]
- Somero GN. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integrative and Comparative Biology. 2002;42:780–789. doi: 10.1093/icb/42.4.780. [DOI] [PubMed] [Google Scholar]
- Somero GN. The physiology of global climate change: linking patterns to mechanisms. Annual Review of Marine Science. 2012;4:39–61. doi: 10.1146/annurev-marine-120710-100935. [DOI] [PubMed] [Google Scholar]
- Steinberg PD, De Nys R, Kjelleberg S. Chemical cues for surface colonization. Journal of Chemical Ecology. 2002;28:1935–1951. doi: 10.1023/a:1020789625989. [DOI] [PubMed] [Google Scholar]
- Swanson RL, De Nys R, Huggett MJ, Green JK, Steinberg PD. In situ quantification of a natural larval settlement cue and recruitment of the Australian sea urchin Holopneustes purpurascens. Marine Ecology Progress Series. 2006;314:1–14. [Google Scholar]
- Swanson RL, Williamson JE, De Nys R, Kumar N, Bucknall MP, Steinberg PD. Induction of settlement of larvae of the sea urchin Holopneustes purpurascens by histamine from a host alga. Biological Bulletin. 2004;206:161–172. doi: 10.2307/1543640. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan V. A review of the role of chemical cues in habitat selection by barnacles: New insights from larval proteomics. Journal of Experimental Marine Biology and Ecology. 2010;392:22–36. [Google Scholar]
- Trussell GC, Ewanchuk PJ, Bertness MD. Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecology Letters. 2002;5:241–245. [Google Scholar]
- Trussell GC, Ewanchuk PJ, Bertness MD. Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology. 2003;84:629–640. [Google Scholar]
- Trussell GC, Ewanchuk PJ, Bertness MD, Silliman BR. Trophic cascades in rocky shore tide pools: distinguishing lethal from nonlethal effects. Oecologia. 2004;139:427–432. doi: 10.1007/s00442-004-1512-8. [DOI] [PubMed] [Google Scholar]
- Trussell GC, Ewanchuk PJ, Matassa CM. The fear of being eaten reduces energy transfer in a simple food chain. Ecology. 2006;87:2979–2984. doi: 10.1890/0012-9658(2006)87[2979:tfober]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Turner AM, Bernot RJ, Boes CM. Chemical cues modify species interactions: the ecological consequences of predator avoidance behavior by fresh water snails. Oikos. 2000;88:148–158. [Google Scholar]
- West L. Interindividual variation in prey selection by the snail Nucella (= Thais) emarginata. Ecology. 1986;67:798–809. [Google Scholar]
- Zimmer RK, Ferrer RP. Neuroecology, chemical defense, and the keystone species concept. Biological Bulletin. 2007;213:208–225. doi: 10.2307/25066641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.